Abstract
To investigate CHD1L’s impacts and molecular processes in hypoxic cutaneous squamous cell carcinoma. Monoclonal proliferation assays and CCK-8 were used to detect the proliferation capacity of A431 cells and Colon16 cells; wound healing experiments and Transwell assays were used to examine the migration and invasion capacity of A431 cells and Colon16 cells; angiogenesis experiments were conducted to assess the influence of A431 cells on angiogenesis; a nude mouse tumor xenograft experiment and HE staining were utilized to evaluate the impact of CHD1L on the progression of cutaneous squamous cell carcinoma; western blot analysis was performed to detect the expression of p-PI3K, p-AKT, and PD-L1 in A431 cells, as well as CD9, TSG101, PD-L1 in exosomes, and CD206, Arginase-1, iNOS, IL-1β, p-AKT, p-mTOR, VEGF, COX-2, MMP2, MMP9, p-ERK1/2 in tumor-associated macrophages. Under hypoxic conditions, CHD1L promoted the proliferation, migration, invasion, and angiogenesis of cutaneous squamous cell carcinoma. Furthermore, CHD1L facilitated the progression of cutaneous squamous cell carcinoma. CHD1L also increased the relative protein expression of p-PI3K, p-AKT, and PD-L1 in A431 cells, as well as CD9, TSG101, PD-L1 in exosomes, CD206, Arginase-1, p-AKT, p-mTOR, VEGF, COX-2, MMP2, MMP9, and p-ERK1/2 in tumor-associated macrophages, while inhibiting the relative protein expression of iNOS and IL-1β. Under hypoxic conditions, CHD1L can promote the proliferation and migration of cutaneous squamous cell carcinoma.
Similar content being viewed by others
Introduction
The second most prevalent skin cancer, after basal cell carcinoma, is cutaneous squamous cell carcinoma (SCC). Even though most SCC patients have a good prognosis with the right care, 5–10% of cases have a poor prognosis that includes disease-specific death, lymph node metastases, and local recurrence. SCC often occurs in sun-exposed areas such as the face and backs of the hands. It is more prevalent in males, older individuals, and is rare in adolescents. Lesions can manifest as nodules, ulcers, or plaques. Common causes include UV radiation, chronic inflammation, chronic infection, exposure to carcinogens, and human papillomavirus (HPV) infection. Nevertheless, there are few trustworthy indicators to forecast the biological behaviour and clinical consequences of SCC1,2,3. Therefore, in order to create efficient treatment strategies for the condition, it is essential to identify biomarkers and get a deeper comprehension of the molecular processes behind SCC.
A prevalent characteristic of solid tumours, particularly skin cancers, is intratumoral hypoxia. Low oxygen tension causes cellular reactions that accelerate the growth of cancer by triggering biological processes like glycolysis and angiogenesis that are essential for the survival of cancer cells4. Most often, moderately hypoxic cells in the skin’s epidermal layer are the source of cutaneous malignancies. In glioma cells, PD-L1 expression is induced under hypoxic conditions, and knockdown of HIF-1α or treatment with HIF-1α inhibitors eliminates the enhanced PD-L1 expression. In a mouse glioma model, the combined treatment of HIF-1α inhibitors and anti-PD-L1 antibodies showed a more pronounced inhibitory effect on tumor growth compared to any single-agent treatment. Immunologically, the combination treatment improved the activation of dendritic cells (DC) and CD8 T cells. Overall, PD-L1 and HIF-1α are positively correlated in gliomas, and tumor-secreted exosomes carry PD-L1 on their surface5,6,7,8,9.
CHD1L belongs to the SNF2-like family and has a conserved SNF2_N ___domain, a helicase superfamily structural ___domain that might be crucial for DNA repair, chromosomal integrity maintenance, and transcriptional control. Therefore, studying the CHD1L transcriptional regulation network helps to elucidate the oncogenic molecular mechanisms of CHD1L, and may further provide new therapeutic targets for cancer treatment. CHD1L exhibits oncogenic properties in the process of malignant transformation and has been identified as a novel oncogene. In transgenic CHD1L mouse models, transgenic CHD1L expression induces spontaneous tumor formation. Overexpression of the CHD1L protein is considered an adverse prognostic biomarker in many solid tumors such as ovarian cancer, pancreatic cancer, colorectal cancer, and gastric cancer. Overexpression of the CHD1L gene has also been observed in 40%-50% of cutaneous squamous cell carcinoma patients. The overexpression of CHDIL has been associated with more aggressive tumor behavior and poorer prognosis in cutaneous squamous cell carcinoma10,11,12. However, the mechanism by which CHD1L promotes the progression of cutaneous squamous cell carcinoma is not fully understood. Therefore, in this study, we explored the effects and molecular mechanisms of CHD1L in cutaneous squamous cell carcinoma under hypoxic conditions.
Methods
Cell culture
The Cell Biology Research Institute of Shanghai provided the human cutaneous squamous cell carcinoma cell lines A431 and Colon16, the monocyte macrophage cell line THP-1, and the endothelial cell line HUVEC. The cell lines were cultured in DMEM medium supplemented with 10% foetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin. The cells were cultivated at 37 °C in an incubator that was humidified and contained 94% N2, 5% CO2, and 1% O2. After seeding A431 cells (5 × 104 cells/well) in 6-well plates, Lipofectamine 2000 was added when the cell confluency reached 70%. The cells were separated into four groups: NC, CHD1L-OE, NC, and CHD1L-KD. They were then incubated for 24 h at 37 ℃ with 5% CO2. For the tests that followed, the cells with an 80% transfection rate were used.
Co-culture system
THP-1 cells were seeded in cell culture plates. A permeable polycarbonate membrane was placed at the bottom of the Transwell chamber, and A431 cells and Colon16 cells were cultured in separate Transwell chambers. The Transwell chambers were then placed in the culture plate to establish a co-culture system of A431 cells and THP-1 cells.
Xenograft tumor experiment
Six-week-old BALB/c nude mice were obtained from Henan SCBS Biotechnology Co., Ltd. They are housed in SPF-grade, constant-temperature, constant-humidity clean rooms using an individually ventilated cage system (IVC) with four mice per cage. Temperature/humidity is controlled at (26 ± 2)ºC/40–60% with a light/dark cycle of 12 h/12 h. The mice have access to autoclaved SPF-grade food and ultrafiltered purified water. Nude mice were randomly divided into four groups: the NC group, the CHD1L-OE group, the NC group, and the CHD1L-KD group, with 6 nude mice in each group, for a total of 24 mice. Co-cultured A431 and THP-1 cells were inoculated into the mice at a volume of 1 × 107/200 μl/mouse. During the inoculation process, the needle was inserted from the upper lateral part of the mice with a distance less than the length of the needle from the inoculation site, without piercing the skin or muscle layer. The inoculation process was completed within 1 h. Tumor volumes were measured twice weekly and mice were euthanized using CO2 30 days after inoculation. During euthanasia, the environment was kept quiet and warm and additional stressors were avoided to minimize the suffering of the nude mice. Animal experiments were performed according to the guidelines laid down by the Laboratory Animal Ethical and Welfare Committee of Affiliated Hospital of Hebei Engineering University(IACUC-Hebeu-2023–0019). The procedures used in this experiment adhered to THE RULES OF 3R and ARRIVE standards, as well as all applicable laws and regulations.
HE staining
Tumor tissue sections were dewaxed, stained in hematoxylin solution, differentiated in acidic water and ammonia water, rinsed in running water, dehydrated in alcohol, stained in eosin, dehydrated in alcohol and xylene, and then mounted with neutral gum for observation under a light microscope.
Flow cytometry
Tumor tissues were aseptically separated, cut into 1 mm3 tissue blocks, digested in a mixture of 0.2% type IV collagenase and 0.25% trypsin at 37 °C, filtered through a 200-mesh sieve, and resuspended in PBS. After that, the cells were examined and sorted in a sterile environment using BD FACSAria™ III.
Extracellular vesicle extraction
To generate medium without extracellular vesicles, extracellular vesicles from fetal bovine serum were centrifuged at 10,000 g overnight and then filtered through a 0.2 μm filter attached to a syringe. This vesicle-depleted fetal bovine serum was used for cell culture. For extracellular vesicle isolation, cell culture supernatant was sequentially centrifuged at 2,000xg and 10,000xg for 30 min each. The final supernatant was filtered through a 0.22 μm filter and ultra-centrifuged at 120,000xg for 70 min. The pellet was then washed with phosphate-buffered saline (PBS) and ultra-centrifuged again at 120,000xg for 70 min. The isolated extracellular vesicles were characterized.
Clonal proliferation assay
After being planted in 6-well plates with 800 μL per well, A431 cells were cultivated for a full day. After then, new medium containing 10% serum was added to the old media. The media was withdrawn after two weeks, and the cells were counted, stained, fixed, and photographed.
CCK-8 assay
2,000 cells per well of 96-well plates were used to seed A431 and Colon16 cells. Following the designated incubation periods of 24, 48, and 72 h, 90 μL of DMEM was mixed with 10 μL of CCK-8 solution, and the cells were allowed to incubate for three hours at room temperature. Next, a microplate reader was used to measure the absorbance at 450 nm. Every sample underwent three tests.
Transwell assay
Matrigel was applied to the bottom of Transwell inserts for the invasion experiment, and cells were planted in the top chamber before being incubated for 48 h. Following their invasion through the membrane, the cells were counted, dyed, and preserved.Experiment on migration: The steps were the same as in experiment on invasion, with the exception that Matrigel was not employed.
Wound healing experiment
A431 and Colon16 cells were seeded in sterilized culture dishes and allowed to grow to confluence. A scratch wound was created, and the healing process was monitored and analyzed using ImageJ software.
Tube formation assay
Matrigel was added to 24-well plates and allowed to solidify. HUVEC cells were then added along with the co-culture supernatant of A431 cells and THP-1 cells, and the tube formation was observed after 8 h. The formed tubes were analyzed and the number of nodes was measured.
Western blotting
Total proteins were extracted, and their concentrations were determined using the Bradford method. Protein samples were separated by SDS-PAGE, transferred to PVDF membranes, and then subjected to antibody probing and chemiluminescent detection. Band intensities were analyzed using Image J software.
Statistical analysis
Data were presented as mean ± standard deviation and analyzed using t-tests for two-group comparisons and one-way ANOVA for multiple-group comparisons. Statistical significance was set at P < 0.05 or P < 0.01 using GraphPad Prism 9.0 software.
Results
CHD1L can promote the progression of SCC
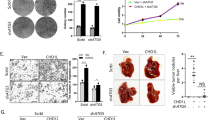
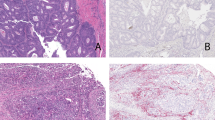
To explore the impact of CHD1L on the progression of SCC, we conducted xenograft experiments in nude mice and observed using HE staining. It was discovered that the CHD1L-OE group’s tumour area had greatly risen in comparison to the NC group, whereas the CHD1L-KD group’s tumour area had significantly reduced. This indicates that CHD1L can promote the progression of SCC (Fig. 1).
CHD1L can promote the proliferation of cutaneous squamous cell carcinoma cells in a hypoxic environment
In cutaneous squamous cell carcinoma, the rapid tumor growth and disrupted tissue structure often lead to hypoxic environments. The rapid proliferation of tumor cells leads to increased demand for oxygen and nutrients. However, due to the disordered blood vessel structure and uneven blood supply, the ability to supply oxygen and nutrients cannot meet the demands of tumor cells, resulting in the formation of hypoxic regions. The results of the clonal proliferation assay indicated that, in our hypoxic cell experiments with A431 and Colon16 cells were co-cultured with THP-1 cells after respectively, the clone number was significantly higher in the CHD1L-OE group and significantly lower in the CHD1L-KD group when compared to the NC group. The CCK-8 data showed that the OD value in the CHD1L-OE group was much higher than in the NC group, whereas in the CHD1L-KD group it was significantly lower. This suggests that in a hypoxic tumor environment, CHD1L can promote the proliferation of cutaneous squamous cell carcinoma cells (Fig. 2).
CHD1L promotes the proliferation of cutaneous squamous cell carcinoma cells in a hypoxic environment. (A) Clonal proliferation assay results for A431 and Colon16 cells; (B) Plot of statistical results of clone formation in A431 cells and Colon16 cells; (C) Results of CCK-8 assay on A431 cells and Colon16 cells. Data are expressed as mean ± standard deviation; N = 3; **P < 0.01.
CHD1L can promote the migration and invasion of cutaneous squamous cell carcinoma cells in a hypoxic environment
The results of the wound healing experiment showed that at 48 h, the healing rate was significantly increased in the CHD1L-OE group relative to the NC group, while it was significantly decreased in the CHD1L-KD group. Transwell results indicated that relative to the NC group, the migration and invasion of A431 cells were significantly increased in the CHD1L-OE group and significantly decreased in the CHD1L-KD group. This suggests that in a hypoxic environment, CHD1L can promote the migration and invasion of cutaneous squamous cell carcinoma cells (Fig. 3).
CHD1L promotes the migration and invasion of cutaneous squamous cell carcinoma cells in a hypoxic environment. (A) Results of 0 h and 48 h wound healing tests with A431 cells and Colon16 cells; (B) Wound closure rate statistics for A431 cells and Colon16 cells; (C) Results of Transwell assay of A431 and Colon16 cells; D: Statistics on the amount of cell migration and invasion in A431 and Colon16 cells. Data are expressed as mean ± standard deviation; N = 3; **P < 0.01.
CHD1L can promote angiogenesis of cutaneous squamous cell carcinoma cells in a hypoxic environment
The limited angiogenic capacity required for tumor growth cannot meet the oxygen supply needs of the tumor. Some tumor cells may be located far from existing blood vessels, leading to insufficient oxygen supply. The angiogenesis experiment’s findings showed that the angiogenesis rate in the CHD1L-OE group was much higher than in the NC group, while it was significantly lower in the CHD1L-KD group. This indicates that in a hypoxic environment, CHD1L can promote the angiogenesis of cutaneous squamous cell carcinoma cells (Fig. 4).
CHD1L can promote the expression of PD-L1 in cutaneous squamous cell carcinoma cells in a hypoxic environment and promote the M2 polarization of TAMs through exosomes
We first used flow cytometry to isolate A431 cells and tumor-associated macrophages from the tumor tissues, and then used western blotting to detect the expression of p-PI3K, p-AKT, and PD-L1 in A431 cells, the expression of CD9, TSG101, PD-L1 in exosomes, and the expression of CD206, Arginase-1, iNOS, IL-1β, p-AKT, p-mTOR, VEGF, COX-2, MMP2, MMP9, p-ERK1/2 in tumor-associated macrophages. The findings demonstrated that the CHD1L-OE group had significantly higher relative protein expression levels of p-PI3K, p-AKT, and PD-L1 in A431 cells and significantly lower relative protein expression levels of iNOS and IL-1β in tumor-associated macrophages, as well as CD206, Arginase-1, p-AKT, p-mTOR, VEGF, COX-2, MMP2, MMP9, and p-ERK1/2 in tumor-associated macrophages, as compared to the NC group (Fig. 5). In the CHD1L-KD group, the relative protein expression levels of p-PI3K, p-AKT, and PD-L1 in A431 cells, CD9, TSG101, PD-L1 in exosomes, and CD206, Arginase-1, p-AKT, p-mTOR, VEGF, COX-2, MMP2, MMP9, p-ERK1/2 in tumor-associated macrophages were significantly decreased, while the relative protein expression levels of iNOS and IL-1β were significantly increased. Similar results were obtained in our cell experiments (Fig. 6). These results indicate that in a hypoxic environment, CHD1L can promote the expression of PD-L1 in cutaneous squamous cell carcinoma cells and promote the M2 polarization of tumor-associated macrophages through exosomes (Fig. 7).
CHD1L promotes the expression of PD-L1 in cutaneous squamous cell carcinoma cells and promotes M2 polarization in TAMs. (A) p-PI3K, p-AKT, and PD-L1 protein bands and relative protein expression levels in A431 cells; (B) CD206, Arginase-1, iNOS, IL-1β, p-AKT, p-mTOR, VEGF, COX-2, MMP2, MMP9, and p-ERK1/2 protein bands and relative protein expression levels in tumor-associated macrophages. GAPDH as control protein; Data are expressed as mean ± standard deviation; N = 6; **P < 0.01.
CHD1L promotes the expression of PD-L1 in cutaneous squamous cell carcinoma cells in a hypoxic environment and promotes M2 polarization in TAMs through exosomes. (A) p-PI3K, p-AKT, and PD-L1 protein bands and relative protein expression levels in A431 cells; (B) CD9, TSG101, and PD-L1 protein bands and protein expression levels in exosomes; (C) CD206, Arginase-1, iNOS, IL-1β, p-AKT, p-mTOR, VEGF, COX-2, MMP2, MMP9, and p-ERK1/2 protein bands and relative protein expression levels in tumor-associated macrophages. GAPDH as control protein; Data are expressed as mean ± standard deviation; N = 3; **P < 0.01.
In the hypoxic environment of cutaneous squamous cell carcinoma, CHD1L can promote the secretion of PD-L1 by cancer cells, thus promoting the activation of the ERK/AKT/mTOR signaling pathway and M2 polarization in tumor-associated macrophages, inhibiting M1 polarization and leading to the proliferation, migration, invasion, and angiogenesis of cutaneous squamous cell carcinoma cells.
Discussion
Cutaneous squamous cell carcinoma is characterized by active proliferation of epidermal keratinocytes, which can lead to an increased demand for oxygen by the cells, limited oxygen supply, and the formation of a hypoxic microenvironment within the tissue. Additionally, the keratinocytes themselves exhibit some degree of hypoxia. Tumor cells, in order to adapt to the hypoxic environment, increase glycolysis rates and develop a network of blood vessels13.
CHD1L (Chromodomain Helicase DNA Binding Protein 1-Like) is a gene that encodes a protein involved in chromatin remodeling and transcription regulation. The CHD1L protein contains a structured ___domain with a helical structure, which can bind to DNA and regulate gene expression. Studies have shown that CHD1L plays an important role in various cancers. In certain tumors, the CHD1L gene may undergo abnormalities through mechanisms such as gene amplification and mutations, leading to abnormally high levels of CHD1L protein expression. Overexpression of CHD1L is associated with the onset and progression of tumors and is considered an important factor in promoting tumor cell proliferation, metastasis, and resistance to treatment. Furthermore, CHD1L is also involved in other biological processes such as cell cycle regulation, DNA repair, and genomic stability. Further research on CHD1L will contribute to a deeper understanding of its specific mechanisms in cancer development, providing new targets and strategies for the diagnosis and treatment of cancer. However, it should be noted that the role of CHD1L in different types of cancer may vary, and specific research is ongoing11,14,15,16.
Research has shown that CHD1L has a variety of roles in the development of HCC, including stimulating cell invasion and migration in breast cancer via the PI3K/AKT/ARK5/mTOR/MMP pathway. CHD1L may stimulate cell migration and the advancement of the cell cycle via the MDM2/p53 pathway. Through an imbalance in the p1-cyclinE-CDK53 pathway in gliomas, CHD1L may cause the G2/S transition. By triggering the Wnt/β-catenin/TCF pathway, CHD1L influences the proliferation of cells in pancreatic cancer. The inhibition of PI3K/AKT pathway glycolysis in esophageal squamous cell carcinoma cells by downregulation of CHD1L increases the cytotoxicity of cisplatin11,17.
PD-L1 is the ligand of PD-1, expressed widely on both immune and non-immune cells. Similar to other molecules in the B7 family, PD-L1 not only exists as an intracellular form in human serum and other fluids but also has a soluble form. As a key immune inhibitory regulator, PD-L1 is linked to exosome secretion, PI3K/AKT, and T cell activation during immunological responses via binding to PD-1 on T cells18,19,20. In this work, we discovered that CHD1L may stimulate exosome release, the growth of PD-L1 in A431 cells, and the activation of the PI3K/AKT signalling pathway. In conclusion, the PI3K/AKT pathway in tumours is mediated by CHD1L, which promotes exosome secretion.
Tumor-associated macrophages (TAMs) are a type of macrophage found in the tumor microenvironment. They are an important part of the immune system, involved in regulating inflammation, cell proliferation, and tissue repair. TAMs play a critical role in tumor growth and development. They can originate from monocytes in the peripheral blood circulation around tumors or from local macrophages within the tissue. Once in the tumor tissue, TAMs undergo polarization and functional changes under the influence of the tumor microenvironment, affecting the progression of the tumor. TAMs can be classified into M1 and M2 types.M1 macrophages have pro-inflammatory and anti-tumour functions. They enhance killing of pathogens and tumour cells by producing high levels of pro-inflammatory cytokines (e.g. IL-1β, iNOS) and reactive oxygen metabolites (e.g. NO). In addition, M1-type macrophages promote Th1-type immune responses and activate T-cells to attack tumours.M2-type macrophages normally have anti-inflammatory and tissue-repair-promoting functions, but in the tumour environment they mainly promote tumour growth, angiogenesis, stromal remodelling, and immune escape.M2-type macrophages support tumour growth and immune escape through the secretion of CD206 (mannose receptor), arginase-1, and other anti-inflammatory factors to inhibit the immune response and support the survival and expansion of tumour cells.TAMs are one of the important sources of VEGF, and through the secretion of VEGF, TAMs are able to promote the formation of neovasculature inside the tumour, providing the tumour with the necessary oxygen and nutrients.TAMs also secrete MMPs, especially MMP9, which further promote angiogenesis and stromal remodelling, thus enhancing the expansion of the tumour TAMs also secrete MMPs, especially MMP9, which further promote angiogenesis and stromal remodelling, thus enhancing tumour expansion21,22,23,24. VEGF (vascular endothelial growth factor) is a potent angiogenic factor that provides sufficient oxygen and nutrients to the tumour by stimulating the formation of new blood vessels.Cutaneous squamous cell carcinoma is often hypoxic, and the high expression of VEGF can contribute to the formation of a rich vascular network within the tumour microenvironment, which enables the tumour to grow and expand rapidly. VEGF can promote angiogenesis. By promoting angiogenesis, VEGF is also involved in the formation and maintenance of the tumour microenvironment, which in turn supports the continued growth and survival of cutaneous squamous cell carcinoma.COX-2 (cyclooxygenase-2) is a key inflammatory mediator enzyme, and the overexpression of COX-2 promotes tumourigenesis and development. COX-2 is a key inflammatory mediator enzyme, and overexpression of COX-2 can promote tumourigenesis and progression, and COX-2 can inhibit apoptosis and enhance the viability of tumour cells.COX-2 has also been associated with enhanced invasiveness and increased metastatic potential of tumour cells, which can be achieved through the promotion of MMP expression, increased angiogenesis, and alteration of the extracellular matrix. MMPs (Matrix Metalloproteases) are a class of enzymes that are capable of degrading extracellular matrix (ECM), and they play a critical role in the invasion process of tumour cells. They play a key role in tumour cell invasion. By degrading the ECM, MMPs enable tumour cells to break through the basement membrane, invade surrounding tissues and enter blood vessels and lymphatic vessels, thereby promoting metastasis.MMPs (e.g., MMP2 and MMP9) enhance the ability of tumour cells to migrate through remodelling of the extracellular matrix and modulation of cellular adhesion molecules, which are essential for tumour spread and metastasis. Certain MMPs (e.g. MMP9) are also involved in the regulation of angiogenesis and further promote neovascularisation by releasing VEGF and other growth factors from the ECM. Together, these molecules form an important part of the tumour microenvironment, enabling effective tumour growth, invasion and metastasis25,26,27. In this work, we discovered that CHD1L may stimulate the production of VEGF, COX-2, and MMP family members as well as the activation of the ERK/AKT/mTOR signalling pathway, hence boosting M2 polarisation and suppressing M1 polarisation in TAMs. Moreover, CHD1L may encourage A431 cell motility, angiogenesis, invasion, and multiplication.
Conclusion
In conclusion, in the hypoxic environment of cutaneous squamous cell carcinoma, CHD1L can promote the secretion of PD-L1 by cancer cells, thus promoting the activation of the ERK/AKT/mTOR signaling pathway and M2 polarization in tumor-associated macrophages, inhibiting M1 polarization and leading to the proliferation, migration, invasion, and angiogenesis of cutaneous squamous cell carcinoma cells. We will also conduct clinical trials to supplement the findings of this study, thus providing new targets for immunotherapy of cutaneous squamous cell carcinoma.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Zuo, S. et al. Pre-mRNA processing factor 3 enhances the progression of keratinocyte-derived cutaneous squamous cell carcinoma by regulating the JAK2/STAT3 pathway. Sci. Rep. 10(1), 8863 (2020).
Zou, S., Gao, Y. & Zhang, S. lncRNA HCP5 acts as a ceRNA to regulate EZH2 by sponging miR-138-5p in cutaneous squamous cell carcinoma. Int. J. Oncol. 59(2), 56 (2021).
Zou, D. D. et al. Identification of key genes in cutaneous squamous cell carcinoma: A transcriptome sequencing and bioinformatics profiling study. Ann. Transl. Med. 9(19), 1497 (2021).
McLean, L. S. et al. A phase II study of tarloxotinib (a hypoxia activated prodrug of a pan-erb tyrosine kinase inhibitor) in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or skin. Invest. New Drugs 40(4), 782–788 (2022).
Martínez-Nieto, G. A. et al. Upregulated integrin α11 in the stroma of cutaneous squamous cell carcinoma promotes skin carcinogenesis. Front Oncol. 12, 981009 (2022).
Ding, X. C. et al. The relationship between expression of PD-L1 and HIF-1α in glioma cells under hypoxia. J Hematol. Oncol. 14(1), 92 (2021).
Tamura, R. et al. Alterations of the tumor microenvironment in glioblastoma following radiation and temozolomide with or without bevacizumab. Ann. Transl. Med. 8(6), 297 (2020).
Zhu, S. et al. An effective dendritic cell-based vaccine containing glioma stem-like cell lysate and CpG adjuvant for an orthotopic mouse model of glioma. Int. J. Cancer 144(11), 2867–2879 (2019).
Zhou, Z. et al. PD-L1 in combination with CD8(+)TIL and HIF-1α are promising prognosis predictors of head and neck squamous cell carcinoma. Cancer Manag. Res. 12, 13233–13239 (2020).
Hu, K. L. et al. Effect of chromodomain helicase/ATPase DNA binding protein 1-like gene on the invasion and metastasis of tongue squamous cell carcinoma CAL27 cells. Hua Xi Kou Qiang Yi Xue Za Zhi 39(1), 81–87 (2021).
Li, F. et al. ALC1 knockdown enhances cisplatin cytotoxicity of esophageal squamous cell carcinoma cells by inhibition of glycolysis through PI3K/Akt pathway. Life Sci. 232, 116679 (2019).
He, L. R. et al. Overexpression of CHD1L is positively associated with metastasis of lung adenocarcinoma and predicts patients poor survival. Oncotarget 6(31), 31181–31190 (2015).
Zwiebel, S. & Baron, E. PDT in squamous cell carcinoma of the skin. G Ital Dermatol. Venereol. 146(6), 431–444 (2011).
Zhang, X. et al. CHD1L augments autophagy-mediated migration of hepatocellular carcinoma through targeting ZKSCAN3. Cell Death Dis. 12(10), 950 (2021).
Zhang, L. et al. The high expression of CHD1L and its clinical significance in human solid tumors: A meta-analysis. Medicine (Baltimore) 100(10), e24851 (2021).
Xu, X. et al. Cell adhesion induces overexpression of chromodomain helicase/ATPase DNA binding protein 1-like gene (CHD1L) and contributes to cell adhesion-mediated drug resistance (CAM-DR) in multiple myeloma cells. Leuk Res. 47, 54–62 (2016).
Mu, Q. J. et al. Chromodomain helicase/ATPase DNA-Binding Protein 1-Like Gene (CHD1L) expression and implications for invasion and metastasis of breast cancer. PLoS ONE 10(11), e0143030 (2015).
Zouein, J., Kesrouani, C. & Kourie, H. R. PD-L1 expression as a predictive biomarker for immune checkpoint inhibitors: between a dream and a nightmare. Immunotherapy 13(12), 1053–1065 (2021).
Zhu, T. et al. Sequential targeting hybrid nanovesicles composed of chimeric antigen receptor T-cell-derived exosomes and liposomes for enhanced cancer immunochemotherapy. ACS Nano 17(17), 16770–16786 (2023).
Zhu, L. et al. Quantification-promoted discovery of glycosylated exosomal PD-L1 as a potential tumor biomarker. Small Methods 6(9), e2200549 (2022).
Zhang, X. et al. Pan-cancer analysis of PARP1 alterations as biomarkers in the prediction of immunotherapeutic effects and the association of its expression levels and immunotherapy signatures. Front. Immunol. 12, 721030 (2021).
Zhang, H. et al. The molecular feature of macrophages in tumor immune microenvironment of glioma patients. Comput. Struct. Biotechnol. J. 19, 4603–4618 (2021).
Yan, X. et al. Stromal expression of cathepsin K in squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 25(3), 362–365 (2011).
Takahashi, T. et al. Rejection of intradermally injected syngeneic tumor cells from mice by specific elimination of tumor-associated macrophages with liposome-encapsulated dichloromethylene diphosphonate, followed by induction of CD11b(+)/CCR3(-)/Gr-1(-) cells cytotoxic against the tumor cells. Cancer Immunol. Immunother. 58(12), 2011–2023 (2009).
Mu, G. et al. Calmodulin 2 facilitates angiogenesis and metastasis of gastric cancer via STAT3/HIF-1A/VEGF-A mediated macrophage polarization. Front Oncol. 11, 727306 (2021).
Mohan, C. D. et al. Crocetin imparts antiproliferative activity via inhibiting STAT3 signaling in hepatocellular carcinoma. IUBMB Life 73(11), 1348–1362 (2021).
Jung, Y. Y. et al. Brucein D imparts a growth inhibitory effect in multiple myeloma cells by abrogating the Akt-driven signaling pathway. IUBMB Life 75(2), 149–160 (2023).
Acknowledgements
Not applicable.
Funding
The study was supported by the Youth Science and Technology Project of Hebei Provincial Health Commission (No. 20240523).
Author information
Authors and Affiliations
Contributions
Mei Liu: responsible for the design of the experiments, data collection and analysis, and contributed significantly to the writing and revision of the paper. Chao Lv, Haiping Dong, Meng Zhou: participated in the design and execution of the experiment, interpreted the results through data analysis. Yao Yao, Huanrong Hu, Na Shen: Participated in the design and execution of the experiments, responsible for the quality control and analysis of the data. Baoguo Liu, Guoying Miao: participated in the lab work and provided technical support, contributed to the data analysis and interpretation of the results. Yaling Liu: responsible for the design and management of the research project, participated in the data analysis and interpretation of the results, and contributed significantly to the writing and revision of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Animal experiments were performed according to the guidelines laid down by the Laboratory Animal Ethical and Welfare Committee of Affiliated Hospital of Hebei Engineering University (IACUC-Hebeu-2023-0019).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, M., Lv, C., Dong, H. et al. CHD1L accelated the progression of cutaneous squamous cell carcinoma via promoting PI3K/PD-L1 signaling pathway induced M2 polarization of TAMs. Sci Rep 14, 31231 (2024). https://doi.org/10.1038/s41598-024-82594-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82594-2