Abstract
Previous studies have reported that senolytic drugs can reverse obesity-mediated accumulation of senescent cells in the ovary and protect against cisplatin-induced ovarian injury by removing senescent cells. Early intervention with ABT-263 has been shown to mitigate ovarian aging. However, it remains unknown whether treatment with ABT-263 could rejuvenate the aged ovary in reproductively old females. Therefore, the current study was aimed to investigate whether advanced age intervention with ABT-263 could ameliorate age-related decline in ovarian function. Fourteen 16-month-old mice with a C57/BL6 background were treated with ABT-263 (N = 7) or vehicle (N = 7) for two weeks. Mice were initially treated with ABT-263 (60 mg/kg/d) or vehicle for 7 consecutive days. After a 7-day break, the treatment was repeated for another 7 consecutive days. Six 2-month-old mice with C57BL/6 were used as a young control. The hormonal levels, estrus cycles, ovarian reserve, ovarian cell proliferation and apoptosis, ovarian fibrosis, and steroidogenic gene expression of ovarian stromal cells were evaluated. ABT-263 treatment did not rescue abnormal estrus cycles and sex hormonal levels, or inhibit the formation of multinucleated giant cells and ovarian stromal cell apoptosis in aged ovaries. However, it reduced ovarian fibrosis and preserved the steroidogenic gene expression of ovarian stromal cells in aged ovaries. Importantly, ABT-263 treatment further depleted ovarian follicles in aged mice. In conclusion, ABT-263 treatment accelerated the depletion of ovarian follicles in aged mice, suggesting that senolytic drugs for reproductively old female may adversely affect female fertility.
Similar content being viewed by others
Introduction
The fertility of women declines significantly with advanced age1. Achieving pregnancy remains challenging for infertile women of advanced age, even with the assistance of reproductive technologies worldwide2. The ovary, responsible for producing the female gamete, the oocyte, undergoes a decline in function with age, a process known as ovarian aging3. Remarkably, the ovary ages at a faster rate compared to other organs4. In addition to infertility, ovarian aging is closely associated with an increased risk of age-related diseases, including cardiovascular disease, osteoporosis, and mortality5. Therefore, understanding ovarian aging may reveal potential targets for future interventions aimed at preserving fertility and delaying the onset of age-related diseases.
When defining ovarian aging, two aspects should be considered: a reduction in both the quantity and quality of follicles3. It is known that a certain number of primordial follicles exist within the ovary at birth and are gradually depleted with age after sexual maturation6. However, evidence from clinics indicates that a decreased number of primordial follicles may not be solely reason for decreased fertility. The decline in oocyte quality with age also matters7. Folliculogenesis, a highly intricate process, involves the activation of primordial follicles, follicle development, selection of the dominant follicle, and follicle maturation8. With age, the mechanisms and signaling involved in folliculogenesis become dysregulated, resulting in poor oocyte quality9. However, the exact mechanisms for these age-related changes are not well understood.
Senescent cells are characterized by permanent cell growth arrest and resistance to apoptosis10. Recent studies have indicated that the accumulation of senescent cells within aging organs contributes to aging and the development of age-related diseases11. Numerous studies have shown that removing senescent cells can be an effective strategy to counteract aging and delay age-related diseases12.The ovary ages more rapidly than other organs, and accumulated senescent cells are also observed in aged ovaries4,13,14,15. Recent studies have reported that senolytic drugs dasatinib and quercetin (D + Q) can reverse obesity-mediated accumulation of senescent cells in the ovary and protect against cisplatin-induced ovarian injury by removing senescent cells16,17. ABT-263, a senolytic drug, could target Bcl-2 family and specifically induce apoptosis of senescent cells18,19,20,21. ABT-263 has been widely used to eliminate senescent cells in various disease models20,21,22,23,24. A recent study showed that early intervention with ABT-263 attenuated ovarian ageing of mice25. However, whether advanced age intervention with.
ABT-263 improves ovarian condition in aged female mice remains largely unexplored. In this study, we used naturally aged female mice treated with ABT-263 to determine whether clearance of senescent cell benefit fertility in aged mice.
Methods
Mice and treatment
The experimental mice were purchased from Cavens Experimental Animal Co., Ltd (Changzhou, China). Fourteen 16-month-old mice with a C57BL/6 background were housed in an SPF laboratory. They were randomly assigned to two groups: Control group (O) and ABT-263 treatment group (A). ABT-263(S1001, Selleck) was dissolved in a formula as previously described21. Mice in group A or O were initially treated with ABT-263 (60 mg/kg/d) or vehicle for 7 consecutive days. After a 7-day break, the treatment was repeated for another 7 consecutive days. Mice were sacrificed one week after the final treatment. Additionally, six 2-month-old mice served as the young control group. All animal procedures and experiments were approved by the ethics committee of Changzhou Maternal and Healthy Care Hospital. All methods were performed in accordance with the relevant guideline and regulations. The animal study is reported in accordance with ARRIVE guidelines.
Estrus period
After the last treatment, the estrus cycle of each mouse was examined for six consecutive days. At 9 am, 10 µl of normal saline was injected into the vagina and suctioned 2–3 times, and the mixture was smeared on slides. Following air drying, the slides were immersed in hematoxylin solution, and the estrus period was determined based on cell morphology, as previously described26.
Hormonal levels
Serum hormones were assessed using ELISA kits purchased from RenJie Biotec, including AMH (RJ-17,440), FSH(RJ-17,024)), LH(RJ-17208) and E2(RJ-17016).All procedures strictly followed the manufacturer’s instructions, and the measured values fell within the standard curve range.
Follicle counts
Paraffin-embedded ovaries were serially sectioned with a thickness of 5 μm. Ovarian sections for follicle counts were collected at intervals of every 5 sections, starting from the presence of ovarian tissue. The other sections were also collected for histological or immune staining. H&E staining was conducted for follicle counting. The criteria for the classification of follicles are as follows as we previously described27: primordial follicle, the oocyte was enclosed by a layer of squamous granulosa cells; primary follicle, the oocyte was enclosed by cuboidal granulosa cells; secondary follicle, the oocyte was enclosed by 2 or more layers of granulosa cells; and antral follicle, an antrum cavity was present. To prevent duplicate counting, only follicles with visible oocyte nuclei were included in the count. The total number of follicles in an ovary was determined using the formula: the count of follicles multiplied by 5, as described in a previous study28.
Picrosirius red staining (PSR)
The Sirius Red Staining kit (R20385, Yuanye Biotechnology Company, China) was employed for PSR staining. Following dewaxing and rehydration, ovarian sections were incubated with the PSR solution at room temperature (RT) for 6 h. After dehydration and transparency steps, the slides were mounted for photography.
Periodic acid-Schiff staining (PAS)
The PAS staining kit (G1008, Servicebio, China) was employed for PAS staining. Following dewaxing and rehydration, ovarian sections were incubated with Solution B for 15 min. Then the slides were rinsed with pure water. The sections were incubated with solution A for 30 min in the dark. The sections were counterstained with hematoxylin. After dehydration and transparency steps, the slides were mounted for photography.
Oil-red O staining
The Saturated Oil-red O solution (G1015, Servicebio, China) was diluted with distilled water at a ratio of 6:4 (v: v). The mixture was stored at 4 °C overnight and then filtered through filter paper. The filtered liquid was stored at 4 °C overnight and filtered again before preparing the working solution of Oil-red O. Frozen ovarian sections were incubated with the Oil-red O working solution for 10 min in the dark. The sections were then immersed in 60% isopropanol for differentiation. Following this, the sections underwent counterstaining with hematoxylin and were mounted for photography.
Immunostaining
After dewaxing and rehydration, ovarian sections underwent antigen retrieval in Tris-EDTA solution (pH = 9.0) under high-pressure conditions. Following natural cooling, for immunohistochemistry staining, the sections were treated with 3% H2O2 for half an hour at room temperature (RT) to eliminate endogenous catalase. Subsequently, ovarian sections were incubated with primary antibodies overnight at 4 °C, including anti p-H2AX (9718T, Cell Signaling Technology) and anti caspase-3 antibodies (ab184787, Abcam). After rinsing in PBS to remove unbound antibodies, the sections were incubated with HRP-conjugated goat anti-rabbit antibody (A0208, Beyotime Biotechnology, China) at RT for 1 h. Positive staining on sections was visualized by DAB, followed by counterstaining with hematoxylin. Following dehydration and transparency steps, the slides were mounted for photography.
For immunofluorescence, ovarian sections were incubated with primary antibodies overnight at 4 °C, including anti-α-SMA (14395-1-AP, Proteintech), anti-PCNA (10205-2-AP, Proteintech), anti-Cyp11a1(ab272494, Abcam), anti-StAR (12225-1-AP, Proteintech), and anti-SF1(18658-1-AP, Proteintech ) antibodies. After rinsing in PBS to remove unbound antibodies, the sections were incubated with Cy3-conjugated goat anti-rabbit antibody (A0516, Beyotime Biotechnology, China) at RT for 1 h. Subsequently, sections were counterstained with DAPI solution. The slides were then mounted for photography.
RNA extraction and qPCR
Frozen-thawed ovaries were homogenized in FreeZol solution (R711-01, Vazyme China), and subsequent procedures strictly followed the manufacturer’s instructions. RNA concentration was determined using Nanodrop, and 500 µg of RNA for each sample was utilized for cDNA synthesis with a reverse transcription kit (R123-01, Vazyme China). SYBR-green kit (Q331-02, Vazyme China) was employed for qPCR. The expression of Gapdh served as the reference gene for each sample. The relative mRNA expression was calculated using the 2−ΔΔCT method.
The primer sequences used in the study are listed below:
-
Gapdh forword-5’ACTTTGGCATTGTGGAAGGG3’;
-
Gapdh reverse-5’CATGCCAGTGAGCTTCCCGTT3’; Cyp11a1 forword-5’ CTGCCTCCAGACTTCTTTCG3’; Cyp11a1 reverse-5’TTCTTGAAGGGCAGCTTGTT;
-
StAR forward 5’ TCGCTACGTTCAAGCTGTG3’;
-
StAR reverse-5’ACGTCGAACTTGACCCATCC3’.
Statistics
The data were presented as mean ± SD. For comparisons between two groups, Student’s t-test was utilized. For comparisons among groups (where the number of groups is three or more), one-way ANOVA followed by Tukey’s multiple comparisons test was employed. A significance level of p < 0.05 was considered statistically significant. Statistical analysis was conducted using Prism software (GraphPad).
Results
ABT-263 treatment did not lead to an improvement in hormonal levels and estrus cycles in aged mice
In the last week before sacrifice, estrus cycles were examined in young, aged and ABT-263-treated aged female mice. Our results indicated that compared to mice in young group, mice in both aged and ABT-263-treated aged groups exhibited irregular estrus cycles (Fig. 1A). Additionally, for half of the mice in the ABT-263-treated group, estrus cycles were arrested in the diestrus phase (Fig. 1A). Comparing to young mice, serum levels of FSH and LH were significantly higher, whereas serum levels of E2 and AMH were significantly lower in both aged and ABT-263 treated groups (Fig. 1B-E). Despite the higher FSH and LH levels and lower AMH and E2 levels in ABT-263-treated mice, no significant differences were observed between aged and ABT-263 treated groups (Fig. 1B-E).
Effects of ABT-263 treatment on hormonal levels and estrus cycles in aged Mice. (A) Detection of the estrus cycle for each mouse over 6 days. The serum hormonal levels of (B) FSH, (C) LH, (D) AMH, and (E) E2. n = 6 in each group. Y: young group; O: old group; A: old group treated with ABT-263. D: diestrus; M: metestrus; E:estrus; P: proestrus. Compared with the Y group: *P < 0.05; **P < 0.01; ***P < 0.001.
ABT-263 treatment resulted in a reduction of ovarian reserve in aged mice
The ovarian size was obviously smaller in aged and ABT-263 treated mice, in contrast to the young mice (Fig. 2A). Hematomas were visible in ovaries of aged and ABT-263 treated mice (Fig. 2A). Furthermore, the ovarian size in the ABT-263 treated group was smaller than that in the aged group (Fig. 2A). One ovary from a mouse treated with ABT-263 was not found and one ovary from another mouse treated with ABT-263 was visibly ruptured (Fig. 2A). Additionally, 2 out of 6 uteri in aged mice displayed atrophy (Fig. 2B). Examination of consecutive ovarian sections revealed numerous follicles in young mice, frequent follicles in aged mice, and scarce follicles in ABT-263 treated mice (Fig. 2C). α-SMA, which labels vessels and is highly expressed surrounding follicles, corpus luteum, and within corpus luteum, effectively distinguishes follicles and corpus luteum29. α-SMA immunofluorescence demonstrated the absence of follicles or corpus luteums in the majority of ovaries from ABT-263 treated mice (Fig. 2D). Statistical analysis revealed that all stages of follicles, including primordial, primary, secondary, and antral follicles, were significantly lower in ABT-263 treated mice compared to those in aged mice.(Fig. 2E-H). Collectively, these results suggested that ABT-263 treatment accelerated the depletion of follicles.
Effects of ABT-263 treatment on ovarian reserve in aged Mice. (A) The left ovary from each mouse was selected for photographs.Black triangle indicates ovary with hematoma. Red triangle indicates a ruptured ovary. (B) Size of uteri. Blue triangle indicates uteri displaying atrophy. (C) Representative images of ovarian sections stained with H&E. (D) Representative fluorescence images of ovarian sections stained with anti-α-SMA antibody. White arrow indicates follicles; green arrow indicates corpus luteum. Number of (E) primordial follicles, (F) primary follicles, (G) secondary follicles and (H) antrum follicles. n = 3 in the Y group, n = 6 in the O group, and n = 4 in the A group. Y: young group; O: old group; A: old group treated with ABT-263. Compared with aged mice: *P < 0.05; **P < 0.01; ***P < 0.001.
ABT263 treatment did not prevent the formation of multinucleated giant cells (MGCs) in aged mice
The presence of MGCs is considered a hallmark of ovarian aging30. A previous report has identified MGCs in ovary from aged female mice by their lightly stained cytoplasm, foamy appearance, autofluorescence, and positive staining of PAS31. Our study confirmed these features (Supplementary Fig. 1A, B). Furthermore, we found that these MGCs also tested positive for Oil-red O staining (Supplementary Fig. 1C), akin to foam cells implicated in atherosclerosis. Interestingly we observed that the blue autofluorescence (AF) could more specifically and accurately mark these cells, while green and red could also identified degenerative follicle (Supplementary Fig. 1D). MGCs were identified in ovaries from both aged mice and those treated with ABT-263(Fig. 3A, B). No significant difference in the area of ovarian MGCs was observed between aged and ABT-263 treated groups (Fig. 3C).
Effects of ABT-263 treatment on the formation of MGCs in aged mice. (A) Ovarian sections stained with H&E. (B) Blue autofluorescence from the corresponding ovarian section stained with H&E. (C) Statistical analysis of the area of MGCs. n = 3 in the Y group, n = 6 in the O group, and n = 4 in the A group. MGCs: multinucleated giant cells; AF: autofluoresence. Y: young group; O: old group; A: old group treated with ABT-263.
ABT263 treatment did not prevent ovarian stromal cell apoptosis in aged mice
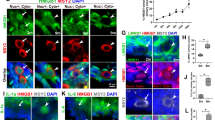
PCNA immunostaining revealed that ovarian granulosa cells within follicles were undergoing normal proliferation across all groups (Fig. 4A, B). MGCs appeared as dark holes in the ovarian sections stained with DAPI, attributable to a large portion of the cytoplasm in MGCs that remained unvisualized by nuclear staining (Fig. 4A). We observed that some nuclei of MGCs were PCNA positive in both aged and ABT-263 treated groups (Fig. 4A). γ-H2AX is a marker that reflects the DNA double-strand damage response, which drives cell apoptosis and senescence32,33. It has been reported higher expression of γ-H2AX in granulosa cells and ovarian stromal cells is associated with ovarian aging14,34. Our findings indicated that γ-H2AX was expressed in the most inner mural granulosa cells and cumulus granulosa cells in young and aged mice, as well as in luteal cells in young mice (Fig. 4C). Notably, no significant difference in the number of γ-H2AX positive ovarian stromal cells was observed among groups (Fig. 4D). However, numerous caspase 3-positive stromal cells were evident in both aged and ABT-263 treated groups (Fig. 4E). No significant difference in the number of caspase 3-positive stromal cells was observed between the aged and ABT-263 treated groups (Fig. 4F).
Effects of ABT-263 treatment on cell proliferation and apoptosis in aged mice. (A) Representative fluorescence images of ovarian sections stained with anti-PCNA antibody. Green rectangle enclosed region were magnified in the insets and indicates MGCs. (B) Statistical analysis of the percentage of PCNA positive cells per follicle. n = 3 in each group. (C) Representative images of ovarian sections stained with anti-γ-H2AX antibody. The boxed regions were magnified in the insets. The red triangle indicates γ-H2AX positive cells. (D) Statistical analysis of the number of γ-H2AX positive cells in the ovarian stroma. n = 3 in theY group, n = 6 in the O group, and n = 4 in the A group. (E) Representative images of ovarian sections stained with anti-caspase 3 antibody. The boxed regions were magnified in the insets. (F) Statistical analysis of the number of caspase 3 positive cells in the ovarian stroma. n = 3 in the Y group, n = 6 in the O group, and n = 4 in the A group. MGCs: multinucleated giant cells; CL: corpus luteum; GF: graafian follicle; SC: stromal cell. Y: young group; O: old group; A: old group treated with ABT-263. Compared with the Y group: *P < 0.05.
ABT263 treatment mitigated ovarian fibrosis in aged mice
Ovarian fibrosis is another hall marker of ovarian aging31. In comparison to young mice, the ovaries from aged mice exhibited intense PSR-positive staining and thick PSR-positive fibers (Fig. 5A, B). However, this effect was notably mitigated by ABT-263 treatment (Fig. 5A, B). Furthermore, a stronger PSR-positive staining in the tunica albuginea region was observed in aged mice compared to young and ABT-263 treated mice (Fig. 5C, D). Moreover, we observed that the majority of MGCs were situated in the center of the PSR region and surrounded by PSR-positive fibers (Fig. 5C).
Effects of ABT-263 treatment on ovarian fibrosis in aged mice. (A) Representative images from ovarian sections stained with PSR. (B) Statistical analysis of the PSR-positive area in the whole ovarian section. n = 3 in the Y group, n = 6 in the O group, and n = 4 in the A group. (C) Representative images from ovarian sections stained with PSR showing tunica albuginea region. (D) Statistical analysis of the PSR-positive area in tunica albuginea region. n = 3 in the Y group, n = 6 in the O group, and n = 4 in the A group. & indicates MGCs. double arrow indicates tunica albuginea region. MGCs: multinucleated giant cells. Y: young group; O: old group; A: old group treated with ABT-263. Compared with the Y group: *P < 0.05; Compared with the O group: #P < 0.05.
ABT263 treatment preserved the steroidogenic gene expression of ovarian stromal cells in aged mice
A previous study utilizing single-cell sequencing has indicated that steroidogenic enzymes such as StAR are expressed in clusters of ovarian stromal cells35. Our observations revealed that Cyp11a1 was expressed in various cell types including corpus luteum (CL), theca cells (TC), graafian follicles (GF) and stromal cells (SC) (Fig. 6A). The expression of Cyp11a1 was similar among the groups (Fig. 6A, B). Steroidogenic factor 1 (SF1), an important steroidogenic gene36, exhibited a similar expression pattern in the ovary as Cyp11a1 (Fig. 6B). However, SF1 expression was down-regulated in ovarian stromal cells from aged mice compared to young mice (Fig. 6C, D). ABT263 treatment enhanced SF1 expression in ovarian stromal cells of aged mice (Fig. 6C, D). Moreover, we observed premature luteinization in a late secondary follicle, and in an early secondary follicle with normal appearance as evidenced by improper and high expression of SF1 and StAR in granulosa cells, from aged mice (Supplementary Fig. 2A-C). We further examined the mRNA expression of Cyp11a1 and StAR. ΔCT, calculated as CT value of target gene minus CT value of reference gene, reflects the abundance of gene expression in cells or tissues. We found that ΔCT value of Cyp11a1 and StAR, particularly Cyp11a1, were highly expressed in ovaries regardless of age and treatment (Fig. 6E, F). In comparison to young mice, there was a trend towards lower StAR expression in the ovaries from aged mice, while a trend towards higher StAR expression was observed in the ovaries from ABT-263 treated mice (Fig. 6F). However, no significant difference in the mRNA expression levels of Cyp11a1 and StAR was observed among the groups (Fig. 6G, H).
Effects of ABT-263 treatment on the steroidogenic gene expression of ovarian stromal cells in aged mice. (A) Representative fluorescence images of ovarian sections stained with anti-Cyp11a1 antibody. (B) Statistical analysis of the percentage of Cyp11a1-positive cells in the ovarian stroma. n = 3 in the Y group, n = 6 in the O group, and n = 4 in the A group. (C) Representative fluorescence images of ovarian sections stained with anti-SF1 antibody. (D) Statistical analysis of the percentage of SF1-positive cells in the ovarian stroma. n = 3 in the Y group, n = 6 in the O group, and n = 4 in the A group. qPCR analysis of the expression of Cyp11a1 and StAR on ovaries among groups, ΔCT value of (E) Cyp11a1 and (F) StAR, the relative expression of (G) Cyp11a1 mRNA and (H) StAR mRNA. n = 6 in each group. Y: young group; O: old group; A: old group treated with ABT-263. CL: corpus luteum; GF: graafian follicle; SC: stromal cell; TC: theca cell. Compared with the Y group: *P < 0.05; Compared with the O group: #P < 0.05.
Discussion
It has been reported that senolytic drugs can enhance ovarian function in female mice affected by obesity and cisplatin by preventing the accumulation of senescent cells in the ovaries16,17. A recent study indicated that early intervention with a senolytic drug (ABT-263) extended the reproductive lifespan of mice25. It has been established that advanced age intervention with senolytic drugs can delay aging and age-related diseases37. Early intervention is inclined towards prevention, whereas advanced age intervention can be viewed as therapy. It remains unclear whether advanced age intervention with senolytic drugs can improve ovarian function. In the present study, we comprehensively evaluated the effects of ABT-263, a well-known senolytic drug, on the aged ovary in vivo. Our results revealed that ABT-263 treatment accelerated the depletion of ovarian follicles in aged mice, suggesting that the administration of senolytic drugs in aged women may expedite the process of reproductive aging.
The follicle, the fundamental functional unit in the ovary, is crucial for generating mature oocyte. In this study, we utilized mice at the age of 16 months, equivalent to approximately 45 human years (a time point close to menopause). At this age, the ovaries harbored few follicles. A previous study using data from an in vitro fertilization center indicated that premature luteinization of GCs is more likely to occur in older infertile women (43–47 years old) than in younger women (21–29 years old)38. Consistently, we clearly observed premature luteinization of follicles in ovaries from aged mice in the present study, suggesting that premature luteinization could be a contributing factor to impaired folliculogenesis and diminished oocyte quality in reproductively old females. We expected an increase, or at least maintenance, in the number of follicles in aged ovaries by ABT263 treatment. Unexpectedly, ABT-263 treatment decreased the ovarian function of aged mice evidenced by alternations of hormonal levels, ovarian size and estrus cycles. Particularly, the serum AMH level, a classic marker of ovarian reserve, was further reduced in ABT-263-treated mice compared to aged mice, although this reduction did not reach statistical significance. Consistent with this observation, ABT-263 treatment further depleted all stages of follicles, including primordial, primary, secondary, and antral follicles, in the ovaries of reproductively old mice. However, it seemed that the existing follicles in aged and ABT263-treated mice proliferated normally. A recent paper reported an increase in senescent primary oocytes with age, and ABT-263 was shown to eliminate these senescent primary oocytes25. In that study, around 15% of primordial follicles (approximately 200 primordial follicles in the ovary) were senescent at the age of 9 months. In the present study, the mice at the age of 16 months were treated with ABT-263. At that age, only a small number of primordial follicles remained in the ovary, and the majority of primordial oocytes may be senescent. Therefore, it is possible that ABT263 treatment eliminates the remaining senescent primordial follicles, leading to an accelerated depletion of follicles.
The presence of MGCs in the ovary is considered as a hallmark of ovarian aging30,39. A previous study has showed that that MGCs exhibited various characteristics, including light staining of cytoplasm, autofluorescence and positive staining of PAS31. In the study, we validated these features. In addition, we showed that these cells were positive for oil red o, indicating a high storage of lipid droplets for MGCs. Intriguingly, only blue autofluorescence specifically and accurately labeled MGCs, suggesting the existence of specific materials in MGCs that distinguish them from other cells in the ovary. Experimental evidence suggests that the formation of MGCs in the aged ovary is a result of macrophage fusion31. In addition to cell fusion, acytokinetic cell division is also a mechanism contributing to the multinucleation of MGCs40,41. In line with this, some nuclei of MGCs were observed to be proliferating in both aged and ABT-263 treated mice. Therefore, the multinucleation of MGCs in the ovary is, in part, attributed to acytokinetic cell division. The formation of MGCs is presumed to remove large debris from tissues42. It has been proposed that MGCs in the aged ovary may be involved in the degradation of large fibrotic regions or the removal of cellular debris39. Our study revealed that the most of MGCs were situated in the center of the PSR-rich region and surrounded by PSR-positive fibers, suggesting that MGCs may help to clean fibrotic region in the aged ovary. However, ABT-263 did not prevent the formation of MGCs, indicating that MGCs were not in senescent state and providing evidence to support the perspective that ABT263 treatment did not mitigate the ovarian aging in aged mice.
Fibrosis is another hallmark of ovarian aging31. In this study, intense PSR-positive staining was exclusively observed in ovaries from aged mice. Additionally, fibrosis in the tunica albuginea region was evident only in aged mice. Therefore, ABT-263 treatment decreased fibrosis in the aged ovary. Single-cell sequence data revealed an elevated proportion of senescent fibroblasts that may be involved in SASP secretion in the aged ovary43. It has been reported that ABT263 treatment reverses established fibrosis by inducing the apoptosis of stiffness-primed myofibroblasts44. Therefore, it is possible that ABT-263 treatment targets senescent fibroblasts to mitigate fibrosis in the aged ovary. It has been demonstrated that targeted removal of fibrotic collagen extends the reproductive lifespan of female mice45. AlthoughABT-263 treatment reduced fibrosis, it accelerated ovarian aging, indicating that the detrimental effects of ABT-263 treatment on follicles in aged mice cannot be prevented by reducing fibrosis.
Ovarian stromal cells exhibit significant heterogeneity46. The role of stromal cells in ovarian aging is less understood. It is established that an expanded stromal cell compartment is a key characteristic of the aged ovary47. In this study, we observed a clear shift during ovarian aging, with the ovary transitioning from being follicle-occupied to stromal cell-occupied. Furthermore, numerous apoptotic stromal cells were observed in both the aged ovaries and those treated with ABT263. It has been reported that granulosa cells from small antral follicles contribute to the survival of ovarian stromal cells48. Therefore, the increased apoptosis of stromal cells in the aged and ABT-263 treated ovary may result from insufficient follicles. To our surprise, no increase in γ-H2AX positive stromal cells was observed in aged or ABT-263-treated aged mice compared to young mice, indicating that stromal cell apoptosis is independent of DNA damage. Markers of DNA damage are recommended only as auxiliary indicators of cell senescence in vivo49. To our knowledge, only one published paper has so far shown an ovarian stromal cell extracted from aged mice that is positive for both SA-βgal and γ-H2AX14. Therefore, whether all the senescent ovarian stromal cells exhibit high levels of γ-H2AX remains to be investigated.
Previous studies have shown that ovarian stromal cells can produce steroid hormones, and single-cell sequence data revealed that clusters of stromal cells can be marked by steroidogenic enzymes such as StAR46,50. In our study, we found that the expression of Cyp11a1 was nearly equivalent to that of the housekeeping gene (while StAR was less expressed) in the ovary, regardless of age or treatment. Despite a significantly reduced number of follicles in the aged ovary, particularly in ABT263-treated ovaries, the expression of Cyp11a1 and StAR was not affected. These results strongly suggest that Cyp11a1 could serve as a broad marker of stromal cells, and aging did not evidently alter the identity of ovarian stromal cells.
SF1, as a steroidogenic gene, is crucial for steroid hormone synthesis51. A previous study has shown the expression of SF1 in ovarian stromal cells of postmenopausal women with ovarian mucinous adenocarcinoma52. In the present study, we showed high expression of SF1 in ovarian stromal cells in young mice, supporting steroid hormone synthesis capability of ovarian stromal cells. It has been reported that ovarian granulosa cells promote the steroidogenesis of ovarian stromal cells48. Consistently, we found that the expression of SF1 in ovarian stromal cells was decreased in the aged ovary. Surprisingly, ABT-263 treatment enhanced the expression of SF1 in ovarian stromal cells from aged mice, suggesting that ABT263 treatment preserved the steroidogenic activity of ovarian stromal cells from aged mice. This action of ABT-263 may be attributed to its role against senescent cells. The exact mechanism remains to be investigated. Taken together, ABT-263 treatment could not reduce apoptosis but could preserve the steroidogenic gene expression of ovarian stromal cells from aged mice. Moreover, our results also underscore the steroidogenic role of ovarian stromal cells.
A previous study demonstrated that intracellular levels of reactive oxygen species (ROS) correlate with ABT-263 sensitivity in non-small cell lung cancer cells53. Similarly, another study showed that targeting antioxidant enzymes enhances the therapeutic efficacy of ABT-263 in KRAS-mutant colorectal cancers54. These studies suggest that ABT-263 tends to kill cells with high levels of ROS. It has been reported that ROS accumulates in oocytes as well as somatic cells within the aged ovary55,56. On one hand, it is possible that oocytes with high ROS levels are sensitive to ABT-263, and that intervention with ABT-263 in advanced age may lead to the accelerated depletion of ovarian reserve by killing the remaining oocytes with high ROS levels. On the other hand, somatic cells with high ROS levels, including senescent cells, could also be killed by ABT-263, leading to an improved overall ovarian environment. This may contribute to the decreased fibrosis and increased expression of steroidogenic genes in the ovaries of aged mice treated with ABT-263.
In summary, we reported the multifaceted effects of ABT-263 treatment on the aged ovary: (1) ABT-263 treatment accelerated the depletion of follicles in the ovary of reproductively old females. (2) ABT-263 treatment could not prevent the formation of MGCs and apoptosis of ovarian stromal cells in the aged ovary. (3) ABT-263 treatment mitigated ovarian stromal fibrosis in the aged ovary. (4) ABT-263 treatment may help preserve the steroidogenic gene expression of ovarian stromal cells from aged mice. This study suggests that the strategy of clearing senescent cells may adversely affect female fertility in reproductively old females and underscores the importance of early intervention for preserving female fertility.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Garcia, D., Brazal, S., Rodriguez, A., Prat, A. & Vassena, R. Knowledge of age-related fertility decline in women: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 230, 109–118 (2018).
O’Brien, Y. M., Ryan, M., Martyn, F. & Wingfield, M. B. A retrospective study of the effect of increasing age on success rates of assisted reproductive technology. Int. J. Gynaecol. Obstet. 138, 42–46 (2017).
Vollenhoven, B. & Hunt, S. Ovarian ageing and the impact on female fertility. F1000Res 7 (2018).
Yang, Q. et al. NADase CD38 is a key determinant of ovarian aging. Nat. Aging 4, 110–128 (2024).
Christensen, M. W., Kesmodel, U. S., Christensen, K., Kirkegaard, K. & Ingerslev, H. J. Early ovarian ageing: Is a low number of oocytes harvested in young women associated with an earlier and increased risk of age-related diseases? Hum. Reprod. 35, 2375–2390 (2020).
Wallace, W. H. & Kelsey, T. W. Human ovarian reserve from conception to the menopause. PLoS ONE 5, e8772 (2010).
Moghadam, A. R. E., Moghadam, M. T., Hemadi, M. & Saki, G. Oocyte quality and aging. JBRA Assist. Reprod. 26, 105–122 (2022).
Yang, D. Z., Yang, W., Li, Y. & He, Z. Progress in understanding human ovarian folliculogenesis and its implications in assisted reproduction. J. Assist. Reprod. Genet. 30, 213–219 (2013).
Esencan, E., Beroukhim, G. & Seifer, D. B. Age-related changes in Folliculogenesis and potential modifiers to improve fertility outcomes—A narrative review. Reprod. Biol. Endocrinol. 20, 156 (2022).
Hu, L. et al. Why senescent cells are resistant to apoptosis: An insight for senolytic development. Front. Cell. Dev. Biol. 10, 822816 (2022).
Katzir, I. et al. Senescent cells and the incidence of age-related diseases. Aging Cell. 20, e13314 (2021).
Childs, B. G., Durik, M., Baker, D. J. & van Deursen, J. M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015).
Dai, X. et al. PQQ dietary supplementation prevents alkylating agent-induced ovarian dysfunction in mice. Front. Endocrinol. 13, 781404 (2022).
Maruyama, N. et al. Accumulation of senescent cells in the stroma of aged mouse ovary. J. Reprod. Dev. 69, 328–336 (2023).
Xu, Z. et al. The role of cellular senescence in cyclophosphamide-induced primary ovarian insufficiency. Int. J. Mol. Sci. 24 (2023).
Du, D. et al. Senotherapy protects against cisplatin-induced ovarian injury by removing senescent cells and alleviating DNA damage. Oxid. Med. Cell Longev. 9144644 (2022).
Hense, J. D. et al. Senolytic treatment reverses obesity-mediated senescent cell accumulation in the ovary. Geroscience 44, 1747–1759 (2022).
Tse, C. et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Mylonas, K. J. et al. Cellular senescence inhibits renal regeneration after injury in mice, with senolytic treatment promoting repair. Sci. Transl. Med. 13 (2021).
Saleh, T. et al. Clearance of therapy-induced senescent tumor cells by the senolytic ABT-263 via interference with BCL-X(L)-BAX interaction. Mol. Oncol. 14, 2504–2519 (2020).
Kim, H. et al. Attenuation of intrinsic ageing of the skin via elimination of senescent dermal fibroblasts with senolytic drugs. J. Eur. Acad. Dermatol. Venereol. 36, 1125–1135 (2022).
Yang, H. et al. Navitoclax (ABT263) reduces inflammation and promotes chondrogenic phenotype by clearing senescent osteoarthritic chondrocytes in osteoarthritis. Aging 12, 12750–12770 (2020).
Pan, J. et al. Inhibition of Bcl-2/xl with ABT-263 selectively kills senescent type II pneumocytes and reverses persistent pulmonary fibrosis induced by ionizing radiation in mice. Int. J. Radiat. Oncol. Biol. Phys. 99, 353–361 (2017).
Yan, H. et al. Primary oocytes with cellular senescence features are involved in ovarian aging in mice. Sci. Rep. 14, 13606 (2024).
Zenclussen, M. L., Casalis, P. A., Jensen, F., Woidacki, K. & Zenclussen, A. C. Hormonal fluctuations during the estrous cycle modulate heme Oxygenase-1 expression in the uterus. Front. Endocrinol. 5, 32 (2014).
Liu, F. et al. Loss of p16 does not protect against premature ovarian insufficiency caused by alkylating agents. BMC Pregnancy Childbirth 23, 151 (2023).
Meng, T. G. et al. Oocyte-specific deletion of furin leads to female infertility by causing early secondary follicle arrest in mice. Cell. Death Dis. 8, e2846 (2017).
Hall, A. P. et al. PDGFR inhibition results in pericyte depletion and hemorrhage into the corpus luteum of the rat ovary. Toxicol. Pathol. 44, 98–111 (2016).
Foley, K. G., Pritchard, M. T. & Duncan, F. E. Macrophage-derived multinucleated giant cells: Hallmarks of the aging ovary. Reproduction 161, V5–V9 (2021).
Briley, S. M. et al. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction 152, 245–260 (2016).
Mah, L. J., El-Osta, A. & Karagiannis, T. C. gammaH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 24, 679–686 (2010).
Rogakou, E. P., Nieves-Neira, W., Boon, C., Pommier, Y. & Bonner, W. M. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J. Biol. Chem. 275, 9390–9395 (2000).
Liu, W. et al. Epg5 deficiency leads to primary ovarian insufficiency due to WT1 accumulation in mouse granulosa cells. Autophagy 19, 644–659 (2023).
Wagner, M. et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat. Commun. 11, 1147 (2020).
Parker, K. L. & Schimmer, B. P. Steroidogenic factor 1: A key determinant of endocrine development and function. Endocr. Rev. 18, 361–377 (1997).
Robbins, P. D. et al. Senolytic drugs: Reducing senescent cell viability to extend health span. Annu. Rev. Pharmacol. Toxicol. 61, 779–803 (2021).
Wu, Y. G. et al. Aging-related premature luteinization of granulosa cells is avoided by early oocyte retrieval. J. Endocrinol. 226, 167–180 (2015).
Kloc, M. et al. Giant multinucleated cells in aging and senescence—An abridgement. Biology 11 (2022).
Ariizumi, T. et al. Multinucleation followed by an acytokinetic cell division in myxofibrosarcoma with giant cell proliferation. J. Exp. Clin. Cancer Res. 28, 44 (2009).
Maros, M. E. et al. Cell cycle regulatory protein expression in multinucleated giant cells of giant cell tumor of bone: Do they proliferate?? Pathol. Oncol. Res. 27, 643146 (2021).
Milde, R. et al. Multinucleated giant cells are specialized for complement-mediated phagocytosis and large target destruction. Cell. Rep. 13, 1937–1948 (2015).
Landry, D. A. et al. Metformin prevents age-associated ovarian fibrosis by modulating the immune landscape in female mice. Sci. Adv. 8, eabq1475 (2022).
Lagares, D. et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci. Transl Med. 9 (2017).
Umehara, T. et al. Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Sci. Adv. 8, eabn4564 (2022).
Kinnear, H. M. et al. The ovarian stroma as a new frontier. Reproduction 160, R25–R39 (2020).
Wei, Y. et al. Single-cell profiling of mouse and primate ovaries identifies high levels of EGFR for stromal cells in ovarian aging. Mol. Ther. Nucleic Acids 31, 1–12 (2023).
Qiu, M. et al. Effects of granulosa cells on steroidogenesis, proliferation and apoptosis of stromal cells and theca cells derived from the goat ovary. J. Steroid Biochem. Mol. Biol. 138, 325–333 (2013).
Ogrodnik, M. et al. Guidelines for minimal information on cellular senescence experimentation in vivo. Cell 187, 4150–4175 (2024).
Wang, S. et al. Single-cell transcriptomic Atlas of primate ovarian aging. Cell 180, 585–600e519 (2020).
Caron, K. M., Clark, B. J., Ikeda, Y. & Parker, K. L. Steroidogenic factor 1 acts at all levels of the reproductive axis. Steroids 62, 53–56 (1997).
Hattori, Y. et al. Ovarian mucinous adenocarcinoma with functioning stroma in postmenopausal women: Aromatase and SF-1 expressions. J. Ovarian Res. 8, 73 (2015).
Ohgino, K. et al. Intracellular levels of reactive oxygen species correlate with ABT-263 sensitivity in non-small-cell lung cancer cells. Cancer Sci. 111, 3793–3801 (2020).
Oh, Y. et al. Targeting antioxidant enzymes enhances the therapeutic efficacy of the BCL-X(L) inhibitor ABT-263 in KRAS-mutant colorectal cancers. Cancer Lett. 497, 123–136 (2021).
Sasaki, H. et al. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front. Endocrinol. 10, 811 (2019).
Lu, J., Wang, Z., Cao, J., Chen, Y. & Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 16, 80 (2018).
Funding
This work was supported by the Top Talent of Changzhou “The 14th Five-Year Plan” High-Level Health Talents Training Project (2022CZBJ090) to X.D., the Key Project funded by Changzhou Clinical Medical Center (CMCM202203) to L.C., Applied Basic Research Program of Changzhou Science and Technology Bureau, General Program (CJ20230077) to P.X., the Young Project Funded by Nanjing Medical University (NMUB20210055) to X.X., and the China Reproductive Public Welfare Fund “Pilotage Plan” (SZ202412) to Y.W.
Author information
Authors and Affiliations
Contributions
X.D.,P.X. and Y.W. conceived the project, designed the experiments and analysed data. Y.W. and X.D. wrote the manuscript. X.X., Y.Y., and P.L. conducted all the experiments. L.C. supervised the experiments, reviewed and edited the manuscript. All authors reviewed, read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xia, X., Yang, Y., Liu, P. et al. The senolytic drug ABT-263 accelerates ovarian aging in older female mice. Sci Rep 14, 23178 (2024). https://doi.org/10.1038/s41598-024-73828-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73828-4