Abstract
According to the Centers for Disease Control and Prevention (CDC) estimates that 576 to 740 million people globally are infected with hookworms. It remains a significant public health threat in tropical and subtropical regions. Especially in low-income countries, hookworm infection continues to affect millions, even with the availability of modern medical advancements. The present study is based on the transmission dynamics of hookworm infection in a population by using the strategy of mathematical modeling with computational methods. The population has been categorized into the following subpopulations such as susceptible humans, infectious humans, infectious humans with heavy infection, humans recovered, worm eggs, non-infective larvae, and infectious larvae and exposed humans. Firstly, the fundamental properties like positivity and boundness are studied. The equilibrium points like hookworm-endemic equilibrium (HEE), hookworm-free equilibrium (HFE), and basic reproduction numbers for the model were computed. Secondly, the stochastic formation of the model was studied with well-known properties like positivity, and the boundedness of the hookworm model. The model has no analytical solution due to the highly complex nonlinearity of the stochastic delay differential equation (SDDEs) of the model. Methods like Euler Maruyama, stochastic Euler, stochastic Runge Kutta, and stochastic nonstandard finite difference are used for its solution and visualization of results. Also, the comparison of standard with nonstandard methods is presented to verify the efficiency of the computational method. Furthermore, the stochastic nonstandard finite difference approximation is a good agreement to restore the dynamical properties of the model like positivity, boundedness, and dynamical consistency. Also, it is shown as efficient, low-cost, and independent of the time step size. In conclusion, the theoretical and numerical results support understanding the transmission dynamics of hookworm infection in the population.
Similar content being viewed by others
Introduction
Hematophagous nematode parasites known as hookworms have infected over between 576 and 740 million people globally. Since anthelmintic medications are not very effective at preventing reactivation, preventive vaccines are highly sought after. Since whole parasite vaccines are insecure and intolerant, research into substitute subunit vaccines seemed appropriate1. Hamidu et al. 2024, demonstrated the beneficial effects of a second intervention per year in keeping hookworm infection prevalences low and lowering them even more2. Walker et al. 2023, constructed a unique A. ceylanicum multi-host (human and dog) transmission model and evaluated the efficacy of human-only and “One Health” (human plus dog) MDA techniques under various eco-epidemiological hypotheses3. Puchner et al. 2023, examined the unique tool’s further benefit in addition to the vaccine’s biology and implementation viability4. Trinos et al. 2023, investigated the costs and benefits of mass medicine delivery in Dak Lak province, Vietnam, in comparison to school-based targeted preventative chemotherapy for the treatment of hookworms5. Santos et al. 2023, investigation verified a statistically significant drop in the prevalence of hookworm infection between the two time periods studied6. Ramlal et al. 2023, provided a general overview of the potential use of plant-based compounds, or botanicals, made from a variety of medicinal herbs to treat important parasites that cause the condition, systemic hookworm infections that cause the disease and infections, and ultimately death in humans. In contrast, conventional treatments are much less effective and have a great deal of adverse consequences7. Tiremo et al. 2023, analyzed that the diagnosis of unexpected parasitic infections, such as hookworms, can be made with a meticulous endoscopic examination of the small intestine mucosa in patients with IDA who have experienced gastrointestinal bleeding8. Mustapha et al. 2020, established and evaluated an analytical representation of the dynamics of hookworm propagation involving two independent infection categories and stages of parasite development9. Qureshi et al. 2020, constructed and investigated a model utilizing the Caputo fractional order differential operator to simulate the dynamics of Hookworm passing on infection in a human individual10. Sangari et al. 2020, provided an analytical study and modeling of the fluctuations of hookworm transmission in the Obi Local Government Area of Nasarawa State, Nigeria. In an attempt to control the disease, a numerical model of hookworm transmission was created11. Malizia et al. 2024, created a structure-based statistical approach that took into consideration low hemoglobin levels resulting from different sources to simulate individual hemoglobin concentrations in hookworm infections12. Ajjampur et al. 2021, observed that it is necessary for a community-based strategy to address the high prevalence of hookworm in adults in the present scenario13. Grolimund et al. 2022, recognized the uncertainty of the suggested Kato-Katz thick smear diagnostic procedure, a Bayesian model was created to compare the “true” CR and egg reduction rate of various treatment regimens for infections with hookworms14. Clements et al. 2022, conducted two comprehensive evaluations of research on the distribution of hookworms separated by species and genus across different parts of the globe, as well as the relationships between infections caused by hookworm species and clinical results, especially severe anemia15. Haldeman et al. 2020, analyzed that mostly affecting the world’s impoverished communities, human hookworm is an essential cause of mortality worldwide and is a soil-transmitted helminth (STH) ailment triggered by either Nectar americanus duodenale16. İlhan et al. 2022, utilized the fractional derivative and integral operator proposed by Caputo and Fabrizio, and the Hookworm infection model is analyzed17. Koopman et al. 2021, operated human infections with Schistosoma and hookworm are an important tool in the production of vaccines18. Colella et al. 2021, determined and compared, particularly to a species remission and egg diminution incidences of single-dose albendazole (400 mg) versus hookworm infections at the household level employing standard fecal flotation (SFF) and a multiplex qPCR technique19. The authors studied backward bifurcation and control in transmission dynamics of arboviral diseases in20. The authors made a comparative study of machine learning and deep learning methods for flood forecasting in the Far-North region, Cameroon, and fractional dynamics of a Chikungunya transmission model in21,22 respectively. The authors studied projections and fractional dynamics of typhoid fever: a case study of Mbandjock in the Centre Region of Cameroon23. Chazuka et al. studied strategic approaches to mitigating Hookworm infection: an optimal control and cost-effectiveness analysis in24.
Stochastic analysis in epidemiology incorporates randomness into models to correctly replicate disease spread while accounting for human behavior variations and environmental influences. This approach improves prediction accuracy and informs disease management efforts.
-
A stochastic delay model for the propagation of diseases is deduced from epidemiological assumptions.
-
The reproductive number, and equilibria of the deterministic systems are calculated.
-
Feasible Properties of the model are studied rigorously.
-
An NSFD scheme to solve the stochastic delay system is proposed and theoretically analyzed.
-
The simulations show that the scheme is epidemiologically more robust than other approaches.
The paper is structured as follows: A brief analysis of hookworm infections Sect. 1 provides a thorough overview of the literature. Section 2 focuses on developing the delayed model and doing the subsequent mathematical analysis. In addition, reproduction numbers and equilibria are investigated. Sections 3 and 4 describe the stochastic conceptualization processes. The numerical approach to the NSFD technique is provided in Sect. 5. Section 6 focuses explicitly on numerical simulations and the presentation of results. The final opinions provide a comprehensive summary of the work in Sect. 7.
Formulation of model
The model is based on monitoring the dynamics of hookworm and human populations at any time t of \(\:S\left(t\right)\) susceptible humans, \(\:E\left(t\right)\) exposed humans, \(\:{I}_{1}\left(t\right)\) infectious humans, \(\:{I}_{2}\left(t\right)\) infectious humans with heavy infection, \(\:R\left(t\right)\) humans recovered and \(\:F\left(t\right)\) worm eggs, \(\:{L}_{1}\left(t\right)\) non-infective larvae, and \(\:{L}_{2}\left(t\right)\) infectious larvae. (See Fig. 1)10.
The human population is being recruited at a rate of \(\:\pi\:\) (by migration or birth) and a rate of \(\:\gamma\:\) due to the progression of persons from the recovery class. When susceptible individuals \(\:S\left(t\right)\) come into touch with infectious larvae, they become infected at a rate of \(\:\lambda\:S\left(t\right){L}_{2}\left(t\right)\). \(\:S\left(t\right)\) does not instantly become infected upon infection instead it enters an exposed class. Individuals who are exposed to infection proceed to the infectious class of either heavy infection or moderate infection at a rate of, respectively, \(\:\left(1-\epsilon\:\right)\sigma\:\) and \(\:\epsilon\:\sigma\:\). An individual with moderate infectiousness advances at a pace of \(\:{\tau\:}_{1}\) to acquire heavy illnesses. Recovery from a moderate infection occurs at a pace of \(\:{\theta\:}_{1}\) (awareness and improvement of personal cleanliness), whereas chemotherapy treatment causes a heavy infection to recover at a rate of \(\:{\theta\:}_{2}\). Eggs in feces are excreted at rates of \(\:\alpha\:\) by moderately and heavily infected people, and after \(\:\omega\:\) days, the eggs hatched to become \(\:{L}_{1}\left(t\right)\) and \(\:{L}_{2}\left(t\right)\), respectively. \(\:\mu\:\) and \(\:\delta\:\) represent the natural death rate of humans and the disease-induced mortality rate, respectively, whereas \(\:\varphi\:\), \(\:v\), and \(\:k\) represent the death rates for eggs, non-infective larvae, and infectious larvae. Also, \(\:\tau\:\) is delay parameter for this particular model. Hookworm transmission dynamics in human populations are described by the following differential equations with artificial delay parameter \(\:{e}^{-\mu\:\tau\:}\) as follows:
with initial conditions; \(\:S\left(0\right)\ge\:0,\:E\left(0\right)\ge\:0,\:{I}_{1}\left(0\right)\ge\:0,\:{I}_{2}\left(0\right)\ge\:0,\:R\left(0\right)\ge\:0,\)\(\:F\left(0\right)\ge\:0,\:{L}_{1}\left(0\right)\ge\:0,\:{L}_{2}\left(0\right)\ge\:0\) for all \(\:t\ge\:0,\:\tau\:<t\).
Model properties
In this section, we discussed the positivity and boundedness of solutions of system (1) with initial conditions.
For positivity and boundedness, we used the following results.
Theorem 1
For any \(t\ge 0\), the solutions of system (1) with initial conditions are positive.
Proof
The following can be determined from the system (1):
as desired.
Theorem 2
Solutions of the system (1) with initial condition are bounded.
Proof
Let’s examine the function in this particular way:
For detailed proof see appendix A.
Model equilibria
In this section, we evaluate two different types of equilibria for the system (1), as follows:
Hookworm-free equilibrium (HFE), \(\:{\mathcal{H}}^{0}=\left({S}^{0},{E}^{0},\:{I}_{1}^{0},\:{I}_{2}^{0},{R}^{0},{F}^{0}\:{L}_{1}^{0},\:{L}_{2}^{0}\right)=\left(\frac{\pi\:}{\mu\:},\text{0,0},\text{0,0},\text{0,0},0\right)\) and Hookworm endemic equilibrium (HEE), \(\:{\mathcal{H}}^{*}=\left({S}^{*},{E}^{*},\:{I}_{1}^{*},\:{I}_{2}^{*},{R}^{*},{F}^{*},\:{L}_{1}^{*},\:{L}_{2}^{*}\right)\) where
where \(\:{A}_{1}=\sigma\:+\mu\:\), \(\:{A}_{2}=\left({\tau\:}_{1}+\mu\:+{\theta\:}_{1}\right)\), \(\:{A}_{3}=\left(\delta\:+\mu\:+{\theta\:}_{2}\right)\), \(\:{A}_{4}=\left(\psi\:+\omega\:\right)\), \(\:{A}_{5}=\left(v+\varphi\:\right)\), \(\:Q=\left(1-\epsilon\:\right)\sigma\:\).
Reproduction number is of vital importance for epidemiology as a critical threshold that potentially influences the spread of disease. It is the mean number of secondary infections transmitted by an infected person in a fully susceptible population. In the context of the Hookworm model, we estimate this threshold to understand and predict the infection behavior in the population. The reproduction number for the model system (1) is computed using a systematic methodology that is founded upon the next-generation matrix method as described in24. The transmission matrix (F) and transition matrix (G) are derived by substituting the Hookworm-free equilibrium and taking into account the affected classes from the system (1). The largest eigenvalue of \(\:F{G}^{-1}\) represents the reproduction number.
Therefore, Reproduction number is;
Transition probabilities of the model
Let us consider the vector \(\:A={\left[S\left(t\right),\:E\left(t\right),{I}_{1}\left(t\right),{I}_{2}\left(t\right),R\left(t\right),F\left(t\right),{L}_{1}\left(t\right),{L}_{2}\left(t\right)\right]}^{T}\) and the number of chances of an event is presented in Table 1. For the drift and diffusion coefficients of the system (1), we shall calculate the expectation and variance as follows:
Therefore,
The Eq. (4), is called the stochastic delay differential equation with \(\:B\left(t\right)\) is the Brownian.
In this section, we use a conventional numerical technique for approximating a stochastic delayed model’s result of (4). In this regard, we admitted \(\:{I}_{q}=\{\text{0,1},\text{2,3},\dots\:,q\}\) for each q ∈ \(\:\mathbb{N}\). Let \(\:N\in\:\mathbb{N}\), and with the effect of time ∆t divides the partition into equal intervals [0, T] with constant delay as
for each n ∈ \(\:{I}_{N}\). Needless to mention \(\:{t}_{n}={\uptau\:}_\text{n}\), for each n ∈ \(\:{I}_{N}\). Moreover, we agreed that \(\:{U}^{n}=U\left({t}_{n}\right)\), whenever n ∈ \(\:{I}_{N}\) and U = \(\:S,\:E,{I}_{1},{I}_{2},R,F,{L}_{1},{L}_{2}\). Also, we set
∆\(\:{B}_{n}\) = B (\(\:{t}_{n+1}\)) − B (\(\:{t}_{n}\)), ∀n ∈ \(\:{I}_{N-1}\). The mean of each ∆\(\:{B}_{n}\) follows a normal distribution with a variance of one and an average of zero.
The Euler-Maruyama technique to simulate the outcomes of Eq. (4) as follows:
Where the value of \(\:\varDelta\:t\) indicates the discretization parameter.
Stochastic delayed model
The system of stochastic delay differential equations (SDDEs) is a mathematical model that describes the evolution of a set of variables over time, where the equations involve both deterministic time delays and stochastic (random) components. Where the stochastic term \(\:{\sigma\:}_{i}:\left(i=1,\:2,\:3,\:\text{4,5},\text{6,7},8\right),\:\left(B\left(t\right)\right)\) introduces randomness into the system of differential equations as follows:
where \(\:B\left(t\right)\) participation in the Brownian motion and the unpredictability of each compartment are indicated by \(\:{\sigma\:}_{i};i=\text{1,2},\text{3,4},\text{5,6},\text{7,8}\). Also, the initial conditions of the model (6) as follow: \(\:S\left(0\right)\ge\:0,E\left(0\right)\ge\:0,{I}_{1}\left(0\right)\ge\:0,{I}_{2}\left(0\right)\ge\:0,R\left(0\right)\ge\:0,F\left(0\right)\ge\:0,{L}_{1}\left(0\right)\ge\:0,{L}_{2}\left(0\right)\ge\:0\).
For positivity and boundedness of system (6), we assume the following vector, let’s
and norm
Moreover, let \(\:{D}_{1}^{\text{7,1}}\left({\mathbb{R}}^{8}x\left(0,\infty\:\right):{\mathbb{R}}_{+}\right)\) represents the set of all positive functions \(\:{U}_{1}\left(V,t\right)\) that are subsequently defined on \(\:{\mathbb{R}}^{8}x\left(0,\infty\:\right)\). Furthermore, in V the function is once differentiable and twice differentiable. The differentiable operator \(\:{T}_{1}\), associated with eight-dimensional stochastic delay differential equations (SDDEs), has been developed.
As,
If \(\:{T}_{1}\) acts on function \(\:{V}^{*}\in\:{D}_{1}^{\text{7,1}}\left({\mathbb{R}}^{8}x\left(0,\infty\:\right):{\mathbb{R}}_{+}\right)\) then we denote
Where Transportation is represented by T.
Theorem 3
For model (6) and any given initial value \((S(0), E(0),I_{1}(0),I_{2}(0),R(0),F(0),L_{1}(0), L_{2}(0)) \in\:{\mathbb{R}}_{+}^{8}\), there is a unique solution \((S(t), E(t),I_{1}(t),I_{2}(t),R(t),F(t),L_{1}(t), L_{2}(t)) \in\:{\mathbb{R}}_{+}^{8}\) and will remain in \({\mathbb{R}}_{+}^{8}\) with probability one.
Proof
Considering that every model parameter meets the local Lipschitz constraints. Consequently, the above model has a positive solution locally on the interval \([0,\:\tau_e\:]\)according to Ito’s formula, where \(\tau_e\) represents the explosion time. When \(\tau_e\) equals infinity, it may be demonstrated that the model has a global solution.
If \(\:{n}_{0}=\:0\), then a sufficiently large number is required such that \(\:S\left(0\right),E\left(0\right),{I}_{1}\left(0\right),{I}_{2}\left(0\right)R\left(0\right),F\left(0\right),\)\(\:{L}_{1}\left(0\right),{L}_{2}\left(0\right)\) fall inside the interval \(\:\left\{\frac{1}{{n}_{0}},{n}_{0}\right\}\).
For each positive integer \(\:n\), let’s define a series in the following manner:
Here, \(\:\phi\:\) is the empty set, and we set \(\:inf\phi\:\) =\(\:\infty\:\). Since \(\:n\) approaches \(\:\infty\:\) without reducing \(\:{\tau\:}_{n}\),
According to the inequality, \(\:{\tau\:}_{\infty\:}\) is either equal to or smaller than \(\:{\tau\:}_{e}\).
Our goal now is to show that, as we expected, \(\:{\tau\:}_{\infty\:}\) equals infinity.
If this condition fails to be satisfied, then there exist values T > 0 and \(\:{b}_{1}\in\:\:(0,\:1)\) that satisfy the statement.
By using Ito’s formula, we calculate
To simplify, we assume \(\:{M}_{1}=(\pi\:+5\mu\:+\sigma\:+{\tau\:}_{1}+{\theta\:}_{1}+\delta\:+{\theta\:}_{2}+\gamma\:+\psi\:+\omega\:+v+\varphi\)\(\:+k+\frac{{\sigma\:}_{1}^{2}+{\sigma\:}_{2}^{2}+{\sigma\:}_{3}^{2}+{\sigma\:}_{4}^{2}+{\sigma\:}_{5}^{2}+{\sigma\:}_{6}^{2}+{\sigma\:}_{7}^{2}+{\sigma\:}_{8}^{2}}{2})\)
Then Eq. (13) could be written as:
Following the integration from 0 to \(\:{\tau\:}_{n}\wedge\:\tau\:\), where \(\:{M}_{1}\) is a positive constant,
We obtain,
When \(\:{\tau\:}_{n}\wedge\:\tau\:=\text{min}\left({\tau\:}_{n},T\right)\), applying the assumptions results in
Set \(\:{{\Omega\:}}_{n}=\{{\tau\:}_{n}\le\:T\}\) for \(\:n>{n}_{1}\) and from (11), we have \(\:X\left({{\Omega\:}}_{n}\ge\:b\right).\:\)
There are certain indices such that \(\:{V}_{i}\left({\tau\:}_{n},{a}_{1}\right)=n\) or \(\:\frac{1}{n}\) for each member \(\:{a}_{1}\) in the collection \(\:{{\Omega\:}}_{n}\), where has the values 1, 2, 3,4,5,6,7, and 8.
Hence, \(\:{V}^{*}(\left(S\left({\tau\:}_{n},{a}_{1}\right),E\left({\tau\:}_{n},{a}_{1}\right),{I}_{1}\left({\tau\:}_{n},{a}_{1}\right),{I}_{2}\left({\tau\:}_{n},{a}_{1}\right)\right.,\) \(R\left({\tau\:}_{n},{a}_{1}\right),F\left({\tau\:}_{n},{a}_{1}\right),\)\(\left. \left.{L}_{1}\left({\tau\:}_{n},{a}_{1}\right),{L}_{2}\left({\tau\:}_{n},{a}_{1}\right),\right)\right)\) is less than \(\:\text{min}\left\{n-1-\text{ln}n,\frac{1}{n}-1-\text{ln}\frac{1}{n}\right\}\)
Next, we obtain
Within the set \(\:{{\Omega\:}}_{n}\), the indicator function is represented by the notation \(\:{I}_{{{\Omega\:}}_{n}\left({a}_{1}\right)}\).
The contradiction arises when n gets closer to infinity: infinity is equivalent to \(\:{V}^{*}\left(S\left(0\right),E\left(0\right),{I}_{1}\left(0\right),{I}_{2}\left(0\right),R\left(0\right),F\left(0\right),{L}_{1}\left(0\right),{L}_{2}\left(0\right)\right)+{M}_{1}T\), which has a finite value. as desired.
Numerical methodology
Assume that \(\:{\mathcal{U}}_{n}\) is the set that \(\:{\mathcal{U}}_{e}=\left\{\text{0,1},2\dots\:,\it{e}\right\}\) defines for every \(e\,\epsilon\:\mathbb{N}\). In this part, we will identify and examine the system’s discretization (6). To accomplish our goal, we take into account the temporal interval when \(\:T>0\). Make a consistent division of the time interval [0, T] into n subintervals, each having a length of \(\:k=\frac{T}{e}\). With \(\:{t}_{a}=ak\), for every \(a\:\epsilon{I}_e\), where \(I_e\) is the set of indices. The functions \(\:{S}^{m},{E}^{m},{I}_{1}^{m},\:{I}_{2}^{m},{R}^{m},{F}^{m},\:{L}_{1}^{m}\) and \(\:{L}_{2}^{m}\) correspond to the numerical approximations for S, E, \(\:{I}_{1}\), \(\:{I}_{2}\), R, F, \(\:{L}_{1}\) and \(\:{L}_{2}\). \(\:\left({S}^{0},{E}^{0},\:{I}_{1}^{0},\:{I}_{2}^{0},{R}^{0},{F}^{0}\:{L}_{1}^{0},\:{L}_{2}^{0}\right)\) are the discrete starting data. It is defined so that \(\:{S}^{0}=S\left(0\right),{E}^{0}=E\left(0\right),\:{I}_{1}^{0}={I}_{1}\left(0\right),{I}_{2}^{0}={I}_{2}\left(0\right),\:{R}^{0}=R\left(0\right),{F}^{0}=F\left(0\right),\:{L}_{1}^{0}={L}_{1}\left(0\right),\:{L}_{2}^{0}={L}_{2}\left(0\right)\) as required.
Stochastic nonstandard computational method
A stochastic non-standard finite difference methodology might be used to solve model (6) in our parametric perturbation model. The susceptible class from the model (6) can be expressed using an unusual computing method.
The equation for the stochastic NSFD technique looks like this:
The stochastic NSFD process, as demonstrated in (18), may be used to decompose the system (6), and the resultant whole system can be expressed as follows:
Here, \(\:n=\text{0,1},2,\dots\:\) and \(\:\varDelta\:{\text{B}}_{n}=\varDelta\:{\text{B}}_{{t}_{n+1}}-\varDelta\:{\text{B}}_{{t}_{n}}\) represents a generic normal distribution that is, \(\:\varDelta\:{\text{B}}_{\text{n}}\sim\text{N}(0,\:1\)).
Convergence analysis of nonstandard computational method
The following theorems are stated concerning the convergence analysis.
Theorem 4
For all initially values of \((S(0), E(0),I_{1}(0),I_{2}(0),R(0),F(0),L_{1}(0), L_{2}(0)) \in\:{\mathbb{R}}_{+}^{8}\), there is only one positive solution \((S(t), E(t),I_{1}(t),I_{2}(t),R(t),F(t),L_{1}(t), L_{2}(t)) \in\:{\mathbb{R}}_{+}^{8}\:\forall\:n > 0\).
Proof
The proof is easily demonstrable, due to the non-negative nature of the biological problems’ restriction.
Theorem 5
For the \({\cal{G}}=(S^n,E^n,I_1^n,I_2^n,R^n,F^n,L_1^n,L_2^n ) \in \mathbb{R}_+^8\)\(:S^n+E^n+I_1^n+I_2^n+R^n+F^n+L_1^n+L_2^n\) \(=N \leq \frac{\pi}{\mu}, S^n \geq 0,E^n\geq 0,I_1^n\geq 0,I_2^n\geq 0,R^n\geq 0,\)\(F^n\geq 0,L_1^n\geq 0,L_2^n\geq 0,\) region. For every \(n \ge 0\), is an area of equations that is feasible and positive invariant (34) to (41).
Proof
The deconstruction of the system (19) looks like this:
Once the aforementioned equation system is included, we obtain
Therefore, for any \(\:n>0\), the non-standard computing approach that we suggest is restricted.
Theorem 6
If the unit circle contains the eigenvalue, the computational approach that has been given is stable for any \(\:n>0\) with \(\triangle\:B_{n} = 0.\)
Proof
Assume that the functions A, B, C, D, F, G, H, and P correspond to the right-hand sides of the following equations: (19).
Here,
\(\:A=\frac{S+h\left[\pi\:+\gamma\:R\right]}{1+h\left(\lambda\:{L}_{2}{e}^{-\mu\:\tau\:}+\mu\:\right)}\), \(\:B=\frac{E+h\left[\lambda\:S{L}_{2}{e}^{-\mu\:\tau\:}\right]}{1+h\left(\mu\:+\epsilon\:\sigma\:+\left(1-\epsilon\:\right)\sigma\:\right)}\) ,\(\:C=\frac{{I}_{1}+h\left[\left(1-\epsilon\:\right)\sigma\:E\right]}{1+h\left({\tau\:}_{1}+\mu\:+{\theta\:}_{1}\right)}\), \(\:D=\frac{{I}_{2}+h\left[\epsilon\:\sigma\:E\right]}{1+h\left(\delta\:+\mu\:+{\theta\:}_{2}\right)}\) ,
It is commonly understood that a system of the forms (6) converges to the model’s optimal state if and only if the Jacobian’s spectral radius, (J),
For the model’s stability. It complies with the requirements:
The equilibrium of the model is stable for \(\:\rho\:\left(J\right)<1\). Whether \(\:\rho\:\left(J\right)>1\) determines whether the equilibria of the model are stable. The equilibrium states of the model are inherently stable when \(\:\rho\:\left(J\right)=1\).
The following is an expression for the elements of the method-related Jacobian:
Hookworm-free equilibrium (HFE), \(\:{\mathcal{H}}^{0}=\left({S}^{0},{E}^{0},\:{I}_{1}^{0},\:{I}_{2}^{0},{R}^{0},{F}^{0}\:{L}_{1}^{0},\:{L}_{2}^{0}\right)=\left(\frac{\pi\:}{\mu\:},\text{0,0},0,\text{0,0},\text{0,0}\right)\)
Thus, the Jacobian’s eigenvalues at \(\:{\mathcal{H}}^{0}\) are as follows:
\(\:{\lambda\:}_{1}=\frac{1}{1+h\left(\mu\:\right)}<1\), \(\:{\lambda\:}_{3}=\frac{1}{1+h\left({\tau\:}_{1}+\mu\:+{\theta\:}_{1}\right)}\), \(\:{\lambda\:}_{4}=\frac{1}{1+h\left(\delta\:+\mu\:+{\theta\:}_{2}\right)}\), \(\:{\lambda\:}_{5}=\frac{1}{1+h\left(\mu\:+\gamma\:\right)}\), \(\:{\lambda\:}_{6}=\frac{1}{1+h\left(\psi\:+\omega\:\right)}\), \(\:{\lambda\:}_{7}=\frac{1}{1+h\left(v+\varphi\:\right)}\), \(\:{\lambda\:}_{8}=\frac{1}{1+hk}\) provided that \(\:{R}_{0}<1\) and \(\:{\lambda\:}_{2}=\frac{1}{1+h\left(\mu\:+\epsilon\:\sigma\:+\left(1-\epsilon\:\right)\sigma\:\right)}<1\).
Thus, all the eigenvalues lie in the unit circle at hookworms’ free equilibrium point. As desired.
Computational results
This section contrasts a non-standard computing method with established numerical methodologies (see Table 2).
Discussion
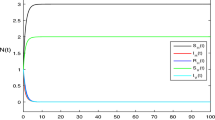
This section provides a discussion of the graphical presentation of the standard and nonstandard methods like Euler Maruyama, stochastic Euler, stochastic Runge Kutta, and nonstandard finite difference (NSFD) methods. Figure 2a,b present a comparative analysis of infected human populations under heavy infection scenarios using the Stochastic Non-Standard Finite Difference (NSFD) method and the Euler-Maruyama method. At a step size of \(\:h=0.01\), both methods exhibited convergence as shown in Fig. 2a. However, when the step size was increased to \(\:h=1\), the Euler-Maruyama method diverged while the Stochastic NSFD method-maintained convergence, as illustrated in Fig. 2b. Figure 3a,b analyze the population of infected humans with heavy infections using the Stochastic NSFD and Stochastic Euler Method. Both methods showed convergence at \(\:h=0.01\) in Fig. 3a. However, when the step size increased to \(\:h=2\) at the hookworm endemic point, the Stochastic Euler Method diverged, whereas the Stochastic NSFD method continued to converge, as depicted in Fig. 3b. Figure 4a,b analyze the population of infected humans under heavy infections using the Stochastic NSFD and Stochastic RK methods. Convergence is observed in both methods at \(\:h=0.01\) in Fig. 4a. However, at the hookworm endemic point, increasing the step size to \(\:h=3\) results in divergence for the Stochastic RK Method, whereas the Stochastic NSFD method maintains convergence, as illustrated in Fig. 4b. In Fig. 5a, the graph illustrates how varying delays \(\:\tau\:=1,\:2,\:3,\:4,\:5\) affect the susceptible class of the model. Figure 5b demonstrates the impact of these delays on the infected class across τ values \(\:1,\:2,\:3,\:4,\:and\:5\), showing a gradual reduction in disease prevalence over time. Figure 6 compares the delay terms \(\:\tau\:\) with the basic reproduction number \(\:{\:R}_{0}\). As \(\:\tau\:\) increases, \(\:{\:R}_{0}\) shows a decreasing trend. Specifically, at \(\:\tau\:=1.80\) days, \(\:{\:R}_{0}\) equals 1.042, indicating a diminishing disease presence in the host population over time.
Conclusion
In this paper, we developed and analyzed a model for the transmission dynamics of Hookworm infection in a human population using stochastic delay differential equations. The feasible properties of the model like positivity and boundedness studied. Also, we discuss two states of the model Hookworm free equilibrium and hookworms endemic equilibrium. Also, the reproduction number of the delayed model is investigated. After that, we studied the stochastic formation of the model in two ways transition probabilities and non-parametric perturbations, and verified the positivity and boundedness of the model. Due to nonlinearity and nondifferentiable terms of Brownian motion, these stochastic systems have no analytical solutions. So, we have used some standard and nonstandard numerical methods for its results. The implementation of standard methods like Euler Maruyama, Stochastic Euler, and stochastic Runge Kutta many discrepancies such as negative and unbounded results for the different values of time size, and did not apprehend a long-term behavior of the disease. To overcome such discrepancies, we used a stochastic nonstandard method for its results which restored the dynamical properties of the model and free of-time step size. Results from our modeling suggest that chemotherapy is administered to individuals infected with Hookworm, awareness campaigns of prevention methods, and well-known personal hygiene procedures including bathing and public toilets.
In future work, we will also seek optimal control strategies to minimize the emergence of moderate and severe infections in our future work. These strategies will also aim to reduce the cost of management within an optimally-designed Hookworm control system.
Data availability
The references for the data used to support the findings of this study are cited within the article.
References
Pearson, M. S. et al. Molecular mechanisms of hookworm disease: stealth, virulence, and vaccines. J. allergy Clin. Immunol. 130 (1), 13–21 (2012).
Hamidu, B. A. et al. The effectiveness of albendazole against hookworm infections and the impact of bi-annual treatment on anemia and body mass index of school children in the Kpandai district of northern Ghana. Plos one, 19(3), e0294977. (2024).
Walker, M. et al. Modeling the effectiveness of One Health interventions against the zoonotic hookworm Ancylostoma ceylanicum. Front. Med. 10, 1092030 (2023).
Puchner, K. P. et al. Vaccine value profile for Hookworm. Vaccine (2023).
Delos Trinos, J. P. C. et al. Cost and cost-effectiveness analysis of mass drug administration compared to school-based targeted preventive chemotherapy for hookworm control in Dak Lak province, Vietnam. The Lancet Regional Health–Western Pacific, 41 (2023).
Santos, M. C. S., de Oliveira, G. L., Mingoti, S. A. & Heller, L. Sewerage as a protective factor for the prevalence of hookworm infection in schoolchildren in Brazil: A multilevel ecological analysis of national prevalence surveys (1950–2018). Sci. Total Environ. 895, 164621 (2023).
Ramlal, A. et al. B. Botanicals against some important nematode diseases: Ascariasis and hookworm infections. Saudi J. Biol. Sci. 30(11), 103814 (2023).
Tiremo, S. N. & Shibeshi, M. S. Endoscopic Diagnosis of Hookworm Disease in a Patient with Severe Iron Deficiency Anemia: A Case Report. Int. Med. Case Rep. J. 841–845. (2023).
Mustapha, U. T. & Hincal, E. An optimal control of hookworm transmission model with differential infectivity. Phys. A: Stat. Mech. its Appl. 545, 123625 (2020).
Mustapha, U. T., Qureshi, S., Yusuf, A. & Hincal, E. Fractional modeling for the spread of Hookworm infection under Caputo operator. Chaos Solitons Fractals. 137, 109878 (2020).
Sangari, J. S. et al. Mathematical Modeling and Analysis of Transmission Dynamics and Control of Hookworms in Obi Local Government Area of Nasarawa State, Nigeria. S. Asian J. Parasitol. 7(3), 234–249 (2024).
Malizia, V., Giardina, F., de Vlas, S. J. & Coffeng, L. E. Appropriateness of the current parasitological control target for hookworm morbidity: a statistical analysis of individual-level data. PLoS Negl. Trop. Dis. 16(6), e0010279. (2022).
Ajjampur, S. S. et al. Epidemiology of soil-transmitted helminths and risk analysis of hookworm infections in the community: Results from the DeWorm3 Trial in southern India. PLoS Negl. Trop. Dis. 15(4), e0009338 (2021).
Grolimund, C. M., Bärenbold, O., Utzinger, J., Keiser, J. & Vounatsou, P. Modeling the effect of different drugs and treatment regimen for hookworm on cure and egg reduction rates taking into account diagnostic error. PLoS Negl. Trop. Dis., 16(10), e0010810. (2022).
Clements, A. C. & Alene, K. A. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 3 (1), e72–e79 (2022).
Haldeman, M. S., Nolan, M. S. & Ng’habi, K. R. Human hookworm infection: is effective control possible? A review of hookworm control efforts and future directions. Acta Trop. 201, 105214 (2020).
İlhan, E. Analysis of the spread of Hookworm infection with Caputo-Fabrizio fractional derivative. Turkish J. Sci. 7 (1), 43–52 (2022).
Koopman, J. P. R., Driciru, E. & Roestenberg, M. Controlled human infection models to evaluate schistosomiasis and hookworm vaccines: where are we now? Expert Rev. Vaccines. 20 (11), 1369–1371 (2021).
Colella, V. et al. Risk profiling and efficacy of albendazole against the hookworms Necator americanus and Ancylostoma ceylanicum in Cambodia to support control programs in Southeast Asia and the Western Pacific. The Lancet Regional Health–Western Pacific, 16, 01–18 (2021).
Abboubakar, H., Kamgang, J. C. & Tieudjo, D. Backward bifurcation and control in transmission dynamics of arboviral diseases. Math. Biosci. 278, 100–129 (2016).
Dtissibe, F. Y. et al. A comparative study of Machine Learning and Deep Learning methods for flood forecasting in the Far-North region, Cameroon. Sci. Afr. 23, e02053 (2024).
Yangla, J. et al. Fractional dynamics of a Chikungunya transmission model. Sci. Afr. 21, e01812 (2023).
Abboubakar, H., Kombou, L. K., Koko, A. D., Fouda, H. P. E. & Kumar, A. Projections and fractional dynamics of the typhoid fever: A case study of Mbandjock in the Centre Region of Cameroon. Chaos Solitons Fractals. 150, 111129 (2021).
Chazuka, Z., Chukwu, C. W., Mathebula, D. & Mudimu, E. Strategic approaches to mitigating Hookworm infection: An optimal control and cost-effectiveness analysis. Results Control Optim. 17, 100477 (2024).
Acknowledgements
All authors reviewed the results and approved the final version of the manuscript. The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through large Research Project under Grant Number RGP2/302/45. Also, this work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [KFU242541].
Funding
There is no specific funding for the present study.
Author information
Authors and Affiliations
Contributions
Ali Raza: Conceptualization, Investigation, Data curation, Writing-original draft. Mohammed Mahyoub Al-Shamiri: Project administration, Conceptualization, Investigation, Funding acquisition. Emad Fadhal: Data curation, Resources, Investigation, Funding acquisition. Muhammad Rafiq, Nauman Ahmed and Baboucarr Ceesay: Resources, Supervision, Project administration. Umar Shafique: Software, Writing-original draft & review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A
Appendix A
By the Gronwall’s inequality, we get
It demonstrates that the system (1)’s solution is bounded and falls inside the appropriate region \(\:{\beta\:}_{1}\).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shafique, U., Al-Shamiri, M.M., Raza, A. et al. Computational analysis of a mathematical model of hookworm infection. Sci Rep 15, 6668 (2025). https://doi.org/10.1038/s41598-024-83123-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83123-x