Abstract
Observational studies and clinical trials have suggested the relationship between the gut microbiome and respiratory diseases, but the causality between them remains unclear. Firstly, we selected eight respiratory diseases Genome-wide association study (GWAS) datasets mainly from the FinnGen collaboration as outcomes. The exposure was based on GWAS statistics about the gut microbiome, sourced from the MiBioGen consortium, including gut microbial taxa. The causal link between the gut microbiome and respiratory illnesses was then estimated using a Two-sample Mendelian randomization (MR) analysis, including the inverse-variance weighted (IVW), weighted median, MR-Egger, simple mode, and weighted mode. To ensure reliability, F-statistics and sensitivity tests were conducted. Furthermore, we performed a reverse MR analysis of the pre-Mendelian positive findings to possible reverse causality. For the 196 gut microbe taxa, the IVW analysis suggested 88 potential associations with eight clinically prevalent respiratory diseases. Among them, 30 causal associations were found in more than one MR method. Multiple statistical corrections have confirmed three causal associations: genus Holdemanella was a risk factor for chronic obstructive pulmonary disease (COPD) (P = 1.3 × 10−4, OR = 1.18), family FamilyXIII was a protective factor for COPD (P = 1.3 × 10−3, OR = 0.75), and genus Oxalobacter was a risk factor for asthma (P = 2.1 × 10−4, OR = 1.09). Our MR analysis results indicate that there would be a causal relationship between the gut microbiome and respiratory diseases, contributing to the gut-lung axis. This finding offers new insights into the gut microbiome’s roles in respiratory diseases’ clinical prevention, pathogenesis, and improvement of clinical symptoms. Further randomized controlled trials are necessary to clarify the protective effect of probiotics and fecal microbial transplantation on respiratory health.
Similar content being viewed by others
Introduction
With the aging of the population and urbanization, respiratory diseases will continue to occupy a crucial position in morbidity and mortality, especially lung cancer and chronic respiratory disease1. Since 2010, the number of cancer deaths worldwide has increased by over 20%2. As the second most prevalent cancer, lung cancer is also the primary cause of cancer-related globally3. Regarding chronic respiratory disease, patients often only become aware when their lung function declines significantly and pronounced respiratory symptoms emerge. Furthermore, recent research indicates that chronic respiratory diseases are the third leading cause of death, trailing only behind cardiovascular diseases and cancer4. Therefore, how to prevent and treat respiratory diseases has gradually become one of the hot spots.
The human body is settled by a large number of microbes, with the intestine being the most densely populated organ5. The symbiotic relationship between microbes and their hosts is closely related to human health and disease. Similarities exist in the microbes that colonize the digestive and respiratory systems in their early stages due to their shared embryonic origin. Several epidemiological studies have demonstrated the potential for respiratory morbidity and increased mortality in patients with inflammatory bowel disease (IBD)6,7,8, which suggests that there is some connection between the gut and the lungs. Since the initial amplification and sequencing of genes using 16 S ribosomal Ribonucleic Acid (rRNA) a decade ago, the functions of the gut microbiota, particularly in host metabolism and immunology, have been gradually investigated9,10,11.
Alterations in the gut microbiome and the diversity of microbiota metabolites affect the immune response in the gastrointestinal tract and distant organs, such as the lung, through the mucosal immune system, thereby influencing lung function and respiratory disease. The crosstalk between the gut and lung is known as the gut-lung axis. However, the exploration of the mechanisms of the gut-lung axis is still in its infancy, and it is now largely considered to be a long-distance interaction between the gut and lung on immune function through commensal microorganisms, which may be related to the stimulation of the production of biologically active substances and probiotics by gut microorganisms.
Since most earlier studies used case-control designs and metagenomic sequencing to observe the variations between each gut microbe, the relationship between the gut microbiome and respiratory disorders has been susceptible to residual confounding and reverse causality. Therefore, it remains unclear whether there is a direct causal relationship between the gut microbiome and respiratory diseases. It is essential to determine whether the gut microbiome plays a causal role in the development of respiratory diseases. Mendelian randomization (MR), a practical approach for investigating causal links in epidemiology, can overcome the limitations of observational research12. Using genetic variation highly correlated with exposure, MR could investigate the causal relationship between exposure and outcome13. Since genetic variation is randomized to individuals before disease onset, similar to a randomized controlled trial, the influence of confounding factors and reverse causality can be minimized12,14. Several studies have utilized MR to explore the causal association between the gut microbiome and clinical diseases, including metabolic diseases15and blood metabolites16.
Based on the sample size of respiratory diseases in the public database, we screened for eight clinically prevalent respiratory diseases, including four subgroups of lung cancer, three subgroups of chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, chronic bronchitis, interstitial lung disease (ILD), sleep apnoea, and idiopathic pulmonary fibrosis (IPF). Undoubtedly, determining whether a causal association exists between the gut microbiome and respiratory diseases contributes to the gut-lung axis. Furthermore, it provides novel insights into the role of gut microbiomes in the pathogenesis, prevention, clinical improvement, treatment, and prognosis of respiratory diseases17,18.
Materials and methods
Study overview
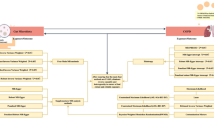
Briefly, the exposure was 196 gut microbes, and eight clinical respiratory diseases were outcomes. The causal link between the gut microbiome and respiratory illnesses was estimated using MR analysis, and a series of sensitivity analyses were performed to determine the robustness of the results. Finally, a reverse MR analysis was conducted to obtain positive results from the pre-MR and assess the possibility of reverse causality. The overall design of this study is illustrated in Fig. 1. To ensure the accuracy and validity of the research, Single Nucleotide Polymorphisms (SNPs) used as instrumental variables (IVs) in this study must comply with the following three significant assumptions19: (1) the relevance assumption: IVs are closely associated with the exposure factors; (2) the exclusion assumption: IVs only affect the outcome through the exposure factors, which means that IVs can not directly influence the outcome; (3) the independence assumption: IVs are not associated with confounders.
Overview of present MR analysis and assumptions. IVs, instrumental variables; GWAS, Genome-wide association study; LUSC, lung squamous cell carcinoma; SCLC, small cell lung carcinoma; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; SNP, Single Nucleotide Polymorphism; MR, Mendelian randomization; IVW, inverse variance weighted.
Exposure sources
The full summary statistics of the genetics of the host gut microbiome were obtained from the MiBioGen consortium (https://mibiogen.gcc.rug.nl/), which organized a large-scale, multi-ethnic microbiome Genome-wide association study (GWAS) meta-analysis20. This microbiome GWAS analysis encompassed 24 cohorts, comprising 18,340 participants, 85% European. Data were used as processed by Kurilshikov et al., which included rarefying to sample depths of 10,000 and utilizing a Ribosomal Database Project (RDP) classifier to bin reads to a reference database.
Utilizing a standardized and rigorous framework, microbiome trait loci (mbTL) were systematically identified, enabling the precise localization of genetic loci that regulate the relative abundance (designated as microbiome quantitative trait loci, mbQTL) or the presence (termed microbiome binary trait loci, mbBTLs) of distinct microbial taxa. Meanwhile, the MiBioGen consortium also performed the rigorous Gene Set Enrichment Analysis and comprehensive Phenome-wide Association Studies to interpret the biological implications of the findings from the GWAS. Overall, 211 gut microbial taxa, collapsed across five different taxonomic levels, along with 122,110 loci of variation, were included in the analysis to investigate the relationship between the host and gut microbiome.
Outcome sources
Our study used eight types of clinical respiratory diseases as outcomes. Among them, genetic associations with lung cancer were obtained from a large-scale meta-analysis21, which combined the imputed genotypes of 14,803 cases and 12,262 controls from the OncoArray dataset, along with 14,436 cases and 44,188 controls from the prior lung cancer GWAS. Our study included four subgroups of lung cancer: overall lung cancer, lung adenocarcinoma, squamous cell lung carcinoma, and small cell lung carcinoma. The genetic GWAS of the rest of the seven types of clinical respiratory diseases were acquired from the FinnGen consortium22. It comprises COPD (16,410 cases and 283,589 controls), early onset COPD (6,371 cases and 326,794 controls), later onset COPD (9,334 cases and 135,491 controls), asthma (37,253 cases and 187,112 controls), bronchiectasis (1,967 cases and 283,589 controls), chronic bronchitis (1,180 cases and 283,589 controls), ILD (3,715 cases and 338,784 controls), sleep apnoea (33,423 cases and 307,648 controls), IPF (1,812 cases and 338,784 controls).
IVs selection
First, the IVs selected for analysis should comply with the relevance assumption. For the gut microbiome, we screened for a genome-wide significance threshold (P < 5 × 10−8). In this way, there were exceptionally rare SNPs. Therefore, we adopted a locus-wide significance threshold (P < 1 × 10−5) to select SNPs related to the gut microbiome15. Secondly, this study established a low linkage disequilibrium correlation coefficient (r2 < 0.001 and clumping distance = 10,000 kb) to ensure the absence of linkage disequilibrium among the included IVs. Thirdly, when we merged the statistics of exposure and outcome, we excluded SNPs related to exposure that could not be fitted to the outcome’s GWAS summary statistics. Additionally, we harmonized the statistics of exposure and outcome, removing palindromic and incompatible SNPs. Finally, to fulfill the relevance assumption and acquire a more precise causal relationship, we calculated F statistics (F = β2exposure / se2exposure) and excluded the weak IVs with F< 1023.
MR analysis
This study performed MR analysis to estimate the causal effect of the gut microbiome on the eight clinical respiratory diseases. MR analysis utilized three popular MR methods to explore causal relationships: the inverse-variance weighted (IVW), weighted median, and MR-Egger methods. The IVW method is based on the hypothesis that all assumptions of MR are effective, and the weighted median method is appropriate for the hypothesis that less than 50% of Vs have horizontal pleiotropy24. Conversely, the MR-Egger is suitable for the hypothesis that more than 50% of IVs have horizontal pleiotropy25. This study also included simple and weighted modes. With the other four methods as complements, the IVW method was used as the primary statistical method to estimate the causal effect. If the result of the MR analysis was nominally significant (P< 0.05), we inferred that there might be a causal relationship between the gut microbiota and the outcome26. The causal relationship between the gut microbe taxa and outcome was considered more reliable if two or more MR methods were suitable for nominal significance. Furthermore, this study implemented a multiple-testing correction at each taxonomic level, with separate MR analyses performed for each level (e.g., genus, family). The threshold for statistical significance was set at P < 0.05/n, where n represents the effective number of bacterial taxa at the relevant taxonomic level.
Sensitivity analysis and reverse MR analysis
We employed Cochrane’s Q test to assess the heterogeneity because heterogeneity between the IVs can potentially influence the results of the MR analysis. P < 0.05 indicated signified the presence of heterogeneity. Horizontal pleiotropy indicated that IVs are associated with outcomes in other ways, which is not allowed in the MR analysis. Therefore, the intercept test of MR Egger regression and MR Pleiotropy Residual Sum were used to test the horizontal pleiotropy of the selected IVs and the outcome, with P> 0.05 indicating no horizontal pleiotropy27. To ensure the reliability of causality between IVs and outcome, we used leave-one-out analysis to identify whether the causality was strongly affected by an SNP. In addition, we performed a reverse MR analysis of pre-Mendelian positive results for possible reverse causality. In the reverse MR analysis, we set the range (P < 5 × 10−8) for IVs of eight kinds of clinically respiratory diseases. The screening of remaining IVs was consistent with previous MR analysis. The entire statistical analysis was performed using the “TwoSampleMR” package (version 0.5.6) in R software (version 4.2.1).
Results
Selection of IVs of the gut microbiome related to eight respiratory diseases
After implementing multiple quality filtering control steps (P < 5 × 10−5, r2 < 0.001, and clumping distance = 10,000 kb), a total of 2601 SNPs were identified as strongly associated and independent SNPs with the gut microbiome (124 SNPs in 9 phyla, 223 SNPs in 16 classes, 279 SNPs in 20 orders, 444 SNPs in 32 families, and 1531 SNPs in 119 genera). Notably, no weak associations were detected, as the F-statistics for the SNPs ranged from 14.59 to 88.43, with all F-statistics exceeding the threshold of 10. Comprehensive results are presented in Supplementary Table 1. After merging and harmonizing the statistics of exposure and outcome, a total of 2219 SNPs were identified as IVs for.
lung cancer. Likewise, 2211 SNPs were identified as IVs for lung adenocarcinoma, 2213 SNPs for squamous cell lung carcinoma, and 2199 SNPs for small cell lung carcinoma. Furthermore, 2247 SNPs were identified as IVs for COPD, 2245 SNPs for early onset COPD, 2247 SNPs for later onset COPD, 2246 SNPs for asthma, 2245 SNPs for bronchiectasis, 2247 SNPs for chronic bronchitis, 2245 SNPs for ILD, 2246 SNPs for sleep apnoea, 2245 SNPs for IPF. Comprehensive results are shown in Supplementary Table 2.
MR analysis results of the gut microbiome and eight respiratory diseases
We conducted MR analysis on 196 gut microbe taxa and eight respiratory diseases, as depicted in Supplementary Fig. 2 and Supplementary Fig. 3. The IVW results suggested 88 possible connections with eight clinical respiratory diseases for 196 different gut microbes, including the genus Streptococcus. The results are presented in detail in Supplementary Table 3. As illustrated in Fig. 2and Figs. 3 and 30 causal associations were demonstrated in more than one MR method. Additionally, multiple testing corrections, serving as IVs, were considered in this study. The significance thresholds for five taxonomic levels were set to the following: P = 5.56 × 10−3 (0.05/9) for the phylum, P = 3.13 × 10−3 (0.05/16) for the class, P = 2.5 × 10−3 (0.05/20) for the order, P = 1.56 × 10−3 (0.05/32) for the family, and P = 4.20 × 10−4 (0.05/119) for the genus. However, despite the biological plausibility, the multiple statistical corrections were so strict and conservative that only three causal associations emerged: genus Holdemanella as exposure with COPD as the outcome (P = 1.3 × 10−4, OR = 1.18), family FamilyXIII as exposure with COPD as the outcome (P = 1.3 × 10−3, OR = 0.75), and genus Oxalobacter as exposure with asthma as the outcome (P = 2.1 × 10−4, OR = 1.09).
Forest plot of 30 causal relationships between the gut microbiome and eight respiratory diseases. The fifth column is the forest plot, greater than one means increased risk, and less than 1 means protective risk. NSNPs, the number of single nucleotide polymorphisms; CI, confidence interval; IVW, inverse variance weighted; LUSC, lung squamous cell carcinoma; SCLC, small cell lung carcinoma; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis.
Circular heatmap depicting the P for the associations between the gut microbiome and eight respiratory. In this heatmap, the P serves as an indicator of the statistical significance of the potential causal relationships inferred through the MR method. Specifically, P < 0.05 is considered to suggest potential statistical significance. IVW, inverse variance weighted; LUSC, lung squamous cell carcinoma; SCLC, small cell lung carcinoma; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis.
Sensitivity analysis and reverse MR analysis
Supplementary Table 4 contains complete results for Cochrane’s Q test (MR Egger and IVW). All 30 potential associations with outcome in MR Egger are in Supplementary Table 5, with P > 0.05 certifying no heterogeneity or horizontal pleiotropy. Furthermore, the leave-one-out analysis of 30 causalities indicated the stability and reliability of our results. Comprehensive findings are in Supplementary Fig. 3. We conducted a reverse MR analysis of 30 positive results from the pre-MR analysis. Unfortunately, the IVW method only suggested that when IPF was an exposure and order Rhodospirillales was an outcome, the result was significant (P = 0.03, OR = 1.04). Nevertheless, it does not satisfy the criteria for multiple statistical corrections with P > 0.025 (P = 0.05/2) and other methods, so it could only be considered a potential risk factor. The comprehensive results are presented in Supplementary Table 6.
Discussion
In this study, the gut microbiome and eight respiratory diseases were causally linked using a bidirectional two-sample MR analysis. To the best of our knowledge, this is the first large-scale MR analysis of the association between the gut microbiome and eight clinical respiratory diseases. As shown in Figs. 4and 30 causal relationships were found in more than one MR method. Among them, three causal relationships satisfy multiple statistical correction criteria. These causal findings contribute to the gut-lung axis, which aligns with previous studies. Many microorganisms colonize the gut, and specific microbial communities have been found in lungs that were previously considered sterile28. Although the mechanisms underlying the influence of gut microbiota on lung microbiota remain incompletely understood, accumulating evidence suggests there was a crosstalk between gut microbes and the lungs, also known as the gut-lung axis. Clinical trials have found that the decrease in gut microbial abundance following antibiotic administration increases susceptibility to respiratory diseases29,30. Studies have shown that the gut microbes protect against bacterial and viral pulmonary infections by activating inflammasomes31. These studies confirmed that there was a relationship between intestinal diseases and respiratory diseases. However, whether there was a causal or influencing mechanism remains unknown. Our study provided the causal relationship between gut microbes and eight respiratory diseases, further reinforcing the gut-lung axis and providing novel insights into clinical prevention, pathogenesis, and alleviation of clinical symptoms.
The gut-lung axis: gut microbes associated with eight respiratory diseases identified by the current MR analysis. The pink bottom indicates that the gut microbes are the protective factors of the outcome, and the blue bottom indicates that the gut microbes are the risk factors of the outcome. LUSC, lung squamous cell carcinoma; SCLC, small cell lung carcinoma; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis.
Metagenomic sequencing has gradually been used to identify gut microbes in the context of respiratory diseases. Research on the gut microbiome in respiratory diseases has primarily concentrated on COPD, lung cancer, and asthma. Bowerman et al. used metagenomic sequencing to compare the fecal microbiomes between COPD patients and healthy controls, discovering that Streptococcuslevels were significantly elevated in the COPD population32, which was consistent with our findings. On this basis, our investigation into 88 potential associations suggested that the genus Streptococcusis a risk factor for COPD. As the third leading cause of death globally, COPD primarily arises from prolonged smoking and air pollution33. In addition, inflammation and immune responses contribute to its pathogenesis34. Studies have identified an imbalance between the Type 1 helper T cell (Th1) and Type 2 helper T cell (Th2) immune responses in COPD patients, possibly caused by changes in cytokine levels, specifically an increase in IL-12 levels and a decrease in IL-4 levels35. The gut microbiome plays a crucial role in maintaining the balance between Th1 and Th2 immune responses. Therefore, future research endeavors are required to examine whether the genus Streptococcus and other microbes could promote Th1 immune responses to cause COPD-associated inflammation and structural remodeling of the lungs by elevating IL-12 levels. Analogous to COPD, ILD is a respiratory disease with an inflammatory reaction as the pathological manifestation. Previous studies have supported the idea that the alteration in the abundance of intestinal Bacteroidescould potentially trigger inflammation36. Observations have indicated that patients with a high intestinal Bacteroidesload suffer from a longer treatment time for sepsis37. This research was consistent with our findings that family Bacteroides was considered a risk factor for early onset COPD, and genus Bacteroides was also revealed as a risk factor for ILD.
Immunodeficiency is an essential mechanism in the development of lung cancer, contributing not only to cancer progression but also to an inadequate response to cancer treatment38. Our study supported that the genus Prevotellais a risk factor for lung cancer, which has also been established in gastric cancer39, psoriasis40, and colorectal cancer41. The potential mechanism involves the overexpression of Mucin 13, Cell Surface Associated (MUC13), which could promote tumor cell proliferation, migration, and invasion through the activation of multiple signaling pathways. In our study, the genus Collinsellawas a protective factor for lung cancer, possibly by producing ursodeoxycholate42, which has also been supported in the fecal microbiota of patients. The cancer-immunity cycle posits that pro-inflammatory cytokines produced by gut microbiota metabolites may activate effector T-cells that target cancer-specific antigens, effectively eradicating malignant lung cancer cells43. Additionally, short-chain fatty acids produced by gut microbes are the most prevalent immunomodulatory metabolites in the gastrointestinal tract and are implicated in the potential mechanisms of the gut-lung axis44. Notably, butyrate has been demonstrated to have a significant impact on a range of lung conditions, encompassing asthma, COPD, and pulmonary fibrosis45. In our study, Rumnococcaceae, the leading producer of butyrate46, suggested a protective effect on ILD and sleep apnoea.
Immunologically, asthma is characterized by an allergic inflammatory response, dominated by the Th2 immune response. The “hygiene hypothesis” proposes that a lack of bacterial exposure early in life and dysbiosis leads to an enhanced Th2 immune response. Furthermore, the gut-lung axis suggests that altered diversity and dysbiosis of gut microbes may cause lung disease by altering the immune response47. Our study found order Clostridium to be a protective factor for asthma. It has been discovered that Clostridium butyricum has unique immunomodulatory effects. Experiments have confirmed that Clostridium butyricumcould reverse the imbalance between Th1 and Th2 to suppress airway inflammation and remodeling in asthmatic mice48. Another study showed that Clostridium butyricumalleviated allergic airway inflammation, reduced mucus secretion, and restored the balance between Th1 and Th250. Therefore, we hypothesize that Clostridium butyricum is protective against asthma by promoting the Th1 immune response and inhibiting the Th2 immune response to ameliorate the immune imbalance. In addition, the butyrate produced by Clostridium butyricum can nutritionally repair intestinal mucosa, enhance intestinal barrier function, and reduce the absorption of allergens.
Notably, altering the composition of gut microbes has been shown to achieve specific therapeutic efficacy in the treatment of respiratory diseases50. Research has confirmed that lipopolysaccharide derived from Parabacteroides goldsteiniiexhibits anti-inflammatory properties, suggesting the potential development of relevant probiotics as a therapeutic option for COPD patients51. Additionally, the potential role of probiotics treating asthma52, lung cancer53, and sleep apnoea54was initially confirmed. Another novel therapeutic approach, Fecal microbiota transplantation (FMT) has also been gradually explored for the treatment of respiratory diseases. Existing studies have confirmed that FMT and diet modification could reduce the severity of emphysema by changing the intestinal microbial composition and improving inflammation55. Our research would provide new perspectives for the subsequent treatment of respiratory diseases by probiotics and FMT. The treatment of respiratory diseases by associated probiotics and FMT is worthy of further exploration. It should also be noted that probiotics and FMT carry certain risks as treatment options, and these potential risks should be considered during their application56.
Undoubtedly, our research has the following advantages. Firstly, conducting bidirectional MR analysis of the gut microbiome and eight clinical respiratory diseases can avoid confounding factors and reverse causal relationships, which contribute to the gut-lung axis. This provides a theoretical basis for subsequent research and treatments of specific gut microbes in respiratory diseases and helps to find new biomarkers. Meanwhile, comprehensive exposure statistics were obtained from the large-scale GWAS, and outcomes statistics of the outcomes were sourced from the most extensive lung cancer GWAS and the FinnGen GWAS, with the large sample size providing reliable support for our results. Additionally, compared to several small randomized controlled studies, it consolidates the reliability of these research results.
However, there are also several limitations to this research. Firstly, the data sources for exposure are predominantly from a broad representation of European ancestry, whereas the outcomes data are mostly derived from FinnGen individuals. To a certain extent, this similarly restricts the generalizability of our findings, potentially making them inapplicable to other populations. Secondly, although we selected outcome subgroups for analysis based on summary statistics, which inherently lack stratification, such as specific clinical characterizations, treatment details, and disease severity, this prevented us from conducting further analyses. Therefore, translating these results to other populations may be challenging. Thirdly, the application of multiple statistical corrections may be overly rigorous and conservative, potentially overlooking potential causal relationships between the gut microbiome and respiratory diseases57. Therefore, while obtaining multiple test results, we retained results that satisfied two or more MR methods based on their biological plausibility. Lastly, the widespread use of 16 S RNA sequencing in gut microbiota research is accompanied by limitations, including quantitative imprecision, database incompleteness, limited species resolution, the potential for bias in bioinformatic processing58, and inadequate sequencing depth. The foundational understanding of the causal relationship between gut microbiota and respiratory diseases heavily relies on gut microbiota GWAS data. Consequently, the adoption of advanced shotgun metagenomic techniques in future microbiota GWAS endeavors promises to significantly augment the precision MR relationships.
Conclusion
In summary, this bidirectional MR analysis found the causal relationships between gut microbes and respiratory diseases, supporting the gut-lung axis. It will offer new insights into the gut microbiome’s roles in respiratory diseases’ clinical prevention, pathogenesis, and amelioration of clinical symptoms. Further randomized controlled trials and mediation MR analysis are required to identify the protective effect and possible immune mechanisms of probiotics and FMT on respiratory diseases.
Data availability
The datasets analyzed during the current study are available in the MiBioGen consortium (https://mibiogen.gcc.rug.nl/) 21, European Bioinformatics Institute , and the FinnGen consortium (https://r9.finngen.fi) 22.
Abbreviations
- GWAS:
-
Genome-wide association study
- MR:
-
Mendelian randomization
- IVW:
-
inverse variance weighted
- COPD:
-
chronic obstructive pulmonary disease
- IBD:
-
inflammatory bowel disease
- rRNA:
-
ribosomal Ribonucleic Acid
- ILD:
-
interstitial lung disease
- IPF:
-
idiopathic pulmonary fibrosis
- SNPs:
-
Single Nucleotide Polymorphisms
- RDP:
-
Ribosomal Database Project
- mbTL:
-
microbiome trait loci
- mbQTL:
-
microbiome quantitative trait loci
- mbBTLs:
-
microbiome binary trait loci
- CI:
-
confidence interval
- LUSC:
-
lung squamous cell carcinoma
- SCLC:
-
small cell lung carcinoma
- Th1:
-
Type 1 helper T cell
- Th2:
-
Type 2 helper T cell
- MUC13:
-
Mucin 13,Cell Surface Associated
- FMT:
-
Fecal microbiota transplantation
References
Zhou, M. et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 394, 1145–1158 (2019).
GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010-19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 400, 563–591 (2022).
Liao, W. et al. Predicting the future risk of lung cancer: development, and internal and external validation of the CanPredict (lung) model in 19·67 million people and evaluation of model performance against seven other risk prediction models. Lancet Respir Med. 11, 685–697 (2023).
GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet Respir Med. 8, 585–596 (2020).
Lepage, P. et al. A metagenomic insight into our gut’s microbiome. Gut. 62, 146–158 (2013).
Bernstein, C. N., Wajda, A. & Blanchard, J. F. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 129, 827–836 (2005).
Sato, H. et al. Chest high-resolution computed tomography findings in 601 patients with inflammatory Bowel diseases. Acad. Radiol. 25, 407–414 (2018).
Vutcovici, M. et al. Inflammatory bowel disease and risk of mortality in COPD. Eur. Respir J. 47, 1357–1364 (2016).
Adak, A. & Khan, M. R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 76, 473–493 (2019).
Miyauchi, E., Shimokawa, C., Steimle, A., Desai, M. S. & Ohno, H. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat. Rev. Immunol. 23, 9–23 (2023).
Shim, J. A., Ryu, J. H., Jo, Y. & Hong, C. The role of gut microbiota in T cell immunity and immune mediated disorders. Int. J. Biol. Sci. 19, 1178–1191 (2023).
Smith, G. D. & Ebrahim, S. Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 32, 1–22 (2003).
Sekula, P., Del Greco, M., Pattaro, F., Köttgen, A. & C. & Mendelian randomization as an Approach to assess causality using Observational Data. J. Am. Soc. Nephrol. 27, 3253–3265 (2016).
Davey Smith, G. & Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23, R89–98 (2014).
Sanna, S. et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51, 600–605 (2019).
Liu, X. et al. Mendelian randomization analysis support causal relationships between blood metabolites and the gut microbiome. Nat. Genet. 54, 52–61 (2022).
Gould, A. L. et al. Microbiome interactions shape host fitness. Proc. Natl. Acad. Sci. U S A. 115, E11951–E11960 (2018).
Heintz-Buschart, A. & Wilmes, P. Human gut microbiome: function matters. Trends Microbiol. 26, 563–574 (2018).
Gupta, V., Walia, G. K. & Sachdeva, M. P. Mendelian randomization’: an approach for exploring causal relations in epidemiology. Public. Health. 145, 113–119 (2017).
Kurilshikov, A. et al. Large-scale association analysis identify host factors influencing human gut microbiome composition. Nat. Genet. 53, 156–165 (2021).
McKay, J. D. et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat. Genet. 49, 1126–1132 (2017).
Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 613, 508–518 (2023).
Burgess, S., Thompson, S. G. & CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in mendelian randomization studies. Int. J. Epidemiol. 40, 755–764 (2011).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in mendelian randomization with some Invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314 (2016).
Burgess, S. & Thompson, S. G. Interpreting findings from mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32, 377–389 (2017).
Li, P. et al. Association between gut microbiota and preeclampsia-eclampsia: a two-sample mendelian randomization study. BMC Med. 20, 443 (2022).
Morrison, J., Knoblauch, N., Marcus, J. H., Stephens, M. & He, X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat. Genet. 52, 740–747 (2020).
Bassis, C. M. et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio. 6, e00037 (2015).
Russell, S. L. et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 13, 440–447 (2012).
Russell, S. L. et al. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes. 4, 158–164 (2013).
Ichinohe, T. et al. Microbiota regulates immune defense against respiratory tract influenza a virus infection. Proc. Natl. Acad. Sci. U S A. 108, 5354–5359 (2011).
Bowerman, K. L. et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat. Commun. 11, 5886 (2020).
Fricker, M. et al. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI Insight. 3, e94040. 94040 (2018).
Brightling, C. & Greening, N. Airway inflammation in COPD: progress to precision medicine. Eur. Respir J. 54, 1900651 (2019).
Chen, G., Mu, Q. & Meng, Z. J. Cigarette smoking contributes to Th1/Th2 cell dysfunction via the Cytokine Milieu in Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct Pulmon Dis. 18, 2027–2038 (2023).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 490, 55–60 (2012).
Sun, S. et al. Altered intestinal microbiome and metabolome correspond to the clinical outcome of sepsis. Crit. Care. 27, 127 (2023).
Gopalakrishnan, V., Helmink, B. A., Spencer, C. N., Reuben, A. & Wargo, J. A. The influence of the gut microbiome on Cancer, immunity, and Cancer Immunotherapy. Cancer Cell. 33, 570–580 (2018).
Oosterlinck, B. et al. Mucin-microbiome signatures shape the tumor microenvironment in gastric cancer. Microbiome. 11, 86 (2023).
Zhao, Q. et al. Intestinal dysbiosis exacerbates the pathogenesis of psoriasis-like phenotype through changes in fatty acid metabolism. Signal. Transduct. Target. Ther. 8, 40 (2023).
Shi, F. et al. Altered gut microbiome composition by appendectomy contributes to colorectal cancer. Oncogene. 42, 530–540 (2023).
Hirayama, M. et al. Intestinal Collinsella may mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate. PLoS One. 16, e0260451 (2021).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 39, 1–10 (2013).
Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166 (2014).
Theiler, A. et al. Butyrate ameliorates allergic airway inflammation by limiting eosinophil trafficking and survival. J. Allergy Clin. Immunol. 144, 764–776 (2019).
Vital, M., Karch, A. & Pieper, D. H. Colonic Butyrate-Producing Communities in Humans: an Overview Using Omics Data. mSystems. 2, e00130-17 (2017).
Frati, F. et al. The role of the Microbiome in Asthma: the gut–lung Axis. Int. J. Mol. Sci. 20, 123 (2018).
Juan, Z. et al. Oral administration of Clostridium butyricum CGMCC0313-1 reduces ovalbumin-induced allergic airway inflammation in mice. Respirology. 22, 898–904 (2017).
Li, L. et al. Aerosol Inhalation of Heat-Killed Clostridium butyricum CGMCC0313-1 Alleviates Allergic Airway Inflammation in Mice. J Immunol Res. 8447603 (2022).
Marchesi, J. R. et al. The gut microbiota and host health: a new clinical frontier. Gut. 65, 330–339 (2016).
Lai, H. C. et al. Gut microbiota modulates COPD pathogenesis: role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut. 71, 309–321 (2022).
Liu, A. et al. Adjunctive Probiotics alleviates asthmatic symptoms via modulating the gut microbiome and serum metabolome. Microbiol. Spectr. 9, e0085921 (2021).
Tomita, Y. et al. Association of Probiotic Clostridium butyricum Therapy with survival and response to Immune Checkpoint Blockade in patients with Lung Cancer. Cancer Immunol. Res. 8, 1236–1242 (2020).
Badran, M. et al. Gut microbiota mediate vascular dysfunction in a murine model of sleep apnoea: effect of probiotics. Eur. Respir J. 61, 2200002 (2023).
Jang, Y. O. et al. Fecal microbial transplantation and a high fiber diet attenuates emphysema development by suppressing inflammation and apoptosis. Exp. Mol. Med. 52, 1128–1139 (2020).
Hill, C. Balancing the risks and rewards of live biotherapeutics. Nat. Rev. Gastroenterol. Hepatol. 17, 133–134 (2020).
Liu, K., Zou, J., Fan, H., Hu, H. & You, Z. Causal effects of gut microbiota on diabetic retinopathy: a mendelian randomization study. Front. Immunol. 13, 930318 (2022).
Nearing, J. T. et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 13, 342 (2022).
Acknowledgements
The authors express their gratitude to the participants and investigators of the FinnGen consortium and the European Bioinformatics Institute. The authors also appreciate the MiBioGen consortium for releasing the gut microbiome GWAS summary statistics.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No: 82174302).
Author information
Authors and Affiliations
Contributions
XQ-Z and SY-S designed the study and analyzed and interpreted the data. XQ-Z drafted the manuscript, and SY-S performed the picture. Z-W conceived and designed the study and revised the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
For this study, consortia offered publicly accessible summary statistics and published papers. The relevant ethical review board has agreed on all original studies, and participants have given their informed consent. Additionally, this study did not use any data at the person level. Therefore, there was no need for approval from the new ethical review board.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, X., Shen, S. & Wang, Z. Genetic evidence of bidirectional mendelian randomization study on the causality between gut microbiome and respiratory diseases contributes to gut-lung axis. Sci Rep 14, 25550 (2024). https://doi.org/10.1038/s41598-024-77273-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77273-1