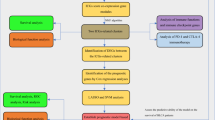
Abstract
Gremlin 1 (GREM1) can regulate the development of many cancers. However, a few studies have revealed the role of GREM1 in bladder cancer (BC). To evaluate the expression and potential function of GREM1 in bladder cancer, we used R version 3.6.3 and related packages to analyze the data from common databases. Samples from our institution were assessed by immunohistochemical staining (IHC), which was approved by the Institutional Ethics Committee (K20220830). GREM1 was highly expressed in BC tissues according to the TCGA and IHC data. Data from TCGA, GSE31684, GSE32894, and IHC showed that GREM1 has significant prognostic value for BC patients. GREM1 is involved in immune and metabolism-related pathways. According to the TIDE algorithm, 61.0% of patients with low GREM1 expression responded well to immunotherapy, compared to only 13.3% in the high GREM1 expression group. High GREM1 expression was associated with sensitivity to cisplatin, docetaxel, gemcitabine, and vinblastine. Thus, GREM1 can predict prognosis and responses to immunotherapy and chemotherapy in BC patients, making it a potential biomarker and therapeutic target.
Similar content being viewed by others
Introduction
Bladder cancer (BC) ranks as the 6th most common cancer in men1. Several factors contribute to the carcinogenesis of the bladder, including smoking2, employment in dye factories3, arsenic exposure4, and so on. According to the pathological manifestations, BC is clinically divided into nonmuscle-invasive BC and muscle-invasive BC5,6,7. Although multiple molecular subtypes have been reported, there is no consensus on the classification of molecular subtypes in clinical classes because BC is a highly heterogeneous disease8,9. With the continuous efforts of researchers, various treatments (including surgery, chemotherapy, radiotherapy, and immunotherapy) have been used to improve the prognosis of patients with BC10,11. Many problems appear with the application of these treatments. Radical cystectomy brings heavy economic, physical, and mental burdens to BC patients12,13. Many patients are resistant to chemotherapy and immunotherapy due to the high heterogeneity of BC14,15. Meanwhile, the efficiency of these treatments is still unsatisfactory. These puzzles promote the emergence of precision medicine16,17. Under the instruction of precision medicine, researchers find new medicine by screening targets18,19,20. On the other hand, doctors can choose the optimal therapy plan for each BC patient and reduce the cost of treatment while treating BC efficiently21,22.
Gremlin1 (GREM1), a highly conserved secreted protein, is required in the process of embryogenesis23,24,25. GREM1 can inhibit the activation of BMP signaling by binding to BMP2, BMP4 or BMP7 to form heterodimers26. Recently, some studies reported that GREM1 was highly expressed in tumor tissues and could regulate the progression of tumors. The epithelial-mesenchymal transition (EMT) induced by GREM1 was the cause of poor prognosis in patients with high GREM1 expression27,28. GREM1 acts as an oncogene in many cancers, such as prostate cancer29, glioma30, colorectal cancer31,32,33, lung cancer34, and esophageal squamous cell carcinoma28. However, in pancreatic ductal adenocarcinoma, GREM1 is required to maintain cellular heterogeneity in tumors and can restrict EMT by inhibiting the expression of the EMT transcription factors Snai1 and Snai235. Loss of GREM1 promotes the metastasis of pancreatic ductal adenocarcinoma, and GREM1 is a tumor suppressor gene in pancreatic ductal adenocarcinoma35. Furthermore, a study identified that knockdown of GREM1 could improve the chemotherapy sensitivity of cervical squamous carcinoma cells36. Zhu et al.29 reported that GREM1 was highly expressed in prostate cancer and could induce castration resistance by regulating the lineage plasticity of prostate cancer. These studies indicated the key role of GREM1 in tumors. However, the role of GREM1 in BC need to been further explored.
Therefore, in this study, we collected and analyzed BC data from an online database to identify the role of GREM1 in BC. Meanwhile, we performed immunohistochemical analysis (IHC) to validate the results of bioinformatics analysis by collecting the clinical samples in our hospital with the approval of the Institutional Ethics Committee. Furthermore, we also explored the function of GREM1 in immunotherapy and chemotherapy.
Materials and methods
Data acquisition from the online database
The expression data and clinical data of BC tissues and adjacent nontumor tissues were extracted from the Cancer Genome Atlas (https://www.gdc.cancer.gov, TCGA). The expression of GREM1 between 19 adjacent nontumor samples and 414 BC samples was compared by the ‘limma’ package. Then, we excluded patients with postoperative survival of less than 30 days, non-pure bladder urothelial carcinoma, or no survival outcome, as previous description18. We also assessed the clinical value of GREM1 using the GSE31684, and GSE32894 datasets downloaded from the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov, GEO) database.
Patients and clinical specimens
With the approval of the Ethics Committee of Taizhou Hospital (K20220830), we also verified the role of GREM1 in BC patients treated in our hospital. All patients provided written informed consent. A total of 195 BC patients were included in our study during the period between January 2017 and December 2019. Our study did not include patients with other malignancy histories, ages less than 18, nonpure transitional cell carcinoma, and postoperative survival periods less than 30 days. Clinical features were also collected, such as age, sex, TNM stages and so on. Clinical stages were determined according to the criteria of the 8th edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) system37.
Immunohistochemical analysis
Samples from transurethral resection of bladder tumors or radical cystectomy were cut into 3-µm sections, including tumor tissue and tumor-adjacent tissues. The sections from paraffin blocks were deparaffinized and rehydrated. After being autoclaved for antigen repair, endogenous peroxidase was inactivated by treatment of the sections with 3% hydrogen peroxide solution. Then, the sections were incubated with 10% fetal calf serum for 30 min, washed in PBS, and incubated with rabbit anti-GREM1 antibody (1:800, #DF15419, Affbiotech, USA) for 1.5 h at room temperature. This was followed by incubation with appropriate secondary horseradish peroxidase-linked antibodies for 30 min. Then, each section was developed with diaminobenzidine and counterstained with hematoxylin.
To semiquantitatively represent the GREM1 immunostaining results, evaluable sections were classified into seven IHC scores with the following scale: Staining intensity scores: 0, unstained (None); 1, Light yellow (weak); 2, Brownish yellow (medium); 3, Brown (strong). Quantity scores: 0, no staining or nuclear staining in less than 1% of cells; 1, nuclear staining in 1–10% of cells and/or with weak cytoplasmic staining; 2, nuclear staining in 10–50% of cells and/or with distinct cytoplasmic staining; and 3, nuclear staining in more than 50% of cells and/or with strong cytoplasmic staining. All sections were scored independently by two experienced pathologists. According to previous reports38,39,40, the IHC score was calculated by multiplying the staining intensity score and the quantity score. Patients with IHC score of 0 to 3 were divided into low GREM1 expression group, and patients with IHC score of 4 to 9 were divided into high GREM1 expression group.
Clinical value of GREM1
We explored the correlation between clinical parameters and the expression of GREM1 using the Wilcoxon rank-sum test in the TCGA dataset, GSE31684 dataset, GSE32894 dataset and our cohort. Then, Kaplan‒Meier curves were used to evaluate the prognostic value of GREM1 in the TCGA dataset, GSE31684 dataset, GSE32894 dataset and our cohort.
Functional analysis
Based on the TCGA dataset, we performed Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis41. The criteria for selecting the GO terms and KEGG pathways were as follows: P value < 0.05 and Q < 0.0542. The results of GO enrichment analysis are shown in three sections: molecular function (MF), biological process (BP), and cellular component (CC). To explore the potential pathways, Gene Set Enrichment Analysis (GSEA) was utilized to enrich the REACTOME pathways. The criteria for selecting the REACTOME pathways were as follows: P value < 0.05 and FDR < 25%. GeneMANIA (www.genemania.org)43 was employed to screen the interacting proteins of GREM1.
Immune-related analysis
The results of functional analysis revealed that GREM1 could be involved in immune-related pathways. Therefore, we analyzed the performance of GREM1 in immune-related analysis based on the TCGA dataset. First, we compared the immune checkpoints between the low- and high-GREM1 groups. Based on the CIBERSORT algorithm, we compared the contents of infiltrated immune cells between the low- and high-GREM1 groups44. Moreover, an mRNA expression-based stemness index (mRNAsi) was established by a one-class logistic regression machine learning algorithm45. Then, we assessed the relationship between the mRNAsi score and GREM1 expression. The immunotherapy response of each patient was evaluated by the Tumor Immune Dysfunction and Exclusion (TIDE) algorithm46. In terms of chemotherapy prediction, the “pRRophetic” package was used to calculate the half-maximal inhibitory concentration (IC50), which was the main endpoint of the chemotherapy response and drug sensitivity47.
Statistical analysis
Based on the normality and variance characteristics of the data, we employed either one-way ANOVA or the Mann‒Whitney U test to conduct statistical analyses on datasets comprising three or more continuous variables. The comparison of quantitative data between two groups was accomplished through Student’s t test. Standard deviation (SD) was adopted to present all analyzed data. Statistical significance across all analyses was inferred at a significance level of P < 0.05. The statistical procedures were executed using R version 3.6.3 in conjunction with relevant packages. Notations used are as follows: ns, representing P ≥ 0.05; *, representing P < 0.05; **, representing P < 0.01; ***, representing P < 0.001.
Results
Information characteristics of the online database and IHC data
The log2FoldChange of GREM1 was 1.31 (P = 0.011), indicating that GREM1 was highly expressed in BC tissues compared with unpaired (Fig. 1A) and paired (Fig. 1B) adjacent nontumor tissues. Moreover, GREM1 was also more highly expressed in tumor tissues than adjacent nontumor tissues, such as breast invasive carcinoma, cholangiocarcinoma, and head and neck squamous cell carcinoma (Fig. 1C). A total of 390 BC patients in the TCGA dataset were finally included in our study. Patients were divided into low- and high-GREM1 groups based on the median value of GREM1 in the TCGA, GSE31684, and GSE32894 datasets. In the TCGA dataset, patients in the high-GREM1 group were significantly associated with higher age, World Health Organization (WHO) high grade, lymph node metastasis, T3_4 stage, and worse overall survival (OS). Detailed information on the TCGA, GSE31684, and GSE32894 datasets is shown in Table 1, Supplementary Tables 1, and Supplementary Table 2, respectively.
The expression of GREM1 in unpaired (A) and paired (B) tissues. The expression of GREM1 in various tumors (C). The correlation between GREM1 expression and clinical parameters: TCGA: WHO grade (D), T stage (E), and lymph node metastasis (F), and smoking history (G); GSE31684: WHO grade (H), T stage (I), lymph node metastasis (J), and smoking history (K); GSE32894: WHO grade (L), T stage (M), age (N), and sex (O). N: lymph node metastasis; WHO: World Health Organization. ns, P ≥ 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We collected 195 BC patients from our institution to validate the results of the bioinformatics analysis. Patients with IHC scores less than 2 were assigned to the low-GREM1 group, and the rest were assigned to the high-GREM1 group. According to the IHC results, patients in the high GREM1 group were statistically correlated with WHO high grade, T2_4 stage, poor OS, poor cancer-specific survival (CSS), and poor recurrence-free survival (RFS). Table 2 presents the detailed information.
GREM1 has clinical value
As shown in Fig. 1D-G, in the TCGA dataset, the expression of GREM1 was significantly correlated with invasive clinical features, such as WHO high grade, T3_4 stage, lymph node metastasis, and smoking history. In the GSE31684 dataset, GREM1 was highly expressed in patients with WHO high-grade (Fig. 1H), T2_4 (Fig. 1I), lymph node metastasis (Fig. 1J), but there was no difference in GREM1 expression among patients with or without smoking history(Fig. 1K). Similarly, in GSE32894, the expression of GREM1 was significantly higher in WHO high grade (Fig. 1L) or T3_4 (Fig. 1M) patients, while no difference was found in GREM1 expression among patients of different ages (Fig. 1N) and sex (Fig. 1O). These results suggested that the expression of GREM1 was significantly correlated with aggressive clinical features and could be applied to patients regardless of age and sex.
In terms of prognostic value, patients with high GREM1 expression had significantly worse OS than those with low GREM1 expression (Fig. 2A, P = 0.042) in the TCGA dataset. In TCGA subgroup analysis, high GREM1 expression was also significantly associated with poor OS in the Ta_2 (Fig. 2B, P = 0.003), no lymph node metastasis (Fig. 2C, P = 0.019), and no distant metastasis (Fig. 2D, P = 0.023) subgroups. Furthermore, GREM1 could predict the OS of patients in the GSE31684 dataset (Fig. 2E, P = 0.006) and patients in the GSE31684 subgroups, including the Ta_2 (Fig. 2F, P = 0.009), no distant metastasis (Fig. 2G, P = 0.034), and age ≤ 70 years (Fig. 2H, P = 0.046) subgroups. Similarly, GREM1 could predict the OS of patients in the GSE32894 dataset (Fig. 2I, P < 0.001) and patients in the GSE32894 subgroups, including the WHO G3 (Fig. 2J, P = 0.032), Ta_2 (Fig. 2K, P < 0.001), and age ≤ 70 years (Fig. 2L, P = 0.001) subgroups. Together, these results indicated that GREM1 had significant prognostic value in BC patients.
The prognostic value of GREM1: TCGA (A) and TCGA subgroups: Ta_2 (B), no lymph node metastasis (C), and no distant metastasis (D); GSE31684 (E) and GSE31684 subgroups: Ta_2 (F), no distant metastasis (G), and age < = 70 years (H); GSE32894 (M) and GSE32894 subgroups: WHO G3 (J), Ta_2 (K), and age < = 70 years (L). WHO: World Health Organization, OS: overall survival. ns, P ≥ 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Validation of the clinical value of GREM1
To validate the results of bioinformatics analysis, we performed IHC to identify these findings at the protein level. Figure 3A presents GREM1 protein expression in BC tissues and corresponding adjacent nontumor tissues. In accordance with the level of mRNA expression, BC tissues highly expressed GREM1 in both unpaired (Fig. 3B) and paired (Fig. 3C) comparisons at the protein level. In our cohort, patients with WHO high grade (Fig. 3D) or T2_4 stage (Fig. 3E) had higher GREM1 expression than their counterparts. There were no differences in GREM1 expression among patients with or without smoking history (Fig. 3F) and different sex (Fig. 3G), which was consistent with the results of bioinformatics analysis. Furthermore, in terms of prognostic value, patients in the high GREM1 group had significantly worse OS (Fig. 3H, P = 0.001), CSS (Fig. 3I, P = 0.008), and RFS (Fig. 3J, P = 0.024) than those in the low GREM1 group. In subgroups, GREM1 could also predict the OS of patients in the age > 70 years (Fig. 3K, P = 0.013), male (Fig. 3L, P = 0.005), and Ta_1 (Fig. 3M, P = 0.002) subgroups. Taken together, these results supported that GREM1 was significantly associated with clinical parameters and had significant prognostic value for BC patients.
Validation of the bioinformatics results of GREM1: representative IHC staining for GREM1 in BC and adjacent nontumor tissues (A); the expression of GREM1 in unpaired (B) and paired (C) tissues; the correlation between GREM1 expression and clinical parameters: WHO grade (D), Ta_1 (E), smoking history (F), age (G); the prognostic value of GREM1 in IHC data: overall survival (H), cancer-specific survival (I), recurrence-free survival (J); the prognostic value of GREM1 in IHC subgroups: age > 70 years (K), male (L), and Ta_1 (M); WHO: World Health Organization, IHC: Immunohistochemical analysis. ns, P ≥ 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
GREM1 was involved in immune-related and metabolism-related pathways
Based on TCGA data, we performed functional analysis. In the GO and BP results, GREM1 was involved in the process of leukocyte migration, lymphocyte differentiation, and leukocyte chemotaxis. The CC and MF results identified that GREM1 was significantly associated with collagen-containing extracellular matrix, collagen trimer, cytokine activity, and cytokine binding (Fig. 4A). The KEGG results showed that GRME1 was enriched in the chemokine signaling pathway, leukocyte trans-endothelial migration, and drug metabolism-cytochrome P450 pathways (Fig. 4B). Consistent with the results of GO and KEGG analysis, the GSEA results of GREM1 also enriched metabolism-related pathways, such as fatty acid metabolism, synthesis of leukotrienes-lt and -eoxins ex, and cytochromep450_arranged_by_substrate_type pathways (Fig. 4C). Furthermore, the GSEA results also enriched immune-related pathways, including signaling by interleukins, chemokine receptors bind chemokines, and antigen activates B-cell receptor BCR, leading to the generation of second messenger pathways (Fig. 4D). GREM1 protein was correlated with KDR, GREM2, and BAMBI proteins (Fig. 4E).
GREM1 could predict immunotherapy and chemotherapy responses
The results of functional analysis revealed that GREM1 was involved in immune-related pathways. Therefore, we explored the performance of GREM1 in immune-related analysis. Based on TCGA data, many immune checkpoints, including CD274, PDCD1 and CTLA4, were differentially expressed between the low- and high-GREM1 groups (Fig. 5A). In the field of the tumor microenvironment (TME), the low-GREM1 group had significantly higher T follicular helper cell and monocyte cell infiltration infiltration than the high-GREM1 group (Fig. 5B).
Immune-related analysis based on TCGA dataset: immune checkpoints (A), immune infiltration (B), the correlation between stemness index and GREM1 expression (C), the GREM1 expression between the low- and high-GREM1 groups (D), the correlation between TIDE score and GREM1 expression (E), the TIDE score between the low- and high-GREM1 groups (F), the GREM1 expression between the patients with or without response to immunotherapy (G), the number of the patients with or without response to immunotherapy between the low- and high-GREM1 groups (H). The IC50 of chemotherapy between the low- and high-GREM1 groups (I). mRNAsi: stemness index, IC50: half-maximal inhibitory concentration. ns, P ≥ 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
GREM1 expression was negatively associated with the stemness index (Fig. 5C). The MRNAsi score in the low GREM1 group was significantly higher than that in the high GREM1 group (Fig. 5D). According to the results of Spearman analysis, a positive correlation was demonstrated between the TIDE score and GREM1 expression (Fig. 5E). The TIDE score in the low-GREM1 group was significantly lower than that in the high-GREM1 group (Fig. 5F). Meanwhile, GREM1 expression was significantly lower in patients who responded to immunotherapy than in those who did not (Fig. 5G). In further prediction, 61.0% (119/195) of patients in the low GREM1 group were effective for immunotherapy, higher than 13.3% (26/195) in the high GREM1 group (Fig. 5H). In chemotherapy prediction, patients in the high-GREM1 group were sensitive to cisplatin, docetaxel, gemcitabine, and vinblastine (Fig. 5I). The results in this section indicated that GREM1 could predict immunotherapy and chemotherapy responses.
Discussion
BC is widely considered a highly heterogeneous disease, leading to treatment difficulty8. Recently, some studies have reported that GREM1 is required to maintain cellular heterogeneity in tumors27,29,35. Meanwhile, GREM1 can regulate carcinoma development and response to chemotherapy30,31. However, a few studies have reported the role of GREM1 in BC. Thus, our study identified that GREM1 was highly expressed in BC tissues and had significant clinical value for BC patients. Furthermore, GREM1 also presented predictive value for the response to immunotherapy and chemotherapy. Taken together, we suggest that GREM1 may be a biomarker and target of BC.
Our study confirmed that GREM1 was highly expressed in BC tissues at both the mRNA and protein levels. This finding was consistent with many studies that reported that GREM1 expression levels were significantly increased in prostate cancer48, lung cancer34, cervical cancer49, and so on. Furthermore, Cheng et al.50 reported that GREM1 was highly expressed in BC tissues than in adjacent non-tumor tissues in mRNA and protein levels, which was consisted to our results. In clinical value analysis, the expression of GREM1 was statistically associated with poor clinical features. Cao et al.30 also found that glioma patients with high histological stage had higher GREM1 expression than those with low histological stage. EMT induced by GREM1 might be the cause of aggressive clinical features in patients with high GREM1 expression27,28. Age plays a key role in human disease51. Some studies have reported the significant role of aging in cancer development52,53,54. Thus, we need to consider the correlation between GREM1 expression and age. There was no significant correlation between GREM1 expression and basic clinical features (including age, sex, and smoking history), indicating that GREM1 could be applied to BC patients regardless of age, sex, and smoking history. In glioma, there was also no significant correlation between GREM1 expression and age and sex30. These results broaden the clinical application of GREM1. In terms of prognostic value, GREM1 could predict the prognosis of BC patients in both the online dataset and our IHC data. Consistent with our results, Holien et al.55 also reported that ER-negative breast cancer patients with high GREM1 expression had a worse prognosis than those with low GREM1 expression. Moreover, GREM1 could predict the prognosis of patients with colorectal cance31, prostate cancer29, oral squamous cell carcinoma27, and clear cell renal cell cancer56. GREM1 could induce EMT by activating ATF6 and inhibiting ATF4 pathways, which would enhance the motility and invasion of colorectal cancer cells57. In prostate cancer, as a ligand for FGFR1, GREM1 could cause castration resistance by promoting the lineage plasticity of prostate cancer cells29. These findings might explain why patients with high GREM1 expression had poor prognosis. Consistent with these studies, this research also found that GREM1 could predict the prognosis of BC patients. Together, these results suggest that GREM1 is highly expressed in BC tissues and has significant clinical value for BC patients.
GREM1 was a secreted protein, which was consistent with the CC results; that is, GREM1 was associated with collagen-containing extracellular matrix and collagen trimer. Recently, Zhu et al.29 reported that GREM1 could regulate the lineage plasticity of prostate cancer cells by binding to FGFR1. Consistent with the literature, our study found that GREM1 was involved in growth factor binding according to the GO results. Furthermore, in KEGG analysis, GREM1 enriched the extracellular matrix (ECM)-receptor interaction pathway, which could regulate cellular activities, such as migration, differentiation, and proliferation. These enriched results identified a secreted protein role of GREM1 in BC and supported the findings of previous studies. Meanwhile, functional analysis, including GO, KEGG, and GSEA analyses, indicated that GREM1 was significantly correlated with metabolism-related and immune-related pathways. In immune-related pathways, GREM1 could affect the regulation of immunoregulatory factors (including signaling by interleukins and chemokine receptors that bind chemokines) and can also participate in the regulation of immune cells (including leukocyte migration, lymphocyte differentiation, and B-cell-related pathways). The interacting proteins of GREM1, kinase insert ___domain receptor (KDR), one of the two receptors of VEGF58, could affect the effectiveness of bevacizumab therapy in colorectal cancer59 and inhibit BC angiogenesis and growth in vivo60. Conclusively, the results of functional analysis identified that GREM1 might regulate BC development and the efficiency of treatments.
There was a strong relationship between GREM1 and immune-related pathways according to the results of functional analysis. Thus, we explored the role of GREM1 in BC by immune-related analysis. Compared with samples in the low-GREM1 group, tissues in the high-GREM1 group had higher immune scores for many checkpoints, such as CD274, PDCD1, and CTLA4. However, there is no agreement on the predictive role of immune checkpoints for immunotherapy in BC61,62. We could only speculate that the immune status between the high- and low-GREM1 groups was different. The next analysis assessed the TME. Since the application of single-cell sequencing, our understanding of the TME has significantly deepened. The infiltrating cells, including cancer cells, immune cells, and other stromal cells, significantly influence the progression of cancer, novel drug development and treatment responses63,64,65. TME analysis showed that the low-GREM1 group had significantly higher T follicular helper cell infiltration than the high-GREM1 group. T follicular helper cells were positively associated with OS in patients with breast cancer66. In accordance with the study, with high T follicular helper cell infiltration, patients in the low-GREM1 group were correlated with long-term survival. Meanwhile, T follicular helper cells are required in cytotoxic transcriptional programs67. CD8 + T cells expressing T follicular helper cell-associated genes respond most robustly to PD1 immunotherapy67. Therefore, patients in the low GREM1 group might be more likely to benefit from immunotherapy. In stemness analysis, patients with a high mRNAsi score usually had a higher response rate to immunotherapy than those with a low mRNAsi score45. In our study, a significant positive correlation was identified between the mRNAsi score and GREM1 expression. The MRNAsi score was higher in the low-GREM1 group than in the high-GREM1 group, which supported that patients in the low-GREM1 group might be more likely to benefit from immunotherapy. Referring to the results of TIDE analysis, 61.0% (119/195) of patients in the low-GREM1 group were effective for immunotherapy, higher than 13.3% (26/195) in the high-GREM1 group. Despite no significant statistical difference in CD8 + T cell infiltration between high and low GREM1 expression groups, the low GREM1 expression group may exhibit a better response to immunotherapy. This could be due to the activation and suppression states of CD8 + T cells. According to the study by Meng et al.68, CD8 + T cells within the TME can exist in both activated and suppressed states. Patients tend to respond better to immunotherapy when these cells are activated, and worse when suppressed. Therefore, we should focus not only on the quantity of immune cell infiltration (which can help predict immune response69) but also on the state of the immune cells. Together, we suggest that GREM1 could predict the response rate to immunotherapy and that patients in the low GREM1 group might be more likely to benefit from immunotherapy. Chemoresistance is a clinical difficulty in BC management70. In chemotherapy prediction, the results indicated that chemotherapy was more suitable for patients in the high-GREM1 group, which was sensitive to cisplatin, docetaxel, gemcitabine, and vinblastine.
Cheng et al.50 found that GREM1 is overexpressed in BC and, based on the TCGA database, discovered that GREM1 expression is negatively correlated with the prognosis of BC patients. They also identified that GREM1 could promote BC progression through the PI3K/AKT pathway. Compared to the study by Cheng et al., our paper not only contrasts multiple external validation datasets but also utilizes actual patient data and specimens from our center, demonstrating that GREM1 can predict the prognosis of BC patients in the real world. Furthermore, through additional bioinformatics analysis, it has been shown that GREM1 may play a significant role in the TME and immunotherapy. It can also predict patients’ responses to immunotherapy and chemotherapy, facilitating the development of personalized treatment plans. With the advent of the aging era, the burden of medicine is increasingly heavy. Precision medicine is attracting the attention of researchers because it can effectively alleviate or even solve the problem at a lower cost. These results are based on online databases and clinical samples, which are more reliable and have strong clinical relevance.
Conclusion
GREM1 can predict the prognosis, immunotherapy and chemotherapy responses of BC patients, indicating that it is a biomarker and target of BC. Of course, the specific mechanism of GREM1 in BC remains to be further studied, which is our next step.
Data availability
Since human data are involved, you should contact the corresponding authors to obtain relevant information. The datasets utilized and examined in the present study are accessible in the TCGA database, specifically the Cancer Genome Atlas (www.gdc.cancer.gov, TCGA). Additionally, the GSE31684and GSE32894 datasets were acquired from the Gene Expression Omnibus database repository (https://www.ncbi.nlm.nih.gov, GEO).
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Kim, H. J. et al. Familial risk and interaction with smoking and alcohol consumption in bladder cancer: A population-based cohort study. World J. Oncol. 14, 382–391 (2023).
Walker, B. Jr. & Gerber, A. Occupational exposure to aromatic amines: benzidine and benzidine-based dyes. Natl. Cancer Inst. Monogr. 11–13 (1981).
Bakare, S. O. et al. Arsenicosis in bladder pathology and schistosomiasis in Eggua, Nigeria. Trans. R Soc. Trop. Med. Hyg. 112, 230–237 (2018).
Li, D. X. et al. Efficiency of bladder-sparing strategies for bladder cancer: An umbrella review. Ther. Adv. Med. Oncol. 16, 17588359241249068 (2024).
Li, D. X. et al. Efficiency of transurethral en-bloc resection vs. conventional transurethral resection for non-muscle-invasive bladder cancer: An umbrella review. Cancer Med. 13, e7323 (2024).
Jin, Y. H. et al. Treatment and surveillance for non-muscle-invasive bladder cancer: A clinical practice guideline (2021 edition). Mil Med. Res. 9, 44 (2022).
EAU Guidelines. Edn. Presented at the EAU Annual Congress Paris 2024. ISBN 978-94-92671-23-3.
Li, D. et al. Clinical characteristics and prognosis of rare histological variants of bladder cancer: A single-center retrospective study from China. Cancer Manag. Res. 12, 9635–9641 (2020).
Feng, D., Li, D., Wu, R. & Han, P. Scientific advancements in drug development and trials for urothelial carcinoma: Insights from the 2023 ASCOGU cancers symposium. Aging Dis. (2023).
Li, D. et al. Natural products and derivatives in renal, urothelial and testicular cancers: targeting signaling pathways and therapeutic potential. Phytomedicine Int. J. Phytother. Phytopharmacol. (2024).
Zhong, W. et al. Long-term survival after female pelvic organ-sparing radical cystectomy versus standard radical cystectomy: A multi-institutional propensity score-matched analysis. Int. J. Surg. 109, 2742–2750 (2023).
Bicchetti, M. et al. A novel pathway to detect muscle-invasive bladder cancer based on integrated clinical features and VI-RADS score on MRI: Results of a prospective multicenter study. Radiol. Med. 127, 881–890 (2022).
Li, D. X. et al. Prognostic value of preoperative neutrophil-to-lymphocyte ratio in histological variants of non-muscle-invasive bladder cancer. Investig. Clin. Urol. 62, 641–649 (2021).
Chen, J. et al. The complementary and alternative roles of elemene injection in cancer: An umbrella review. Pharmacol. Res. 198, 107007 (2023).
Yuan, M. et al. mRNA nanodelivery systems: targeting strategies and administration routes. Biomater. Res. 27, 90 (2023).
Li, G. et al. Small-molecule nanoprodrug with high drug loading and EGFR, PI3K/AKT dual-inhibiting properties for bladder cancer treatment. Explor. (Beijing China). 3, 20220141 (2023).
Yu, Q. X. et al. Adhesion-regulating molecule 1 (ADRM1) can be a potential biomarker and target for bladder cancer. Sci. Rep. 13, 14803 (2023).
Tuo, Z. et al. Unveiling clinical significance and tumor immune landscape of CXCL12 in bladder cancer: Insights from multiple omics analysis. Chin. J. Cancer Res. 35, 686–701 (2023).
Fan, Z. et al. Diagnostic and therapeutic effects of fluorescence cystoscopy and narrow-band imaging in bladder cancer: A systematic review and network meta-analysis. Int. J. Surg. 109, 3169–3177 (2023).
Ruiz de Porras, V. Natural bioactive compounds: A potential therapeutic strategy to sensitize bladder cancer to cisplatin treatment? Cancer Drug Resist. 5, 339–343 (2022).
Xue, X., Qu, H. & Li, Y. Stimuli-responsive crosslinked nanomedicine for cancer treatment. Explor. (Beijing China). 2, 20210134 (2022).
Verheyden, J. M. & Sun, X. An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature. 454, 638–641 (2008).
Costello, C. M., Cahill, E., Martin, F., Gaine, S. & McLoughlin, P. Role of gremlin in the lung: Development and disease. Am. J. Respir. Cell Mol. Biol. 42, 517–523 (2010).
Bénazet, J. D. et al. A self-regulatory system of interlinked signaling feedback loops controls mouse limb patterning. Sci. (New York NY). 323, 1050–1053 (2009).
Hsu, D. R., Economides, A. N., Wang, X., Eimon, P. M. & Harland, R. M. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol. Cell. 1, 673–683 (1998).
Wang, Y., Jiang, Y. & Chen, L. Role of mir-218-GREM1 axis in epithelial-mesenchymal transition of oral squamous cell carcinoma: An in vivo and vitro study based on microarray data. J. Cell. Mol. Med. 24, 13824–13836 (2020).
Hong, D. et al. Gremlin1 delivered by mesenchymal stromal cells promoted epithelial-mesenchymal transition in human esophageal squamous cell carcinoma. Cell. Physiol. Biochemistry: Int. J. Experimental Cell. Physiol. Biochem. Pharmacol. 47, 1785–1799 (2018).
Cheng, C. et al. Gremlin1 is a therapeutically targetable FGFR1 ligand that regulates lineage plasticity and castration resistance in prostate cancer. Nat. Cancer. 3, 565–580 (2022).
Guan, Y., Cheng, W., Zou, C., Wang, T. & Cao, Z. Gremlin1 promotes carcinogenesis of glioma in vitro. Clin. Exp. Pharmacol. Physiol. 44, 244–256 (2017).
Davis, H. et al. Aberrant epithelial GREM1 expression initiates colonic tumorigenesis from cells outside the stem cell niche. Nat. Med. 21, 62–70 (2015).
Mitroi, A. F. et al. Association of TCF7L2, CASC8 and GREM1 polymorphisms in patients with colorectal cancer and type II diabetes mellitus. Genes (Basel) 13 (2022).
Kobayashi, H. et al. The balance of stromal BMP signaling mediated by GREM1 and ISLR drives colorectal carcinogenesis. Gastroenterology. 160, 1224–1239e1230 (2021).
Kan, J. et al. He-Chan Pian inhibits the metastasis of non-small cell lung cancer via the mir-205-5p-mediated regulation of the GREM1/Rap1 signaling pathway. Phytomed. Int. J. Phytother. Phytopharmacol. 94, 153821 (2022).
Lan, L. et al. GREM1 is required to maintain cellular heterogeneity in pancreatic cancer. Nature. 607, 163–168 (2022).
Miao, H., Song, W. B., Zhu, H., Wang, Q. & Tian, Y. Effect of GREM 1 gene on chemoradiotherapy sensitivity of cervical squamous carcinoma cells. Eur. Rev. Med. Pharmacol. Sci. 24, 1072–1080 (2020).
Amin, M. B. et al. The Eighth Edition AJCC Cancer staging Manual: continuing to build a bridge from a population-based to a more personalized approach to cancer staging. CA Cancer J. Clin. 67, 93–99 (2017).
Hayashi, R. et al. Expression of placenta-specific 1 and its potential for eliciting anti-tumor helper T-cell responses in head and neck squamous cell carcinoma. Oncoimmunology. 10, 1856545 (2020).
Bi, C. et al. High Beclin-1 and ARID1A expression corelates with poor survival and high recurrence in intrahepatic cholangiocarcinoma: A histopathological retrospective study. BMC Cancer. 19, 213 (2019).
Dai, F. et al. Liposomal curcumin inhibits hypoxia-induced angiogenesis after transcatheter arterial embolization in VX2 rabbit liver tumors. Onco Targets Ther. 8, 2601–2611 (2015).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–d592 (2023).
Feng, D. et al. Developing an immune-related gene prognostic index associated with progression and providing new insights into the tumor immune microenvironment of prostate cancer. Immunology. 166, 197–209 (2022).
Warde-Farley, D. et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 38, W214–220 (2010).
Zhang, F. et al. Development and validation of a hypoxia-related signature for predicting survival outcomes in patients with bladder cancer. Front. Genet. 12, 670384 (2021).
Malta, T. M. et al. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell. 173, 338–354e315 (2018).
Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 24, 1550–1558 (2018).
Li, D. X. et al. A novel endothelial-related prognostic index by integrating single-cell and bulk RNA sequencing data for patients with kidney renal clear cell carcinoma. Front. Genet. 14, 1096491 (2023).
Sena, L. A., Brennen, W. N. & Isaacs, J. T. There are gremlins in prostate cancer. Nat. cancer. 3, 530–531 (2022).
Sun, Q., Qi, X., Zhang, W. & Li, X. Knockdown of circRNA_0007534 suppresses the tumorigenesis of cervical cancer via miR-206/GREM1 axis. Cancer Cell. Int. 21, 54 (2021).
Jiang, P. C., Xu, L. Z., Ning, J. Z. & Cheng, F. GREM1 is a potential biomarker for the progression and prognosis of bladder cancer. World J. Surg. Oncol. 21, 255 (2023).
Fakhri, S., Moradi, S. Z., Ash-Rafzadeh, A. & Bishayee, A. Targeting cellular senescence in cancer by plant secondary metabolites: A systematic review. Pharmacol. Res. 177, 105961 (2022).
Schwartz, A. G. Dehydroepiandrosterone, cancer, and aging. Aging Dis. 13, 423–432 (2022).
Wang, J., Wei, J. & Inuzuka, H. Aging and cancer hallmarks as therapeutic targets. Acta Mater. Med. 2 (2023).
Jia, Z. et al. Immune-ageing evaluation of peripheral T and NK lymphocyte subsets in Chinese healthy adults. Phenomics. 3, 360–374 (2023).
Neckmann, U. et al. GREM1 is associated with metastasis and predicts poor prognosis in ER-negative breast cancer patients. Cell. Commun. Signal. 17, 140 (2019).
van Vlodrop, I. J. H. et al. A four-gene promoter methylation marker panel consisting of GREM1, NEURL, LAD1, and NEFH predicts survival of clear cell renal cell cancer patients. Clin. Cancer Res. 23, 2006–2018 (2017).
Li, R., Zhou, H., Li, M., et al. Gremlin-1 Promotes Colorectal Cancer Cell Metastasis by Activating ATF6 and Inhibiting ATF4 Pathways. Cells. 11(14), 2136 (2022).
Peng, F. W., Liu, D. K., Zhang, Q. W., Xu, Y. G. & Shi, L. VEGFR-2 inhibitors and the therapeutic applications thereof: A patent review (2012–2016). Expert Opin. Ther. Pat. 27, 987–1004 (2017).
Wang, F. & Liu, G. Influence of KDR genetic variation on the effectiveness and safety of Bevacizumab in the first-line treatment for patients with advanced colorectal cancer. Int. J. Gen. Med. 15, 5651–5659 (2022).
Zhang, Z. et al. Extracellular ___domain of kinase ___domain region mediated by adeno-associated virus inhibits growth and angiogenesis of bladder cancer in Balb-c mice. Chin. Med. J. 115, 1209–1212 (2002).
Frostberg, E. et al. The prevalence of pathogenic or likely pathogenic germline variants in a Nationwide Cohort of Young Colorectal Cancer patients using a panel of 18 genes associated with colorectal cancer. Cancers (Basel) 13 (2021).
Butler, S. A., Staite, E. M. & Iles, R. K. Reduction of bladder cancer cell growth in response to hCGbeta CTP37 vaccinated mouse serum. Oncol. Res. 14, 93–100 (2003).
Zhang, J., Guo, F., Li, L., Zhang, S. & Wang, Y. Immune evasion and therapeutic opportunities based on natural killer cells. Chin. J. Cancer Res. 35, 283–298 (2023).
Chen, Q., Guo, X. & Ma, W. Opportunities and challenges of CD47-targeted therapy in cancer immunotherapy. Oncol. Res. 32, 49–60 (2023).
Yoon, W. H., DeFazio, A. & Kasherman, L. Immune checkpoint inhibitors in ovarian cancer: Where do we go from here? Cancer Drug Resist. 6, 358–377 (2023).
Bindea, G. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 39, 782–795 (2013).
Im, S. J. et al. Defining CD8 + T cells that provide the proliferative burst after PD-1 therapy. Nature. 537, 417–421 (2016).
Meng, J. et al. Tumor immune microenvironment-based classifications of bladder cancer for enhancing the response rate of immunotherapy. Mol. Ther. Oncol.. 20, 410–421 (2021).
Huang, M. et al. Identification of immune-related subtypes and characterization of tumor microenvironment infiltration in bladder cancer. Front. Cell. Dev. Biol. 9, 723817 (2021).
Wang, X. et al. Targeted inhibition of P4HB promotes cell sensitivity to Gemcitabine in urothelial carcinoma of the bladder. Onco Targets Ther. 13, 9543–9558 (2020).
Acknowledgements
We appreciate Figdraw (www.figdraw.com) for their assistance in the drawing process.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Interpretation of the data was performed by QXY, SSX, SXW, LXY, HHZ and DXL. QXY, SSX and DXL planned and performed IHC experiments. The first draft of the manuscript was written by QXY and SSX. The final version of the manuscript was revised by HHZ and DXL. All authors commented on previous versions of the manuscript, and all authors had access to the data and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical considerations
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Taizhou Hospital (K20220830), and informed consent was obtained from all patients.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, Q., Xu, S., Weng, S. et al. GREM1 may be a biological indicator and potential target of bladder cancer. Sci Rep 14, 23280 (2024). https://doi.org/10.1038/s41598-024-73655-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73655-7