Abstract
Adipose tissue (AT) metabolism involves coordinating various cells and cellular processes to regulate energy storage, release, and overall metabolic homeostasis. Therein, macrophage and its cytokine are important in controlling tissue homeostasis. Among cytokines, the role of transforming growth factor-β1 (Tgf-β1), a cytokine abundantly expressed in CD206+ M2-like macrophage and correlated with the expansion of AT and fibrosis, in AT metabolism, remains unknown. We used CD206CreERT2; Tgf-β1f/f mouse model in which the Tgf-β1 gene was conditionally deleted in CD206+ M2-like macrophages followed by tamoxifen administration, to investigate the role of the Tgf-β1 gene in glucose and insulin metabolism. Our data demonstrated that lack of CD206+ M2-like macrophages derived Tgf-β1 gene improved glucose metabolism and insulin sensitivity by enhancing adipogenesis via hyperplasia. The Tgf-β1 gene, specifically from CD206+ M2-like macrophages, deletion stimulated APs’ proliferation and differentiation, leading to the generation of smaller mature adipocytes, therefore enhancing insulin sensitivity and improving glucose metabolism under normal chow conditions. Our study brings a new perspective that Tgf-β1 gene deletion specific from CD206+ M2-like macrophage promotes adipocyte hyperplasia, improving glucose homeostasis and insulin sensitivity in the lean state.
Similar content being viewed by others
Introduction
Adipose tissue (AT) metabolism involves coordinating various cells and cellular processes to regulate energy storage, release, and overall metabolic homeostasis. AT includes adipocytes, a stromal vascular fraction (SVF), and an extracellular matrix. Excess nutrients are associated with an expansion of white adipose tissue (WAT)1,2,3, leading to AT dysfunction, insulin resistance, type 2 diabetes, and other metabolic disorders4,5. WAT expands in two ways: adipocyte hypertrophy (increased adipocyte size) or adipocyte hyperplasia (de novo adipogenesis)6,7. The expansion of adipocytes is closely related to SVF. SVF, which is located in the connective tissue surrounding adipocytes, contains heterogeneous cell populations such as adipocyte progenitors (APs) and immune cells. A large number of APs, which give rise to mature adipocytes with a higher number of smaller adipocytes, preserves AT function and enhances insulin sensitivity8,9. Epididymal WAT (eWAT), a major visceral WAT depot, has the function of energy storage and expands through cellular hypertrophy, increasing inflammation and inflammatory cytokine and leading to insulin resistance10. Thus, ameliorating the expansion of eWAT brings a promising therapy for obesity-related disorders.
WAT is a source of adipokines, including immune modulatory cytokines, chemokines, and growth hormones. M1-polarized macrophages provide acute pro-inflammatory effector functions by expressing reactive oxygen species, nitric oxide, and secretion of type-1 cytokines such as TNF-α, IFN-γ, and interleukin 1 beta (IL-1β). Previous studies demonstrated that the upregulation of M1 marker genes such as tumor necrosis factor-alpha (TNFα), C–C motif chemokine receptor (CCR) 2, or CD11c resulted in the development of insulin resistance11,12,13,14. In contrast, macrophages are activated towards M2 polarization characterized by the relatively high expression of CD206, arginase-1, Mgl1, and IL-10, which are involved in the repair or remodeling of tissues15,16. A recent study reported that depletion of CD206+ M2-like macrophage improves glucose and insulin metabolism8. Another study showed that ablation of IL10 secreted from macrophages protects from obesity development17. However, the role of transforming growth factor-β1 (Tgf-β1), a cytokine abundantly expressed in CD206+ M2-like macrophage within AT and correlated with the expansion of AT and fibrosis18,19,20, in AT metabolism remains unknown.
Precious study reported that Tgf-β signaling induces hibernation of tissue stem cells such as hematopoietic and melanocyte stem cells21,22, suggesting the essential role of Tgf-β1 in regulating APs to respond with adipocyte expansion. Thus, we hypothesize that deletion of Tgf-β1 gene-specific from CD206+ M2-like macrophages might improve glucose metabolism and insulin sensitivity by enhancing adipogenesis via stimulating APs proliferation and differentiation.
Results
The Tgf-β1 gene was successfully deleted in our mouse model
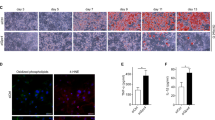
To investigate the role of the Tgf-β1 gene in CD206+ M2-like macrophage, we used the mice CD206CreERT2; Tgf-β1f/f in which Tgf-β1 gene was conditionally deleted from CD206+ M2-like macrophage by tamoxifen (TAM) treatment as schema in Fig. 1A. To investigate the role of the Tgf-β1 gene, 18-week-old mice were administered TAM for 5 consecutive days. After 1 week of recovery, we performed GTT and ITT. Finally, mice were sacrificed at 22 weeks as the schematic protocol described in Fig. 1B. Before and after 5 times TAM treatment, the body weights of both groups were comparable (Supplementary Fig. 1A, B). From 6 to 18 weeks of normal chow diet, both food intake and body weight also showed no significant difference between the two groups (Supplementary Fig. 1C, D). When sacrificed, body weight, eWAT, and inguinal white adipose tissue (iWAT) were also comparable between both groups (Supplementary Fig. 2A–C). To evaluate our mouse model, we examined Tgf-β1 gene expression and found it downregulated significantly in the eWAT whole tissue of Tgf-β1 KO mice (Fig. 1C). For further confirmation, we performed immunohistochemistry stained with anti-CD206 and anti-Tgf-β1. The result revealed that CD206 and Tgf-β1 double-positive expression was reduced significantly in Tgf-β1 KO mice (Fig. 1D, E); however, the circulatory Tgf-β1 remained unchanged (Supplementary Fig. 2D). Collectively, our data confirmed that the Tgf-β1 gene was successfully deleted in CD206+ M2-like macrophage in our mouse model.
Tgf-β1 gene successfully deleted in our mouse model. (A) Mouse model. (B) Schematic of the experiment protocol. (C) Relative Tgf-β1 expression in eWAT whole tissue (n=5; n=5). (D) Representative confocal images indicates Tgf-β1 co-localization with CD206 (scale bar = 20 μm, n=4; n=4). (E) Quantification of Tgf-β1+ CD206+/total CD206+ (n=4; n=4). Data represent mean ± SEM. Statistical analysis was performed using a two-tail Student’s t-test (**p < 0.01, ****p < 0.0001).
Deleting the Tgf-β1 gene in CD206+ M2-like macrophage improves glucose metabolism and insulin sensitivity
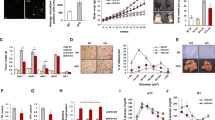
We next investigate whether deleting the Tgf-β1 gene derived from CD206+ M2-like macrophage affects glucose and insulin metabolism. As expected, in GTT, we found glucose levels were lower in Tgf-β1 KO mice (Fig. 2A), and the area under the GTT curve was also significantly lower in Tgf-β1 KO mice (Fig. 2B). We also found that ITT was improved in Tgf-β1 KO mice (Fig. 2C, D), suggesting Tgf-β1 KO enhances glucose metabolism and insulin sensitivity. Consistent with this, gene analysis revealed that several metabolically favorable genes’ expressions were upregulated significantly in Tgf-β1 KO mice (Fig. 2E). In addition, we also found that the expression of adipogenesis-related genes was elevated significantly in Tgf-β1 KO mice (Fig. 2F). Moreover, the lipogenesis-related genes (such as Fasn, Acaca, and Srebp1c) and lipolysis-related genes (such as Atgl and Hsl) were also upregulated in Tgf-β1 KO mice (Supplementary Fig. 3A). Insulin signaling-related genes were also upregulated in liver and eWAT, but remained unchanged in skeletal muscle (Supplementary Fig. 3B–D). Collectively, our data demonstrated that Tgf-β1 deletion in CD206+ M2-like macrophage improved glucose metabolism and insulin sensitivity in lean state.
Deleting the Tgf-β1 gene derived from CD206+ M2 macrophage improved glucose metabolism and insulin sensitivity. (A) Glucose tolerance test (n=11; n=11). (B) Area under the curve (AUC) of glucose tolerance test (n=11; n=11). (C) Insulin tolerance test (n=11; n=11). (D) Area under the curve (AUC) of insulin tolerance test (n=11; n=11). (E) Relative mRNA expression of metabolically favorable genes (n=6; n=6). (F) Relative mRNA expression of adipogenesis-related genes in eWAT whole tissue (n=6; n=6). (A, C) Data represent mean ± SEM. Statistical analysis was performed using two-way ANOVA (*p<0.05, **p < 0.01, ****p < 0.0001). (B, D, E, F) Data represent mean ± SEM. Statistical analysis was performed using a two-tail Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001, ***p < 0.0001).
Lack of CD206+ M2-like macrophage-derived Tgf-β1 gene generates smaller adipocytes
We further aimed to find the mechanism behind the lack of the Tgf-β1 gene to promote glucose and insulin tolerance. Previous studies reported that M1 pro-inflammatory marker genes are involved in insulin resistance11,12,13. M2 macrophages are reported to maintain homeostasis and adapt to energy surplus conditions23,24. Thus, we examine whether macrophage remodeling was responsible for the improvement. However, we found no significant difference in M1 and M2 macrophage-related gene expression between both groups (Fig. 3A). We next confirmed the CD206+ signal by immunohistochemistry and found that there were no significant differences between both groups (Supplementary Fig. 4), suggesting that there was no macrophage remodeling in response to Tgf-β1 gene deletion under normal chow conditions. Another report demonstrated that hyperplasia was involved in insulin sensitivity7,8. Our data reported that the adipogenesis marker (C/EBPα, Pparγ) was elevated significantly in Tgf-β1 KO mice (Fig. 2E). We next investigated small adipocyte-related gene expression and found it elevated dramatically in Tgf-β1 KO mice (Fig. 3B). As expected, we found that Tgf-β1 KO increased smaller adipocytes with an elevated number of adipocytes and reduced average adipocyte size (Fig. 3C–E). Collectively, our data demonstrated that deletion of Tgf-β1 in CD206+ M2-like macrophage stimulated adipogenesis and generated smaller adipocytes.
Lack of CD206+ M2 macrophage-derived Tgf-β1 gene generated smaller adipocytes. (A) Relative mRNA expression of M1, M2 macrophages-related genes in eWAT whole tissue (n=5; n=5). (B) Relative mRNA expression of small adipocyte-related genes in eWAT whole tissue (n=6; n=6). (C) Representative images stain with hematoxylin and eosin (H&E) from eWAT (scale bar = 200 μm, n=4; n=4), and the quantification of adipocyte cells between Tgf-β1f/f and Tgf-β1 KO (n=4; n=4). (D) Frequency distribution of adipocyte size (area μm2) in Tgf-β1f/f and Tgf-β1 KO (n=4; n=4). (E) Quantification of adipocyte size compared Tgf-β1f/f and Tgf-β1 KO (n=4; n=4). Data represent mean ± SEM. Statistical analysis was performed using a two-tail Student’s t-test (*p < 0.05, ***p < 0.001).
CD206+ M2 macrophage-derived Tgf-β1 gene deletion enhanced APs proliferation. (A) Relative mRNA expression analysis of cell cycle-related genes in SVF from eWAT (n=6; n=6). (B) Schematic for magnetic activated cell sorting Pdfgrα+ cells from SVF of eWAT. (C) Relative mRNA expression analysis of cell cycle-related genes in PDGFRα+ cells from eWAT (n=3; n=3). (D) Representative confocal images indicate PDGFRα co-localization with mKi67 (scale bar = 20 μm, n=4; n=4). (E) Quantification of PDGFRα+ mKi-67+ DAPl+ (n=4; n=4). Data represent mean ± SEM. Statistical analysis was performed using a two-tail Student’s t-test (*p < 0.05, **p<0.01). PDGFRα, Platelet-derived growth factor receptor alpha.
Impact of CD206+ M2 macrophage-derived Tgf-β1 gene on APs differentiation. (A) Relative mRNA expression analysis of adipocyte progenitors-related genes (APs) in SVF from eWAT (n=6; n=6). (B) Relative mRNA expression analysis of adipocyte progenitors (APs) in Pdfgrα+ cells from eWAT (n=3; n=3). (C) Flow cytometry analysis of adipocyte progenitors-related genes in SVF from eWAT (n=5; n=5). (D) Percentage of adipocyte progenitors in SVF from eWAT (n=5; n=5). Data represent mean ± SEM. Statistical analysis was performed using a two-tail Student’s t-test (*p < 0.05, **p < 0.01).
CD206+ M2-like macrophage-derived Tgf-β1 gene deletion enhanced APs proliferation
APs are the population that gives rise to mature adipocytes. TGF-β signaling was reported to control cell proliferation25,26,27. Other studies also reported that TGF-β signaling induces hibernation of tissue stem cells such as hematopoietic and melanocyte stem cells21,22. Therefore, we hypothesize that the APs pool was hibernated by CD206+ M2-like macrophage-derived Tgf-β1 gene; thus, Tgf-β1 gene deletion stimulated APs proliferation. We investigated the cell cycle-related genes in SVF of eWAT and found that the expression of mKi-67 and Cyclin d1 were increased significantly (Fig. 4A). Platelet-derived growth factor receptor alpha (PDGFRα) is a marker of APs and preadipocytes that can differentiate into functional adipocytes in vivo28,29,30. We next isolated PDGFRα+ cells using magnetic-activated cell sorting (MACS) (Fig. 4B) from eWAT, then examined cell cycle-related gene expression and found that almost all genes elevated dramatically (Fig. 4C). We next examined immunohistochemistry by staining with anti-Ki-67 and anti-PDGFRα and found that PDGFRα+Ki-67+DAPI+ signals were significantly elevated in Tgf-β1 KO mice (Fig. 4D, E). Our data demonstrated that deletion of the Tgf-β1 gene in CD206+ M2-like macrophage stimulated APs proliferation.
Impact of CD206+ M2-like macrophage-derived Tgf-β1 gene on APs differentiation
A previous study reported that the TGF-β family inhibits Dpp4+ stem cell-like progenitors from differentiating into Icam1+ committed pre-adipocytes31,32. Thus, we hypothesize that Tgf-β1 also inhibited APs’ differentiation. We examined APs-related gene expression and found that almost all genes were increased significantly in the SVF of Tgf-β1 KO mice (Fig. 5A). We further confirmed the expression of APs-related genes and also found that almost all genes were elevated significantly in Pdgfrα+ cells of Tgf-β1 KO mice (Fig. 5B). We next performed flow cytometry using the strategy described in Supplementary Fig. 5. Consistent with our data, we found the significant upregulation of Dpp4 and Icam1 double-positive cells in Tgf-β1 KO mice, suggesting the shifting from stem cell-like progenitors to committed pre-adipocytes, resulting in generating more small adipocytes (Fig. 5C, D). Collectively, our data demonstrated that Tgf-β1 gene deletion stimulated stem cell-like progenitors to differentiate into committed pre-adipocytes, thus generating smaller mature adipocytes, stimulating the expression of metabolically favorable genes, and finally improving glucose metabolism and insulin sensitivity in the lean state.
Discussion
AT metabolism involves coordinating various cells and cellular processes to regulate energy storage, release, and overall metabolic homeostasis. These include adipocytes, preadipocytes/APs, endothelial cells, and immune cells. Therein, macrophages have an essential role in regulating homeostasis and AT metabolism. AT macrophages (ATMs) maintain tissue homeostasis by scavenging debris, pathogens, and apoptotic or necrotic cells; this efferocytotic process maintains an anti-inflammatory environment33. ATMs show highly heterogeneous characteristics and include at least two major populations called the classically activated, or M1-ATMs and alternatively activated, or M2-ATMs34. M1 macrophages are mainly induced by Th1 signaling and express high levels of inflammatory cytokines such as TNFα and IL-6, While M2 macrophages are induced by Th2 signaling and are associated with anti-inflammatory reactions35. Previous reports showed that TNFα, a cytokine released from M1 macrophage, increases phosphorylation of PLIN1, promoting basal lipolysis and thus releasing fatty acids (FAs) into plasma36,37,38. These FAs activate the JNK pathway to increase releasing pro-inflammatory factors, resulting in insulin resistance39. Another cytokine from the M1 macrophage, IL-6, was also reported to increase insulin resistance by promoting lipolysis40,41. M2 macrophages, on the other hand, were reported to be involved in maintaining insulin sensitivity through their anti-inflammatory functions23,24. However, recent reports demonstrated that depletion of CD206+ M2-like macrophage or ablation IL10 derived from M2 macrophage leads to improved glucose metabolism and insulin sensitivity8,17, suggesting that not all M2 macrophages were good. From the current study perspective, our data demonstrated that deletion of the Tgf-β1 gene, a cytokine mainly secreted from CD206+ M2-like macrophage, improves glucose and insulin sensitivity without macrophage remodeling.
Previous studies reported that TGF-β signaling was involved in inducing hibernation of tissue stem cells such as hematopoietic and melanocyte stem cells21,22. In addition, Merrick et al. reported that TGF-β signaling inhibits the differentiation from stem cell-like progenitors APs (Dpp4+APs) into committed preadipocytes (Icam1+APs)31. Consistent with them, our data demonstrated that CD206+ M2-like macrophage-derived Tgf-β1 inhibits proliferation and differentiation into mature adipocytes of APs. By inhibiting the proliferation of APs, they can avoid unnecessary cell division, thus preventing cellular senescence and contributing to preserving the quality of APs and maintaining AT homeostasis. However, when we deleted the Tgf-β1 gene specifically in the M2-like macrophage, the inhibition of APs proliferation and differentiation was released. APs start to proliferate and then differentiate into smaller mature adipocytes, readily taking up free fatty acids and glucose, leading to improved glucose tolerance. Obese conditions bring excess nutrients. Thus, AT expand strongly via hyperplasia or hypertrophy. During HFD, Tgf-β1 is also upregulated in AT and robustly inhibits the proliferation and differentiation of APs, which will promote adipocyte hypertrophy, thus secreting inflammation cytokines, resulting in insulin resistance. There may be another mechanism for how increased Tgf-β1 expression worsens insulin resistance in AT in obese conditions. Moreover, the expression of lipogenesis-related genes (such as Fasn, Acaca, and Srebp1c) or lipolysis-related genes (such as Atgl and Hsl) in eWAT of Tgf-β1KO mice were upregulated compared to control mice in lean state. Taken together, the AT metabolism was improved, followed by the deletion of Tgf-β1-derived from CD206+ M2-like macrophages. Tgf-β1 transmits its signal to the nucleus through a transcriptional factor called SMAD42,43. Roh et al.44 reported that the TGF-β activated SMAD repressor complex downregulates Pparγ at the transcriptional level via histone deacetylation after binding to a novel TGF-β inhibitory element and canonical SMAD-binding elements. Agreed with them, we also found that Pparγ, the master regular of adipogenesis45,46, expression was elevated in response to Tgf-β1 gene deletion, suggesting that Tgf-β1 controls the APs pool via interacting with Pparγ.
In conclusion, our study brings a new perspective that Tgf-β1 gene deletion specific from CD206+ M2-like macrophage promotes adipocyte hyperplasia, improving glucose homeostasis under physiological conditions. Tgf-β1 in CD206+ M2-like macrophages may play a role in the inhibition of unnecessary cell division and cellular senescence of APs, thereby keeping APs young and maintaining adipose tissue homeostasis in lean mice.
Material and methods
Mice
We generated CD206CreERT2; Tgf-β1f/f mice by crossing CD206 CreERT2 mice and Tgf-β1 flox/flox (Tgf-β1f/f) as previously described by Nawaz et al.47. All animals were housed, 6 mice in one cage, in a room with the temperature (24 \(\pm\) 2 degrees and humidity (55 \(\pm\) 5 percentage) were controlled automatically, and the cycle of light/dark was maintained at 12:12 h. The mice had free access to water ad libitum and food (a normal chow diet: CE-2 CLEA, Japan). The mice tissues were collected after euthanizing the mice. The mice were first anesthetized by sevoflurane (Nikko Pharmaceuticals Co., Ltd.) and then followed by cervical dislocation. All the experiments were performed in accordance with the relevant guidelines and regulations and were approved by the Committee for Institutional Animal Care and Use of the University of Toyama (Toyama, Japan).
Genotyping
Whole genomic DNA was obtained by lysis tail tissue with Direct PCR (Tail) lysis solution (Viagen Biotech) and proteinase K (Roche Diagnostics), following the manufacturer’s instructions. We performed PCR by using a Tks Gflex DNA polymerase kit from TAKARA (Shiga, Japan) with this crude DNA.
PCR conditions for CD206 CreERT2 included segment 1: 1 cycle of 94 degrees for 1 min, segment 2: 30 cycle includes 98 degrees for 10 s; 58 degrees for 30 s; and 68 degrees for 30 s. Then PCR productions were kept at 4 degrees. The expected DNA fragment size is 299 bp. The primers used for PCR had the sequence GGTCGATGCAACGAGTGATGAG (primer 1) and GTGAAACAGCATTGCTGTCACTTGG (primer 2).
The PCR condition for Tgf-β1f/f included segment 1: 1 cycle of 94 degrees for 1 min, segment 2: 40 cycle including 98 degrees for 10 s; 54 degrees for 30 s; and 68 degrees for 30 s. Then PCR productions were kept at 4 degrees. The expected DNA fragment sizes of WT and f/f mice were 210 bp and 338 bp, respectively. The primers’ sequences were AAGACCTGGGTTGGAAGTG (primer 1) and CTTCTCCGTTTCTCTGTCACCCTAT (primer 2). Both primers for PCR were purchased from Invitrogen™ Life Technology (Tokyo, Japan). Then PCR products were separated using 1.5% Agarose gel (Nippon gel) electrophoresis for 30 min. Ethidium bromide (1:1000) was added to visualize DNA on the gel.
Tamoxifen administration
We used sunflower oil (WAKO) to dissolve tamoxifen (TAM: sigma-Aldrich) incubated at 55 degrees and vortexed every 5 min until dissolved. After dissolved, TAM was administered to both Tgf-β1f/f and Tgf-β1 KO at the dose of 225 mg/kg body weight for five consecutive days, as previously described10 at the 18-week-old following schematic protocol in Fig. 1B.
Glucose tolerance and insulin tolerance test
For the intraperitoneal glucose tolerance test (ip-GTT), the mice were fasted for 5 h. Glucose was injected into both Tgf-β1 KO and Tgf- β1f/f at a dose of 1 mg/g body weight. The blood glucose level was measured at 0, 15, 30, 60, 90 and 120 min. For the intraperitoneal insulin tolerance test (ip- ITT), mice were fasting for 4 h. Both Tgf-β1 KO and Tgf- β1f/f mice were injected with human insulin (Humalin R) with a dose of 0.8 units/Kg. The blood glucose level was measured at 0, 15, 30, 45, 60, 90, and 120 min. In both ip-GTT and ip-ITT, the blood glucose level was taken from the tail vein using the STAT STRIP Express 900 (Nova Biomedical, Waltham, MA).
Real-time polymerase chain reaction (RT-PCR)
eWAT whole tissue was collected and extracted using the Qiagen RNeasy kit following the manufacturer’s instructions. The TaKaRa PrimerScript RNA Kit was used following the company’s guidance for reverse transcription. The quantitative PCR amplification reaction was performed using gene-specific primers (provided in Supplementary Table S2) and TB Green Fast Premix (Takara, Shiga Japan), followed by the manufacturer’s instructions. The relative mRNA expression levels were calculated by \(\Delta \Delta\) Ct value and normalized by internal control TF2B or RPL13a.
Flow cytometry analysis
To isolate and prepare stromal vascular fraction (SVF) of eWAT48,49. Tissue was collected and digested in collagenase (Sigma) for 45 min at 37 degrees before filtering through a 100- µm strainer to harvest a single cell. The 7AAD− population was gated to analyze lineage-negative (CD31−CD45−) populations, followed by Sca1+, then separated into Dpp4+, Icam1+, and Dpp4+ Icam1+ populations. For justification of the gating strategy, unstained and fluorescence minus one (FMO) were used. All this experiment and cell sorting were performed using BD FACS Aria™ SORP II machine and the FlowJo offline software (v10) to analyze data.
Magnetic-activated cell sorting study
SVF was dissociated from eWAT tissue as previously described8,48. The SVF was processed for magnetic cell sorting with anti-Pdgfr\(\alpha\) microbeads, then we collected a positive population, extracted RNA, and performed qPCR analysis of adipocyte progenitors and cell cycle. All incubation and procedure were performed at 4 degrees for 10–15 min following the manufacturer’s instructions. Microbead Kit was purchased from Miltenyi Biotech.
Histology
After collection, tissue was fixed in 4%PFA, and paraffin sections were prepared with 5–10 µm thickness and then mounted on the slide.
For Hematoxylin and Eosin (H/E staining), the slide was stained with hematoxylin and eosin. Hematoxylin eosin was captured using Keyence BZ-X800 with a 20× lens (scale bar 200 µm).
Immunohistochemistry
After collection, tissue was fixed in 4%PFA, and paraffin sections were prepared with 5–10 µm thickness and then mounted on the slide. As described previously, paraffin-embedded tissue sections were used in immunohistochemical staining. The primary and secondary antibodies are used following the manufacturer’s instructions, with the ratio for primary antibody being 1:100, the secondary antibody being 1:250, and DAPI being 1:400. Primary antibodies included CD206, Tgf-β1, PDGFRα, and mKi-67. Secondary antibodies included anti-rabbit, anti-mouse, and anti-goat. All primer sources were provided in Supplementary Table S1. All images were taken by an LSM 900 with an Arycan confocal microscope.
Quantification of adipocyte size
The number of adipocytes was counted at 3.9 × 105 µm2 (area). The multi-point tool in ImageJ 1.53a (National Institute of Health, USA) was used for adipocyte counting. The “Set Scale” function in ImageJ adipocyte size was used to analyze adipocyte size manually. We measured 4 random fields/specimens, with 4 specimens in each group.
Statistical analysis
Statistical significance between the Tgf-β1 KO and Tgf-β1f/f group was performed using two-way ANOVA followed by the Sidak multiple comparison test for GTT and ITT. Other data used two-tail unpaired Student’s t-test, *p < 0.05, ** p < 0.01, ***p < 0.001, ****p < 0.0001. Data are expressed as mean ± SEM.
Data availability
The raw data generated for all figures and supplementary figures of this study are provided in the Source data file.
References
Blüher, M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298. https://doi.org/10.1038/s41574-019-0176-8 (2019).
Sakers, A., De Siqueira, M. K., Seale, P. & Villanueva, C. J. Adipose-tissue plasticity in health and disease. Cell 185, 419–446. https://doi.org/10.1016/j.cell.2021.12.016 (2022).
Kahn, C. R., Wang, G. & Lee, K. Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Invest. 129, 3990–4000. https://doi.org/10.1172/jci129187 (2019).
Sun, K., Kusminski, C. M. & Scherer, P. E. Adipose tissue remodeling and obesity. J. Clin. Invest. 121, 2094–2101. https://doi.org/10.1172/jci45887 (2011).
Klein, S., Gastaldelli, A., Yki-Järvinen, H. & Scherer, P. E. Why does obesity cause diabetes?. Cell Metab. 34, 11–20. https://doi.org/10.1016/j.cmet.2021.12.012 (2022).
Wang, Q. A., Tao, C., Gupta, R. K. & Scherer, P. E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344. https://doi.org/10.1038/nm.3324 (2013).
Ghaben, A. L. & Scherer, P. E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 20, 242–258. https://doi.org/10.1038/s41580-018-0093-z (2019).
Nawaz, A. et al. CD206(+) M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat. Commun. 8, 286. https://doi.org/10.1038/s41467-017-00231-1 (2017).
Joe, A. W., Yi, L., Even, Y., Vogl, A. W. & Rossi, F. M. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 27, 2563–2570. https://doi.org/10.1002/stem.190 (2009).
Bilal, M. et al. Fate of adipocyte progenitors during adipogenesis in mice fed a high-fat diet. Mol. Metab. 54, 101328. https://doi.org/10.1016/j.molmet.2021.101328 (2021).
Uysal, K. T., Wiesbrock, S. M., Marino, M. W. & Hotamisligil, G. S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389, 610–614. https://doi.org/10.1038/39335 (1997).
Weisberg, S. P. et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124. https://doi.org/10.1172/jci24335 (2006).
Patsouris, D. et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8, 301–309. https://doi.org/10.1016/j.cmet.2008.08.015 (2008).
Kamei, N. et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 281, 26602–26614. https://doi.org/10.1074/jbc.M601284200 (2006).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184. https://doi.org/10.1172/jci29881 (2007).
Lumeng, C. N., DelProposto, J. B., Westcott, D. J. & Saltiel, A. R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57, 3239–3246. https://doi.org/10.2337/db08-0872 (2008).
Rajbhandari, P. et al. IL-10 signaling remodels adipose chromatin architecture to limit thermogenesis and energy expenditure. Cell 172, 218-233.e217. https://doi.org/10.1016/j.cell.2017.11.019 (2018).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338. https://doi.org/10.1038/nrneph.2016.48 (2016).
Samad, F. et al. Tumor necrosis factor alpha is a key component in the obesity-linked elevation of plasminogen activator inhibitor 1. Proc. Natl. Acad. Sci. U. S. A. 96, 6902–6907. https://doi.org/10.1073/pnas.96.12.6902 (1999).
Samad, F., Yamamoto, K., Pandey, M. & Loskutoff, D. J. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol. Med. 3, 37–48 (1997).
Yamazaki, S. et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158. https://doi.org/10.1016/j.cell.2011.09.053 (2011).
Nishimura, E. K. et al. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell 6, 130–140. https://doi.org/10.1016/j.stem.2009.12.010 (2010).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117. https://doi.org/10.1172/jci57132 (2011).
Pollard, J. W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 9, 259–270. https://doi.org/10.1038/nri2528 (2009).
Huang, S. S. & Huang, J. S. TGF-beta control of cell proliferation. J. Cell Biochem. 96, 447–462. https://doi.org/10.1002/jcb.20558 (2005).
Zhang, Y., Alexander, P. B. & Wang, X. F. TGF-β Family signaling in the control of cell proliferation and survival. Cold Spring Harb. Perspect. Biol. 9, 78. https://doi.org/10.1101/cshperspect.a022145 (2017).
Moustakas, A., Pardali, K., Gaal, A. & Heldin, C. H. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol. Lett. 82, 85–91. https://doi.org/10.1016/s0165-2478(02)00023-8 (2002).
Feng, X. H. & Derynck, R. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693. https://doi.org/10.1146/annurev.cellbio.21.022404.142018 (2005).
Iwayama, T. et al. PDGFRα signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev. 29, 1106–1119. https://doi.org/10.1101/gad.260554.115 (2015).
Lee, Y. H., Petkova, A. P., Mottillo, E. P. & Granneman, J. G. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491. https://doi.org/10.1016/j.cmet.2012.03.009 (2012).
Merrick, D. et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364, 859. https://doi.org/10.1126/science.aav2501 (2019).
Ignotz, R. A. & Massagué, J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 82, 8530–8534. https://doi.org/10.1073/pnas.82.24.8530 (1985).
Fischer-Posovszky, P., Wang, Q. A., Asterholm, I. W., Rutkowski, J. M. & Scherer, P. E. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology 152, 3074–3081. https://doi.org/10.1210/en.2011-1031 (2011).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686. https://doi.org/10.1016/j.it.2004.09.015 (2004).
Fujisaka, S. The role of adipose tissue M1/M2 macrophages in type 2 diabetes mellitus. Diabetol. Int. 12, 74–79. https://doi.org/10.1007/s13340-020-00482-2 (2021).
Yang, X., Zhang, X., Heckmann, B. L., Lu, X. & Liu, J. Relative contribution of adipose triglyceride lipase and hormone-sensitive lipase to tumor necrosis factor-α (TNF-α)-induced lipolysis in adipocytes. J. Biol. Chem. 286, 40477–40485. https://doi.org/10.1074/jbc.M111.257923 (2011).
Langin, D. & Arner, P. Importance of TNFalpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol. Metab. 17, 314–320. https://doi.org/10.1016/j.tem.2006.08.003 (2006).
Rydén, M. & Arner, P. Tumour necrosis factor-alpha in human adipose tissue—from signalling mechanisms to clinical implications. J. Intern. Med. 262, 431–438. https://doi.org/10.1111/j.1365-2796.2007.01854.x (2007).
Nguyen, M. T. et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 282, 35279–35292. https://doi.org/10.1074/jbc.M706762200 (2007).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808. https://doi.org/10.1172/jci19246 (2003).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758. https://doi.org/10.1016/j.cell.2015.01.012 (2015).
Shi, Y. & Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700. https://doi.org/10.1016/s0092-8674(03)00432-x (2003).
Massagué, J. & Sheppard, D. TGF-β signaling in health and disease. Cell 186, 4007–4037. https://doi.org/10.1016/j.cell.2023.07.036 (2023).
Roh, H. C. et al. Adipocytes fail to maintain cellular identity during obesity due to reduced PPARγ activity and elevated TGFβ-SMAD signaling. Mol. Metab. 42, 101086. https://doi.org/10.1016/j.molmet.2020.101086 (2020).
Lefterova, M. I., Haakonsson, A. K., Lazar, M. A. & Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 25, 293–302. https://doi.org/10.1016/j.tem.2014.04.001 (2014).
Mota de Sá, P., Richard, A. J., Hang, H. & Stephens, J. M. Transcriptional regulation of adipogenesis. Compr. Physiol. 7, 635–674. https://doi.org/10.1002/cphy.c160022 (2017)
Nawaz, A. et al. Depletion of CD206(+) M2-like macrophages induces fibro-adipogenic progenitors activation and muscle regeneration. Nat. Commun. 13, 7058. https://doi.org/10.1038/s41467-022-34191-y (2022).
Takikawa, A. et al. HIF-1α in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes 65, 3649–3659. https://doi.org/10.2337/db16-0012 (2016).
Fujisaka, S. et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58, 2574–2582. https://doi.org/10.2337/db08-1475 (2009).
Acknowledgements
This research was supported by Moonshot R&D (Grant numbers JPMJMS2021). This study was also supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) (24K02502 to K. T, 22K203737 and 24K19282 to M.B, 21K16338 and 23KJ1022 to Y.I, 22K16423 and 24K19303 to A.Nishimura, 22K16424 to Y.W.). Research Grants from The Uehara Memorial Foundation 2023 to K.T, Eli Lilly Japan KK Innovation Research Grant 2023 to K.T., and a grant from Suzuken Memorial Foundation to M.B. This work was also supported by the Young Research Grant from the Japan Diabetes Society (to A.N., T.K. and A. Nishimura), and a grant from the Japan Foundation for Applied Enzymology (a grant for Front Runner of Future Diabetes Research to M.B., A.N. and T.K.). This work was also supported by Grant from The Naito Foundation (2021-2023) to Y.I., Japan Diabetes Foundation to S.F., T.K. and A. Nishimura, Japan Society for the Study of Obesity (JASSO) to S.F., First Bank of Toyama Scholarship Foundation to S. F., Yamaguchi Endocrine Research Foundation to S.F., Japan Association for Diabetes Education and Care to S.F., a grant from Boehringer Ingelheim to T.K., a grant from Novo Nordisk Pharma to A. Nishimura., a grant from Lotte Foundation to Y.W., Hokugin Young Researchers Grant to Y.W., ONO Medical Research Foundation to T.K., and a grant for Young Researchers from Japan Association for Diabetes Education and Care to T.K.
Author information
Authors and Affiliations
Contributions
N.Q.P and M.B. contributed equally, performed all experiments, acquired the data, and wrote the manuscript. M.B., A.N., and K.T. generate the hypothesis. T.K., Y.I., and H.M., generated mice. M.M., S.K., L.D.A., M.R.A., Ay.N. Y.W., Y.I. helped perform genotype and RT-qPCR analysis. K.O., A.N., I.U., and S.F. helped with manuscript writing. S.Y., K.H., help in performing histology. T.N, H.M. and R.H. helped in the discussion. K.T. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures were conducted in accordance with ARRIVE guidelines. All experiments and procedures were performed in accordance with the relevant guidelines and regulations and were approved by the Animal Care Committee of the University of Toyama (Approved number A2023MED-16).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Phuong, N.Q., Bilal, M., Nawaz, A. et al. Role of transforming growth factor-β1 in regulating adipocyte progenitors. Sci Rep 15, 941 (2025). https://doi.org/10.1038/s41598-024-81917-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81917-7