Abstract
This study investigated the adsorption of Oxytetracycline (OTC) from pharmaceutical wastewater using a kappa carrageenan based hydrogel (KPB). The aim of the present study was to explore the potential of KPB for long-term pharmaceutical wastewater treatment. A sustainable adsorbent was developed to address oxytetracycline (OTC) contamination. The hydrogel’s structural and adsorption characteristics were examined using various techniques like Scanning Electron Microscope (SEM), Fourier Transform Infrared (FTIR), X-ray powder diffraction (XRD), and kinetic models. The results revealed considerable changes in the vibrational modes and adsorption bands of the hydrogel, suggesting the effective functionalization of Bentonite nano-clay. Kappa carrageenan based hydrogel achieved the maximum removal (98.5%) of OTC at concerntration of 40 mg/L, pH 8, cotact time of 140 min and adsorbent dose of 0.1 g (KPB-3). Adsorption of OTC increased up to 99% with increasing initial concentrations. The study achieved 95% adsorption capacity for OTC using a KPB film at a concentration of 20 mg/L and a 0.1 g adsorbent dose within 60 min. It also revealed that chemisorptions processes outperform physical adsorption. The Pseudo-Second-Order model, which emphasized the importance of chemical adsorption in the removal process, is better suited to represent the adsorption behavior. Excellent matches were found that R2 = 0.99 for KPB-3, R2 = 0.984 for KPB-2 and R2 = 0.989 for KPB-1 indicated strong chemical bonding interactions. Statisctical analysis (ANOVA) was performed using SPSS (version 25) and it was found that pH and concentration had significant influence on OTC adsorption by the hydrogel, with p-values less than 0.05. The study identified that a Kappa carrageenan-based hydrogel with bentonite nano-clay and polyvinyl alcohol (PVA) can efficiently remove OTC from pharmaceutical effluent, with a p-value of 0.054, but weak positive linear associations with pH, temperature, and contact time. This research contributed to sustainable wastewater treatment and environmental engineering.
Similar content being viewed by others
Introduction
Water is a vital resource that is necessary for maintaining ecosystems, regular human activity, and survival of living organisms. Unfortunately, a wide range of pollutants have been released into the environment which pollute the water and endangered both human health and aquatic life1. Concern over water pollution has grown across the globe, and further materials or technologies are required to properly remove contaminants from water and associated hazards. Water contaminants including pesticides, heavy metals, phenols, dyes and antibiotics are removed from wastewater by a variety of technologies including adsorption, catalytic degradation, biological methods, flocculation, demulsification and filtration2,3,4,5.
Recently, there is increase in pharmaceutical demand due to rapid population growth and technological advancements. As a result, pharmaceutical companies have focused on research and development. This has led to the development of novel drugs and treatments that have increased the accessibility and affordability of healthcare6. In 2018, the market was estimated at 1.2 trillion US dollars, and expected to reach 1.77 trillion US dollars by 2030. The pharmaceutical industry uses a significant amount of water, and then turned into wastewater. This wastewater contains toxic contaminants and antibiotics, which have negative effects on human health and aquatic ecosystem. In recent years, the effects of pharmaceutical wastewater on the environment has grown especially due to the presence of antibiotics like OTC. Antibiotic-resistant bacteria can arise from the inappropriate disposal of pharmaceutical waste containing antibiotics, which poses a major risk to the environment and public health7. There is a growing demand for non-biodegradable plastic polymers, such as Carrageenan, a flexible polymer from Irish Moss. Pharmaceutical products (PPs) are ubiquitous in environmental compartments, making it difficult to identify efficient removal strategies8.
Water from pharmaceutical plants contains biodegradable organic matter, including antibiotics, lipid regulators, and anti-inflammatory chemicals that lead to bacterial resistance, allergies, and the growth of aquatic plants9. Antibiotics account for 70% of all drugs produced annually, with a significant amount found in the South China Sea reservoir10. The discovery of penicillin in 1928 marked the peak of antibiotic discovery in the mid-1950s11,12. Scientists and researchers have been investigating long-term ways to remove antibiotics from pharmaceutical effluent in order to solve this critical issue. The adsorption of oxytetracycline using hydrogels based on kappa carrageenan has generated considerable interest as a promising method13,14. Carrageenan has potential bioactive properties and is used in various sectors like wound healing, medication delivery, tissue engineering, and aqueous pollutants removal. Thermo-reversible gels are created through two phases involving gel-inducing chemicals and temperature15,16. OTC a widely used antibiotic due to its low cost and antimicrobial properties, is found in human excretion, animal products, hospital waste, pharmaceutical industries, and manure-fertilized soil17,18. Human life is endangered by excessive concentrations of OTC in water and when it translocates to plants also deteriorate the quality of water. However, poor absorption and metabolism in the digestive system can lead to antibiotic resistance, disrupting ecosystems19,20. The increasing amount of OTCs poses a global concern for removing them from pharmaceutical wastewater21. Adsorption is a promising technique for removing OTCs from pharmaceutical wastewater due to its economic viability, eco-friendliness, and efficacy22,23. Carrageenan, a high-biodegradable, non-toxic, and biocompatibility adsorbent, is suitable for hydrogel adsorption. Hydrogels, three-dimensional, cross-linked polymeric networks, can store large amounts of water and are hydrophilic24,25.
Carrageenan has potential bioactive properties and is used in various sectors like wound healing, medication delivery, tissue engineering, and aqueous pollutants removal. Thermo-reversible gels are created through two phases involving gel-inducing chemicals and temperature15,16. The present study was conducted to develop biocompatible aerogel microparticles using commercial carrageenan as a precursor. Supercritical carbon dioxide extraction transforms the gel into an aerogel, with analyzed FTIR, SEM, particle density and particle size distribution26.
In addition to its high hydrophilicity, Kappa carrageenan (KC) exhibits poor stability and low gel strength, which make it less suitable for pharmaceutical wastewater treatment than other hydrogels. Kappa carrageenan has been blended with other resilient polymers like agar, gelatin and Polyvinyl Alcohol (PVA)27. In this study, kappa carrageenan hydrogel along with PVA gel is used to remove antibiotics from pharmaceutical wastewater. The objectives include eliminating OTC, characterizing the hydrogel's physical and chemical properties, and analyzing its ability to remove contaminants from the wastewater.
Material and methods
Chemical reagents
Different chemical reagents were used in the experiment. These include Kappa-carrageenan (22048-100G-F) a sulphated plant polysaccharide, Polyvinyl Alcohol (PVA) 87–90% hydrolyzed (average mol wt. 30,000–70,000), γ-Aminopropyltriethoxysilane (C9H23NO3Si) CAS.NO: 919-30-2, Bentonite Nano clay AL-SIAT-02NCLAY (Al2O3.2SiO2.H2O) CAS # 1302-78-9, distilled water and the Oxytetracycline salt (C22H24N2O9).
Study area
Present study focused on a renowned pharmaceutical firm in Faisalabad, Pakistan, known for its expertise in producing antibiotics, antidepressants, syrups, and analgesics, making it an ideal ___location for pharmaceutical industrial research.
Functionalization of bentonite nanoclay
Bentonite nano clay (3 g) and ethanol (250 mL) were dispersed in 500 mL beaker and stirred continuously for 1 h with the help of magnetic stirrer. After that 500 µL of APTES (3-Aminopropyltriethoxysilane) was dissolved into 20 mL of ethanol and then added to Bentonite nano clay mixture. Then in a glass reactor at 60 °C, the suspension was mechanically stirred for 2 h. After filtering, the functionalized clay was washed with ethanol. The functionalized Bentonite Nano clay (FBNC) was dried into a vacuum oven. For cross linking, varying amounts of FBNC (0.5, 0.10, 0.15, 0.20 and 0.25 g) were dispersed in 10 mL water and sonicated for one hr at ambient temperature28.
Preparation of kappa carrageenan/ polyvinyl alcohol/ bentonite nano clay hydrogel film
The preparation of KC-based hydrogel involved the preparation of a PVA solution and a KC solution. The PVA solution was prepared by adding 0.3 g of PVA and 25 mL of distilled water in a 250 mL beaker. Solution was placed on a hot plate in the laboratory for one hr at a mixing speed of 300 rpm. The temperature was maintained at < 50 °C with constant stirring. The KC solution was prepared by adding 0.7 g of KC and 25 mL of distilled water in a 250 mL beaker. The PVA solution was added to the KC solution in a 1:1 ratio, stirring at a mixing speed of 300 rpm for 2 h. The hydrogel films were created using functionalized Bentonite nano clay, which was added drop by drop to the KC/PVA blend. KP1was a controlled sample that contains PVA/KC but no clay. KPB-2, KPB -3, KPB -4, KPB-5, and KPB -6 were assigned that had 0.05, 0.1, 0.15, 0.2, and 0.25 g of Bentonite Nano clay, respectively (Table 1). Prepared solution was placed on hot plate with 70 °C temperature until the solution became viscous. Then, solution was poured to petri dishes and was baked in oven at a temperature of 50 °C. The hydrogel films were peeled off from the dishes and were stored in bags for further processing29.
Characterization of hydrogel
Fourier transfer infrared spectroscopy
Fourier Transform Infrared (FTIR) spectroscopy is a cost-effective and non-destructive method for determining clay mineral composition, structure, and interactions with inorganic or organic molecules. It was used to characterize surface functional groups in synthesized hydrogel films30. The study employed FTIR (JASCO, FT/IR-6600) to characterize the attached surface functional groups and their interactions with the constituents of the synthesized hydrogel films.
Scanning electron microscope
Samples of hydrogel were characterized by Scanning Electron Microscope (SEM) that provided information about the sample composition and surface topography. The SEM images were also used to compare the properties of the samples.
X-Ray diffraction analysis (XRD)
In this study, X-ray diffraction (XRD) was used to analyze the crystalline structure of synthesized hydrogel films in according to Bragg's Law. XRD patterns showed composition of crystalline and amorphous phases of the hydrogel films. The crystalline phases were further analyzed to revealed the chemical composition using pKw, 200 mA, 45 kV radiation, and a 5–70° angle31.
Swelling ratio measurement
Adsorption capacity is influenced by the shrinking and swelling properties of the adsorbent. The pre-weight dry hydrogel was submerged in distilled water, and the swelling degree was tested after every 10 min. The swollen adsorbent was separated and weighed, with the average value determined after three replicates. The swelling behavior of hydrogel was calculated using a given equation.
where, “S” is the swelling ratio, “Ws” is the weight of swelling hydrogel at a particular time and “Wd” is the weight of dry hydrogel at t = 0.
Different parameters effects on adsorption
Effect of initial concentration
The impact of initial concentration on OTC was investigated at a predetermined time interval of 40 min on a magnetic stirrer at room temperature 25 °C and optimal pH 8 with the adsorbent dose (0.05) at various concentration (10, 20, and 40 mg/L in 25 mL of the pharmaceutical solution. Later, the solution was strained and the concentration of OTC in the filtrate residual was examined by using UV spectrophotometer. The similar method described above was used to create all samples with various adsorbent dosages.
Effect of pH
The adsorption process is influenced by pH, which can alter the surface charge of adsorbents and the separation of functional groups. This study examined the effect of pH on the adsorption of OTC at different pH levels. The pH of the solutions was maintained by using 0.1 M NaOH and 0.1 M HCl. At room temperature, 25 mL of an antibiotic solution was taken with a 0.05 g adsorbent dose for 40 min. The decrease in H+ ion concentration by pharmaceutical ions raises pH, while the increase in hydroxyl ions and negatively charged active sites between the adsorbent and the medicinal solution results in a minor pH shift.
Effect of temperature
The study examined the effect of temperature on OTC adsorption at different temperatures (10, 15, 20 and 25 °C). The pH of the solution was maintained using 0.1 M NaOH and 0.1 M HCl. The solution was filtered and the amount of OTC in the filtrate residual was analyzed using a UV spectrophotometer.
Effect of contact time
OTC adsorption was examined at various contact times ranging from 5 to 240 min, whereas the remaining parameters (temperature 25 °C, adsorbent dosage 0.05, and pH 8) remained unchanged. The pH of the solution was maintained using 0.1 M NaOH and 0.1 M HCl. After that, the solution was filtered and the amount of OTC in the filtrate residual was analyzed with the help of UV spectrophotometer. All the samples with different adsorbent dose were prepared by same procedure mentioned above. Because there are so many active sites on the adsorbent's surface the pharmaceutical compounds adsorbed immediately. The specific adsorpent dose, pH and temperature conditions were set on the basis of preliminary experiments and economical considerations to ensure representative results with optimimum sample size.
Adsorption experiment
Antibiotic removal efficiency
A study was conducted to evaluate the synthetic hydrogel film's ability to absorb 40 ppm of OTC from pharmaceutical effluent. The drug was ingested in a 50 mL Erlenmeyer flask, and 0.05 g of the adsorbent dosage remained in contact with it. The mixture was shaken in an incubator for 240 min at 25 °C and 120 rpm. The residual amount of OTC was examined using a UV spectrophotometer to confirm the concentration change was due to the adsorbent.
Then the adsorption ability and removal efficacy of OTC was estimated through the variation in the concentration before and after the adsorption. The OTC concentration at equilibrium was calculated using Eqs. (2) and (3):
where, “qe” is the adsorption capacity at equilibrium, “Co” is the initial concentration of Oxytetracycline, “Ce” is equilibrium in concentration of Oxytetracycline (mg/L), “m” is the mass of adsorbent (g) and “V” is the volume of the solution (L).
Statistical analysis
SPSS (version 25) was used to statistically analyze the data obtained from the experiments. The data was subjected to analysis of variance (ANOVA) for the level of significance of difference. Correlation analysis between pH, temperature, contact time and swelling was also carried out for data interpretation.
Results and discussion
Functionalization of bentonite nano clay
The FTIR analysis of Bentonite nano-clay's functionalization revealed significant changes in the clay's vibrational modes and absorption bands, indicating the effective attachment of functional groups, as shown in Fig. 1. These findings demonstrate the efficiency of the functionalization method in improving the clay's surface qualities and reactivity, which is crucial for industrial applications like adsorption, catalysis, and nano-composite materials.
Hydrogel characterization
Fourier transform infrared spectroscopy (FTIR)-test
The study used Fourier Transform Infrared Spectroscopy (FTIR) to investigate the surface functionalization and chemical properties of nano-composite films made of Carrageenan-based hydrogel, Bentonite Nano-clay, and Polyvinyl Alcohol (PVA) as shown in, Table 2 and Fig. 2. Interestingly, almost all the hydrogels have exhibited prominent peaks around 3690 cm−1 indicating O–H stretching. These hydroxyl groups on the designed hydrogels can form hydrogen bonding with hydroxyl and amine groups present in OTC pollutant. Therefore, it is anticipated that the designed gydrogels will have higher adsorption capacity due to the presence of these bonding sites. In addition, the carboxyl groups (C=O) at 1581.6 cm−1 and 1352.1 cm−1, alcohol groups (C–O) present at at 1382.9 cm−1 and 1027.4 cm−1 and siloxane groups (Si–O) present at around 1004.9 cm−1 and 914.2 cm−1 can also form hydrogen bonding or van der Waal interactions with OTC, thereby providing more active sites for the adsorption.
SEM analysis of hydrogel
The study used Scanning Electron Microscopy (SEM) to analyze the surface shape of hydrogel films, as shown in Fig. 3, which affects their adsorption capacity. The hydrogel's higher surface area allows for better interactions with contaminants. The addition of nano fillers increased the mechanical strength of the nano-composite films, demonstrating the power of hydrogen bonding between oxygen-containing groups and hydroxyl groups of chitosan and polyvinyl alcohol. Findings of our study are crucial for applications like adsorption, medication administration, and tissue engineering.
Hydrogel X-ray diffraction analysis
The X-ray Diffraction (XRD) technique was used to evaluate the crystallinity of K-carrageenan (KC)-based hydrogel. The results showed a semi-crystalline structure, with higher crystallinity correlated with higher intensities, as shown in Fig. 4. Modifications with Nano clay and cross-linker reduced crystallinity, affecting the material's crystalline structure. As crystallinity increases, these characteristics also tend to increase.
Properties of hydrogel
Swelling behavior of K-carrageenan (KC) hydrogel in distilled water
Hydrogels grow gradually over time, with swelling occurring at later periods. Increased PVA concentrations increase swelling percentages, indicating PVA's water-absorbing ability, as shown in Fig. 5. Stability and equilibrium occurs after 70–80 min, allowing hydrogels to absorb as much water as possible. Understanding these tendencies is crucial for modifying hydrogel properties. Table 3 displays the swelling behavior of K-carrageenan hydrogel in distilled water at different time intervals and polyvinyl alcohol concentrations. The swelling percentage, indicates water absorption and is crucial for evaluating its effectiveness in drug delivery and tissue engineering applications.
Figure 5 shows the equilibrium swelling, which stabilizes after 70–80 min, indicating that the hydrogel has decomposed. This information assists the researchers in better understanding the kinetics and properties of the hydrogel's swelling behavior, which is useful for applications like as medication delivery, wound dressings, and tissue engineering. Overall, the Table 3 is useful for comprehending the swelling behavior of kappa carrageenan hydrogels and their prospective applications in various disciplines.
Table 3 shows the swelling ratio (Ws–Wd) of kappa carrageenan KC hydrogel with time compared to distilled water. The table comprises columns for time intervals (minutes) and distinct KC hydrogel compositions, each with differing PVA concentrations or formulations. The table's key findings include time-dependent swelling, which normally rises with time, and the influence of PVA concentration on swelling ratios. Larger PVA concentrations result in larger swelling ratios, demonstrating that PVA improves the hydrogel's ability to absorb water.
Effect of pH on adsorption capacity
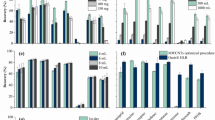
The effect of pH on the adsorption capacity of KC hydrogel is shown in the Fig. 6. The ability of a material to attract and hold molecules or chemicals from its surroundings is referred to as its adsorption capacity. The ideal pH range for maximizing KC hydrogel adsorption capability is typically near-neutral to slightly alkaline (about pH-6 to pH-8). KPB-1, for instance, has greater adsorption capability at pH-6 and pH-8 than at pH-2 and pH-4. This behaviour can be explained by swelling behavior of KPB-1 hydrogel which might swell at higher pH i.e. 6 to 8. This excessive swelling increases the surface area and active sites for adsorption.
The reaction to pH changes is also concentration dependent, depending on the starting concentration of KC hydrogel. Understanding these pH-dependent adsorption properties is critical for optimizing the hydrogel performance in various applications such as water treatment, drug delivery, and adsorption of pollutants from aqueous solutions.
Figure 7 shows that pH has a substantial impact on the OTC adsorption capacity of KC hydrogel. The adsorption capability across multiple pH levels, the hydrogel, especially KC/PVA/0.05, consistently outperforms other hydrogel formulations.
Effect of initial concentration on K-carrageenan hydrogel
The ideal adsorbent dose is 0.05 g with a pH of 6 over 120 min on a magnetic stirrer at 35 °C. At an initial concentration of 40 mg/L, the KC/PVA/0.1 (H) Bentonite Nano clay nano-composite adsorbent displays outstanding capacity to adsorb OTC, with an excellent adsorption of 99%. The adsorbent's effectiveness rises as the initial oxytetracycline concentration increases. The adsorbent still has 98% adsorption capacity at a lower starting concentration of 20 mg/L.
However, Fig. 8 shows that when the initial OTC concentration rises to 40 mg/L, the adsorbent reaches its maximum potential, obtaining a adsorption capacity of more than 95%. This is due to the fact because initially at higher OTC concentrations, the driving force is maximum and more active sites are available for adsorption. As the adsorption proceeds, the gydrogel has reached its maximum potential and the active sites are nearly fully occupied by OTC molecules.
Overall removal of OTC from hydrogel
The removal efficiency for OTC from pharmaceutical wastewater by kappa carrageenan/ polyvinyl alcohol/ bentonite nanoclay (KPB) hydrogel was evaluated under various conditions and the results are demonstrated in Fig. 9. It was observed that removal effectiveness of most adsorbent compositions drecreases, as the initial OTC content increases. Thus, the adsorbent composition greatly influence adsorption capacity, with KPB-3 consistently demonstrating the best removal percentages, even at increasing OTC concentrations. This result can be explained by the fact that KPB-3 hydrogel had the optimum composition and it had provided synergistic effect of kappa carrageenan, polyvinyl alcohol, and bentonite nanoclay. Moreover, at initial concentrations, the hydrogel’s adsorption sites are abundantly available that make it suitable for handling higher OTC loads. The best performance of KPB-3 hydrogel can be explained by excellent swelling behaviour of the hydrogels that provide excessive surface area and active sites for adsorption.
Significant differences and correlation
The ANOVA test revealed that pH and concentration significantly influence OTC adsorption by a hydrogel (Table 4). The adsorption capacity of OTC is marginally higher than 0.054, suggesting that pH and concentration are more important. The sum of squares for pH fluctuations affecting OTC adsorption is 8.867, with a p-value of 0.01.
ANOVA test revealed that pH and concentration significantly influence the hydrogel's efficacy in removing OTC. The F-statistic is 6.697, with a p-value of 0.01, and the SS for OTC removal efficacy is 3.585, indicating some difference but not substantial, as shown in Table 4. Therefore, pH and concentration have a greater influence on OTC adsorption. In summary, the ANOVA findings show that pH and concentration have a greater influence on OTC adsorption by the hydrogel.
The offered correlation (Table 5) demonstrates the links between several factors associated with KC/ PVA/ BNC hydrogel. The degree and direction of a linear link between two variables are measured by correlation.
The study found no significant relationships between pH, temperature, contact time, or swelling. Initial concentration had strong positive correlations with pH, temperature, and contact time, indicating weak positive linear associations.
Reaction kinetics model
Adsorption is a crucial process used in environmental remediation and wastewater treatment. Researchers used OTC solutions in a mixture of Kappa-carrageenan, polyvinyl alcohol, and Bentonite clay (Table 6).
Comparative analysis
Environmental concerns have led to a growing demand for biodegradable polymers, such as Carrageenan, a flexible polymer from Irish Moss. Pharmaceutical products (PPs) are ubiquitous in environmental compartments, making efficient removal strategies difficult to identify8. A composite material with a cadmium adsorption capacity of 20.6 mg/g is selective in removing lead ions. Clay minerals, the oldest and least expensive adsorbents, potentially extract pharmaceutical products from wastewater effluents32. Research gaps exist in determining the full potential of clay-based adsorbents33,34. A recent research aims to develop biocompatible aerogel microparticles using commercial carrageenan as a precursor. Supercritical carbon dioxide extraction transform the gel into an aerogel, with analyzed FTIR, SEM, particle density and particle size distribution26. Three different carrageenans were used to create biodegradable aerogel micro-spherical particles with varying surface areas and average pore volume and size. The surface area varied from 33 to 174 m2/g, with an average pore volume and size of 0.35 0.11 cm3/g and 12.34 3.24. The porous material can be used in medication delivery applications26. Hybrid aerogel monoliths from alginate and -carrageenan were created by heating a carrageenan solution to 90 °C and adding a KSCN solution as a cross-linker. Cylindrical -carrageenan aerogels were created by gently dripping a -carrageenan solution into a -carrageenan solution35. Contact time is an important factor in oxytetracycline removal from pharmaceutical wastewater, with extended contact duration from 20 to 120 min, improving removal effectiveness with a 0.05 g adsorbent dosage.
Researchers are exploring the economic viability of applying kappa carrageenan hydrogels in wastewater treatment and other applications by utilizing conditions, such as temperature, different pH levels and desorbing agents. The reusability of kappa carrageenan hydrogel combined with nano-composites has been explored and significant adsorption capabilities have been found36. A study on a specific contaminant showed a remarkable reusability of the hydrogel-nanocomposite with up to 90% retention of initial capacity even after five to ten cycles37. The adsorption process was endothermic, and the hydrogel was more reusable after five cycles and with over 70% clearance rate37,38. This study presented a novel carrageenan hydrogel for removing cationic methylene blue (MB) from aqueous solutions. PG hydrogel with reactive function groups enhanced the hydrogels adsorption capacity and stability. The adsorption was well-fitted using the Langmuir isotherm and the pseudo-second order model39. The beads could be easily regenerated and reused for at least five cycles efficiently. The material demonstrated excellent adsorption ability in various pH ranges, with a maximum capacity of 80.28 mg g−1 at 45 °C. Thermal, chemical, and pH level changes can be used to renew Kappa carrageenan hydrogel, with the effectiveness of regeneration determined by the adsorbate and applied technique40.
Research on the economic feasibility of kappa carrageenan hydrogels for water treatment and other applications suggests possible cost effective methods of wastewater treatment. Studies on the reusability of nano-composites mixed with kappa carrageenan hydrogel showed strong adsorption capacities across several cycles. By using these nanocomposites in water purification systems, the requirement for single-use materials can be decreased, improving sustainability initiatives. The results highlight the vital significance that accurate pH management plays in optimizing the adsorption capacity for pollutants in pharmaceutical wastewater treatment regimens. The study also emphasizes the wider significance of OTC removal efficiency in adsorption processes and its implications for public health and environmental protection. In recent years, numerous different plant extracts, including Azadirachta indica leaf extract, have been utilized to remediate industrial effluent. Plant-based remediation has gained popularity for the efficient cleanup of polluted water41,42. Recent breakthroughs in materials science and green chemistry have resulted in the production of nanomaterials with large specific surface areas and diverse functionalities, making them effective in removing heavy metals from wastewater. According to the research, the most efficient, effective, clean, and sustainable technique for removing heavy metals from wastewater is by the adsorption of these metals onto green nanomaterials derived from plant extracts43,44,45.
Conclusion
Present study investigated the adsorption of oxytetracycline from pharmaceutical wastewater using a Carrageenan-based hydrogel. The hydrogel, consisting of bentonite nano-clay and polyvinyl alcohol, showed outstanding oxytetracycline removal efficiency, with a maximum removal rate of 98.5% in 120 min. The adsorption process was pH-dependent, with higher initial oxytetracycline levels improving adsorption capacity. The Pseudo-Second-Order kinetic model accurately exhibited the adsorption behaviour with significant chemical bonding interactions between the hydrogel and oxytetracycline. The study highlighted the need for accurate pH management in pharmaceutical wastewater treatment operations and the impact of oxytetracycline adsorption capacity on adsorption. Future research should focus on Carrageenan-based hydrogels for the removal of pharmaceutical pollutants specifically and generally elimination of water pollutants for environmental sustainability.
Data availability
All the data of this study is contained in the manuscript. This paper is part of MPhil. thesis of third author (Saddam Hussain). Thesis of the said author is submitted to Higher Education Commission Repository (https://www.turnitin.com/download_file.asp?r=1.93288050797698&svr=6&lang=en_us&type=&oid=2198334280&fn=312-R-MPHIL-ENV-21_Saddam.docx&session-id=&p=1). For any additional data or information needed regarding this research, please contact the corresponding author Muhammad Afzal: [email protected].
References
Wang, T., Jiang, M., Yu, X., Niu, N. & Chen, L. Application of lignin adsorbent in wastewater Treatment: A review. Sep. Purif. Technol. 302, 122116. https://doi.org/10.1016/j.seppur.2022.122116 (2022).
Das, P. P., Sharma, M. & Purkait, M. K. Recent progress on electrocoagulation process for wastewater treatment: A review. Sep. Purif. Technol. 292, 121058. https://doi.org/10.1016/j.seppur.2022.121058 (2022).
Irshad, M. A. et al. Enhancing chromium removal and recovery from industrial wastewater using sustainable and efficient nanomaterial: A review. Ecotoxicol. Environ. Saf. 263, 115231 (2023).
Irshad, M. A. et al. Application of nanomaterials for cadmium adsorption for sustainable treatment of wastewater: A review. Water Air Soil Pollut. 234(1), 54 (2023).
Li, Y., Dong, X. & Zhao, L. Application of magnetic chitosan nanocomposites modified by graphene oxide and polyethyleneimine for removal of toxic heavy metals and dyes from water. Int. J. Biol. Macromol. 192, 118–125. https://doi.org/10.1016/j.ijbiomac.2021.09.202 (2021).
Zou, R. et al. When microbial electrochemistry meets UV: The applicability to high-strength real pharmaceutical industry wastewater. J. Hazard. Mater. 423, 127151. https://doi.org/10.1016/j.jhazmat.2021.127151 (2022).
Haque, M. Aggressive medicine promotional activity of pharmaceutical industry all over the globe: Who will do the highly risky job to defy the monstrous financial tyrant?. Bangl. J. Med. Sci. 56, 159–201. https://doi.org/10.3329/bjms.v19i4.46610 (2020).
Thiebault, T. Raw and modified clays and clay minerals for the removal of pharmaceutical products from aqueous solutions: State of the art and future perspectives. Crit. Rev Environ Sci Technol. 50(14), 1451–1514 (2019).
Zhang, Y. et al. Microbial community functional structure in response to antibiotics in pharmaceutical Wastewater treatment systems. Water Res. 47(16), 6298–6308. https://doi.org/10.1016/j.watres.2013.08.003 (2018).
Bengtsson-Palme, J. et al. Industrial wastewater treatment plant enriches antibiotic resistance genes and alters the structure of microbial communities. Water Res. 162, 437445. https://doi.org/10.1016/j.watres.2019.06.073 (2019).
Gaynes, R. P. The discovery of penicillin—new insights after more than 75 years of clinical use. Emerg. Infect. Dis. 23(5), 849–853. https://doi.org/10.3201/eid2305.161556 (2017).
Chen, Y. et al. Comprehensive insights into the occurrence, distribution, risk assessment and indicator screening of antibiotics in a large drinking reservoir system. Sci. Total Environ. 716, 137060. https://doi.org/10.1016/j.scitotenv.2020.137060 (2020).
Jian, J., Fan, X., He, P., Xiong, H. & Shen, H. The effects of energy consumption, economic growth and financial development on CO2 emissions in China: A VECM approach. Sustainability 11(18), 4850 (2019).
Minale, M. et al. Enhanced removal of oxytetracycline antibiotics from water using manganese dioxide impregnated hydrogel composite: Adsorption behavior and oxidative degradation pathways. Chemosphere 280, 130926 (2021).
Ashfaq, M. et al. Ecological risk assessment of pharmaceuticals in the receiving environment of pharmaceutical wastewater in Pakistan. Ecotoxicol. Environ. Saf. 136, 31–39. https://doi.org/10.1016/j.ecoenv.2016.10.029 (2017).
Mirzaei, A., Esmkhani, M., Zallaghi, M., Nezafat, Z. & Javanshir, S. Biomedical and environmental applications of carrageenan-based hydrogels: A review. J. Polym. Environ. 31(5), 1679–1705. https://doi.org/10.1007/s10924-022-02726-5 (2022).
Karpov, M. et al. Transformation of Oxytetracycline by redox-active Fe(III)- and Mn(IV)-containing minerals: Processes and mechanisms. Water Res. 145, 136–145. https://doi.org/10.1016/j.watres.2018.08.015 (2018).
Liang, G. et al. Efficient removal of Oxytetracycline from aqueous solution using magnetic montmorillonite-biochar composite prepared by one step pyrolysis. Sci. Total Environ. 695, 133800. https://doi.org/10.1016/j.scitotenv.2019.133800 (2019).
Liang, X. et al. 2017, May). Provchain: A blockchain-based data provenance architecture in cloud environment with enhanced privacy and availability. In 2017 17th IEEE/ACM International Symposium on Cluster, Cloud and Grid Computing (CCGRID) 468–477 (IEEE, 2017).
Veiga, N., Diesendruck, Y. & Peer, D. Targeted lipid nanoparticles for RNA therapeutics and immunomodulation in leukocytes. Adv. Drug Deliv. Rev. 159, 364–376 (2020).
Başkan, G., Açıkel, Ü. & Levent, M. Investigation of adsorption properties of oxytetracycline hydrochloride on magnetic zeolite/Fe3O4 particles. Adv. Powder Technol. 33(6), 103600 (2022).
Liu, D. et al. Core shell Zn/Co MOFs derived CO3O4/CNT sasan efficient magnetic heterogeneous catalyst for persulfate activation and oxytetracycline degradation. J. Chem. Eng. 387, 124008. https://doi.org/10.1016/j.cej.2019.124008 (2020).
Saman, N., Othman, N. S., Chew, L., Setapar, S. H. M. & Mat, H. Cetyltrimethyl ammonium bromide functionalized silica nanoparticles (MSN) synthesis using a combined sol-gel and adsorption steps with enhanced adsorption performance of Oxytetracycline in aqueous solution. J. Taiwan Inst. Chem. Eng. 112, 67–77. https://doi.org/10.1016/j.jtice.2020.07.008 (2020).
Dai, Y., Li, J. & Shan, D. Adsorption of tetracycline in aqueous solution by biochar derived from waste Auriculariaauricula dregs. Chemosphere 238, 124432. https://doi.org/10.1016/j.chemosphere.2019.124432 (2020).
Xue, JiaJia et al. Antibiotic residue and toxicity assessment of wastewater during the pharmaceutical production processes. Chemosphere 291, 132837. https://doi.org/10.1016/j.chemosphere.2021.132837 (2022).
Alnaief, M., Obaidat, R. & Mashaqbeh, H. Effect of processing parameters on preparation of carrageenan aerogel microparticles. Carbohydr. Polym. 180, 264–275 (2018).
Ahmed, E. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 6(2), 105–121. https://doi.org/10.1016/j.jare.2013.07.006 (2015).
Rehmat, S. et al. Novel stimuli-responsive pectin-PVP-functionalized clay based Smart hydrogels for drug delivery and controlled release application. Front. Mater. Sci. 9, 545. https://doi.org/10.3389/fmats.2022.823545 (2022).
Ghafelebashi, A., Khosravani, S., Kazemi, M., Rajabi, F. & Amiri, M. A novel fabricated polyvinylalcohol/ bentonitenano composite hydrogel generated into colloidal gas aphron. Colloids Surf. A 650, 129580. https://doi.org/10.1016/j.colsurfa.2022.129580 (2022).
Thürmer, M. B., Diehl, C. E., Brum, F. J. B. & Santos, L. A. D. Preparation and characterization of hydrogels with potential for use as biomaterials. Mater. Res. 17(suppl 1), 109–113. https://doi.org/10.1590/1516-1439.223613 (2014).
Chen, J. et al. The synergistic gelation of okra polysaccharides with kappa-carrageenan and its influence on gelrheology, texture behaviour and micro structures. Food Hydrocoll. 87, 425–435. https://doi.org/10.1016/j.foodhyd.2018.08.003 (2019).
Karimi, M. H., Mahdavinia, G. R., Massoumi, B., Baghban, A. & Saraei, M. Ionically crosslinked magnetic chitosan/κ carrageenan bio adsorbents for removal of an ionic eriochrome Black-T. Int. J. Biol. Macromol. 113, 361375. https://doi.org/10.1016/j.ijbiomac.2018.02.102 (2018).
Krajišnik, D. et al. Properties of diclofenac sodium sorption onto natural zeolite modified with cetylpyridinium chloride. Colloids Surf. B. 83(1), 165–172. https://doi.org/10.1016/j.colsurfb.2010.11.024 (2011).
Alshameri, A., Yan, C. & Lei, X. Enhancement of phosphate removal from water by TiO2/Yemeni natural zeolite: Preparation, characterization and thermodynamic. Microporous Mesoporous Mater. 196, 145–157. https://doi.org/10.1016/j.micromeso.2014.05.008 (2014).
Lara, M. et al. Exploitation of κ-carrageenan aerogels as template for edible oleogel preparation. Food Hydrocoll. 71, 68–75. https://doi.org/10.1016/j.foodhyd.2017.04.021 (2017).
Ajayan, A. & Jude, M. M. Natural nanofillers family, their properties, and applications in polymers. Nanofillers 233, 23 (2023).
Yang, B. et al. Polyvinylpyrolidone functionalized magnetic graphene-based composites for highly efficient removal of lead from waste water. Colloids Surf. 582, 123927. https://doi.org/10.1016/j.colsurfa.2019.123927 (2019).
Tanhaei, B., Ayati, A., Iakovleva, E. & Sillanpää, M. Efficient carbon interlayer magnetic chitosan adsorbent for anionic dye removal: Synthesis, characterization and adsorption study. Int. J. Biol. Macromol. 164, 36213631. https://doi.org/10.1016/j.ijbiomac.2020.08.207 (2020).
Zheng, Y. & Wang, A. Superadsorbent with three-dimensional networks: From bulk hydrogel to granular hydrogel. Eur. Polym. J. 72, 661–686. https://doi.org/10.1016/j.eurpolymj.2015.02.031 (2015).
Lapwanit, S., Sooksimuang, T. & Trakulsujaritchok, T. Adsorptive removal of cationic methylene blue dye by kappa carrageenan/poly (glycidyl methacrylate) hydrogel beads: Preparation and characterization. J. Environ. Chem. Eng. 6(5), 62216230. https://doi.org/10.1016/j.jece.2018.09.050 (2018).
Irshad, M. A. et al. Sustainable and safe treatment of wastewater of paint industry using Azadarachta indica leaf extract combined with silver nitrate solution. Sustainability 15(4), 3592 (2023).
Irshad, M. A. et al. Green and eco-friendly treatment of textile wastewater by using Azadirachta indica leaf extract combined with a silver nitrate solution. Sustainability 15(1), 81 (2022).
Irshad, M. A. et al. Green synthesis and characterization of silver and copper nanoparticles and their use as an effective adsorbent for chromium removal and recovery from wastewater. Environ. Sci. Pollut. Res. 30(52), 112575–112590 (2023).
Irshad, M. A. et al. Enhancing chromium removal and recovery from industrial wastewater using sustainable and efficient nanomaterial: A review. Ecotoxicol. Environ. Saf. 263(115231), 10–1016 (2023).
Masood, N. et al. Green synthesis, characterization and adsorption of chromium and cadmium from wastewater using cerium oxide nanoparticles; reaction kinetics study. J. Mol. Struct. 1294, 136563 (2023).
Acknowledgements
The authors express their gratitude to the Deanship of Scientific Research, Imam Mohammad Ibn Saud Islamic University, Saudi Arabia to support this research work. As this paper is extracted from MPhil thesis of the third author (Saddam Hussain), thanks are extended to supervisor (first author) and the research student (third author).
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU).
Author information
Authors and Affiliations
Contributions
M.A.: Conceptualization, Supervision, Writing—original draft, Writing—review & editing, S. H.: Writing—original draft Resources, Methodology, Writing—review & editing; R. N.: Conceptualization, Supervision, Writing—original draft, Writing—review & editing; M. N.: Writing—original draft, Investigation, Writing—review & editing; , M. A. I.: Writing—original draft, Investigation, Writing—review & editing.; A. I.: Data curation, Visualization, Funding acquisition, Writing—review & editing; H. A. M.; Formal Analysis, Methodology, Investigation, Validation; A. A. A. ; Data Curation, Formal analysis, Writing—review & editing; At. I.; Resources, Validation, Formal analysis, Writing—review & editing; M. R.; Formal analysis, Validation, Visualization, Resources,; S. A. A.; Validation, Funding acquisition, Visualization, Data curation. Methodology, Writing—review & editing.; M. E. A. Z.: Funding acquisition, Project Administration, Investigation, Formal analysis, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Afzaal, M., Nawaz, R., Hussain, S. et al. Removal of oxytetracycline from pharmaceutical wastewater using kappa carrageenan hydrogel. Sci Rep 14, 19687 (2024). https://doi.org/10.1038/s41598-024-69989-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69989-x