Abstract
Clinical phenotypes of COVID-19, associated with mortality risk, have been identified in the general population. The present study assesses their applicability in solid organ transplant recipients (SOTR) hospital-admitted by COVID-19. In a cohort of 488 SOTR, nonvaccinated (n = 394) and vaccinated (n = 94) against SARS-CoV-2, we evaluated 16 demographic, clinical, analytical, and radiological variables to identify the clinical phenotypes A, B, and C. The median age was 61.0 (51–69) years, 330 (67.6%) and 158 (32.4%) were men and women, respectively, 415 (85%) had pneumonia, and 161 (33%) had SpO2 < 95% at admission. All-cause mortality occurred in 105 (21.5%) cases. It was higher in nonvaccinated versus vaccinated SOTR (23.4% vs 13.8%, P = 0.04). Patients in the entire cohort were classified into phenotypes A (n = 149, 30.5%), B (n = 187, 38.3%), and C (n = 152, 31.1%), with mortality rates of 8.7%, 16.6%, and 40.1%, respectively, which were similar to those of nonvaccinated SOTR (9.5%, 16.7%, and 52.0%) and lower in vaccinated SOTR (4.4%, 15.8%, and 17.3%, respectively), with difference between nonvaccinated and vaccinated in the phenotype C (P < 0.001). In conclusion, COVID-19 clinical phenotypes are useful in SOTR, and all-cause mortality decreases in vaccinated patients.
Similar content being viewed by others
Introduction
The coronavirus disease (COVID-19) pandemic has led to significant morbidity and mortality, leading to the global collapse of the healthcare system. As of May 12, 2024, there were 775,481,326 confirmed cases and 7,049,376 deaths worldwide1. Throughout this global health crisis, a broad spectrum of disease severity has been observed in affected individuals. Understanding the heterogeneity of clinical presentation and underlying pathogenic mechanisms is crucial for optimizing patient management and resource allocation. Extensive epidemiological, clinical, and therapeutic studies have contributed to our understanding of pathogens and diseases. This wealth of information includes insights into the risk factors for unfavorable outcomes in the general population, focusing primarily on demographics, comorbidities, symptom severity, and laboratory findings2,3,4. In addition, factors related to immunology, virology5,6,7,8, and treatment9 have been explored.
The global number of vaccinated individuals has been steadily increasing, with 13.5 billion vaccine doses administered as of November 26, 2023. In Western countries, particularly Andalusia and southern Spain, a remarkably high percentage of people have completed vaccination, reaching 100% in those over 60 years of age and 90% in the 40–60 age group10. Within the vaccinated population, COVID-19 predominantly affects vulnerable individuals, including those with either pluripathology or frailty, or both, and those with compromised immune responses. Furthermore, individuals receiving solid organ transplantation (SOT) or hematopoietic stem cell transplantation (HSCT) are at an elevated risk of developing severe forms of COVID-19, as seroconversion after immunization remains low11,12. Therefore, understanding the factors influencing clinical outcomes, especially in patients who have received a transplant, is crucial for optimizing care. Age, underlying comorbidities, immunosuppressive regimens, and time since transplantation have been identified as risk factors associated with unfavorable outcomes, as analyzed in a prospective multicenter study involving patients who have received a solid organ transplant4 conducted before widespread vaccination. Moreover, pre-transplant prognostic profiles have been identified in patients with hematologic profiles13.
The clinical phenotypes of COVID-19 were initially defined in adult patients on hospital admission at the onset of the pandemic14. In a multicenter cohort study, three clinical phenotypes with different mortality rates were identified and validated in patients admitted to hospitals with COVID-19. To identify the phenotypes, this study developed a multinomial logistic regression model with 16 variables including age, sex, chronic lung disease, obesity, diastolic blood pressure, oxygen saturation, white blood cell and neutrophils counts, hematocrit, coagulation international normalized ratio (INR), C-reactive protein (CRP), glucose, creatinine, sodium, potassium, and type of lung infiltrate on chest radiograph. The 30-day mortality rates for phenotypes A, B, and C were 2.6%, 31.0%, and 53.4%, respectively14. This study provides valuable insights into the prognoses associated with these phenotypes and reveals potential disease severity and mortality risk indicators. It also guides personalized treatment strategies to enhance patient outcomes. However, it is essential to note that the utility of these clinical phenotypes has not yet been validated for SOT or HSCT recipients.
Data analysis from kidney transplant recipients has revealed distinct respiratory and gastrointestinal phenotypes associated with COVID-1915. Certain patients primarily display respiratory symptoms similar to the typical manifestations of COVID-19 observed in the general population. In contrast, the other patients manifested prominent gastrointestinal symptoms, a more prevalent pattern in the immunocompromised cohort. Disease severity differs based on these phenotypes, generally with patients exhibiting gastrointestinal symptoms facing a higher risk of severe outcomes.
The present study aimed to assess and validate the applicability of the clinical phenotypes identified in the general population at the onset of the pandemic14 in SOT recipients (SOTR) admitted to the hospital with COVID-19. This evaluation was conducted in two distinct cohorts of SOTR: nonvaccinated and vaccinated against SARS-CoV-2. This study aimed to identify the most accurate predictors and current clinical phenotypes within this population, serving as a valuable tool for optimal management of outpatients and inpatients.
Methods
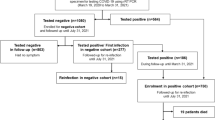
We conducted an international retrospective and observational cohort study across 18 participating centers in Spain, Italy, and Brazil (COVIDSOT Study, clinicaltrials.gov ID: NCT04319172, first posted on March 24, 2020; EU PAS Register Number: EUPAS34349, first posted on March 31, 2020). These centers were asked to include all consecutive adult SOT recipients (SOTR) who were hospitalized for confirmed COVID-19, by a positive SARS-CoV-2 reverse transcription-PCR (RT-PCR) test or rapid antigen detection test (RADT) targeting the SARS-CoV-2 nucleocapsid protein from nasopharyngeal (NP) swabs, between January 2020 and October 2022 (nonvaccinated patients from January 2020 to March 2022, and vaccinated patients from May 2021 to October 2022), and with the availability of data in the 16 variables required to assign patients with a specific COVID-19 phenotype14.
Data collection
Anonymized data were collected through an electronic Case Report Form and entered into a database constructed explicitly for this study using the Research Electronic Data Capture (REDCap) tools16. The recorded variables included demographics, comorbidities, transplant type, and date, signs and symptoms upon admission, baseline laboratory tests, chest radiography findings, complications during hospitalization, immunosuppression management, therapeutics with purported activity against COVID-19, adjunctive strategies to modulate the host inflammatory response, and clinical outcomes. Vaccinated SOTR against SARS-CoV-2 were considered if they had received at least two vaccine doses. The clinical severity at hospital admission (basal WHO scale) and at the end of follow-up (final WHO scale) was evaluated using the WHO clinical progression scale17.
Event of interest
The clinical outcomes of the patients after a 30-day follow-up were categorized as survival or death. In cases where patients were discharged and subsequently readmitted to the hospital during the study period, only the initial hospital admission episode was considered for the analysis.
The primary stratification strategy was based on phenotypes A, B, and C, utilizing 16 demographic, clinical, analytical, and radiological variables (age, sex, chronic lung disease, obesity, diastolic blood pressure, oxygen saturation, white blood cell and neutrophils counts, hematocrit, coagulation INR, CRP, glucose, creatinine, sodium, potassium, and type of lung infiltrate on chest radiograph) of the FEN-COVID probability calculator to assign patients with a specific COVID-19 phenotype (http://fen-covid.com/index.html)14. The secondary stratification strategy was vaccination status (yes or no).
Statistical analysis
Two analyses were conducted. First, a descriptive analysis encompassing all patients and separate assessments of nonvaccinated and vaccinated individuals was performed using the probabilistic phenotype method. Second, the bivariate analysis focused on describing and examining the characteristics associated with 30-day mortality. Categorical variables are presented as n (%), and continuous variables as mean (standard deviation [SD]) or median (interquartile range [IQR]) based on the normality of the distribution. Appropriate statistical tests, such as the χ2-test, the Yates Correction for Continuity, Student’s t test, or Mann–Whitney U test, were employed to assess between-group differences. Interactions, confusion, and collinearity were thoroughly explored. Variables associated with the outcome and those considered clinically relevant were incorporated into a multivariate regression analysis to assess the real impact of every exposure factor in the 30-day mortality. Survival analysis was performed using the Kaplan–Meier method. All analyses were performed using the software package SPSS (26.0. Armonk, NY: IBM Corp.). The p-values were derived from two-tailed tests, and those < 0.05 were considered significant.
Ethics approval
The Ethics Committee of Virgen del Rocío and Virgen Macarena University Hospitals approved the study protocol (C.I. 0842-N-20), as well as by the appropriate institutional review board of each participating center (Supplementary Information), and adhered to the Helsinki Declaration. An informed consent was established as a mandatory requirement for all patients.
Results
Clinical characteristics and outcomes of all SOTR, and according to vaccination
Four hundred and eighty-eight SOTR with COVID-19, confirmed by RT-PCR and RADT in 467 and 21 cases, respectively, were included in the study from January 2020 to October 2022, with a median age of 61.0 (51–69) years, 330 (67.6%) were men and 158 (32.4%) were women (Table 1). The most frequently transplanted organs were the kidneys (n = 309, 63.3%) and the liver (n = 84, 17.2%). At the time of COVID-19 diagnosis, 186 (38.1%) patients experienced dyspnea, 415 (85.0%) had pneumonia, and 161 (33.0%) had peripheral oxygen saturation (SpO2) < 95%. The median number of days from symptom onset to diagnosis was 4 (2–9).
Regarding the analytical parameters, 47 (9.6%) patients had white blood cell counts > 11,000/µL, 285 (58.3%) had lymphocytes < 1000/µL, 72 (14.8%) had C-reactive protein (CRP) values ≥ 100 mg/L, and 212 (43.4%) had D-dimer levels ≥ 600 ng/mL. Only 88 (18%) and 80 (16.4%) patients received tocilizumab and dexamethasone, respectively. The clinical outcome, as assessed using the WHO clinical progression scale, was severe (final WHO scale 7–10) in 131 (26.8%) patients, and all-cause mortality (final WHO scale 10) occurred in 105 (21.5%). Table 1 summarizes the clinical characteristics and outcomes of all patients, including the differences between the nonvaccinated and vaccinated populations.
The nonvaccinated group (n = 394, 80.7%) compared to the vaccinated group (n = 94, 19.3%) of SOTR were younger (60 [50–68] vs. 64 [57–71], p < 0.05) and more frequently exhibited a temperature > 37.5 °C (25.4% vs. 10.6%, P = 0.002), systolic pressure < 90 mmHg (9.1% vs. 0%, p = 0.04), and diastolic pressure < 60 mmHg (8.1% vs. 2.1%, p = 0.03), with no differences in the frequency of SpO2 < 95% (Table 1). Creatinine > 1.3 mg/dL (83.0% vs. 69.5%, p = 0.013) and C-reactive protein ≥ 100 mg/L (30.9% vs. 10.9%, p < 0.05) were more common in vaccinated SOTR. Vaccinated patients received dexamethasone upon hospital admission more frequently than nonvaccinated patients (61.7% vs. 5.6%, p < 0.001). Severe clinical outcomes (WHO scale 7–10) and all-cause mortality were higher in nonvaccinated patients than in vaccinated patients (29.7% vs. 14.9%, p = 0.003 and 23.4% vs. 13.8%, p = 0.04, respectively) (Table 1).
Clinical phenotypes, distribution, and mortality in all SOTR and according to vaccination status
In the entire cohort of 488 SOTR, the clinical phenotypes at admission were A in 149 (30.5%) patients, B in 187 (38.3%) patients, and C in 152 (31.1%) patients, with increasing mortality rates of 8.7% (n = 13), 16.6% (n = 31), and 40.1% (n = 61), respectively (p < 0.001). The association between higher mortality rates and the phenotypes remained in the 394 nonvaccinated SOTR (p < 0.001) from A to C: 9.5% (n = 12), 16.7% (n = 28), and 52% (n = 52), respectively. However, in vaccinated SOTR, the mortality rate, although lower in phenotype A (4.4% [n = 1]), did not differ between the three phenotypes (p = 0.11) or between phenotypes B (15.8% [n = 3]) and C (17.3% [n = 9]) (p = 0.59). Finally, mortality rates were comparable for phenotypes A and B in vaccinated and nonvaccinated patients when assessing the SOTR phenotypes according to vaccination status. However, it differed between the nonvaccinated and vaccinated SOTR belonging to phenotype C (52% vs. 17.3%, p < 0.001) (Supplementary Fig. S1). In the Kaplan-Meier analysis, survival at day 30 was lower in SOTR with phenotype B or C than in those with phenotype A (p < 0.01), regardless of vaccination. Overall survival was also lower in nonvaccinated SOTR with phenotypes B or C compared to phenotype A (p < 0.01), but we found no differences in survival at day 30 for vaccinated patients, irrespective of the phenotype they belonged to (p = 0.311) (Fig. 1).
Factors independently associated with mortality in all SOTR and according to vaccination
In the subsequent analysis, we conducted a bivariate examination of the factors associated with all-cause mortality at hospital admission on day 30 after COVID-19 diagnosis (Table 2). Deceased patients were more likely to be aged ≥ 70 years (36.2% vs. 19.8%, p < 0.001), have diabetes mellitus (48.6% vs. 33.9%, p = 0.006), and have not received the COVID-19 vaccination (87.6% vs. 78.9%, p = 0.04). Regarding clinical characteristics, deceased patients exhibited a higher frequency of pneumonia (92.4% vs. 83.0, p = 0.017), SpO2 < 95% (48.6% vs. 28.7%, p < 0.001), and elevated inflammatory parameter values (neutrophils > 7500/µL [p = 0.016], CRP > 100 mg/dL [p = 0.008], lactate dehydrogenase [LDH] > 300 U/L [p < 0.001], and higher median D-dimer [p = 0.025]).
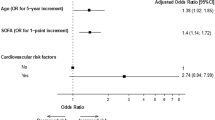
Multivariate logistic regression analysis was performed to assess potential independent predictors of death. Age ≥ 70 years (odds ratio [OR] 2.5, p < 0.001), SpO2 < 95% (OR 2.35, p < 0.001), creatinine > 1.3 mg/dL (OR 2.35, p = 0.004), and not having received COVID-19 vaccination (OR 2.21, p = 0.018) were identified as independent risk factors associated with all-cause mortality at day 30 (Table 3). A receiver operating characteristic (ROC) curve plot was generated for the model, with an area under the curve of 0.71 (95% CI 0.650–0.767), with a standard error of 0.03 (under the non-parametric assumption), and p < 0.001 (being the null hypothesis a true area = 0.50) (Fig. 2).
Discrimination power of the final multivariable model: receiving operative curve (ROC) plot (including age ≥ 70 years, SpO2 < 95%, neutrophils > 7500/µL, non-vaccination, and time from transplant onset to COVID-19 diagnosis < 6 months), expressed by an area under the ROC of 0.71 (95% CI 0.65–0.767), SE = 0.03 (under the non-parametric assumption), and p < 0.001 (being the null hypothesis a true area = 0.50).
Concerning the nonvaccinated SOTR, all-cause mortality at day 30 occurred in 92 patients (23.4%) and was more frequent in those with age ≥ 70 years (p < 0.001), diabetes mellitus (p = 0.006), pneumonia (p = 0.038), dyspnea (p < 0.001), and SpO2 < 95% (p < 0.001) at diagnosis (Supplementary Table S1). In the multivariable logistic regression analysis, age ≥ 70 years (OR 3.32, p < 0.001), diabetes mellitus (OR 2.01, p = 0.037), SpO2 < 95% (OR 1.90, p = 0.045), creatinine > 1.3 mg/dL (OR 2.12, p = 0.041), and LDH > 300 U/L (OR 3.28, p < 0.001) were independently associated with all-cause mortality at day 30 (Supplementary Table S2).
Finally, in vaccinated SOTR all-cause mortality at day 30 occurred in 13 patients (13.8%) and was more frequent in those with combined transplantation (15.4% vs. 1.2%, p = 0.007), chronic heart disease (53.8% vs. 25.9%, p = 0.041), and dyspnea at hospital admission (84.6% vs. 39.5%, p = 0.001) (Supplementary Table S1). In the multivariable logistic regression analysis, dyspnea was the only factor independently associated with mortality (OR 15.7, p = 0.011) (Supplementary Table S2).
Discussion
The study findings revealed a higher all-cause mortality rate for COVID-19 in SOTR among nonvaccinated individuals than in their vaccinated counterparts. Additionally, the clinical phenotypes14 initially identified in the general population during the early stages of the COVID-19 pandemic apply to the SOTR cohort. This trend was particularly notable among nonvaccinated patients, with all-cause mortality rates progressively increasing from phenotypes A to C. In the vaccinated SOTR, phenotype A was associated with a low mortality rate, which also increased in phenotypes B and C, although with less difference between the two, owing to a three-fold decrease in patients with phenotype C in the vaccinated SOTR compared with their nonvaccinated counterparts. In a secondary analysis, independent risk factors associated with all-cause mortality at day 30 included age ≥ 70 years, SpO2 < 95%, creatinine > 1.3 mg/dL, and absence of COVID-19 vaccination in the overall SOTR cohort. Among nonvaccinated SOTR, age ≥ 70 years, diabetes mellitus, SpO2 < 95%, creatinine > 1.3 mg/dL, and LDH > 300 U/L were independently associated with all-cause mortality at day 30, and in vaccinated patients, the sole factor independently linked to mortality was the presence of dyspnea at the time of COVID-19 diagnosis.
This study built on the clinical phenotypes established by analyzing immunocompetent adult patients with COVID-19 in 202014. Sixteen demographic, clinical, analytical, and radiological variables were identified to evaluate the likelihood of death during the COVID-19 diagnosis (http://fen-covid.com/index.html). In the validation cohort of the general population14, a pronounced contrast in mortality rates was observed among patients with phenotypes A (3.7%), B (23.7%), and C (51.4%). The present study noted a more gradual increase in mortality rates across phenotypes, ranging from phenotype A (9.5%) to phenotype B (16.7%). This gradual increase indicates higher clinical applicability, although the mortality rate for nonvaccinated SOTR with phenotype C remained elevated (52.0%), similar to the general population. Notably, the mortality rate of phenotype C in vaccinated SOTR (17.3%) was lower than in nonvaccinated SOTR and the general population (52.0% and 51.4%, respectively).
In a previous study conducted before the availability of vaccinations, four baseline factors were identified as independent predictors of unfavorable outcomes, defined as the need for intensive care or death: advanced age, high respiratory rate, lymphopenia, and elevated LDH level4. In the current study, which encompassed the entire SOTR cohort, advanced age and SpO2 < 95% potentially influenced respiratory rate, emerged as independent risk factors for mortality. The absence of COVID-19 vaccination was identified as an independent risk factor. Moreover, the mortality rate among vaccinated SOTR in this study (13.8%) was lower than that among nonvaccinated SOTR (23.4%), which, in turn, mirrored that in the general population (28.1%)14. This result underscores the need to vaccinate immunocompromised patients, including those with SOTR.
In the present study, the vaccinated status of patients was recorded depending on having received at least two vaccine doses, without considering if they might have received additional doses or the time after the last vaccine administration. A longitudinal study involving 129 kidney transplant recipients, after three doses of mRNA vaccine, revealed that only 41.1% of patients had significant neutralizing antibody titers. Moreover, analysis of the concerned variants indicated a diminished binding affinity towards the Omicron variants BA1 and BA218. Other study carried out in 101 SOTR, found that 1-month after a third dose of mRNA vaccine 68% of patients had anti–SARS-CoV-2 antibodies, higher than the 40% after the second dose, and with increased antibody titers in those with positive antibodies after the second dose19. However, some studies in immunocompetent patients, demonstrating varied immune responses of individuals following an Omicron outbreak20, and in SOTR, showing that some patients may remain at high risk for Omicron infection in spite of receiving a fourth vaccine dose21, underline the heterogeneity of SARS-CoV-2 immune responses between individuals, including after vaccination. Moreover, the durability of SARS-CoV-2 antibodies has been evaluated over six-month after the second dose of mRNA vaccine in 312 SOTR, using semiquantitative anti-spike antibody testing. Among the 198 patients with positive titers at one-month, 7.1% were negative at six months, and 87 (27.2%) patients of the entire cohort had negative titers at six-month22. Thus, although the vaccinated SOTR criteria in the present study was only to have received at least two vaccine doses, the inter-individual variability of the immune response and its durability decrease the risk of bias in the analysis of the 94 vaccinated SOTR in our study. Besides, stratifying the vaccinated SOTR by two vs. three or more vaccine doses, or the time from the last shot, would decrease the ability to achieve the main objective, aimed to assess and validate the applicability of the clinical phenotypes, identified in the general population, in SOT recipients with COVID-19.
The present study has several limitations. First, our cohort of SOTR was smaller than the general population cohort14, where clinical phenotypes were identified. Furthermore, our focus on hospitalized patients may restrict the generalizability of our conclusions regarding SOTR treatment in outpatient settings. Second, the period of SOTR inclusion does not exactly represent the current mortality rate of COVID-19 in these patients, which has decreased because of the COVID-19 vaccine and the antivirals availability in the clinical practice, although this is reflected in the significant lesser mortality found in the vaccinated than in the nonvaccinated patients in the present study. However, considering the current changes in the prevalence of the SARS-CoV-2 variants of interest and variants under monitoring1, with increased vaccine dosing and availability of antivirals, future analyses to validate clinical phenotypes would be desirable. Finally, the size of the vaccinated patient cohort was smaller than that of the nonvaccinated cohort, and the limited number of deaths hampered the ability to identify independent risk factors associated with mortality robustly.
However, this study had some notable strengths. It has a robust design that involves a multicenter and international approach to enhance the generalizability and comparability of the results. Standardized and anonymous data collection contributed to the reliability of the study. The 30-day follow-up period provides a comprehensive perspective. Importantly, this study represents the first analysis of a prospective cohort of SOTR vaccinated against COVID-19 to predict mortality-related factors.
In conclusion, clinical phenotypes are valuable in assessing the risk of mortality among nonvaccinated and vaccinated SOTR, and therefore useful in guiding the level of care in patients with COVID-19. In vaccinated SOTR, dyspnea, a readily identifiable clinical symptom, emerged as an independent risk factor for increased mortality, highlighting the importance of early treatment and close monitoring of individuals exhibiting this symptom. Moreover, vaccination against SARS-CoV-2 decreases the all-cause mortality of COVID-19 by half in the SOTR, which underlies the crucial role of vaccination against SARS-CoV-2 in the SOTR and serves as a pivotal factor in reducing COVID-19 mortality.
Data availability
All relevant data are within the paper and its Supporting information files. The raw data are accessible upon reasonable request to the corresponding author.
References
Covid-19 cases | WHO COVID-19 Dashboard. World Health Organization. (2024). https://data.who.int/dashboards/covid19/ (accessed 6 Nov 2024).
Berenguer, J. et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin. Microbiol. Infect. 26, 1525–1536. https://doi.org/10.1016/j.cmi.2020.07.024 (2020).
Lu, Q. B. et al. Comorbidities for fatal outcome among the COVID-19 patients: a hospital-based case-control study. J. Infect. 82, 159–198. https://doi.org/10.1016/j.jinf.2020.07.026 (2021).
Salto-Alejandre, S. et al. Risk factors for unfavorable outcome and impact of early post-transplant infection in solid organ recipients with COVID-19: a prospective multicenter cohort study. PLoS One. 16, e0250796. https://doi.org/10.1371/journal.pone.0250796 (2021).
Benotmane, I., Risch, S., Doderer-Lang, C., Caillard, S. & Fafi-Kremer, S. Long-term shedding of viable SARS-CoV-2 in kidney transplant recipients with COVID-19. Am. J. Transpl. 21, 2871–2875. https://doi.org/10.1111/ajt.16636 (2021).
Kaul, D. R. et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am. J. Transpl. 21, 2885–2889. https://doi.org/10.1111/ajt.16532 (2021).
Kemlin, D. et al. Humoral and cellular immune correlates of protection against COVID-19 in kidney transplant recipients. Am. J. Transpl. 23, 649–658. https://doi.org/10.1016/j.ajt.2023.02.015 (2023).
Yang, J. et al. Augmented humoral and cellular immunity against severe acute respiratory syndrome coronavirus 2 after breakthrough infection in kidney transplant recipients who received 3 doses of coronavirus disease 2019 vaccine. Am. J. Transpl. 23, 565–572. https://doi.org/10.1016/j.ajt.2022.12.022 (2023).
Masotti, L. et al. Predictors of poor outcome in tocilizumab treated patients with Sars-CoV-2 related severe respiratory failure: a multicentre real-world study. Int. Immunopharmacol. 107, 108709. https://doi.org/10.1016/j.intimp.2022.108709 (2022).
Portal de datos estadísticos y Geoespaciales de Andalucía: Inicio. Portal de Datos Estadísticos y Geoespaciales de Andalucía | Inicio. http://www.juntadeandalucia.es/institutodeestadisticaycartografia/ (accessed 21 Mar 2024).
Lee, A. R. Y. B. et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ 376, e068632. https://doi.org/10.1136/bmj-2021-068632 (2022).
Strasfeld, L. COVID-19 and HSCT (hematopoietic stem cell transplant). Best Pract. Res. Clin. Haematol. 35, 101399. https://doi.org/10.1016/j.beha.2022.101399 (2022).
Faucheux, L., Bassolli de Oliveira Alves, L., Chevret, S. & Rocha, V. Comparison of characteristics and laboratory tests of COVID-19 hematological patients from France and Brazil during the pre-vaccination period: identification of prognostic profiles for survival. Hematol. Transfus. Cell. Ther. 45, 306–316. https://doi.org/10.1016/j.htct.2022.05.003 (2023).
Gutiérrez-Gutiérrez, B. et al. Identification and validation of clinical phenotypes with prognostic implications in patients admitted to hospital with COVID-19: a multicentre cohort study. Lancet Infect. Dis. 21, 783–792. https://doi.org/10.1016/S1473-3099(21)00019-0 (2021).
Crespo, M. et al. Respiratory and gastrointestinal COVID-19 phenotypes in kidney transplant recipients. Transplantation 104, 2225–2233. https://doi.org/10.1097/TP.0000000000003413 (2020).
Harris, P. A. et al. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42, 377–381. https://doi.org/10.1016/j.jbi.2008.08.010 (2009).
WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 20, e192–e197. https://doi.org/10.1016/S1473-3099(20)30483-7 (2020).
Murali, T. M. et al. Analyzing COVID-19 vaccine responses in transplant recipients. Immunohorizons 7, 708–717. https://doi.org/10.4049/immunohorizons.2300071 (2023).
Kamar, N. et al. Three doses of an mRNA Covid-19 vaccine in Solid-Organ transplant recipients. N. Engl. J. Med. 385, 661–662. https://doi.org/10.1056/NEJMc2108861 (2021).
Wu, J. et al. Heterogeneity of SARS-CoV-2 immune responses after the nationwide Omicron wave in China. Microbiol. Spectr. e0111724. https://doi.org/10.1128/spectrum.01117-24 (2024).
Karaba, A. H. et al. A fourth dose of COVID-19 vaccine does not induce neutralization of the Omicron variant among solid organ transplant recipients with suboptimal vaccine response. Transplantation 106, 1440–1444. https://doi.org/10.1097/TP.0000000000004140 (2022).
Alejo, J. L., Palacios-Baena, R. & Torre-Cisneros. Six-month antibody kinetics and durability in SARS-CoV-2 mRNA vaccinated solid organ transplant recipients. Transplantation. 106, e109–e110. https://doi.org/10.1097/TP.0000000000003975 (2022).
Acknowledgements
This study was supported by Plan Nacional de I+D+i 2013-2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades, Spanish Network for Research in Infectious Diseases (REIPI, RD16/0016/0001 [JR-B, BG-G], RD16/0016/0005 [NS], and RD16/0016/0009 [JP, EC, JSC]), co-financed by European Development Regional Fund “A way to achieve Europe”, Operative Program Intelligence Growth 2014-2020. EC, JSC and JR-B received grants from the Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, Proyectos de Investigación sobre el SARS-CoV-2 y la enfermedad COVID-19 (COV20/00370, COV20/00580, COV20/01031). EC and JSC also were supported by the grant IM22/INF/13 from the CIBERINFEC, Instituto de Salud Carlos III, Spain. This study was also supported by CIBERINFEC - Consorcio Centro de Investigación Biomédica en Red, Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea – NextGenerationEU (EC and JS-C [CB21/13/00006], BG-G and JR-B (CB21/13/00012). JSC is a researcher belonging to the program “Nicolás Monardes” (RC-0002–2022), Servicio Andaluz de Salud, Junta de Andalucía, Spain. The ORCHESTRA project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 101016167.
Author information
Authors and Affiliations
Author notes
A list of authors and their affiliations appears at the end of the paper.
Consortia
Contributions
Conceptualization, E.C., J.P., J.R.-B., and B.G.-G.; methodology, E.C., J.P., J.R.-B., B.G.-G., C.I.-D., and S-S-A.; software, S.J.-J.; validation, J.P., C.I.-D., S.S.-A., and S.J-J.; formal analysis, J.P., C.I.-D., and S.S.-A.; data curation, R.A.-M., N.S., A.R., A.M., K.M., Z.P.-B., P.M., M.F.-R., M.B., C.F., E.V., E.M.-L., M.H., R.H.-G., M.B., A.M., A.G.-D., M.R., S.J.-J., M.B., L.F.A.-C., M.V., and J.S.-C.; writing-original draft preparation, C.I.-D. and S.S.-A.; writing-review and editing, J.P., E.C., J.R.-B., B.G.-G., and M.G.; visualization, R.A.-M., N.S., A.R., A.M., K.M., Z.P.-B., P.M., M.F.-R., M.B., C.F., E.V., E.M.-L., M.H., R.H.-G., M.B., A.M., A.G.-D., M.R., S.J.-J., M.B., L.F.A.-C., M.V., and J.S.-C.; supervision, J.P., E.C., J.R.-B., B.G.-G., and M.G.; project administration, J.P., J.S.-C., E.C., J.R.-B., and M.G.; funding acquisition, J.S.-C., E.C., J.R.-B., and M.G. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement
The views expressed in this publication are the sole responsibility of the authors and the Commission is not responsible for any use that may be made of the information it contains.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Infante-Domínguez, C., Salto-Alejandre, S., Álvarez-Marín, R. et al. COVID-19 clinical phenotypes in vaccinated and nonvaccinated solid organ transplant recipients: a multicenter validation study. Sci Rep 14, 30021 (2024). https://doi.org/10.1038/s41598-024-81099-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81099-2