Abstract
It is now understood that hematological diseases can have detrimental effects on the retina, reducing retinal capillaries, compromising visual function, and potentially causing irreversible visual impairment. Over the years, there has been limited research on macular microvascular abnormalities, such as changes in vessel density and the foveal avascular zone (FAZ) and variations in the severity of these effects across different types of blood disorders. This study aims to quantitatively assess the impact of various hematological disorders on the retina using optical coherence tomography angiography (OCTA). Compared with healthy eyes, patients with different blood diseases exhibited reductions in linear vessel density (LVD), perfusion vessel density (PVD), FAZ area, and FAZ perimeter. Notably, patients with erythrocyte diseases showed more significant abnormalities in LVD and PVD, while patients with lymphocytic diseases demonstrated more pronounced abnormalities in the FAZ area and perimeter. OCTA imaging could potentially reflect changes of the retinal microvascular of patients with hematological diseases and may serve as a valuable tool for distinguishing abnormalities affecting different blood cell lines. This approach offers a novel avenue for assessing, treating, and monitoring blood disorders.
Similar content being viewed by others
Introduction
Hematological diseases encompass a wide spectrum of conditions that disrupt the normal morphology and function of blood cells, coagulation factors, or the bone marrow1,2. These diseases have varying global prevalence rates and pose significant health challenges3,4,5. It has been established that anemia, affecting approximately 1.62 billion people, leads to fatigue, reduced productivity, and increased morbidity2,6,7. Thalassemia, impacting 1–2% of the population, requires lifelong care and can potentially result in organ dysfunction8,9. Hemophilia, affecting 1 in 10,000 individuals, leads to spontaneous bleeding and necessitates specialized treatment10,11. Leukemia affects about 437,000 people globally, leading to compromised immune systems and demanding intensive therapies12,13. Lymphoma impacts around 790,000 individuals, causing symptoms and necessitating comprehensive diagnostic and treatment approaches14,15. The above studies overlap in their assertion that hematological disorders impose a substantial burden on individuals and healthcare systems worldwide, with the potential to affect multiple organ systems6,8,16. In recent years, ocular manifestations associated with hematologic disorders have been increasingly recognized, particularly in the retina17,18,19. Retinal abnormalities linked to hematological diseases include hemorrhages in various retinal layers, Roth spots, hard exudates, cotton-wool spots, alterations in retinal vessels, optic disc edema, and pallor20,21. However, the precise mechanisms underlying these fundal abnormalities remain poorly understood.
Anemia induces retinal hypoxia, resulting in vascular dilatation, increased transmural pressure due to hypoproteinemia, and microtrauma to the vessel wall, leading to retinal edema and hemorrhages6,7,22. In many clinical situations, leukemic infiltration in the retina can cause retinal hemorrhages, infiltrates, and optic nerve involvement, affecting visual acuity23,24. Additionally, thrombocytopenia, often associated with anemia, leads to impairment of coagulation and bleeding25,26. These hematological disorders underscore the importance of regular ophthalmic monitoring for the timely detection and management of ocular complications to preserve visual health.
Given the close relationship between retinal tissues and the circulatory system, even minor blood components or vascular integrity disruptions can significantly impact retinal health and visual acuity. Both retinal and choroidal tissues require oxygen and nutrients to maintain normal visual processing19,21,27. Patients with blood diseases experience reduced oxygen delivery to the retinal and choroidal tissues, resulting in retinal and choroidal hypoxia and impairing the oxygen metabolism of the inner and outer retinal layers, consequently causing visual impairment21. For example, retinal hypoxia can induce structural and vascular changes, such as retinal thinning and decreased capillary plexus density, which can be visualized through optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA)28,29. Studies have revealed reduced choroidal and retinal thickness in patients with anemia29 and macular involvement in sickle cell disease can also be detected through (OCT)29,30,31. Nevertheless, these studies have primarily focused on assessing the ocular effects of individual hematological disorders. To the best of our knowledge, no studies have sought to comprehensively explore and compare the impact on the retinal structure in different types of blood diseases.
This paper aims to quantitatively assess the impact of various hematological disorders on the retina using OCTA, comparing the findings with those of age and sex-matched healthy control subjects. Emphasis is placed on the underlying mechanisms, clinical manifestations, and management strategies. By shedding light on the ocular implications of hematologic conditions, we hope to enhance awareness among healthcare providers, facilitate early detection, and prompt timely interventions to optimize visual outcomes for affected individuals.
Methods
This cross-sectional observational study was conducted in accordance with the principles of the Declaration of Helsinki and received approval from the Medical Research and Ethics Committee of Qilu Hospital (Ethical code: KYLL-202309-029). Informed written consent was obtained from all subjects after comprehensively explaining the study’s purpose, procedures, and potential risks. A control group of 30 healthy subjects was established. All patients with blood diseases received treatment at the Department of Hematology and Department of Ophthalmology, Qilu Hospital, Shandong University, Jinan, between October 30, 2022, and March 30, 2023. We initially identified patients with various hematological diseases and subjects in the healthy control group were carefully matched in terms of age, gender, axial length, and refractive error distribution. The subjects were categorized into the following five groups based on their diagnosis and laboratory indicators: Group 1: Patients with myelocyte diseases, such as chronic granulocytic leukemia, leukopenia or acute myelogenous leukemia; Group 2: Patients with erythrocyte diseases, such as iron-deficiency anemia or hemolytic anemia; Group 3: Patients with lymphocyte diseases, such as Hodgkin lymphoma, non-Hodgkin’s lymphoma or chronic lymphocytic leukemia; Group 4: Patients with myelodysplastic syndrome, myelofibrosis or aplastic anemia; Group 5: Patients with platelet diseases such as those with immune thrombocytopenia (ITP). (Figure S1)
Inclusion and exclusion criteria
The diagnosis of blood diseases relied on both blood and bone marrow examination as well as the clinical manifestations of the patients. The inclusion criteria comprised: (1) Patients with a confirmed diagnosis of a specific hematopathy; (2) Patients aged 18 or older; (3) Patients with best-corrected visual acuity (BCVA) of ≥ 60 letters or better in each eye; (4) The time elapsed between the hematological disease diagnosis and the eye examination should not exceed one week. Exclusion criteria included: (1) Clinically significant macular edema; (2) A previous diagnosis of glaucoma and the presence of myopia and chorioretinal atrophy or other ocular conditions that could potentially affect chorioretinal capillaries in the posterior pole; (3) Ocular hypertension; (4) A history of other retinal and choroidal diseases or vitreoretinal surgery; (5) Ocular media opacity; (6) Images of low quality (quality index < 6); 6. Patients with myopia of more than 100 degrees (spherical lens) and the ocular axis of more than 25 mm.
Ophthalmic examinations and blood test
Each patient and healthy subject underwent a comprehensive ophthalmic examination along with blood tests. The ophthalmic examinations encompassed several assessments including: Best-Corrected Visual Acuity (BCVA) measurement after computer optometry using Early Treatment Diabetic Retinopathy Study (ETDRS) units32, measurement of intraocular pressure using a non-contact tonometer, and determination of axial length through IOL (intraocular lens) Master, slit lamp-based biomicroscopy of the anterior segment, and dilated fundus biomicroscopy after full pupil dilation. Additionally, OCTA images were obtained using a commercial OCTA instrument (Zeiss CIRRUS HD-OCT 5000, Germany) with a central wavelength of approximately 1050 nm (990–1100 nm full width). For the quantitative analysis of OCTA scans measuring 3 mm × 3 mm and 6 mm × 6 mm, various retinal parameters were separately calculated in both central and peripheral regions, including the central fovea area (diameter of 1 mm) and the perifoveal concentric ring (3–6 mm). These parameters included linear vessel density (LVD), perfusion vessel density (PVD), area of foveal avascular zone (FAZ), and perimeter of FAZ; calculations were performed using the built-in software version 1.32.9. Additionally, layer segmentation and quantification analyses of vessel density of the retinal capillaries, FAZ area, and FAZ perimeter were carried out using the built-in software. If necessary, manual manipulation of segmentation was performed to ensure accuracy. Vessel density was calculated as the percentage of pixels with a flow signal greater than the threshold (%).33 All OCTA measurement data were extracted using the built-in software by two licensed retinal specialists (ZW. L and BX. S), ophthalmologists with subspecialty training in the retina. For patients with hematological diseases, binocular data were collected, and eyes with lower LVD were selected for further analysis. As for healthy controls, only the right eye data were included in the analysis. Overview of the study was shown in Fig. 1.
Overview of the study. (A) Subject Enrollment: The diagnosis of blood diseases was based on the patients’ clinical manifestations and confirmed through blood and bone marrow data. (B) Data Collection: Subjects were categorized into different groups based on their diagnoses and laboratory disease indicators. Each patient and healthy control underwent a comprehensive ophthalmic examination using slit-lamp and Optical Coherence Tomography Angiography (OCTA). Parameters including Linear Vessel Density (LVD), Perfusion Vessel Density (PVD), and Foveal Avascular Zone (FAZ) were recorded for subsequent analysis. (C) Diagram of blood cell composition, a, normal erythrocyte; b, sickle-shaped erythrocyte; c, Normal leukocyte; d, Normal lymphocyte; e, abnormal lymphocytes. The cell body is larger than normal lymphocytes, the chromatin may be darker, and sometimes vacuoles may appear; f, normal platelets; g, deformation of platelets. OCTA, optical coherence tomography angiography; LVD, linear vessel density; PVD, perfusion vessel density. FAZ, foveal avascular zone.
The image quality of the OCTA scans was automatically graded using the built-in software on a scale from Q1 to Q10. Eyes with poor image quality (quality index < 6) due to refractive media opacity or poor fixation were excluded. All images were captured by experienced technicians who were blinded to the clinical status of the subjects, and this was done between 8:00 AM and 11:00 AM to avoid potential diurnal variations in retinal capillaries. Hematological test data included routine blood examinations, four coagulation tests, erythrocyte sedimentation rate (ESR), and so on. These tests were conducted independently of the two retinal specialists, and no significant differences were observed between their datasets, as indicated by a Pearson correlation coefficient (r ≥ 0.8). Any discrepancies between the two observers were resolved through discussion and consensus.
Outcome measures
The primary outcome measures were the differences in vessel density of the retina in patients with hematological diseases measured by OCTA. Secondary outcomes were the differences in FAZ and the analysis of the correlation between retinal parameters and hematology test data.
Statistical analysis
Statistical analyses were performed using SPSS software (version 19.0 SPSS, Inc, Chicago, IL, USA). The Kolmogorov-Smirnov test was used to assess normality, and normally distributed variables were expressed as mean +/- standard deviation (SD). Analysis of variance (ANOVA) was employed to compare the number of patients enrolled, mean ages, durations, BCVA, axial length, and hematological test data among different groups, including healthy controls, patients with myelocyte diseases, erythrocyte diseases, lymphocyte diseases, pancytopenia diseases or patients with platelet diseases. Furthermore, correlations between hematological test data and retinal characteristics were calculated, with a statistical significance threshold set at P < 0.05.
Results
General clinical characteristics
In our study, we included 81 patients with various hematological diseases, excluding 7 patients who had a history of ocular conditions such as glaucoma and dry age-related macular degeneration, as well as 5 patients whose image quality index was below 6. The study included a total of 69 eyes from 69 patients with blood diseases for further analysis. and there were no significant differences in age, sex composition, spherical equivalent (SE), axial length, or BCVA among the groups (Ps > 0.05). However, significant differences were observed between the groups with blood diseases in terms of hemoglobin (Hb) concentration (Table 1). Both of the independent retinal specialists yielded consistent results for hematological test data and quantitative retinal parameter measurements.
Characteristics of the retinal parameters in 3 × 3 scanning pattern
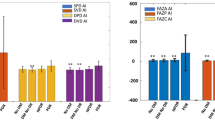
When compared with the control group, patients with hematological disorders (Groups 1 to 5) exhibited significantly thinner LVD and PVD (p < 0.001). Among these groups, Group 2 showed the lowest LVD and PVD. During pairwise comparisons between Groups 1 to 5, Group 2 had significantly lower LVD and PVD than Groups 1 and 3, while no significant differences were observed in other subgroup analyses. Additionally, retinal structural parameters in the macular region, including the area and perimeter of the FAZ, were significantly affected by hematological disorders, with clear decreases in values observed in Groups 1 to 5. Group 3 had the lowest FAZ area, and Group 5 had the lowest FAZ perimeter. During pairwise comparisons between Groups 1 to 5, Group 3 had a significantly lower FAZ area than Group 4, and Group 5 had a significantly lower FAZ perimeter than Groups 1 and 4. (Table 2; Fig. 2)
Comparison of optical coherence tomographical parameters in patients with different hematological diseases and the normal control group. (A-d), (I-L), examples of linear vessel density, perfusion vessel density, FAZ, and B-scan acquired by OCTA in 3 × 3 and 6 × 6 scanning patterns; (E-H) Vessel density and FAZ comparisons of patients with different blood diseases and normal control group in 3 × 3 scanning pattern; (M-P), Vessel density and FAZ comparisons of patients with different blood diseases and normal control group in 6 × 6 scanning pattern. OCTA, optical coherence tomographic angiography; FAZ, foveal avascular zone. * indicates a statistically significant difference (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Characteristics of the retinal parameters in 6 × 6 scanning pattern
Similar to the results in the 3 × 3 scanning pattern, patients in Groups 1 to 5 exhibited significantly thinner LVD and PVD compared with the control group (p < 0.001) with Group 2 showing the lowest LVD and PVD. During pairwise comparisons between Groups 1 to 5, Group 2 had significantly lower LVD and PVD compared with Groups 1 and 3, with no significant differences in other subgroup analyses. Additionally, the FAZ area and perimeter were significantly decreased (p < 0.001) in Groups 1 to 5 compared with healthy controls, and the lowest FAZ area and perimeter were observed in Group 2. There were no significant differences in the FAZ area and perimeter during subgroup analyses. (Table 2; Fig. 2)
Correlation analysis between the hematology test data and retinal parameters
The results of the correlation analysis between hematology test data and retinal parameters are summarized in Tables 3, 4, 5 and 6. Hemoglobin concentration in patients with hematological disorders positively correlated with LVD and PVD in the 3 × 3 scanning pattern (p < 0.001, correlation coefficient = 0.523; p < 0.001, correlation coefficient = 0.543, respectively, Table 3, Figure S2). The White blood cell (WBC) count in patients with hematological diseases exhibited a negative correlation with LVD and PVD in the 3 × 3 scanning pattern (p = 0.027, correlation coefficient = -0.386; p = 0.025, correlation coefficient = -0.390, respectively, Table 4, Figure S3). Lymphocyte counts in patients with blood disorders showed a negative correlation with PVD in the 3 × 3 scanning pattern (p = 0.044, correlation coefficient = -0.328, Table 5, Figure S4), as well as with LVD and PVD in the 6 × 6 scanning pattern (p = 0.031, correlation coefficient = -0.351; p = 0.029, correlation coefficient = -0.354, respectively, Table 5, Figure S4). The platelet count in patients with hematological disorders did not exhibit significant correlations with retinal parameters (Table 6, Figure S5). Furthermore, the duration of patients with hematological disorders displayed a negative correlation with LVD and PVD in the 3 × 3 scanning pattern (p < 0.001, correlation coefficient = -0.421; p < 0.001, correlation coefficient = -0.418, respectively, Table 7) and a positive correlation with FAZ perimeter in the 3 × 3 scanning pattern (p = 0.032, correlation coefficient = 0.258, Table 7).
Discussion
The present study provides important insights into the relationship between blood diseases and retinal vascular health. Our findings substantiated that patients with blood diseases experienced a significant reduction in retinal vessel density and altered FAZ parameters compared with normal control subjects. Notably, the lowest vessel density was observed in patients with various forms of anemia, and the most pronounced abnormalities in FAZ parameters were found in patients with lymphocyte-related diseases and lower hemoglobin concentrations. Furthermore, our correlation analysis between hematological test data and retinal parameters uncovered intriguing associations, including positive correlations between hemoglobin concentration and LVD and PVD and negative correlations between WBC count, lymphocyte count, LVD, and PVD. However, no significant correlations were observed between platelet count and retinal vascular changes.
The retina and choroid, both highly vascularized structures in the posterior segment of the eye, receive their blood supply from different sources. The central retinal artery supplies the inner retinal layers, while the choroid nourishes the outer retinal layers21,33. Fundal photographs of the retina provide a noninvasive means to assess the microvasculature in vivo. Various blood disorders, including anemia, leukemia, Hodgkin’s lymphoma, and alterations in platelet counts, can profoundly impact retinal capillary perfusion17,18,29. However, this study represents the first quantitative comparison of retinal vascular abnormalities among patients with different hematological disorders using OCTA. Our results demonstrate a significant decrease in LVD and PVD across all groups, suggesting that retinal perfusion impairment may precede structural macular changes and visual impairment, especially in patients with anemia-related diseases. Consistent with our study, Koca et al. also showed that the quantification of retinal vessel density was significantly reduced in patients with iron deficiency anemia, so, OCTA may be useful in detecting retinal ischemia before clinically visible signs of retinopathy associated with blood diseases34. Moreover, changes in FAZ parameters reflect structural alterations in microvessels, further underscoring the impact of hematological disorders, particularly those with reduced hemoglobin concentrations, on retinal microvasculature.
To explore the complex relationship between structural changes in retinal capillaries and various blood diseases, we investigated the influence of diverse hematological parameters on retinal blood flow density. Our findings revealed a positive correlation between hemoglobin concentration and retinal blood flow density, aligning with the expected physiological response that higher hemoglobin levels enhance oxygen-carrying capacity, potentially improving tissue perfusion. This direct relationship suggests that systemic hemoglobin levels play a modulatory role in retinal vascular dynamics, emphasizing the significance of hemoglobin in maintaining optimal ocular circulation. In contrast, we observed inverse correlations between WBC counts and retinal vessel density, indicating that elevated WBC levels, including lymphocytes, may contribute to decreased retinal perfusion. Inflammatory and immune responses associated with higher WBC counts could affect retinal microvasculature, reducing blood flow density and highlighting the potential impact of systemic inflammation on ocular vascular health. Furthermore, WBCs are significantly larger than red blood cells (RBCs), possibly associated with leukostasis. Excessive WBCs could potentially slow down RBC flow velocity, and since OCTA relies on detecting the relative motion of RBCs for quantitative measurements of LVD and PVD, this could explain the observed correlations. Interestingly, our study did not find a significant correlation between platelet count and retinal vessel density, suggesting that whether platelet count increases or decreases, both scenarios result in decreased retinal vessel density. However, further investigations into platelet function and their potential role in modulating retinal vascular dynamics are warranted, given our study’s relatively small sample size.
Our study revealed that lymphatic system disorders can significantly impact FAZ area and perimeter. These findings raise questions about the mechanisms underlying these alterations and open avenues for further investigations into the relationship between the lymphatic system and retinal microvasculature. To our knowledge, no previous studies have reported the potential impact of lymphatic system disorders on retinal microvasculature. The absence of prior reports concerning the influence of lymphatic system disorders on retinal blood flow density underscores the underexplored nature of this area. The changes in retinal structural characteristics may be attributed to the intricate interplay between lymphatic system-related inflammation and microvascular dynamics. Further research is warranted to unravel the complex mechanisms linking lymphatic system disorders and retinal microvascular alterations, enhancing our understanding of the systemic-ocular interface and its implications for ocular health.
There are several limitations of the current study. Firstly, the sample size was relatively small, and future studies with larger cohorts are necessary to obtain more robust and reliable results. Additionally, the cross-sectional design of our study limits our ability to establish causal relationships between hematological parameters and retinal capillaries. Longitudinal studies would provide deeper insights into the temporal dynamics of these associations. Moreover, medication usage, comorbidities, and lifestyle factors may contribute to the observed correlations and require further investigation. Finally, we did not account for the staging of different blood disorders and the recovery status of patients, which represents a major limitation of our study. Consequently, our findings may primarily reflect the long-term impact on the retina in patients with relatively stable physical conditions.
In summary, our study underscores the significant influence of hematological parameters on retinal microvasculature. Among these parameters, hematological diseases associated with anemia and reduced hemoglobin concentrations appear to exert the most substantial impact. The correlations identified in the present study shed light on the intricate relationship between systemic hematological factors and retinal perfusion, offering a foundation for further investigations into the mechanisms connecting hematological disorders and retinal vascular alterations. Collaborative efforts between hematologists and ophthalmologists are imperative to comprehensively address the complex interplay between systemic health and ocular vascular integrity.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hemminki, K., Huang, W., Sundquist, J., Sundquist, K. & Ji, J. Autoimmune diseases and hematological malignancies: exploring the underlying mechanisms from epidemiological evidence. Semin Cancer Biol. Aug. 64, 114–121 (2020).
Xu, L. et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J. Hematol. Oncol. Mar. 2 (1), 33 (2018).
Tomasik, J. & Basak, G. W. Inflammasomes-New contributors to Blood diseases. Int. J. Mol. Sci. Jul 23 ;23(15). (2022).
Hu, D. et al. Cellular senescence and hematological malignancies: from pathogenesis to therapeutics. Pharmacol. Ther. Jul. 223, 107817 (2021).
Zhao, E. J., Cheng, C. V., Mattman, A. & Chen, L. Y. C. Polyclonal hypergammaglobulinaemia: assessment, clinical interpretation, and management. Lancet Haematol. May. 8 (5), e365–e375 (2021).
Weiss, G., Ganz, T. & Goodnough, L. T. Anemia of inflammation. Blood Jan. 3 (1), 40–50 (2019).
Stauder, R., Valent, P. & Theurl, I. Anemia at older age: etiologies, clinical implications, and management. Blood Feb. 1 (5), 505–514 (2018).
Zarrabian, D. & Hanna, M. Characterization of pediatric transfusion-dependent thalassemia patients in a large academic center. J. Clin. Lab. Anal. Sep.4, e24962 (2023).
Viana, M. B., Oliveira, E. L. & Belisario, A. R. Severe clinical picture in a cohort of six Brazilian children with hemoglobin sbeta-thalassemia IVS-I-5 G > A. Blood Cells Mol. Dis. Aug. 30, 104:102795 (2023).
Okayama, Y. et al. The safety of the combination therapy of recombinant factor VIIa and plasma-derived factor VIIa and factor X for refractory hemorrhage in acquired hemophilia A. Blood Coagul Fibrinolysis Sep. 1 (6), 419–422 (2023).
Pipe, S. W., Dunn, A. L. & Young, G. Efficacy and safety evaluation of eptacog beta (coagulation factor VIIa [recombinant]-jncw) for the treatment of hemophilia a and B with inhibitors. Expert Rev. Hematol. Aug 21 (2023).
Matarraz, S. et al. Baseline immunophenotypic profile of bone marrow leukemia cells in acute myeloid leukemia with nucleophosmin-1 gene mutation: a EuroFlow study. Blood Cancer J. Sep. 4 (1), 132 (2023).
Awada, H. et al. Gemtuzumab ozogamicin plus standard induction hemotherapy improves outcomes of newly diagnosed intermediate cytogenetic risk acute myeloid leukemia. Blood Cancer J. Sep. 4 (1), 131 (2023).
Ferreri, A. J. M. et al. Burden of illness in follicular lymphoma with multiple lines of treatment, Italian RWE Analysis. Cancers (Basel) Sep. 2 ;15(17). (2023).
Haebe, S. E. et al. Follicular lymphoma evolves with a surmountable dependency on acquired glycosylation motifs in the B cell receptor. Blood. Sep 8. (2023).
Gaudio, A. et al. Hematological diseases and osteoporosis. Int. J. Mol. Sci. May 16 ;21(10). (2020).
Zekavat, S. M. et al. Deep learning of the retina enables phenome- and genome-wide analyses of the microvasculature. Circulation Jan. 11 (2), 134–150 (2022).
Garcia-Castineiras, S. Iron, the retina and the lens: a focused review. Exp. Eye Res. Jun. 90 (6), 664–678 (2010).
Mitani, A. et al. Detection of anaemia from retinal fundus images via deep learning. Nat. Biomed. Eng. Jan. 4 (1), 18–27 (2020).
Xiao, W. et al. Screening and identifying hepatobiliary diseases through deep learning using ocular images: a prospective, multicentre study. Lancet Digit. Health Feb. 3 (2), e88–e97 (2021).
Venkatesh, R. et al. Choroidal and retinal thickness variations in anaemia and anaemic retinopathy. Clin. Exp. Optom. Aug. 105 (6), 602–608 (2022).
Benjamini, E., Shimizu, M., Young, J. D. & Leung, C. Y. Immunochemical studies on the tobacco mosaic virus protein. VII. The binding of octanoylated peptides of the tobacco mosaic virus protein with antibodies to the whole protein. Biochem. Apr. 7 (4), 1261–1264 (1968).
Chollangi, S., Wang, J., Martin, A., Quinn, J. & Ash, J. D. Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol. Dis. Jun. 34 (3), 535–544 (2009).
Chucair-Elliott, A. J. et al. Leukemia inhibitory factor coordinates the down-regulation of the visual cycle in the retina and retinal-pigmented epithelium. J. Biol. Chem. Jul. 13 (29), 24092–24102 (2012).
Velo-Garcia, A., Castro, S. G. & Isenberg, D. A. The diagnosis and management of the haematologic manifestations of lupus. J. Autoimmun. Nov. 74, 139–160 (2016).
Krishnan, A. R. et al. Absence of thrombocytopaenia and/or microangiopathic haemolytic anaemia does not reliably exclude recurrence of atypical haemolytic uraemic syndrome after kidney transplantation. Nephrol. (Carlton) Feb. 22 (Suppl 1), 28–31 (2017).
Zhao, X. et al. Deep-learning-based Hemoglobin Concentration Prediction and Anemia Screening using Ultra-wide Field Fundus images. Front. Cell. Dev. Biol. 10, 888268 (2022).
Duzgun, E., Demir, N., Alkan, A. A., Uslu Dogan, C. & Cakir, A. Retinochoroidal vascular plexuses in patients with iron deficiency anaemia. Clin. Exp. Optom. Apr. 105 (3), 326–332 (2022).
Wu, Y. et al. Optical coherence tomography angiography for the characterisation of retinal microvasculature alterations in pregnant patients with anaemia: a nested case–control study. Br. J. Ophthalmol. Nov 25 (2022).
Han, I. C., Tadarati, M. & Scott, A. W. Macular Vascular Abnormalities identified by Optical Coherence Tomographic Angiography in patients with Sickle Cell Disease. JAMA Ophthalmol. Nov. 133 (11), 1337–1340 (2015).
Martin, G. C. et al. Visual function in asymptomatic patients with homozygous sickle cell disease and temporal Macular Atrophy. JAMA Ophthalmol. Oct. 1 (10), 1100–1105 (2017).
Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmol. May. 98 (5 Suppl), 741–756 (1991).
Xu, F. et al. Retinal microvascular signs in pre- and early-stage Diabetic Retinopathy detected using wide-field swept-source Optical Coherence Tomographic Angiography. J. Clin. Med. Jul 26 ;11(15). (2022).
Koca, S. et al. Evaluation of macular and optic disc radial peripapillary vessel density with optical coherence tomography angiography in iron deficiency anemia. Photodiagnosis Photodyn Ther. 38, 102744 (2022).
Funding
Shandong Province “Double-Hundred Talent Plan” on 100 Foreign Experts and 100 Foreign Expert Teams Introduction (For Team), WST2021004; Youth Fund of the National Natural Science Foundation of China, nos.81,300,383; Novo Nordisk Haemophilia Research Fund (NNHRF) China Clinical Research Center of Shandong University, nos. 2020SDUCRCC015; Shandong Provincial People’s Government, Mount Taishan Scholar Young Expert, tsqn202103167; Natural Science Foundation for Young Scholars of Shandong Province of China (ZR202111120236).
Author information
Authors and Affiliations
Contributions
Author contributions: (I) Conception and design: TZ. J, FB. X, L. Z, JW. S and JP(II) Administrative support: L. Z, JW. S and JP(III) Provision of study materials or patients: FB. X, GH. L, HL. W and XY. Y(IV) Collection and assembly of data: TZ. J, BX. S, ZW. L, Q. F, SQ. X and WB. Z(V) Data analysis and interpretation: TZ. J, FB. X, XC. L(VI) Manuscript writing: TZ. J, FB. X(VII) Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jian, T., Xu, F., Li, G. et al. Quantitative detection of macular microvascular abnormalities identified by optical coherence tomography angiography in different hematological diseases. Sci Rep 14, 25433 (2024). https://doi.org/10.1038/s41598-024-76753-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76753-8