Abstract
The enhancement of soil aggregate size and stability is crucial for mitigating climate change and improving carbon sequestration in forest ecosystems. Biochar, derived from rice husks, has been suggested as an effective mean to increase soil carbon storage. However, isolating biochar’s specific effects on soil aggregate formation and carbon sink capacity can be complex due to the overlapping influences of fertilization and understory vegetation cultivation. Our study circumvented these variables by incorporating different amounts of biochar into plantation soil without any additional cultivation or fertilization, conducting a detailed two–year field experiment. The findings revealed that biochar significantly increased the organic carbon content and density in the uncultivated under–forest Ferralsols, thus enhancing its carbon sink function. Intriguingly, while biochar raised the proportion of small soil aggregates (< 0.25 mm) and their organic carbon levels, it decreased the fraction of larger aggregates (> 0.25 mm), adversely affecting soil aggregate stability. These results suggest that biochar may compromise soil aggregate structure and stability in the absence of plant growth. The positive impact of biochar on soil carbon storage was found to depend more on its inherent inert carbon content than on soil type. Moreover, biochar alone was insufficient to increase the quantity of soil macroaggregates without the binding action of plant root exudates. Biochar’s key function appears to be in enhancing the soil aggregate–forming processes facilitated by plant roots and microorganisms. Therefore, for optimal carbon sequestration in forest soils, integrating biochar application with appropriate agricultural practices is advisable.
Similar content being viewed by others
Introduction
The strategic improvement of soil carbon sequestration is critical for achieving China’s ambitious carbon neutrality goals1. China, with the largest area of plantation forests globally, exerts a significant influence on the soil carbon pool through its forest management practices. Transforming forest maintenance and logging by–products into biochar and applying it to forest soils addresses waste management challenges while crucially enhancing soil carbon stocks, soil quality, and forest productivity2,3.
Biochar is distinguished by its high carbon content, aromaticity, and porosity, produced through the pyrolysis of biomass in an oxygen–limited environment. This results in a versatile material, especially recognized for its carbon sequestration capabilities due to its resistance to decomposition and heterogeneous nature2,4. But the effectiveness of biochar as a carbon sequestration tool is influenced by a variety of factors. First, the type of feedstock, pyrolysis conditions (such as temperature and duration), and soil characteristics, which are inherent properties of biochar, have a significant impact on the carbon sequestration effect. For instance, wood–based biochar is generally superior to straw–based biochar due to its higher carbon content and stability5. The higher the pyrolysis temperature, the greater the stability of the biochar, and a pyrolysis temperature range of 500–700℃ is generally considered to be appropriate6. Additionally, the carbon sequestration potential of biochar may also depend on environmental factors such as soil type, climate conditions, moisture, and temperature, as well as the aging effects of the biochar itself and the duration of the experiment7. In summary, biochar, as a good tool for carbon sequestration, has a sequestration potential that depends on a combination of various factors, including the characteristics of the biochar itself, soil conditions, climate conditions, and agricultural management practices.
Soil aggregates, the essential units of soil structure, play a vital role in safeguarding and capturing soil organic carbon by acting as a defense against microbial breakdown8, thereby boosting the soil’s capacity to store carbon. The size distribution and robustness of these aggregates, especially macroaggregates, are crucial for the enduring stabilization and cycling of organic carbon in the soil9,10.
Biochar application has been shown to significantly enrich the organic carbon content within soil macroaggregates, aiding in their development and structural integrity11,12. It is reported to enhance aggregate stability by 7–20%13 and to facilitate the binding of silt–clay particles within macroaggregates. Nevertheless, there are reports where biochar’s impact on aggregate formation was negligible or even detrimental compared to untreated soil14,15.
Although extensive research exists on biochar’s influence on soil aggregates and nutrient cycles, outcomes vary due to factors like biochar type, soil properties, application duration, and local environmental conditions. Many of these studies involve crop fields with concurrent fertilization, which can independently improve soil structure and organic carbon levels. Our study is unique in that it investigates the effects of biochar on soil aggregate formation and carbon sequestration in undisturbed forest understory soil, aiming to isolate the specific contributions of biochar.
Materials and methods
Experimental site overview
The research was carried out from February 2021 to March 2023 at the rear mountain of the Hunan Vocational College of Environmental Biology (Latitude North: 26°59’48.47”, Longitude East: 112°36’55.90”), located in Shigu District, Hengyang City, Hunan Province. According to the data from Hengyang Meteorological Bureau, the region experiences an average annual temperature of 18.2 °C, with January temperatures typically above 0 °C and July temperatures around 25 °C. Seasonal variations in wind direction are pronounced, with winter and summer showing significant differences. The area receives an average annual rainfall exceeding 1000 mm, predominantly in summer and less in winter. The site was characterized by an osmanthus plantation with 70% canopy cover and no understory vegetation. The predominant soil type was Ferralsols, which was developed from granite, and was raw soil, hence it had low organic carbon and clay content, and was acidic.
Experimental treatments
Rice husk–derived biochar, supplied by the Changsha Biomass Energy Utilization Research Center in Hunan Province, was utilized in this experiment. The biochar was produced through high–temperature pyrolysis in an oxygen–limited environment, with temperatures ranging from 300 to 450 °C. Biochar was incorporated into the understory soil using agricultural tools (hoe, shovel and garden rake) to homogeneously till the top 15 cm layer across each experimental plot. The biochar was thoroughly mixed with the soil in a single application, following predetermined amounts, while the control plots underwent identical tillage without biochar. Four treatment levels were established: B0 (Control), B1 (15 t ha–1), B2 (30 t ha–1), and B3 (45 t ha–1), each with three replicates, resulting in a total of 12 plots, each measuring 2 m by 3 m. The experimental design incorporates a random distribution of treatment plots to eliminate any potential bias related to specific locations within the study area. Post–application, the litter and weeds on the site were left undisturbed. The biochar’s fundamental physicochemical properties are detailed in Table 1.
Sample collection
Soil samples were meticulously gathered from each plot using a five–point sampling technique to acquire 1 kg from the 0–15 cm soil layer. The samples were carefully mixed, packaged, and handled with minimal disturbance to preserve soil aggregate integrity during collection and transport. During the indoor air–drying process, the soil was gently fragmented into small pieces less than 1 cm in size, following the soil’s natural fracture lines.
Screening of soil aggregates
Soil aggregates were fractionated using a dry sieving method. From each plot, 500 g of air–dried soil was placed atop a series of sieves with mesh sizes of 5, 2, 1, 0.5, and 0.25 mm, respectively. The sieves were manually agitated for 2 to 3 min, and the retained soil aggregates on each sieve were collected, weighed, recorded, and stored. Aggregates larger than 0.25 mm were classified as macro–aggregates, while those smaller than 0.25 mm were deemed micro–aggregates. In this experiment, the type of biochar is rice husk biochar, which has a high ash content and very small particles, with sizes ranging from 0.1 mm to 10 mm. During the process of sieving soil aggregates, there was no distinction made, and the biochar was not crushed.
Physical and chemical analysis
Stability Indices Calculation The stability of soil aggregates was assessed using several indices: Mean weight diameter (MWD), geometric mean diameter (GMD), fractal dimension (D), and the proportion of aggregates larger than 0.25 mm (R > 0.25).
Where \(\:{\overline{d}}_{i}\) represents the mean diameter of soil aggregates for each size class, \(\:{w}_{i}\) denotes the percentage of each size class in the total sample, \(\:{m}_{i}\) is the weight of soil aggregates for each size class, \(\:{\overline{d}}_{max}\) is the mean diameter of the largest aggregate size class, and \(\:m\) is the total weight of the soil aggregates. \(\:m<0.25\) is the weight of aggregates smaller than 0.25 mm in diameter.
Soil Organic Carbon Content Determination The organic carbon content within each particle size fraction was measured using the Walkley–Black method. It involves heating soil samples in an acidic environment, using potassium dichromate as an oxidizing agent to convert organic carbon into carbon dioxide, and then titrating the excess potassium dichromate to quantify the organic carbon content in the soil.
The carbon contribution rate of various particle–size aggregates Carbon contribution rate (%) = [carbon content of aggregates of a certain size × percentage of aggregates of that size]/ total soil carbon content ×100.
Soil Organic Carbon Density Calculation The soil organic carbon density (SOCD) is calculated by the following formula:
Where SOCD is the soil organic carbon density (t ha–1), SOC is the soil organic carbon content (g kg–1), Bulk density is the average soil bulk density (g cm–3) measured using the core cutter method, H is the thickness of the soil layer (cm), and Gravel content is the volumetric content of gravel larger than 2 mm.
Statistical analysis
Data were organized with Microsoft Excel 2016, analyzed for statistical significance using IBM SPSS Statistics 18.0, and graphically represented via SigmaPlot 12.5. One–way ANOVA assessed the significance of treatment effects. The Shapiro–Wilk test confirmed the normal distribution of the data set, which met the assumptions required for subsequent statistical analyses.
Results
Influence of biochar on soil aggregate size distribution
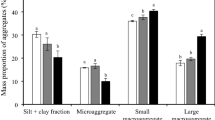
Biochar supplementation significantly altered soil aggregate size distribution (Fig. 1). In the absence of biochar (Control treatment), aggregates larger than 2 mm were predominant, constituting 65.6% of the total. Biochar application inversely affected the size distribution: as biochar levels increased, the proportion of aggregates larger than 2 mm decreased, whereas the fraction of aggregates smaller than 1 mm rose correspondingly. Notably, the B2 and B3 treatments substantially enhanced the presence of 0.25–0.5 mm and smaller than 0.25 mm aggregates (p < 0.05).
Variation in soil aggregate content across different fertilization treatments. The column represents means, and the error bar represents Standard deviation, the same below. Treatments include B0 (Control, CK), B1 (Biochar at 15 t ha–1), B2 (Biochar at 30 t ha–1), and B3 (Biochar at 45 t ha–1). The horizontal axis categorizes soil aggregate particle sizes into six ranges, while the vertical axis quantifies the soil aggregate content for each size range across treatments. Notably, distinct lowercase letters indicate statistically significant differences between treatments (p < 0.05).
Impact of biochar on soil aggregate stability
The incorporation of biochar markedly influenced soil aggregate stability (Fig. 2). Mean weight diameter (MWD), coarse weight diameter (CWD), and the proportion of aggregates with a diameter greater than 0.25 mm (R > 0.25) were elevated, whereas the dispersion ratio (D value) was reduced. Biochar application led to a reduction in the MWD, CWD, and R > 0.25 values, while it increased the D value. This effect was statistically significant only for the B3 treatment (p < 0.05).
Soil aggregate stability indices under varying treatments. Treatments consist of B0 (Control, CK), B1 (Biochar at 15 t ha–1), B2 (Biochar at 30 t ha–1), and B3 (Biochar at 45 t ha–1). The horizontal axis denotes the four indices of soil aggregate stability, and the vertical axis measures the indices’ values for each treatment. Significant differences between treatments are denoted by different lowercase letters (p < 0.05).
Influence of biochar on soil organic carbon distribution
Biochar application considerably altered the distribution of organic carbon within soil aggregates (Fig. 3). It notably enhanced the soil organic carbon content (p < 0.05), with the B2 treatment resulting in the most substantial increase—a 123.9% rise compared to the control (CK). In the control scenario, soil organic carbon was distributed more evenly. However, biochar application uniformly augmented organic carbon levels across all soil aggregate sizes (p < 0.05), with the B1 and B2 treatments particularly increasing the organic carbon in aggregates smaller than 0.5 mm by 231.4–270.0%.
Soil organic carbon (SOC) content and distribution within differently sized aggregate fractions under various treatments. Treatments are B0 (Control, CK), B1 (Biochar at 15 t ha–1), B2 (Biochar at 30 t ha–1), and B3 (Biochar at 45 t ha–1). The horizontal axis displays soil aggregate particle sizes alongside the average SOC content (AVG) for each treatment. The vertical axis shows the SOC content within each size range and the average SOC content per treatment. Statistically significant differences between treatments are marked by different lowercase letters (p < 0.05).
Impact of biochar on organic carbon contribution by soil aggregate size
Biochar application significantly influenced the contribution of organic carbon across different soil aggregate sizes (Fig. 4). In the control group, aggregates larger than 2 mm were responsible for 63.05% of the organic carbon content. However, this contribution diminished progressively with higher rates of biochar application. Conversely, the addition of biochar markedly enhanced the organic carbon contribution from aggregates smaller than 0.25 mm (p < 0.05).
Organic carbon contribution rates in soil aggregates of differing granularities under distinct treatments. Treatments include B0 (Control, CK), B1 (Biochar at 15 t ha–1), B2 (Biochar at 30 t ha–1), and B3 (Biochar at 45 t ha–1). The horizontal axis lists the six soil aggregate particle size ranges, and the vertical axis indicates the organic carbon contribution rate for each size range within each treatment. Different lowercase letters signal significant differences between treatments (p < 0.05).
Impact of biochar on soil organic carbon density
Biochar application had a significant effect on soil organic carbon density, as depicted in Fig. 5. Soil organic carbon density is influenced by factors such as soil organic carbon content, bulk density, soil layer thickness, and the volume of gravel with particles larger than 2 mm. Following biochar application, the B1 and B2 treatments resulted in a marked increase in soil organic carbon density compared to the control (p < 0.05). Although the B3 treatment also led to an increase in organic carbon density, this change was not statistically significant (p > 0.05).
Discussion
Biochar’s influence on soil aggregate stability and size distribution
Our research identified a decrease in soil aggregate stability and the proportion of larger aggregates upon biochar application. Larger aggregates are essential for sustaining soil structure and fertility and are a key metric of soil health16. Soil aggregates are differentiated into macroaggregates (> 0.25 mm) and microaggregates (< 0.25 mm)17. A dominance of macroaggregates generally indicates a more stable soil structure, while a prevalence of microaggregates may lead to soil compaction18. Soil structure stability is gauged by measures such as the macroaggregate ratio (R > 0.25), geometric mean diameter (GMD), mean weight diameter (MWD), and dispersion ratio (D), which are critical for the soil’s erosion resistance. Higher R > 0.25, GMD, and MWD values are associated with increased macroaggregate presence, reflecting a stable soil structure and better erosion resistance. A lower D value, on the other hand, indicates enhanced water retention and reduced nutrient leaching19.
In our study, the addition of biochar increased the microaggregate fraction and significantly reduced soil aggregate stability, which did not translate into improved soil structure. These results are consistent with Sheng, et al. 20 findings, but contrast with other studies21,22. It should be noted that factors such as soil type, biochar properties, and application duration can affect these outcomes. Additionally, studies under crop cultivation conditions have shown that plant root exudates, combined with fertilization and irrigation, can modify soil aggregate distribution and stability.
Previous studies have demonstrated that root exudates can increase the quantity of medium and large soil aggregates by stimulating microbial activity, thus enhancing aggregate stability. This effect is closely linked to the exudates’ concentration and composition23,24. Fresh crop root exudates can quickly bind soil particles, reducing water erosion and strengthening aggregate stability25. In our experiment, without crop cultivation and with mature osmanthus trees spaced 3 to 5 m apart, the soil’s surface layer had sparse roots and lacked significant exudate activity, with only a thin layer of osmanthus leaves on top. In such conditions, particularly in Ferralsols with low inherent organic matter, biochar’s effectiveness is limited. It does not boost macroaggregate proportions but rather decreases them due to its fragmented nature. Our data indicate that the substantial increase in large aggregate proportions seen in other studies with biochar is likely due to the combined effects of biochar with root exudates and fertilization26, where biochar enhances the impact of exudates on aggregate formation27.
Furthermore, biochar’s properties, heavily influenced by the type of biomass and pyrolysis method, dictate its soil amendment efficacy. For example, biochar produced at low temperatures through slow pyrolysis tends to be alkaline, with a high electrical conductivity and ash content but lower surface activity compared to that produced at higher temperatures, which has higher carbon content, lower yield, more developed pore structure, richer aromatic compounds, and a higher pH value28. This type of biochar may not effectively improve soil aggregate stability or mitigate colloidal dispersion. In our case, we used rice husk biochar produced at 300–450 ℃ by slow pyrolysis, which was alkaline and ash–rich, and it did not enhance soil aggregate stability or the proportion of larger aggregates, instead showing a negative impact.
Biochar’s role in enhancing soil organic carbon
Soil organic carbon (SOC) is a key indicator of soil health, sustainable land management, and the potential for carbon sequestration. Biochar, with its carbon–rich and chemically stable composition, is resistant to rapid breakdown, which can enhance the oxidation resistance of soil native organic carbon29, thereby facilitating the accumulation of SOC30,31. Studies across various soil types, including purple, black, and yellow soils, have shown that biochar can significantly increase SOC levels21,32,33,34,35. Our findings support this, demonstrating that biochar application not only increases SOC content but also boosts its association with organic carbon within soil aggregates of different sizes33.
While the B3 treatment had a less marked effect on SOC than B2, it still resulted in a significant increase compared to the control, corroborating Sun’s research36. However, our results differ from other studies in the distribution of SOC enhancements, with the most substantial increases observed in smaller aggregates rather than the larger ones reported elsewhere37. This could be due to a smaller proportion of large aggregates in our study, leading to a decrease in SOC as the mass of large aggregates is reduced. Perhaps, we could also explain this result from the theory of biochar’s priming effect. When applied judiciously, this induced priming effect can promote the swift cycling and sequestration of organic carbon. Conversely, an overabundance of biochar amendment might result in an exaggerated priming effect, potentially diminishing the net sequestration of organic carbon38.
Exogenous organic carbon (organic matter with relatively high C/N) is relatively stable within soil, having long–lasting impacts on soil characteristics39. The integration of such carbon into farmland soil is complex and requires in–depth, long–term research to fully understand the mechanisms at play. The short duration of our study means the results should be viewed as preliminary, with longer–term field trials needed for confirmation. Opinions on the effects of exogenous organic carbon on soil fertility and enzyme activity are mixed, likely due to the variety of carbon sources used and the different soil types and climates where experiments have been conducted.
Despite the two–year span of our study providing some evidence to support our conclusions, this timescale is also a major limitation for answering our research questions, another notable limitation is the experimental design: The lack of a control group for crop planting within the same test area to strengthen the findings. Our team is planning further research to examine the impact of biochar on soil aggregates and SOC in both cultivated and uncultivated soils over a larger time span. Upcoming experiments will involve grass sowing, biochar application, and their combination in a single plot to confirm the initial results. Based on this study and existing literature, we have developed a preliminary model to hypothesize how biochar may contribute to carbon sequestration and affect soil aggregate structure and composition (Fig. 6).
While this study has yielded some persuasive findings, it is not without its limitations. The exclusive use of biochar in our research presents constraints concerning ecological and economic viability. The absence of additional nutrients in pure biochar results in a negligible fertilization effect, thereby restricting our capacity to derive more profound insights into soil amelioration and crop development. Moreover, the study’s duration was relatively brief, spanning only two years, which hampers our evaluation of biochar’s enduring influence on soil organic carbon accumulation and aggregate formation—both critical factors for a thorough understanding of biochar’s environmental implications. Furthermore, the study did not adequately explain the observed consistency of the soil organic carbon (SOC) effect in the treatment with the highest biochar application, despite using twice the amount of biochar. This aspect warrants further investigation to elucidate the correlation between biochar application rates and the soil carbon sequestration effect. We recognize these shortcomings and propose that subsequent research should probe these subjects more deeply to ascertain a more detailed comprehension of biochar’s potential utility in soil stewardship and the pursuit of agricultural sustainability.
Conclusions
This study has elucidated the impact of biochar on soil organic carbon (SOC) dynamics within uncultivated Ferralsols, a significant step towards understanding its role in carbon sequestration and soil quality enhancement. Our findings demonstrate that biochar application leads to a notable increase in both SOC content and SOC density, reinforcing the soil’s carbon sequestration potential with minimal variance across different soil types. A key observation from the study is the preferential effect of biochar on the formation of smaller soil aggregates (< 0.25 mm). Biochar application was found to enrich the SOC content within these smaller aggregates and elevate their contribution to overall carbon sequestration, while inversely influencing the larger aggregates (> 0.25 mm). This suggests that biochar’s efficacy in aggregate formation is size–selective, with a more pronounced effect on the smaller fractions. Interestingly, the study also highlights that biochar alone may not be sufficient to enhance the content of soil macroaggregates in the absence of active plant root and microbial contributions. It appears that biochar acts synergistically with plant root exudates and microbial secretions, which are pivotal in the formation and stabilization of soil macroaggregate structure. Therefore, while biochar is a potent enhancer of soil carbon sequestration, its full potential is realized in conjunction with the biological processes associated with plant and microbial activity. This underscores the importance of a holistic approach to soil amendment practices, where biochar application is integrated with agricultural management practices that support robust plant and microbial activity for optimal soil structure and carbon sequestration.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Ceccherini, G. et al. Abrupt increase in harvested forest area over Europe after 2015. Nature 583, 72–77. https://doi.org/10.1038/s41586-020-2438-y (2020).
Chen, W., Meng, J., Han, X., Lan, Y. & Zhang, W. Past, present, and future of biochar. Biochar 1, 75–87. https://doi.org/10.1007/s42773-019-00008-3 (2019).
Guo, Y. et al. The potential for biochar application in “Shatangju” (Citrus reticulate cv.) orchard on acid red soil: Biochar prepared from its organic waste in an orchard. Front Plant Sci https://doi.org/10.3389/fpls.2022.1001740 (2022).
Wu, P. et al. A scientometric review of biochar research in the past 20 years (1998–2018). Biochar 1, 23–43. https://doi.org/10.1007/s42773-019-00002-9 (2019).
Zhao, N., Lehmann, J. & You, F. Poultry waste valorization via pyrolysis technologies: Economic and environmental life cycle optimization for sustainable bioenergy systems. ACS Sustain. Chem. Eng. 8, 4633–4646. https://doi.org/10.1021/acssuschemeng.0c00704 (2020).
Lefebvre, D. et al. Modelling the potential for soil carbon sequestration using biochar from sugarcane residues in Brazil. Sci. Rep. 10, 19479. https://doi.org/10.1038/s41598-020-76470-y (2020).
Lehmann, J. et al. Biochar in climate change mitigation. Nat. Geosci. 14, 883–892. https://doi.org/10.1038/s41561-021-00852-8 (2021).
Yudina, A. & Kuzyakov, Y. Dual nature of soil structure: The unity of aggregates and pores. Geoderma 434, 116478. https://doi.org/10.1016/j.geoderma.2023.116478 (2023).
Li, S. et al. Distribution and storage of crop residue carbon in aggregates and its contribution to organic carbon of soil with low fertility. Soil Tillage Res. 155, 199–206. https://doi.org/10.1016/j.still.2015.08.009 (2016).
Yang, W. et al. Distribution of soil organic carbon density fractions in aggregates as influenced by salts and microbial community. Land 12, 2024. https://doi.org/10.3390/land12112024 (2023).
Wang, D., Fonte, S. J., Parikh, S. J., Six, J. & Scow, K. M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 303, 110–117. https://doi.org/10.1016/j.geoderma.2017.05.027 (2017).
Xiu, L. et al. Effects of biochar and straw returning on the key cultivation limitations of Albic soil and soybean growth over 2 years. CATENA 173, 481–493. https://doi.org/10.1016/j.catena.2018.10.041 (2019).
Obia, A., Mulder, J., Martinsen, V., Cornelissen, G. & Børresen, T. In situ effects of biochar on aggregation, water retention and porosity in light-textured tropical soils. Soil Tillage Res. 155, 35–44. https://doi.org/10.1016/j.still.2015.08.002 (2016).
Heikkinen, J. et al. Possibilities to improve soil aggregate stability using biochars derived from various biomasses through slow pyrolysis, hydrothermal carbonization, or torrefaction. Geoderma 344, 40–49. https://doi.org/10.1016/j.geoderma.2019.02.028 (2019).
Rahman, M. T., Zhu, Q. H., Zhang, Z. B., Zhou, H. & Peng, X. The roles of organic amendments and microbial community in the improvement of soil structure of a Vertisol. Appl. Soil Ecology 111, 84–93. https://doi.org/10.1016/j.apsoil.2016.11.018 (2017).
Mustafa, A. et al. Soil aggregation and soil aggregate stability regulate organic carbon and nitrogen storage in a red soil of southern China. J. Environ. Manag. 270, 110894. https://doi.org/10.1016/j.jenvman.2020.110894 (2020).
Wang, J. et al. Nutrient characterization in soil sggregate Fractions with different fertilizer treatments in greenhouse vegetable cultivation. Agriculture 12, 440. https://doi.org/10.3390/agriculture12040440 (2022).
Chen, W. Inaugural editorial: pioneering the innovation and exploring the future for biochar technology. Biochar 1, 1–1. https://doi.org/10.1007/s42773-019-00010-9 (2019).
Zhang, Y. et al. Effects of farmland conversion on the stoichiometry of carbon, nitrogen, and phosphorus in soil aggregates on the Loess Plateau of China. Geoderma 351, 188–196. https://doi.org/10.1016/j.geoderma.2019.05.037 (2019).
Sheng, M.-H. et al. Effects of biochar additions on the mechanical stability of soil aggregates and their role in the dynamic renewal of aggregates in slope ecological restoration. Sci. Total Environ. 898, 165478. https://doi.org/10.1016/j.scitotenv.2023.165478 (2023).
Chagas, J. K. M., Figueiredo, C. C. D. & Ramos, M. L. G. Biochar increases soil carbon pools: Evidence from a global meta-analysis. J. Environ. Manag. 305, 114403. https://doi.org/10.1016/j.jenvman.2021.114403 (2022).
Sun, Q. et al. Long-term effects of biochar amendment on soil aggregate stability and biological binding agents in brown earth. CATENA 205, 105460. https://doi.org/10.1016/j.catena.2021.105460 (2021).
Liu, Z. et al. The responses of soil organic carbon mineralization and microbial communities to fresh and aged biochar soil amendments. GCB Bioenergy 11, 1408–1420. https://doi.org/10.1111/gcbb.12644 (2019).
Ma, W. et al. Root exudates contribute to belowground ecosystem hotspots: A review. Front. Microbiol. https://doi.org/10.3389/fmicb.2022.937940 (2022).
Xiao, T., Li, P., Fei, W. & Wang, J. Effects of vegetation roots on the structure and hydraulic properties of soils: a perspective review. Sci. Total Environ. 906, 167524. https://doi.org/10.1016/j.scitotenv.2023.167524 (2024).
Sun, J. et al. Biochar promotes soil aggregate stability and associated organic carbon sequestration and regulates microbial community structures in Mollisols from northeast China. SOIL 9, 261–275. https://doi.org/10.5194/soil-9-261-2023 (2023).
Xiang, Y., Deng, Q., Duan, H. & Guo, Y. Effects of biochar application on root traits: A meta-analysis. GCB Bioenergy 9, 1563–1572. https://doi.org/10.1111/gcbb.12449 (2017).
Sahoo, S. S., Vijay, V. K., Chandra, R. & Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 3, 100101. https://doi.org/10.1016/j.clet.2021.100101 (2021).
Wu, F., Jia, Z., Wang, S., Chang, S. X. & Startsev, A. Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol. Fertil. Soils 49, 555–565. https://doi.org/10.1007/s00374-012-0745-7 (2013).
Plaza, C. et al. Response of different soil organic matter pools to biochar and organic fertilizers. Agric. Ecosyst. Environ. 225, 150–159. https://doi.org/10.1016/j.agee.2016.04.014 (2016).
Yang, Y. et al. Biochar stability and impact on soil organic carbon mineralization depend on biochar processing, aging and soil clay content. Soil Biol. Biochem. 169, 108657. https://doi.org/10.1016/j.soilbio.2022.108657 (2022).
Zhang, S. et al. Biochar significantly reduced nutrient-induced positive priming in a subtropical forest soil. Biol. Fertil. Soils 59, 589–607. https://doi.org/10.1007/s00374-023-01723-7 (2023).
Weng, Z. et al. Biochar built soil carbon over a decade by stabilizing rhizodeposits. Nature Clim. Change 7, 371–376. https://doi.org/10.1038/nclimate3276 (2017).
Weng, Z. et al. Microspectroscopic visualization of how biochar lifts the soil organic carbon ceiling. Nat. Commun. 13, 5177. https://doi.org/10.1038/s41467-022-32819-7 (2022).
Kalu, S. et al. Biochar reduced the mineralization of native and added soil organic carbon: evidence of negative priming and enhanced microbial carbon use efficiency. Biochar 6, 7. https://doi.org/10.1007/s42773-023-00294-y (2024).
Sun, Q., Yang, X., Meng, J., Lan, Y. & Han, X. Effects of biochar on soil aggregate spatial distribution and soil organic carbon in brown earth soil. J. Agro-Environ. Sci. 41, 2515–2524. https://doi.org/10.11654/jaes.2022-0305 (2022).
Lee, M.-H., Chang, E.-H., Lee, C.-H., Chen, J.-Y. & Jien, S.-H. Effects of biochar on soil aggregation and distribution of organic carbon fractions in aggregates. Processes 9, 1431. https://doi.org/10.3390/pr9081431 (2021).
Chen, Y. et al. Priming effect of biochar on the minerialization of native soil organic carbon and the mechanisms: a review. Chin. J. Appl. Ecol. 29, 314–320. https://doi.org/10.13287/j.1001-9332.201801.024 (2018).
Tian, Q. et al. Microbial properties control soil priming and exogenous carbon incorporation along an elevation gradient. Geoderma 431, 116343. https://doi.org/10.1016/j.geoderma.2023.116343 (2023).
Acknowledgements
We extend our gratitude to Youqing Li, Lang Zhang, and the numerous assistants for their invaluable assistance and support in both fieldwork and laboratory analyses. This research was funded by the Dean’s Fund Youth Science and Technology Training Program of Hunan Polytechnic of Environment and Biology (PY2022–03) and the Forestry Science and Technology Innovation Project of the Hunan Provincial Forestry Bureau (XLK202105–2).
Author information
Authors and Affiliations
Contributions
J.W. designed the study, was involved in data acquisition, performed data analysis, and prepared the initial draft of the manuscript; L.Z. conceptualized the study, provided the framework for intellectual content, and critically edited the manuscript. Y.Q.L., W.Y., J.L., Q.Y., H.J., J.J., Z.L., Y.L., and Y.Y. were involved in data acquisition and statistical analysis. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wei, J., Li, Y., Li, J. et al. A 2-year pure biochar addition enhances soil carbon sequestration and reduces aggregate stability in understory conditions. Sci Rep 14, 30122 (2024). https://doi.org/10.1038/s41598-024-81232-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81232-1