Abstract
The increasing prevalence of carbapenem-resistant Enterobacter cloacae complex (CREC) poses great challenges to infection treatment in the clinical setting. In this study, we reported the emergence of carbapenemase in a rare species, Enterobacter chuandaensis, belonging to the Enterobacter cloacae complex (ECC). We elucidated the genetic characteristics of carbapenem-resistant isolate FAHZZU5885, co-harboring blaNDM−1 and blaKPC−2. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and average nucleotide identity (ANI) analysis were used to identify E. chuandaensis. S1 nuclease pulsed-field gel electrophoresis (S1-PFGE) and Southern blotting were used to clarify the number and size of the plasmids in FAHZZU5885. Antimicrobial phenotypes were identified by antimicrobial susceptibility testing (AST), and the characteristics of the strain were examined with whole-genome sequencing (WGS). The conjugation experiment and stability assay were conducted to verify the transferability and stability of the plasmid carrying carbapenemase-encoding genes. E. chuandaensis FAHZZU5885 was isolated from a perianal swab of a patient admitted to the ICU. This strain simultaneously carried blaNDM−1 and two blaKPC−2 genes. FAHZZU5885 was resistant to most of the tested antibiotics except for amikacin, tigecycline, and colistin. Two blaKPC−2 were located separately on two different plasmids, the ~ 120 kb IncFIA-IncFII plasmid and the ~ 80 kb IncR plasmid. Both plasmids shared the conserved sequence klcA-korC-ISkpn6-blaKPC−2-ISkpn27-tnpR-tnpA. The blaNDM−1-bearing plasmid had the potential to transfer and can remain stable after successive passages. In addition, the blaNDM−1 was carried on a ~ 80 kb IncFII plasmid with the conserved sequence ISAba125-blaNDM−1-ble-trpF-dsbD-cutA-groS-groL. In summary, this study marks the first report of the multidrug-resistant E. chuandaensis strain FAHZZU5885 harboring two blaKPC−2-bearing plasmids, indicating the potential for the further dissemination of carbapenemase-encoding genes in novel species. The findings contribute to enhancing our understanding of CREC strains, emphasizing the need for continued and comprehensive surveillance of this species.
Similar content being viewed by others
Introduction
Enterobacter cloacae complex (ECC) is one of the most common pathogens in hospitals and contains the major multi-drug resistant bacterial pathogens, mainly including E. cloacae, E. asburiae, E. hormaechei, E. kobei, E. ludwigii, E. mori, and E. nimipressuralis1. ECC is widely distributed in the environment, animals, and humans, which also poses a huge challenge to effective clinical treatment2,3,4,5. The Enterobacter species/cluster may have different clinical significance6. E. chuandaensis is the ECC first recovered from the blood sample of a patient at West China Hospital, Chengdu, the People’s Republic of China in 20177. Additionally, there have been few global reports on this species. As a newly discovered member of the ECC in recent years, its resistance phenotype and genomic characteristics are still unclear.
Carbapenems, such as meropenem, imipenem, and ertapenem, are typical β-lactam antibiotics with a broad resistance spectrum and have recently been recognized as the last resort for clinical treatment of multi-drug resistant gram-negative bacteria8. Successful expansion of clonal replication and frequent horizontal transfer of carbapenemase genes-bearing plasmids contribute to the increase of carbapenem-resistant Enterobacteriaceae (CRE)9. Due to the limited options of antibiotics, the clinical treatment of CRE infection also faces severe challenges. Notably, carbapenem-resistant Enterobacter cloacae complex (CREC) is among the most common CRE in China10. The high lethality and disability caused by the infection pose a great challenge for clinical treatment. CRE resistance can be induced by carbapenemase, porin, efflux pump, penicillin-binding protein alteration, and biofilm production11. Among them, the increasing prevalence of carbapenemase-producing organisms is particularly concerning given the rapid spread of mobile genetic elements containing carbapenemase genes12. The prevalence of CRE in China is attributed to the dissemination of conservative mobile elements carrying blaNDM or blaKPC−2 on conjugative and non-conjugative plasmids13. It is imperative to further monitor and control the transmission of ECC carrying blaKPC and blaNDM in China, which may be a reservoir of antimicrobial resistance genes.
In China, the proportion of carbapenemase-producing Enterobacter (CP-Ent) in carbapenem-resistant Enterobacter(CR-Ent) was high in comparison to other global regions14. Furthermore, the majority of the CREC strains carried carbapenemase-encoding genes, with blaNDM−1being the most prevalent15. The blaNDM−1-bearing plasmid was strongly related to the antimicrobial agents and biofilm formation, which might be associated with long-term and chronic infections leading to an additional challenge in the treatment of bacterial infections16. Now the reports of CREC strains co-carrying blaNDM−1and other carbapenemase-encoding genes have been gradually common17.
While CREC carrying either blaKPC−2 or blaNDM−1 have been widely identified, CREC with the co-existence of blaKPC−2 and blaNDM−1 have been rarely reported18. Herein, we first report the E. chuandaensis strain harboring blaNDM−1 and two copies of blaKPC−2, suggesting that the carbapenem resistance spectrum may potentially broaden, which is worthy of our attention and further study. Based on this, this study focuses on the resistance characteristics, plasmid features, and potential multi-drug resistant mechanisms in E. chuandaensis.
Results
Multidrug-resistant E. chuandaensis ST2493 isolate from a patient with hospitalizations
FAHZZU5885 was isolated from a perianal swab of a patient admitted to the ICU in the First Affiliated Hospital of Zhengzhou University in 2022. This patient had a history of multiple hospitalizations and resuscitations with the use of meropenem and vancomycin. This strain was identified as E. chuandaensis based on the Matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF-MS) and average nucleotide identity (ANI) analysis, where this strain shared a 98.48% ANI value with the E. chuandaensis reference genome ASM359491v1. Whole-genome sequencing (WGS) revealed that E. chuandaensis FAHZZU5885 belonged to ST2493 (dnaA-fusA-gyrB-leuS-pyrG-rplB-rpoB: 184-433-201-563-277-86-304), which was a novel sequence type first reported in ECC. Twenty-two antibiotics were tested in antimicrobial susceptibility testing (AST), including omadacycline and eravacycline, which are two novel antibacterials within the tetracycline class19. FAHZZU5885 was resistant to most of the tested antibiotics except for amikacin, tigecycline, and colistin (Table 1). This multi-drug-resistant strain posed significant challenges for clinical treatment. The resistance profile of this strain generally aligns with the presence of corresponding resistance genes (Tables 1 and 2).
The confirmation of two bla KPC−2-bearing plasmids in FAHZZU5885
The genome of E. chuandaensis FAHZZU5885 consisted of one chromosome and three plasmids (Fig. 1C). Among them, the size of the chromosome was 4,872,395 bp with an average GC content of 55.5%. p5885-KPC-1 (127,537 bp), p5885-KPC-2 (85,101 bp), and p5885-NDM (77,383 bp) were identified as three different plasmid groups. According to the Resfinder, resistance genes located on different plasmids were detected (Table 2). WGS confirmed the presence of two blaKPC−2-bearing plasmids, which is in line with the result of S1 nuclease pulsed-field gel electrophoresis (S1-PFGE) and Southern blot (Fig. 1C). Two blaKPC−2 genes were located at ~ 120 kb IncFIA-IncFII plasmid p5885-KPC-1 and ~ 80 kb IncR plasmid p55885-KPC-2, respectively. Significantly, IncR-type plasmid p55885-KPC-2 was the main resistance reservoir, which conferred resistance to aminoglycoside, quinolone, beta-lactam, folate pathway antagonist, macrolide, tetracycline, and rifamycin (Table 2).
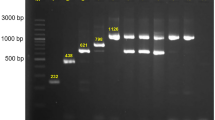
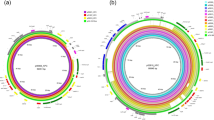
The confirmation of two blaKPC−2-bearing plasmids in FAHZZU5885. (A) Circular comparison between p5885-KPC-1 and three similar blaKPC−2-bearing plasmids in NCBI database (98% query coverage and 99% nucleotide identity). p5885-KPC-1 was used as the reference in the outermost ring. (B) Circular comparison between p5885-KPC-2 and three similar blaKPC−2-bearing plasmids in NCBI database (55% query coverage and 99.9% nucleotide identity). p5885-KPC-2 was used as the reference in the outermost ring. (C) Plasmid size determination by S1-PFGE, with Salmonella enterica serotype Braenderup H9812 as the size marker. The ___location of blaKPC−2- and blaNDM−1- harboring plasmids were shown with Southern blotting hybridization with the specific probes.
Repeated conjugation experiments with both p5885-KPC-1 and p5885-KPC-2 were unsuccessful. Attempts to transfer the plasmid extracted from the isolate using electroporation methods also failed to convey the plasmid to recipient E. coli DH5α cells. OriTFinder analysis revealed that p5885-KPC-1 and p5885-NDM possess complete conjugative systems. However, p5885-KPC-2, identified with only oriT, lacks essential transfer genes such as type IV secretion system (T4SS) and type IV coupling protein (T4CP). This deficiency may explain the unsuccessful conjugation of p5885-KPC-2 (Figure S1).
Tn3 plays a significant role in disseminating bla KPC−2 genes
In the comparative analysis of the two blaKPC−2-bearing plasmids, we examined their structural and genetic features. The 127,537 bp IncF plasmid p5885-KPC-1 possessed an average GC content of 53.6%. The overall structure of p5885-KPC-1 was most similar to pK518_KPC (CP023186) and pK92-KPC (OL828742) from Klebsiella michiganensis and pBKPC18-1 (CP022275) present in Citrobacter freundii (Fig. 1A). The main differences were concentrated in one gap located downstream of the SOS-regulated proteins umuC and umuD. The second blaKPC−2-bearing plasmid, designated as p5885-KPC-2, was an IncR-type plasmid with a size of 85,101 bp and an average GC content of 54.8% (Fig. 1B). This plasmid was a huge antimicrobial reservoir that cointegrated thirteen antimicrobial resistance genes (ARGs). The most similar structures could be seen in Klebsiella pneumoniae unnamed2 (no. CP058868), unnamed1 (no. CP065348), and pWSZBR (no. CP015991). The backbone of the p5885-KPC-2 that was responsible for plasmid replication and maintenance, such as xerC, stbA, and parA, was certainly the same as the above three plasmids. The difference was the profiles of resistance genes and mobile elements (Fig. 1B). The blaKPC−2 gene was situated within a multidrug resistance region (MRR) containing multiple ARGs and insertion sequence (IS) elements.
Compared with the genetic surroundings of the two blaKPC−2, the core platform in Tn3 (klcA-korC-ISkpn6-blaKPC−2-ISkpn27-tnpR-tnpA) was associated with blaKPC−2 (Fig. 2). This conserved sequence was commonly found in Chinese Enterobacteriaceae strains20. The difference in the genetic surroundings of the two blaKPC−2 lay in the insertion of resistance genes aac(3) and blaTEM−1B downstream of tnpA, followed by transposable elements ISkpn27, Tn3-tnpR, and truncated tnpA, which were similar to the conserved sequence of blaKPC−2. Tn3-based blaKPC−2-carrying transposon was associated with several additional resistance modules, forming a unified multi-drug resistance (MDR) region.
Comparison of genes surrounding blaKPC−2 on p5885-KPC-1, p5885-KPC-2, pK518_KPC (accession no. CP023186), and pWSZBR (accession no. CP15991). Open reading frames (ORFs) are indicated by arrows and are colored according to their presumed function. Grey shading indicates shared regions with a high degree of nucleotide identity. Red indicates antimicrobial resistance genes, yellow indicates genes related to mobile elements, blue represents other functional genes, and grey indicates genes encoding hypothetical proteins as well as proteins with unknown functions.
bla NDM−1-bearing IncFII plasmid is a key platform for stable resistance dissemination
S1-PFGE, Southern blot, and WGS showed that blaNDM−1-bearing plasmid p5885-NDM was a 77,383 bp IncFII plasmid, with a GC content of 55.6% (Figs. 1C and 3A). This plasmid carried the blaTEM−1B and blaNDM−1, which can be transferred to E. coli J53. According to the AST, the blaNDM−1-bearing transconjugant FAHZZU5885-J53 exhibited increased resistance mainly to the β-lactam (ceftriaxone, cefotaxime, ceftazidime, cefepime, imipenem, meropenem, and amoxicillin-clavulanic acid) as well as to penicillins (piperacillin/tazobactam) (Table 1). To confirm the stability of p5885-NDM, selected 188 colonies were subjected to PCR validation of blaNDM−1 after 5 days of passage. It was found that the preservation rate was 96.28% (Fig. S2). This phenomenon suggested that this plasmid can stably carry the blaNDM−1 gene in an antibiotic-free environment, providing a robust guarantee for horizontal transmission.
Genomic analyses of blaNDM−1-bearing plasmid. (A) Circular comparison between p5885-NDM and three similar blaNDM−1-bearing plasmids in NCBI database (85% query coverage and 99% nucleotide identity). p5885-NDM was used as the reference in the outermost ring. (B) Comparison of genes surrounding blaNDM−1 on p5885-NDM, pCR-13-36-NDM-1 (accession no. MZ857202), p_dmeb_c388_NDM1 (accession no. CP095673). Grey shading indicates shared regions with a high degree of nucleotide identity. Red indicates antimicrobial resistance genes, yellow indicates genes related to mobile elements, blue represents other functional genes, and grey indicates genes encoding hypothetical proteins as well as proteins with unknown functions.
Combined with the result of a BLASTn search, linear alignment of the plasmid genomes suggests that p5885-NDM was similar to three different plasmids from Enterobacter hormaechei, Citrobacter freundii and Citrobacter braakii, namely pCR-13-36-NDM-1 (no. MZ857202), pF4321-KPC (no. CP137180), and pF217-KPC_86k (no. CP136729), respectively (Fig. 3A). This plasmid carried abundant tra modules encoding a primary pilus for conjugation, which may be the reason for easy transmission21. We analyzed the genetic context of blaNDM−1 on this plasmid and found a conservative structural sequence (ISAba125-blaNDM−1-ble-trpF-dsbD-cutA-groS-groL) located downstream of the blaNDM−1 gene (Fig. 3B).
Discussion
ECC is a group of common opportunistic pathogens that may cause a wide range of infections, such as pneumonia, urinary tract infections, and even bloodstream infections22,23. Therefore, it is necessary and urgent to make the anti-infection treatment for ECC in the clinic. During various hospital outbreaks, the ECC exhibited a multidrug-resistant phenotype, including resistance to the last-resort carbapenems24. The blaNDM and blaKPC comprised the principal resistance mechanism of carbapenemase-producing ECC in the hospital, causing a threat to public health25. In this study, we first report the MDR E. chuandaensis strain harboring blaNDM−1 and blaKPC−2, suggesting the appearance of carbapenemase-encoding genes in new species. Owing to the similar genetic contexts of the two blaKPC−2-bearing plasmids, Tn3 plays a significant role in the dissemination of blaKPC−2 and may be the major reason for forming two copies of blaKPC−2 in this strain. The blaNDM−1 gene was stably persistent in the transconjugant after successive passage, suggesting that this IncFII plasmid p5885-NDM provided a robust platform for the transmission of blaNDM−1.
The efficient spread of carbapenemase-encoding genes in new species poses a significant threat to human health, which raises our attention. Horizontal transfer of plasmids is a key factor mediating the dissemination of resistance genes in different species, and the emerging plasmid-encoded carbapenemases are increasingly reported worldwide26. The blaNDM−1 in FAHZZU5885 was transferred to E. coli J53, and the transconjugant mainly exhibited increased resistance to β-lactams. This IncFII plasmid carrying blaNDM−1 was highly stable and could carry the resistance gene continuously in an antibiotic-free environment. The blaNDM−1 was incorporated into plasmids and stably inherited in animal-borne E. coli strains that can be maintained in animal gut microflora even without carbapenem selection pressure27. The efficient and stable dissemination of conjugative IncFII plasmid is extremely worrisome. Notably, there were two blaKPC−2-bearing plasmids with different sizes that could not be conjugated. p5885-KPC-1 was unable to transfer despite having a complete conjugative system. This is similar to plasmid pBKPC18-1 which was isolated from C. freundii 18 -1 in Shifeng River, Zhejiang Province, China28. It may be the reason that tra genes are far away from the primary transcriptional region, impairing the synthesis of primary RNA transcript28. Simultaneously, most very large plasmids are non-mobilizable, with evidence of ongoing domestication into secondary chromosomes29. p5885-KPC-2 is a typical non-conjugative IncR plasmid, which lacks related conjugative genes and relaxase genes30.
In addition to plasmids, other mobile genetic elements, including transposons and ISs, play a major role in the horizontal gene transfer and rapid spread of antimicrobial resistance genes in Enterobacteriaceae31. In this study, we found that two blaKPC−2 genes located on different plasmids had the same genetic context. The blaKPC−2 genes were all incorporated into the Tn3 transposon. In China, nearly all of the blaKPC genes are located in the Tn3-Tn4401 chimera with the gene order of Tn3 transposase, Tn3 resolvase, ISKpn8, blaKPC, ISKpn6-like element, Tn1721 resolvase, and Tn1721transposase genes32. This structure might have the potential to acquire the blaKPC−2 gene. Additionally, a study of Klebsiella pneumoniae carrying both blaNDM−1 and blaKPC−2 by Gao et al. proposed that KPC-2 and NDM-1-coproducing carbapenem-resistant Klebsiella pneumoniae (KPC-2-NDM-1-CRKP) emerged from a KPC-2-CRKP progenitor which acquired another highly transferable blaNDM−1 plasmid later33. In this study, the conjugative blaNDM−1-plasmid may also be acquired later by the progenitor of FAHZZU5885 only carrying blaKPC−2 genes, and the Tn3 transposon played a significant role in acquiring and spreading blaKPC−2 genes.
FAHZZU5885 was a typical MDR strain that was resistant to almost all commonly used antibiotics in the clinic, including the novel antibiotics within the tetracycline class, leaving patients with very limited options. The transconjugant only carrying blaNDM−1 reduced the MIC values for meropenem and imipenem compared with the wild strain, suggesting that the high MIC values for carbapenems in the wild strain were due to the combined interaction of blaKPC−2 and blaNDM−133,34. Notably, the IncR plasmid is an important reservoir of multidrug resistance in Enterobacteriaceae because the conserved IncR backbones include the MDR regions35. The IncR plasmid p5885-KPC-2 contributes to the formation of multidrug resistance in this strain. A further comprehensive investigation is warranted on the spread of blaKPC-harboring IncR plasmid.
To date, the isolation rates of E. chuandaensis in clinics may be greatly underestimated due to the limitations of the techniques in MALDI-TOF MS and the complexity of ECC. Although reports on CREC are relatively common, studies on carbapenem and colistin resistances of specific strains in this complex group have been relatively scarce36,37. In future studies, more investigations into the prevalence, persistence, and dissemination of carbapenemase-encoding genes in ECC are required. Further research is also needed to gain a better understanding of the role the plasmids and other genetic elements play in the persistence and dissemination of antimicrobial resistance genes. The limitation of this study is that due to only one sample being detected, it was not possible to clarify the resistance mechanism and genomic characteristics of E. chuandaensis more objectively.
Conclusion
In summary, to the best of our knowledge, we present the inaugural case of E. chuandaensis co-harboring blaNDM−1 and two blaKPC−2, located on three different plasmids. The IncR plasmid carrying blaKPC−2 is a huge antimicrobial reservoir, which contributes to the antimicrobial phenotypes of this strain. The similarity of the genetic contexts of the two blaKPC−2 implies a crucial role for Tn3 in the propagation of antimicrobial resistance. The stability and persistence of blaNDM−1-bearing plasmid are conducive to the continuous dissemination of blaNDM−1. These findings underscore the urgency and significance of controlling CREC dissemination, necessitating monitoring programs to prevent the emergence and further spread of antibiotic resistance.
Materials and methods
Bacterial isolation, identification, and antimicrobial susceptibility testing
During the routine surveillance of antimicrobial resistance of bacteria from the First Affiliated Hospital of Zhengzhou University, the major carbapenemase-encoding genes (blaKPC, blaNDM, blaOXA−48, blaVIM, and blaIMP) were identified using PCR, as described previously38. The species were identified by MALDI-TOF MS (Bruker Daltonik GmbH, Bremen, Germany) and ANI. We performed ANI analysis using pyani (https://github.com/widdowquinn/pyani)39. The creation of a database of MALDI-TOF reference spectral profiles with a high number of representatives associated with the performant MSI (https://msi.happy-dev.fr/) software enabled substantial improvement in the identification of ECC strains40. AST was conducted using both the agar dilution method and broth microdilution method41. The results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) standards (https://clsi.org), while colistin and tigecycline were interpreted according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints (https://www.eucast.org/). The omadacycline and eravacycline were interpreted by Food and Drug Administration (FDA) standards (https://www.fda.gov). E. coli ATCC 25922 was used as a quality control strain.
Location of carbapenemase-encoding genes and plasmid transfer experiments
The number and size of plasmids in E. chuandaensis FAHZZU5885 were determined with the S1-nuclease pulsed field gel electrophoresis (S1-PFGE) method. Plasmid DNA fragments were hybridized with digoxigenin-labeled blaNDM−1 or blaKPC−2 specific probes (blaNDM-F: ATGGAATTGCCCAATATTATGCAC, blaNDM-R: TCAGCGCAGCTTGTCGGC; blaKPC-F: ATGTCACTGTATCGCCGTC, blaKPC-R: TTACTGCCCGTTGACGCC) after being transferred to nylon membranes and detected using DIG High Prime DNA Labeling and Detection Starter KitI (Roche, Mannheim, Germany). The ___location of blaNDM−1 and two blaKPC−2were identified by Southern blotting and hybridization with digoxigenin-labeled specific probes42.
The transferability of the plasmids carrying carbapenemase genes was verified by conjugation experiments using NaN3-resistant E. coli J53 as the recipient and strain FAHZZU5885 as the donor. Donor and recipient strains (5 mL each) were cultured overnight shaking at 37 °C and mixed at a donor-recipient ratio of 3:1. The mixed strains were resuspended in 3 mL LB broth. After overnight incubation at 37℃, it was uniformly applied to Mueller-Hinton agar plates containing 200 mg/L azide and 4 mg/L meropenem. The potential transconjugants were verified by PCR (blaNDM-F: ATGGAATTGCCCAATATTATGCAC, blaNDM-R: TCAGCGCAGCTTGTCGGC; blaKPC-F: ATGTCACTGTATCGCCGTC, blaKPC-R: TTACTGCCCGTTGACGCC) and MALDI-TOF MS43. Plasmid extraction was performed using the QIAGEN Large-Contruct Kit (QIAGEN, Hilden, Germany), and the extracted plasmid was electroporated into E. coliDH5α by voltage shock, as described previously44.
Whole-genome sequencing and in silico analyses
Genomic DNA of FAHZZU5885 was extracted using a QIAGEN DNA purification kit (QIAGEN, Hilden, Germany). The DNA sequencing was performed on the Illumina NovaSeq 6000 (Illumina, San Diego, CA, United States) and the Oxford Nanopore (Oxford Nanopore Technologies, Oxford, United Kingdom) platform. The raw llumina reads were assembled using SPAdes3.10.045, and then the short and long reads were hybrid assembled with Unicycler v0.4.7 to get the complete genome sequence46. Prokka was used to annotate the whole genome (https://proksee.ca/). The OriTFinder online tool (https://tool-mml.sjtu.edu.cn/oriTfinder/oriTfinder.html) was used to determine the origin of transfer site (oriT), gene cluster for bacterial type IV secretion system (T4SS), gene encoding type IV coupling protein (T4CP), and relaxase gene. The antimicrobial resistance genes and replicon types of plasmids were determined with the Center for Genomic Epidemiology (https://genomicepidemiology.org/). Additionally, the transposon and IS were detected using the ISFinder database (https://isfinder.biotoul.fr/about.php). The PubMLST (https://pubmlst.org/) was used to determine the MLST of the strain. Then, the figures about the genetic surroundings of antimicrobial resistance genes were made by Easyfig 2.2.547and the BLAST Ring Image Generator (BRIG) was used to compare different plasmid sequences48. A BLASTN search was performed using plasmids p5885-KPC-1, p5885-KPC-2, and p5885-NDM containing blaKPC−2 or blaNDM−1 as a reference sequence against the NCBI database. The selection criteria for comparison plasmids are the three plasmids with the highest nucleotide identity based on the highest query coverage.
Plasmid stability assay
To assess the stability of the plasmid in a bacterial population, FAHZZU5885-J53 was propagated overnight in 5 mL antibiotic-free LB medium at 37 °C while shaking at 200 rpm. Thousand-fold dilutions of the previous cultures were prepared in a fresh 5-mL LB medium until 5 days passage. Cultures from the last passages were serially diluted in saline, and 30 µL of the dilutions (10−5) was spread on plates containing 4 mg/L meropenem. One hundred eighty eight single colonies were verified by PCR to detect blaNDM49.
Data availability
The complete genome sequence of Enterobacter chuandaensis FAHZZU5885 was deposited to GenBank with the accession numbers CP135253-CP135258.
References
Davin-Regli, A., Lavigne, J. P. & Pagès, J. M. Enterobacter spp.: update on taxonomy, clinical aspects, and emerging Antimicrobial Resistance. Clin. Microbiol. Rev. 32 https://doi.org/10.1128/cmr.00002-19 (2019).
Pot, M. et al. Wide distribution and specific resistance pattern to third-generation cephalosporins of Enterobacter cloacae Complex members in humans and in the Environment in Guadeloupe (French West Indies). Front. Microbiol. 12, 628058. https://doi.org/10.3389/fmicb.2021.628058 (2021).
Sato, T., Harada, K., Usui, M., Yokota, S. I. & Horiuchi, M. Colistin susceptibility in Companion Animal-Derived Escherichia coli, Klebsiella spp., and Enterobacter spp. in Japan: frequent isolation of colistin-resistant Enterobacter cloacae Complex. Front. Cell. Infect. Microbiol. 12, 946841. https://doi.org/10.3389/fcimb.2022.946841 (2022).
Chen, J. et al. Carbapenem-resistant Enterobacter cloacae complex in a tertiary hospital in Northeast China, 2010–2019. BMC Infect. Dis. 21, 611. https://doi.org/10.1186/s12879-021-06250-0 (2021).
Dwivedi, A. et al. Detection of clinically relevant carbapenemase encoding genes in carbapenem-resistant Enterobacter cloacae complex and Klebsiella pneumoniae isolated from farmed freshwater fish. J. Appl. Microbiol. 134 https://doi.org/10.1093/jambio/lxad212 (2023).
Chang, C. Y., Huang, P. H. & Lu, P. L. The resistance mechanisms and clinical impact of resistance to the Third Generation cephalosporins in Species of Enterobacter cloacae Complex in Taiwan. Antibiot. (Basel). 11. https://doi.org/10.3390/antibiotics11091153 (2022).
Wu, W., Wei, L., Feng, Y., Kang, M. & Zong, Z. Enterobacter huaxiensis sp. nov. and Enterobacter chuandaensis sp. nov., recovered from human blood. Int. J. Syst. Evol. Microbiol. 69, 708–714. https://doi.org/10.1099/ijsem.0.003207 (2019).
Armstrong, T., Fenn, S. J. & Hardie, K. R. JMM Profile: Carbapenems: a broad-spectrum antibiotic. J. Med. Microbiol. 70 https://doi.org/10.1099/jmm.0.001462 (2021).
Potter, R. F., D’Souza, A. W. & Dantas, G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist. Updat. 29, 30–46. https://doi.org/10.1016/j.drup.2016.09.002 (2016).
Dong, X. et al. Whole-genome sequencing-based species classification, Multilocus sequence typing, and Antimicrobial Resistance mechanism analysis of the Enterobacter cloacae Complex in Southern China. Microbiol. Spectr. 10, e0216022. https://doi.org/10.1128/spectrum.02160-22 (2022).
Ma, J. et al. Global spread of carbapenem-resistant Enterobacteriaceae: epidemiological features, resistance mechanisms, detection and therapy. Microbiol. Res. 266, 127249. https://doi.org/10.1016/j.micres.2022.127249 (2023).
Tamma, P. D. & Simner, P. J. Phenotypic detection of carbapenemase-producing organisms from clinical isolates. J. Clin. Microbiol. 56 https://doi.org/10.1128/jcm.01140-18 (2018).
Zhang, R. et al. Nationwide Surveillance of Clinical Carbapenem-resistant Enterobacteriaceae (CRE) Strains in China. EBioMedicine 19, 98–106, doi: (2017). https://doi.org/10.1016/j.ebiom.2017.04.032
Zhu, Z. et al. Epidemiological characteristics and molecular features of carbapenem-resistant Enterobacter strains in China: a multicenter genomic study. Emerg. Microbes Infect. 12, 2148562. https://doi.org/10.1080/22221751.2022.2148562 (2023).
Yan, Z. et al. Analysis of the transmission chain of carbapenem-resistant Enterobacter cloacae complex infections in clinical, intestinal and healthcare settings in Zhejiang Province, China (2022–2023). Sci. Total Environ. 920, 170635. https://doi.org/10.1016/j.scitotenv.2024.170635 (2024).
Brust, F. R. et al. Macrocolony of NDM-1 Producing Enterobacter hormaechei subsp. Oharae generates subpopulations with different features regarding the response of Antimicrobial agents and Biofilm formation. Pathogens. 8 https://doi.org/10.3390/pathogens8020049 (2019).
Mattioni Marchetti, V. et al. Enterobacter asburiae ST229: an emerging carbapenemases producer. Sci. Rep. 14, 6220. https://doi.org/10.1038/s41598-024-55884-y (2024).
Annavajhala, M. K., Gomez-Simmonds, A. & Uhlemann, A. C. Multidrug-resistant Enterobacter cloacae Complex Emerging as a global, diversifying threat. Front. Microbiol. 10, 44. https://doi.org/10.3389/fmicb.2019.00044 (2019).
Mirza, H. C., Güçlü, A., İnce Ceviz, G. & Başustaoğlu, A. Comparative in vitro activities of omadacycline, eravacycline and tigecycline against non-ESBL-producing, ESBL-producing and carbapenem-resistant isolates of K. pneumoniae. J. Med. Microbiol. 71 https://doi.org/10.1099/jmm.0.001592 (2022).
Li, G. et al. First report of a clinical, multidrug-resistant Enterobacteriaceae isolate coharboring fosfomycin resistance gene fosA3 and carbapenemase gene blaKPC-2 on the same transposon, Tn1721. Antimicrob. Agents Chemother. 59, 338–343. https://doi.org/10.1128/aac.03061-14 (2015).
Chi, X. et al. Emergence of KPC-2-Producing Raoultella ornithinolytica isolated from a Hospital Wastewater Treatment Plant. Antimicrob. Agents Chemother. 64 https://doi.org/10.1128/aac.01983-19 (2020).
Eibach, D. et al. Extended spectrum beta-lactamase producing Enterobacteriaceae causing bloodstream infections in rural Ghana, 2007–2012. Int. J. Med. Microbiol. 306, 249–254. https://doi.org/10.1016/j.ijmm.2016.05.006 (2016).
Sanders, W. E. Jr. & Sanders, C. C. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin. Microbiol. Rev. 10, 220–241. https://doi.org/10.1128/cmr.10.2.220 (1997).
Boyd, D. A. et al. Enterobacter cloacae Complex isolates harboring bla(NMC-A) or bla(IMI)-Type class A carbapenemase genes on novel chromosomal integrative elements and plasmids. Antimicrob. Agents Chemother. 61 https://doi.org/10.1128/aac.02578-16 (2017).
Liu, S. et al. Molecular mechanisms and Epidemiology of Carbapenem-Resistant Enterobacter cloacae Complex isolated from Chinese patients during 2004–2018. Infect. Drug Resist. 14, 3647–3658. https://doi.org/10.2147/idr.S327595 (2021).
Schultsz, C. & Geerlings, S. Plasmid-mediated resistance in Enterobacteriaceae: changing landscape and implications for therapy. Drugs. 72, 1–16. https://doi.org/10.2165/11597960-000000000-00000 (2012).
Lin, D. et al. IncFII Conjugative plasmid-mediated transmission of blaNDM-1 elements among animal-borne Escherichia coli strains. Antimicrob. Agents Chemother. 61 https://doi.org/10.1128/aac.02285-16 (2017).
Zheng, B. et al. Complete nucleotide sequences of two KPC-2-encoding plasmids from the same Citrobacter freundii isolate. J. Antimicrob. Chemother. 73, 531–533. https://doi.org/10.1093/jac/dkx381 (2018).
Smillie, C., Garcillán-Barcia, M. P., Francia, M. V. & Rocha, E. P. Cruz, F. mobility of plasmids. Microbiol. Mol. Biol. Rev. 74, 434–452. https://doi.org/10.1128/mmbr.00020-10 (2010). de la.
Rozwandowicz, M. et al. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 73, 1121–1137. https://doi.org/10.1093/jac/dkx488 (2018).
Beyrouthy, R. et al. IS1R-mediated plasticity of IncL/M plasmids leads to the insertion of Bla OXA-48 into the Escherichia coli chromosome. Antimicrob. Agents Chemother. 58, 3785–3790. https://doi.org/10.1128/aac.02669-14 (2014).
Huang, J. et al. Genetic Factors Associated with enhanced bla(KPC) expression in Tn3/Tn4401 chimeras. Antimicrob. Agents Chemother. 64 https://doi.org/10.1128/aac.01836-19 (2020).
Gao, H. et al. The transferability and evolution of NDM-1 and KPC-2 co-producing Klebsiella pneumoniae from clinical settings. EBioMedicine. 51, 102599. https://doi.org/10.1016/j.ebiom.2019.102599 (2020).
Wang, J. et al. Emergence of Escherichia coli co-producing NDM-1 and KPC-2 carbapenemases from a retail vegetable, China. J. Antimicrob. Chemother. 73, 252–254. https://doi.org/10.1093/jac/dkx335 (2018).
Jing, Y. et al. Genomic diversification of IncR plasmids from China. J. Glob Antimicrob. Resist. 19, 358–364. https://doi.org/10.1016/j.jgar.2019.06.007 (2019).
Zhou, H. et al. Carriage of the mcr-9 and mcr-10 genes in clinical strains of the Enterobacter cloacae complex in China: a prevalence and molecular epidemiology study. Int. J. Antimicrob. Agents. 60, 106645. https://doi.org/10.1016/j.ijantimicag.2022.106645 (2022).
Guh, A. Y. et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae in 7 US communities, 2012–2013. Jama. 314, 1479–1487. https://doi.org/10.1001/jama.2015.12480 (2015).
Liang, G. et al. Co-occurrence of NDM-9 and MCR-1 in a human gut colonized Escherichia coli ST1011. Infect. Drug Resist. 14, 3011–3017. https://doi.org/10.2147/idr.S321732 (2021).
Tsuchida, S., Umemura, H. & Nakayama, T. Current status of Matrix-assisted laser Desorption/Ionization-Time-of-flight Mass Spectrometry (MALDI-TOF MS) in Clinical Diagnostic Microbiology. Molecules. 25 https://doi.org/10.3390/molecules25204775 (2020).
Godmer, A. et al. Revisiting species identification within the Enterobacter cloacae Complex by Matrix-assisted laser desorption ionization-time of Flight Mass Spectrometry. Microbiol. Spectr. 9, e0066121. https://doi.org/10.1128/Spectrum.00661-21 (2021).
Humphries, R., Bobenchik, A. M., Hindler, J. A. & Schuetz, A. N. Overview of changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st Edition. J. Clin. Microbiol. 59, e0021321. https://doi.org/10.1128/jcm.00213-21 (2021).
Dong, H. et al. Genomic epidemiology insights on NDM-Producing pathogens revealed the pivotal role of plasmids on bla(NDM) transmission. Microbiol. Spectr. 10, e0215621. https://doi.org/10.1128/spectrum.02156-21 (2022).
Qiao, J. et al. Detection of IMP-4 and SFO-1 co-producing ST51 Enterobacter hormaechei clinical isolates. Front. Cell. Infect. Microbiol. 12, 998578. https://doi.org/10.3389/fcimb.2022.998578 (2022).
Liu, X. et al. Emergence of plasmid-borne tet(X4) resistance gene in clinical isolate of eravacycline- and omadacycline-resistant Klebsiella pneumoniae ST485. Microbiol Spectr, e0049624, doi: (2024). https://doi.org/10.1128/spectrum.00496-24
Prjibelski, A., Antipov, D., Meleshko, D., Lapidus, A. & Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinf. 70, e102. https://doi.org/10.1002/cpbi.102 (2020).
Wick, R. R., Judd, L. M., Gorrie, C. L. & Holt, K. E. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 13, e1005595. https://doi.org/10.1371/journal.pcbi.1005595 (2017).
Sullivan, M. J., Petty, N. K. & Beatson, S. A. Easyfig: a genome comparison visualizer. Bioinformatics. 27, 1009–1010. https://doi.org/10.1093/bioinformatics/btr039 (2011).
Alikhan, N. F., Petty, N. K., Zakour, B., Beatson, S. A. & N. L. & BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genom. 12, 402. https://doi.org/10.1186/1471-2164-12-402 (2011).
Valle, A. D. Variability of plasmid fitness effects contributes to plasmid persistence in bacterial communities. Nat. Commun. 12, 2653. https://doi.org/10.1038/s41467-021-22849-y (2021).
Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFE0204300); Henan Provincial Science and Technology Research Project (232102310176); National Natural Science Foundation of China (82072314); Shandong Provincial Laboratory Project (SYS202202); Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022011B); Fundamental Research Funds for the Central Universities (2022ZFJH003); and CAMS Innovation Fund for Medical Sciences (2019-I2M-5-045).
Author information
Authors and Affiliations
Contributions
X.G. designed the study. L.F. and Y.S. collected the samples. R.C., C.L., Y.L., and X.L. carried out the laboratory experiments. R.L., H.X., H.G, and J.Q. performed all the bioinformatics analyses and generated the figures. R.C. wrote the manuscript. X.G. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
The study protocol was approved by the First Affiliated Hospital of Zhengzhou University Ethics Committee for Research in Health. All methods were performed in accordance with the relevant guidelines and regulations. Due to the retrospective nature of the study, Institutional Review Board waived the need of obtaining informed consent. All patient data were anonymised prior to analysis (2024-KY-0318).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, R., Li, C., Xu, H. et al. First documentation of a clinical multidrug-resistant Enterobacter chuandaensis ST2493 isolate co-harboring blaNDM-1 and two blaKPC-2 bearing plasmids. Sci Rep 14, 26817 (2024). https://doi.org/10.1038/s41598-024-78163-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78163-2