Abstract
Although smoking is an established risk factor for Mycobacterial infection, the association between smoking and nontuberculous mycobacterial pulmonary disease (NTM-PD) remains unclear. We evaluated the association between smoking and NTM-PD and tuberculosis (TB) using a population-based South Korean nationwide cohort. Using the Korean National Health Insurance Database, we screened individuals over 20 years of age who underwent the national health screening program in 2009. Out of 3,774,308 eligible populations, we identified 2,964 and 26,112 cases of newly developed NTM-PD and TB, respectively. We used multivariate Cox proportional hazards models to estimate the adjusted hazard ratios (aHRs) of risk factors for NTM-PD and TB. The incidence rates for developing NTM-PD and TB were 0.08 and 0.68 per 1,000 person-years, respectively. Current smokers (aHR 0.63, 95% confidence interval [CI] 0.56–0.71) and current heavy smokers (≥ 20 pack-years, aHR 0.74, 95% CI 0.63–0.86) were at lower risk for NTM-PD development than never smokers. On the contrary, current smokers (aHR 1.19, 95% CI 1.15–1.23) and current heavy smokers (aHR 1.27, 95% CI 1.22–1.33) had a higher risk for TB development than never smokers. These trends were augmented if individuals started smoking before age 20 years. In subgroup analyses stratified by age, these trends were prominent in the 40–64 years age range. Current smoking was associated with a decreased risk of NTM-PD and increased risk of TB. These risks were augmented by early smoking initiation and in the middle age population.
Similar content being viewed by others
Introduction
Nontuberculous mycobacteria (NTM) are ubiquitous, opportunistic pathogens that most commonly manifest as pulmonary disease (NTM-PD)1. Recently, the incidence and prevalence of NTM-PD has rapidly increased worldwide, while those of tuberculosis (TB) have slowly decreased2,3. In South Korea, the prevalence of NTM disease increased from 11.4/100,000 in 2010 to 56.7/100,000 in 20214. The prevalence of NTM disease in South Korea has surpassed that of TB, which fell from 131.1/100,000 in 2010 to 52.1/100,000 in 20214. The cause of this trend remains unclear; however, it could be partially explained by several factors, such as increased use of immunosuppressive agents and organ transplantation, alterations in the residential environment, and diminished antimycobacterial immunity5,6,7.
Cigarette smoking is an important risk factor for pulmonary TB8,9. Smoking increases susceptibility to Mycobacterium tuberculosis through ciliary dysfunction, impaired immune response, macrophage abnormalities, and reduced CD4 lymphocyte count10. Based on the relationship between smoking and TB, we could infer that smoking might also be a risk factor for NTM infection. Smoking is also a risk factor for asthma and chronic obstructive pulmonary disease (COPD), which are commonly accompanied by NTM-PD5,11,12. Furthermore, previous studies have suggested that smoking was related with NTM-PD and mortality13,14,15. However, in patients with bronchiectasis, current or past smoking was associated with a lower risk for NTM colonization16. Therefore, unlike in TB, smoking might have a protective effect against NTM-PD.
Previous studies dealing with smoking and NTM-PD have some limitations, including small cohort sizes, single arm studies, and limited populations (such as individuals with human immunodeficiency virus [HIV] infection or bronchiectasis)13,14,15,16,17. Thus, the association between smoking and NTM-PD development remains unclear. In this context, our study evaluated the association between smoking and NTM infection using South Korea national population-based cohort data. Furthermore, we also assessed TB development using the same cohort to compare the association between smoking and TB and NTM independently.
Methods
Data source
The Korean National Health Insurance Service (NHIS) has a comprehensive public database called the National Health Information Database (NHID). The NHID includes socio-demographic data, medical treatment and claims information, health care use, national health screening program, and mortality among the entire Korean population18,19. The NHIS has provided a national health screening program since 199520. Until 2018, individuals aged ≥ 40 years or adult employees with national health insurance underwent a national health screening program biennially (annually for manual workers), which included a simple chest radiograph, laboratory tests, and a questionnaire regarding medical history and lifestyle behaviors19,21.
Study population
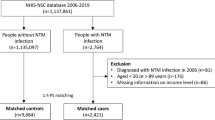
In this study, individuals who aged ≥ 20 years and underwent a national health screening program in 2009 were eligible for inclusion. Among them, 4,234,415 individuals (40% of the total national health screening program recipients) were included. We excluded individuals with a previous diagnosis of NTM-PD (n = 788) or TB (n = 128,555) before enrollment, those with any missing data (n = 319,643), and those diagnosed with NTM-PD or TB within one year of the index date (lag period, n = 11,121). The remaining 3,774,308 eligible individuals were included and followed until the date of death or until December 31, 2020. We identified 2,964 and 26,112 participants who newly developed NTM-PD and TB, respectively (Fig. 1).
Definition of cigarette smoking
Information on cigarette smoking was acquired from self-administered questionnaires during the national health screening program within two years prior to the index date. The following data regarding smoking were included in the questionnaires: daily smoking amount and duration (1 pack-year: 20 cigarettes smoked daily for 1 year), smoking status (never-, former, or current smokers), and smoking initiation age (years). A former smoker was defined as a person who had smoked at least 100 cigarettes or cigars over his/her lifetime but did not smoke at the time of the health screening. The cumulative smoking amount was measured by pack-year and categorized by 10 or 20 pack-years. The smoking initiation age was categorized into four groups (< 20, 20–24, 25–29, ≥ 30 years).
Outcome ascertainment
The outcomes of this study were new NTM-PD and TB development. In South Korea, TB has been monitored in the national TB program, while NTM-PD has not been monitored by national authorities. NTM-PD was identified by insurance claims for the International Classification of Diseases 10th revision (ICD-10) code of A31.0 (pulmonary mycobacterial infection), and at least one hospital visit or admission under the same diagnosis code within one year after the initial claim22. TB was identified by the specific NHIS codes for TB (V206, V246, and V000). The NHIS provides additional insurance benefits (deductible reduction) for all patients with TB according to the national TB care policy. Therefore, the specific diagnostic codes for TB were mandatorily applied to virtually all patients with TB in Korea. Under the current system, these specific codes for TB provides a valid tool to identify individuals with TB in Korea.
Covariates
Information on anthropometric measurements (height, body weight and blood pressure) and lifestyle behaviors (alcohol consumption and regular exercise) were collected from self-reported questionnaires on the national health screening program within two years prior to the index date. Body mass index (BMI) was measured by dividing the body weight by the square of height (kg/m2) and categorized into five groups (< 18.5, 18.5–22.9, 23–24.9, 25–29.9, and ≥ 30 kg/m2) according to the Asian BMI criteria23. Alcohol consumption was classified as none, mild (< 30 g/day), or heavy (≥ 30 g/day). Regular exercise was defined as > 30 min of moderate physical activity at least 5 times per week or > 20 min of strenuous physical activity at least 3 times per week24. The household income level was categorized into quartiles (Q1 = the lowest, Q4 = the highest) based on the subscribers’ annual national health insurance premium. Individuals receiving ‘Medical aid’ benefits (the lowest 3% of the population) were included in the Q1 group. Residential regions were categorized into metropolitan (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, Ulsan, and Sejong) and other regions (Gyeonggi, Gangwon, Chungcheongbuk, Chungcheongnam, Jeollabuk, Jeollanam, Gyeongsangbuk, Gyeongsangnam, and Jeju).
Comorbidities were identified using the NHIS and national health screening program data within one year prior to the index date. The diagnostic definitions were made as follows: (1) diabetes: either an insurance claim for ICD-10 codes E11–14 with a prescription for hypoglycemic medications or a fasting serum glucose ≥ 126 mg/dL in a health screening; (2) hypertension: either an insurance claim for ICD-10 codes I10–13 and I15 with a prescription for antihypertensive medications or high blood pressure (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg) measured during a health screening; (3) dyslipidemia: either an insurance claim for ICD-10 code E78 with a prescription for lipid-lowering medications or serum total cholesterol ≥ 240 mg/dL in a health screening; (4) chronic kidney disease (CKD): either an insurance claim for ICD-10 codes N18–19 or an estimated glomerular filtration rate < 60 mL/min/1.73 m2 calculated by the Modification of Diet in Renal Disease equation in a health screening; (5) bronchiectasis: an insurance claim for ICD-10 code J47; (6) COPD: an insurance claim for ICD-10 codes J43–44.
Statistical analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are expressed as number (percentage). The Student’s t-test and χ2 test were used to compare continuous and categorical variables, respectively. The incidence rate was calculated as the ratio between the number of newly developed NTM-PD or TB cases and the number of person-years (PY) at risk of disease (per 1,000). The multivariate Cox proportional hazards model was used to identify risk factors for NTM-PD and TB development. Model 1 was non-adjusted. The covariates included in Model 2 of the regression models were age and sex. The covariates included in Model 2 plus BMI, alcohol consumption, regular exercise, diabetes, hypertension, dyslipidemia, and CKD were included in Model 3. Model 4, included all covariates included in Model 3 plus COPD and bronchiectasis. Model 5, the main analysis model, included all covariates included in Model 4 plus residential region. For model 5, stratified analyses by age group were conducted to evaluate any interactive effect of smoking on the risk of NTM-PD and TB. All p-values were two-tailed, with statistical significance set at P < 0.05. All statistical analyses were performed using SAS version 9.4 (SAS institute, Cary, NC, United States).
Results
Baseline characteristics of the study population
Table 1 shows the baseline characteristics of study population. The means age was 47.1 ± 14.0 years and 2,043,123 (54.1%) were men. The mean BMI was 23.7 ± 3.2 kg/m2. With regard to smoking status, 2,277,862 (60.4%) were never-smokers, 520,351 (13.8%) were former smokers, and 976,095 (25.9%) were current smokers. By sex, 69.1% of the male population had a smoking history; in contrast, only 4.9% of the female population had a smoking history (Supplementary Table 1). According to the disease category, 29.9% and 41.2% of individuals with NTM-PD and TB had a smoking history, respectively (Supplementary Table 2, 3).
Risk of NTM-PD and TB development according to smoking status and cumulative amounts
The incidence rate of developing NTM-PD was 0.08/1,000 PY. Current smokers had a reduced risk (adjusted hazard ratio [aHR] 0.63, 95% confidence interval [CI] 0.56–0.71) for developing NTM-PD compared to that of never smokers. When considering both smoking status and cumulative amount, current smokers had a lower risk for NTM-PD compared with never smokers. The incidence rate of developing TB was 0.68/1,000 PY. Current smokers had an increased risk (aHR 1.19, 95% CI 1.15–1.23) for developing TB compared to that of never smokers. When considering both smoking status and cumulative amounts, current smokers had a higher risk of TB than never smokers. In a subgroup analysis stratified by age, those aged 40–64 years old showed prominent tendencies for developing both NTM-PD and TB (Fig. 2, Tables 2, 3, 4, and Supplementary Tables 4, 5, 6).
Risk of NTM-PD and TB development according to smoking initiation age
With regard to smoking amount and initiation age, smokers with < 20 pack-years had a lower risk for developing NTM-PD but increased risk of developing TB compared to those of never smokers. These risks were augmented if individuals started smoking before age 20 years. Considering the smoking status and initiation age, current smokers had a reduced risk of developing NTM-PD and increased risk of developing TB, regardless of initiation age. In a subgroup analysis stratified by age, the subpopulation aged 40–64 years showed prominent tendencies to develop both NTM-PD and TB (Supplementary Tables 7, 8, 9, 10).
Discussion
We found that current smoking status was associated with a decreased risk for NTM-PD, but increased risk for TB. These risks were augmented by early smoking initiation and were prominent in the middle age population. As far as we know, this is the first study to evaluate the impact of smoking on NTM-PD in the general population that compared the difference between NTM-PD and TB.
Cigarette smoking is a strong risk factor for pulmonary TB8,9. Previous systematic reviews and meta-analyses have shown that exposure to cigarette smoke is associated with TB infection, active TB development, and TB-related mortality25,26,27. Smoking induces mucociliary dysfunction and macrophage abnormalities, impairs immune response, and reduces CD4 T-cell lymphocyte count10. Tumor necrosis factor-α (TNF-α) is a major cytokine that is involved in granuloma formation, and activates macrophages and dendritic cells. Macrophages release TNF-α after exposure to M. tuberculosis antigens. In current smokers, nicotine reduces TNF-α release by macrophages through the α7 nicotinic receptor, which favors TB development. Cigarette smoke also selectively reduces interleukin-12 and TNF-α, impeding granuloma formation. Through the described mechanisms, cigarette smoking promotes the development of active TB28,29. We found that current smoking was associated with an increased risk for TB development; this risk was positively correlated with cumulative smoking amounts. These results emphasize the importance of smoking cessation in clinical practice and national TB care policy. Notably, selected former smokers (e.g., with smoking history < 20 pack years) in this study demonstrated a decreased risk of developing TB in our results. However, previous studies have shown that former smokers had an increased risk for developing TB, which is consistent with the finding in current smokers25,26,27. Therefore, our results must be interpreted with caution, and further studies are required for clarification.
Most importantly, our results describe a significant relationship between smoking and NTM-PD. Previous studies have described some risk factors for developing NTM-PD. Host factors include immunocompromised condition, underweight, and underlying lung disease, such as COPD, bronchiectasis, cystic fibrosis, primary ciliary dyskinesia, and α1-antitrypsin deficiency30,31,32. Since NTM is a ubiquitous pathogen, environmental exposure has been emphasized, such as that from humidifiers, showerheads, hot tubs, and garden soil33,34,35. However, we have little knowledge of the role of smoking in NTM-PD, unlike in TB. O’Brien et al. showed that smoking was a risk factor for NTM-PD in a previous case series15. Miguez-Burbano et al. showed that smoking was a significant risk factor for hospitalization due to NTM-PD in individuals with HIV infection13. Kotilainen et al. showed that smokers with NTM-PD had a higher risk for mortality compared with non-smokers, although the relationship was not significant after adjusting for underlying conditions14.
Thereafter, Wickremasinghe et al. performed a prospective study of 100 individuals with bronchiectasis to evaluate the prevalence of NTM in their sputum. Among 25 patients with multiple NTM isolates, 52% were never-smokers, 40% were ex-smokers, and only 8% were current smokers17. Shteinberg et al. performed a retrospective cohort study to evaluate the risk factors of NTM infection among 6,274 individuals with bronchiectasis using a registry of Israel's health maintenance organization. Current or past smoking was significantly associated with a lower risk for NTM growth (No NTM 36.7%, NTM single growth 28.0%, NTM colonization 16.7%, adjusted odds ratio 0.60, 95% CI 0.37–0.97)16. These two studies suggested that smoking history was associated with less NTM colonization in the lung of individuals with bronchiectasis. In our results, current smokers had a reduced risk for developing NTM-PD compared to those of never-smokers and former smokers in the general population. Shteinberg et al. said that negative association between smoking and NTM infection might reflect surveillance bias16. However, in our results, current smoking was significantly associated with an increased risk for developing TB, which was consistent with previous studies25,26,27. Previous studies also suggested that smoking inhibits granulomatous inflammation in other granulomatous lung disease, such as hypersensitivity pneumonitis and sarcoidosis36,37, while impaired granuloma formation facilitates active TB28,29. Although smoking might be able to inhibit the development of hypersensitivity pneumonitis and sarcoidosis, it may have detrimental effects on disease severity or prognosis38,39. Further experimental and prospective studies are required to elucidate the role of cigarette smoking in the development of NTM-PD.
This study has several strengths. First, this is the first study to evaluate the effect of smoking on NTM-PD in a general population that compares the difference between NTM-PD and TB. Second, a large size nationwide longitudinal cohort enhanced the statistical power to describe the risks of developing NTM-PD according to smoking history. Third, owing to the national TB care policy, which endowed specific diagnostic codes for TB, virtually all newly developed cases of TB were included in this study.
This study also has some limitations. First, the cases of NTM-PD were identified using diagnostic codes from health insurance claims, and relevant bacteriologic data could not be collected given limitations of the source data. Furthermore, the different definition between TB (national TB registry) and NTM-PD (health insurance claims) may lead to selection bias. In South Korea, cases of TB occurrence have been monitored in the Korean national TB program. However, NTM-PD has not been monitored by national authorities. Considering that the prevalence of NTM-PD has surpassed that of TB in South Korea (as of 2021)4, cases of NTM-PD should be monitored by national authorities. Recently, the nationwide observational cohort for NTM-PD in South Korea (NTM-KOREA) was launched to optimize treatment and understand the treatment outcomes and long-term prognosis of patients with NTM-PD40. Therefore, the relationship between smoking and NTM-PD could be further investigated under this program. A second limitation is that we could not collected information about other clinical risk factors that may affect NTM-PD and TB development, such as HIV infection and organ transplant. Finally, this study was performed in the Korean population; thus, generalizing our results to other ethnicities requires caution.
In conclusion, current smoking was associated with a decreased risk of NTM-PD development and increased risk of TB development. These risks were augmented by early smoking initiation and were prominent in the middle age population.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Griffith, D. E. et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175, 367–416. https://doi.org/10.1164/rccm.200604-571ST (2007).
Dahl, V. N. et al. Global trends of pulmonary infections with nontuberculous mycobacteria: A systematic review. Int. J. Infect. Dis. 125, 120–131. https://doi.org/10.1016/j.ijid.2022.10.013 (2022).
World Health Organization. Global tuberculosis report 2022. World Health Organization https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (2022).
Kim, J. Y., Kwak, N. & Yim, J. J. The rise in prevalence and related costs of nontuberculous mycobacterial diseases in South Korea, 2010–2021. Open Forum Infect. Dis. 9, ofac649. https://doi.org/10.1093/ofid/ofac649 (2022).
Lee, H., Myung, W., Koh, W. J., Moon, S. M. & Jhun, B. W. Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007–2016. Emerg. Infect. Dis. 25, 569–572. https://doi.org/10.3201/eid2503.181597 (2019).
Adjemian, J., Olivier, K. N., Seitz, A. E., Holland, S. M. & Prevots, D. R. Prevalence of nontuberculous mycobacterial lung disease in U.S. medicare beneficiaries. Am. J. Respir. Crit. Care Med. 185, 881–886. https://doi.org/10.1164/rccm.201111-2016OC (2012).
Cowman, S., van Ingen, J., Griffith, D. E. & Loebinger, M. R. Non-tuberculous mycobacterial pulmonary disease. Eur. Respir. J. 54, 1900250. https://doi.org/10.1183/13993003.00250-2019 (2019).
Zhang, H. et al. A dose-response relationship of smoking with tuberculosis infection: A cross-sectional study among 21008 rural residents in China. PLoS One 12, e0175183. https://doi.org/10.1371/journal.pone.0175183 (2017).
Kolappan, C. & Gopi, P. G. Tobacco smoking and pulmonary tuberculosis. Thorax 57, 964–966. https://doi.org/10.1136/thorax.57.11.964 (2002).
van Zyl Smit, R. N. et al. Global lung health: The colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD. Eur. Respir. J. 35, 27–33. https://doi.org/10.1183/09031936.00072909 (2010).
Adeloye, D. et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 10, 447–458. https://doi.org/10.1016/S2213-2600(21)00511-7 (2022).
Thomson, N. C., Chaudhuri, R. & Livingston, E. Asthma and cigarette smoking. Eur. Respir. J. 24, 822–833. https://doi.org/10.1183/09031936.04.00039004 (2004).
Miguez-Burbano, M. J. et al. Non-tuberculous mycobacteria disease as a cause of hospitalization in HIV-infected subjects. Int. J. Infect. Dis. 10, 47–55. https://doi.org/10.1016/j.ijid.2004.11.005 (2006).
Kotilainen, H. et al. Clinical symptoms and survival in non-smoking and smoking HIV-negative patients with non-tuberculous mycobacterial isolation. Scand. J. Infect. Dis. 43, 188–196. https://doi.org/10.3109/00365548.2010.535558 (2011).
O’Brien, D. P., Currie, B. J. & Krause, V. L. Nontuberculous mycobacterial disease in northern Australia: A case series and review of the literature. Clin. Infect. Dis. 31, 958–967. https://doi.org/10.1086/318136 (2000).
Shteinberg, M. et al. Prevalence, risk factors and prognosis of nontuberculous mycobacterial infection among people with bronchiectasis: A population survey. Eur. Respir. J. 51, 1702469. https://doi.org/10.1183/13993003.02469-2017 (2018).
Wickremasinghe, M. et al. Non-tuberculous mycobacteria in patients with bronchiectasis. Thorax 60, 1045–1051. https://doi.org/10.1136/thx.2005.046631 (2005).
Cheol Seong, S. et al. Data resource profile: The national health information database of the national health insurance service in South Korea. Int J Epidemiol 46, 799–800. https://doi.org/10.1093/ije/dyw253 (2017).
Seong, S. C. et al. Cohort profile: The National health insurance service-national health screening cohort (NHIS-HEALS) in Korea. BMJ Open 7, e016640. https://doi.org/10.1136/bmjopen-2017-016640 (2017).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: The national health insurance service-national sample cohort (NHIS-NSC) South Korea. Int. J. Epidemiol. 46, e15. https://doi.org/10.1093/ije/dyv319 (2017).
Shin, D. W., Cho, J., Park, J. H. & Cho, B. National general health screening program in Korea: History, current status, and future direction. Precis. Future Med. 6, 9–31. https://doi.org/10.23838/pfm.2021.00135 (2022).
Choi, H. et al. Female reproductive factors and incidence of nontuberculous mycobacterial pulmonary disease among postmenopausal women in Korea. Clin Infect Dis 75, 1397–1404. https://doi.org/10.1093/cid/ciac134 (2022).
Anuurad, E. et al. The new BMI criteria for asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J. Occup. Health 45, 335–343. https://doi.org/10.1539/joh.45.335 (2003).
Piercy, K. L. et al. The physical activity guidelines for Americans. JAMA 320, 2020–2028. https://doi.org/10.1001/jama.2018.14854 (2018).
Bates, M. N. et al. Risk of tuberculosis from exposure to tobacco smoke: A systematic review and meta-analysis. Arch. Intern. Med. 167, 335–342. https://doi.org/10.1001/archinte.167.4.335 (2007).
Lin, H. H., Ezzati, M. & Murray, M. Tobacco smoke, indoor air pollution and tuberculosis: A systematic review and meta-analysis. PLoS Med. 4, e20. https://doi.org/10.1371/journal.pmed.0040020 (2007).
Jee, S. H. et al. Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am. J. Epidemiol. 170, 1478–1485. https://doi.org/10.1093/aje/kwp308 (2009).
North, R. J. & Jung, Y. J. Immunity to tuberculosis. Annu. Rev. Immunol. 22, 599–623. https://doi.org/10.1146/annurev.immunol.22.012703.104635 (2004).
Cosio, M. G., Saetta, M. & Agusti, A. Immunologic aspects of chronic obstructive pulmonary disease. N. Engl. J. Med. 360, 2445–2454. https://doi.org/10.1056/NEJMra0804752 (2009).
Prince, D. S. et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N. Engl. J. Med. 321, 863–868. https://doi.org/10.1056/NEJM198909283211304 (1989).
Daley, C. L. & Winthrop, K. L. Mycobacterium avium Complex: Addressing gaps in diagnosis and management. J. Infect. Dis. 222, S199–S211. https://doi.org/10.1093/infdis/jiaa354 (2020).
Song, J. H., Kim, B. S., Kwak, N., Han, K. & Yim, J. J. Impact of body mass index on development of nontuberculous mycobacterial pulmonary disease. Eur. Respir. J. 57, 2000454. https://doi.org/10.1183/13993003.00454-2020 (2021).
Falkinham, J. O. 3rd. Ecology of nontuberculous mycobacteria–where do human infections come from?. Semin. Respir. Crit. Care Med. 34, 95–102. https://doi.org/10.1055/s-0033-1333568 (2013).
Feazel, L. M. et al. Opportunistic pathogens enriched in showerhead biofilms. Proc. Natl. Acad. Sci. U S A 106, 16393–16399. https://doi.org/10.1073/pnas.0908446106 (2009).
De Groote, M. A., Pace, N. R., Fulton, K. & Falkinham, J. O. 3rd. Relationships between Mycobacterium isolates from patients with pulmonary mycobacterial infection and potting soils. Appl. Environ. Microbiol. 72, 7602–7606. https://doi.org/10.1128/AEM.00930-06 (2006).
Blanchet, M. R., Israel-Assayag, E. & Cormier, Y. Inhibitory effect of nicotine on experimental hypersensitivity pneumonitis in vivo and in vitro. Am. J. Respir. Crit. Care Med. 169, 903–909. https://doi.org/10.1164/rccm.200210-1154OC (2004).
Newman, L. S. et al. A case control etiologic study of sarcoidosis: Environmental and occupational risk factors. Am. J. Respir. Crit. Care Med. 170, 1324–1330. https://doi.org/10.1164/rccm.200402-249OC (2004).
Ohtsuka, Y. et al. Smoking promotes insidious and chronic farmer’s lung disease, and deteriorates the clinical outcome. Intern. Med. 34, 966–971. https://doi.org/10.2169/internalmedicine.34.966 (1995).
Strom, K. E. & Eklund, A. G. Smoking does not prevent the onset of respiratory failure in sarcoidosis. Sarcoidosis 10, 26–28 (1993).
Kwak, N. et al. Protocol of a nationwide observational study among patients with nontuberculous mycobacterium pulmonary disease in South Korea (NTM-KOREA). Tuberc. Respir. Dis. (Seoul) 83, 141–146. https://doi.org/10.4046/trd.2019.0077 (2020).
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2023R1A2C2006688 and RS-2023-00222687, SWL), the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2022M3A9G8017220).
Author information
Authors and Affiliations
Contributions
Chiwook Chung, Kyu Na Lee, Kyungdo Han, Dong Wook Shin, and Sei Won Lee conceived and designed the study. Kyu Na Lee and Kyungdo Han contributed to the data collection and data analysis. Chiwook Chung, Dong Wook Shin, and Sei Won Lee contributed to data interpretation and drafted the manuscript. All authors revised and approved the final manuscript. All authors accept responsibility for the accuracy of the content in the final manuscript. Generative artificial intelligence was not used in any portion of manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funder did not have any role in the design of the study, in the data collection, analysis, interpretation or in writing the manuscript.
Ethics statement
This study protocol was approved by the Institutional Review Board of Asan Medical Center, Seoul, Republic of Korea (IRB No. 2023–0367). The requirement for informed consent was waived, because it was a retrospective study using anonymized data. This study complied with the guidelines stipulated in the Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chung, C., Lee, K.N., Han, K. et al. The effect of smoking on nontuberculous mycobacterial pulmonary disease and tuberculosis: a nationwide retrospective cohort study. Sci Rep 14, 22653 (2024). https://doi.org/10.1038/s41598-024-72438-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72438-4