Abstract
The triglyceride-glucose (TyG) index is regarded as a surrogate marker of systemic insulin resistance (IR). Studies have substantiated the impact of IR on cardiovascular diseases. Nonetheless, the prognostic value of the TyG index in critical patients with heart failure (HF) with intensive care unit (ICU) admission remains unclear. This study aims to assess the association between the TyG index and all-cause mortality in critically ill patients with HF. Patients with HF requiring ICU admission were identified from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database and subsequently stratified into quartiles based on their TyG index. The primary outcome was 30-day all-cause mortality, and the secondary outcome was 1-year all-cause mortality. The relationship between the TyG index and all-cause mortality in HF patients was analyzed using multivariable Cox proportional hazards models and restricted cubic splines. A total of 1220 patients (62.4% men) were enrolled, with a mean age of 70.6 years. The 30-day and one-year all-cause mortality rate were 15.7% and 34.6%, respectively. Multivariable Cox regression revealed that TyG index was significantly associated with an elevated risk of 30-day all-cause mortality (adjusted HR, 1.360; 95% CI, 1.093–1.694; P = 0.006), but not with one-year mortality (adjusted HR 1.046; 95% CI 0.895–1.222, P = 0.574). Restricted cubic splines showed a progressively increasing risk of 30-day mortality was linearly related to an elevated TyG index. Subgroup analyses indicated a more prominent association between TyG index and 30-day mortality in patients with age ≤ 65, female or BMI > 30 kg/m2. In critically ill patients with HF, the TyG index is significantly associated with short-term all-cause mortality. Our results highlight that the TyG index can be useful in identifying HF patients at high risk of all-cause mortality and require close follow-up after discharge.
Similar content being viewed by others
Introduction
Heart failure (HF) is a serious, life-threatening clinical syndrome characterized by high morbidity, mortality, readmission rates, and significant healthcare costs. HF has been regarded as a global pandemic, with a prevalence of 1.0–2.0% among adults in developed countries1. According to the China Hypertension Survey conducted from 2012 to 2015, the prevalence of HF in adults 35 years and older is 1.3%, corresponding to approximately 13.7 million patients2. Consequently, it has become a critical global public health priority to reduce the heavy socio-economic burden of HF. Accurate diagnosis and timely screening of patients undergoing deteriorated situations are essential prerequisites for the effective treatment of HF.
Insulin resistance (IR) refers to the diminished physiological effect of insulin in the body and is a common pathological mechanism underlying various metabolic disorders3. One of the earliest metabolic disturbances observed in failing hearts is cardiac IR, which is generally defined as a significant reduction in insulin-stimulated glucose oxidation rates. IR only serves as a marker for HF severity, but also contributes to contractile dysfunction4. IR is a predominated metabolic dysfunction in HF, especially among patients with preserved ejection fraction (HFpEF), and the relationship seem to be bidirectional5. Existing evidence indicates that IR is associated with left ventricular (LV) remodeling and adverse outcomes in patients with HF6,7. Therefore, improving IR in HF patients is believed to alleviate the severity of their disease. The triglyceride-glucose (TyG) index, a great surrogate measurement of IR, has been widely utilized in clinical research. Several cross-sectional studies have shown that the TyG index is associated with hypertension8, arterial stiffness9, and stroke10. However, the situation becomes more sophisticated for HF patients admitted to the intensive care unit (ICU). Recently, a study utilizing the MIMIC-IV database, indicated an inverse association between TyG-BMI and all-cause mortality among critically ill patients with HF, while included only 423 participants11. However, other studies have reported the positive relationship between the TyG index and mortality in HF patients12,13,14. The predicative value of the TyG index in critical ill patients with HF remains a topic of ongoing debate. Our study is to evaluate the association between the TyG index and all-cause mortality in critically ill patients with HF utilizing the MIMIC-IV database. The aim of this evaluation is to inform the development of new clinical treatment strategies and to improve the prognosis for HF patients.
Methods
Study population
This is a retrospective observational study utilizing data from the MIMIC-IV database (version 2.2), which includes records of patients admitted to the ICU of Beth Israel Deaconess Medical Center from 2008 to 2019. This publicly available, de-identified database contains detailed patient information such as physiological signals, medication use, laboratory results, and nursing notes. Created by MIT’s Computational Physiology Laboratory, it supports clinical research and medical informatics education. One author (JX) completed NIH training on human research participant protection and passed CITI assessments to access the data. The MIT and Beth Israel Deaconess Medical Center review boards approved the database’s use in research. Informed consent was waived due to the database’s de-identified and anonymous nature.
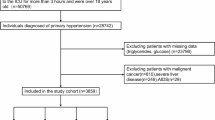
This study included 9383 HF patients from the MIMIC-IV database who were admitted to the ICU for the first time, aged ≥ 18 years, and had an ICU stay exceeding 24 h. ICD-9 and ICD-10 codes were used to identify HF patients. Follow-up began at admission and ended at death. The final study included 1220 patients, excluding patients with incomplete triglyceride (TG) or glucose measurements and patients without other essential information. These patients were divided into four groups based on the quartiles of the TyG index (Fig. 1).
Data collection
A structured query language (SQL) with PostgreSQL (version 13.13) and Navicate Premium 15 was used to extract baseline characteristics, including age, sex, body mass index (BMI) and multiple vital signs. These vital signs encompassed heart rate (HR), systolic and diastolic blood pressure (SBP and DBP), mean blood pressure (MBP), respiratory rate (RR), body temperature (T), and oxygen saturation (SpO2). Various scoring systems were used to assess the severity of each case at the time of admission. These included the systemic inflammatory response syndrome (SIRS), the simplified acute physiological score II (SAPSII), the logistic organ dysfunction system (LODS), the Glasgow coma scale (GCS), the acute physiology scores III (APSIII), and the sequential organ failure assessment (SOFA) score. Patients’ medical histories were also considered, including heart attacks, peripheral vascular disease, strokes, dementia, chronic lung disease, rheumatic disease, peptic ulcers, mild liver disease, paraplegia, kidney disease, cancer, severe liver disease, metastatic solid tumors, AIDS, and diabetes.
The laboratory parameters measured included red blood cells (RBC), white blood cells (WBC), hemoglobin, platelet counts, albumin, bilirubin, liver enzymes (ALT and AST), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), glucose, HbA1c, anion gap, blood urea nitrogen (BUN), calcium, bicarbonate, chloride, creatinine, sodium, potassium, troponin T, CK-MB, NT-pro-BNP and coagulation function indicators (INR, PT, and PTT). The initial recorded value was taken into account when a variable was recorded more than once within the preceding 24 h. In addition, we also included the patients’ left ventricular ejection fraction (LVEF) manually case by case. Outcomes including 30-day and one-year all-cause mortality, as well as hospital and ICU length of stay (LOS), were also collected.
Calculation of TyG index
The TyG index was usually calculated as ln [fasting TG (mg/dl) × fasting glucose (mg/dl)]/215. However, given the difficulty in obtaining accurate fasting blood glucose data from the MIMIC-IV database and considering that initial ICU glucose levels could be affected by acute stressors like pain, infection, or trauma and interventions like epinephrine administration, leading to transient hyperglycemia, we used the average glucose level during the ICU stay as a surrogate measurement of fasting glucose. And to expand the sample size, also to account for the fact that the triglyceride levels cannot fluctuate significantly in the short term, we extracted the patient’s initial triglyceride levels during admission.
Outcomes
The primary outcome was 30-day all-cause mortality, and the secondary outcome was one-year all-cause mortality.
Statistical analysis
Variables with over 20% missing data were excluded from the study. In instances where less than 20% of the data was missing, the gaps were filled using random forest interpolation. Continuous variables are presented as either mean (standard deviation) or median with interquartile range and were analyzed using the Mann–Whitney U test or the student t-test. On the other hand, categorical variables are expressed as frequencies and percentages, and the differences between groups were assessed using the Pearson chi-square test or Fisher’s exact test. We applied Kaplan-Meier survival analysis to evaluate the incidence rate of primary outcome events across groups with different TyG index levels. Differences among these groups were examined using log-rank tests. The association between the TyG index and primary outcomes was determined using Cox proportional hazards models, which provided the hazard ratio (HR) and 95% confidence interval (CI). This was done while adjusting for various models that incorporated clinically relevant variables associated with prognosis. For instance, model 1 adjusted for age, sex, BMI, and race; model 2 further included RR, MBP, hemoglobin, creatinine, potassium, INR, and LVEF; model 3 additionally adjusted for SAPSII, LODS, APSIII, and SOFA scores. The restricted cubic spline method was used to investigate the potential nonlinear relationships between the TyG index and outcomes. The TyG index was included in the models as either a continuous variable or an ordinal variable, with the lowest quartile used as the reference. Further stratified analyses were conducted based on sex, age (≤ 65 and > 65 years), BMI (< 30 and ≥ 30 kg/m²), LVEF (< 40 and ≥ 40%), diabetes, and myocardial infarction to assess the consistency of the prognostic significance of the TyG index for primary outcomes. The interactions between the TyG index and stratification variables were evaluated using likelihood ratio tests.
All data analyses were conducted using R version 4.4.0 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P-value < 0.05 was deemed statistically significant for all analyses.
Results
Baseline characteristics
The study included 1220 critical patients with HF, with an average age of 70.6 ± 13.7 years, and 62.4% were male. During follow-up, 191 (15.7%) patients died within 30 days, and 422 (34.6%) patients died within one year (Table 1).
Patients were categorized into quartiles based on their admission TyG index (Q1: 7.252–8.544; Q2: 8.544–8.937; Q3: 8.937–9.376; Q4: 9.376–12.634). Patients with a higher TyG index (Q4 quartile) were generally younger, had higher BMI, more severe illness scores at admission, and a higher incidence of diabetes and myocardial infarction. These patients also had severer liver dysfunction and elevated levels of WBC, platelets, creatinine, serum potassium, troponin-T, HbA1c, and LVEF, but lower levels of HDL than those in the lower quartiles. Besides, there were no significant differences in RBC, hemoglobin, albumin, bicarbonate, calcium, sodium, BUN, LDL, or coagulation function (P > 0.05). As the TyG index increased, so did the length of stay in the ICU (5.40 days vs. 6.13 days vs. 7.14 days vs. 8.83 days, P < 0.001), while no differences were observed in the morality within 30-day (12.5% vs. 17.6% vs. 15.3% vs. 17.3%, P = 0.426) and one-year (33.4% vs. 40.1% vs. 32.2% vs. 32.6%, P = 0.236).
Comparisons between survivors and non-survivors on 30-day after discharge are detailed in Supplementary Table 1. The non-survivors were older, with elevated levels of BMI, HR, DBP, and temperature at admission, and higher prevalence of cerebrovascular disease, liver disease, renal disease, and malignant cancer (P < 0.05). Regarding the laboratory examinations, patients in the non-survival group exhibited higher levels of WBC, anion gap, creatinine, and BUN, as well as INR and PT, while lower levels of RBC, hemoglobin, albumin, total cholesterol, HDL, and bicarbonate (P < 0.05). Furthermore, non-survivors had higher scores of SOFA, SIRS, APSIII, LODS, and SAPSII compared to survivors, indicating more severe illness (P < 0.05). As for the duration of hospitalization, it was found that the non-survivors experienced a shorter in-hospital stay (16.6 days vs. 12.6 days, P = 0.027) and a longer ICU stay (6.69 days vs. 7.79 days, P < 0.001) in comparison to the survivors.
Relationship between TyG index and prognosis of critical patients with HF
Figure 2 illustrates the Kaplan-Meier survival curves of the incidence of 30-day all-cause mortality stratified by TyG quartiles. It was showed that patients with the higher TyG index were more vulnerable to suffer from mortality within 30 days after discharge, compared to those in the lowest quartile of TyG index (log-rank P < 0.001).
Kaplan–Meier survival analysis curve for 30-day all-cause mortality. TyG index quartiles: Q1 (7.252–8.544), Q2 (8.544–8.937), Q3 (8.937–9.376), Q4 (9.376–12.634). Kaplan–Meier survival curve shows cumulative probability of all-cause mortality according to TyG quartiles at 30 days adjusted for age, gender, BMI, race, RR, MBP, hemoglobin, creatinine, potassium, INR, LVEF, SAPSII, LODS, APSIII, SOFA.
To further investigate the association between the TyG index and all-cause mortality in critical HF patients, multivariable Cox proportional hazards models were constructed (Table 2). It was showed that the TyG index was significantly associated with 30-day mortality in the crude Model (HR 1.585 [95% CI 1.274–1.972], P < 0.001) and the fully adjusted model (HR 1.360 [95% CI 1.093–1.694], P = 0.006) when the TyG index was treated as a continuous variable. Furthermore, the TyG quartiles were also included in rank order, with the lowest quartile (Q1) as the reference group. After adjusting for age, gender, BMI, and race (Model 1), the TyG index was dose-responsibly associated with 30-day mortality (Q1 vs. Q2: HR 1.599 [95% CI 1.054–2.427], P = 0.027; Q3: HR 1.600 [95% CI 1.037–2.468], P = 0.034; Q4: HR 2.25 [95% CI 1.34–3.77], P < 0.001), indicating an increasing trend with rising TyG index levels. After full-adjustment for clinical characteristics and severity scores in Model 3, the TyG index still maintained a strong association with 30-day mortality (Q1 vs. Q2: HR, 1.545 [95% CI 1.012–2.359], P = 0.044; Q3: HR, 1.466 [95% CI 0.943–2.278], P = 0.089; Q4: HR, 1.782 [95% CI 1.145–2.772], P = 0.010), while non-significant association were found between TyG index and one-year mortality (adjusted HR, 1.046; 95% CI, 0.895–1.222; P = 0.574,Tab. S2).
The restricted cubic spline regression indicated that the risk of 30-day all-cause mortality increased linearly with rising TyG index levels (P for nonlinear = 0.351, Fig. 3), as did the risk of one-year all-cause mortality (P for nonlinear = 0.534, Fig. S2).
The relationship between TyG index and the risk of 30-day mortality in critical ill patients with HF by restricted cubic spline regression. Graphs show HR for 30-day mortality according to TyG index adjusted for age, gender, BMI, race, RR, MBP, hemoglobin, creatinine, potassium, INR, LVEF, SAPSII, LODS, APSIII, SOFA. Data were fitted by a Cox regression model, and the model was conducted with 4 knots at the 5th, 35th, 65th, 95th percentiles of TyG index (reference is the 5th percentile). Solid lines indicate ORs, and shadow shape indicate 95% CIs. HR hazard ratio, CI confidence interval, TyG triglyceride-glucose index.
Subgroups analyses
To further explore whether the association between the TyG index and 30-day all-cause mortality persisted under different conditions, subgroup analyses were conducted stratified by age, gender, BMI, LVEF, diabetes, and myocardial infarction (Fig. 4). The results indicated a more prominent association between TyG index and 30-day mortality in patients with age ≤ 65 (HR, 1.94; 95% CI, 1.00–3.77) and female (HR, 2.27; 95% CI, 1.25–4.10), with P for interaction of 0.142, 0.011, respectively.
Discussion
In this study, we explored the association between the TyG index and mortality in critical patients with HF within 30 days and one year. Through a detailed retrospective analysis of the MIMIC database, we found a significant positive association between the TyG index and 30-day all-cause mortality in HF patients requiring ICU admission. Specifically, increasing of the TyG index was corresponded with proportional rising of the mortality risk within 30 days after discharge. This relationship remains robust after careful adjustment for possible confounding factors. However, there was no significant association found between the TyG index and long-term mortality at one year.
While the precise mechanism undermine the TyG index and HF has yet to be clarified, the close relationship between TyG index and insulin resistance (IR) indicates that systemic IR could play a crucial role. It has been reported that IR is closely associated with the increased risk of HF mobility and mortality, no matter complicated with or without diabetes15. Epidemically, patients with HFpEF often combined with a higher prevalence of diabetes, obesity, and metabolic syndrom16. IR is regarded to deeply participate in the pathogenesis of the above metabolic disorders, thus fueling up the onset of cardiac remolding and LV dysfunction17. Studies had shown that IR could impair the nitric oxide biogenesis in vessels wall, leading to endothelia dysfunction and vasodilation disorder, ultimately resulting in cardiac myocyte apoptosis. Additionally, endothelial IR further contributes to the release of endothelin-1, which acts as a strong vasocontractile factor inducing cardiac hypertrophy and fibrosis18. Elevated levels of insulin and aldosterone can reduce the bioavailability of nitric oxide and promote pathological vascular sclerosis19. Although the euglycemia-hyperinsulinemic clamp test is regarded as the benchmark for assessing systemic IR, its complexity and high-expense have disturbed its application under sophisticated clinical conditions. The TyG index is widely used in clinical research as a simpler and more affordable alternative to gauge IR. Previous studies have demonstrated that the TyG index is an effective tool for assessing IR, with a sensitivity of 96.5% and a specificity of 85.0%, compared to the gold standard20.
Recently, numerous studies have investigated the relationship between the TyG index and the occurrence and progression of HF. However, the results remain debated. A large prospective cohort study involving 138,620 participants demonstrated that the TyG index was positively associated with the risk of HF in a J-shaped dose–response relationship, and persisted in both genders and patients with or without abdominal obesity21. Additionally, a high TyG index observed in two large, geographically diverse Chinese cohorts was identified as an independent risk factor for the development of HF among the general population22. Similar findings have been reported in studies involving patients with acute decompensated heart failure (ADHF), where the TyG index was directly linked to poor prognosis in ADHF patients, irrespective of diabetes mellitus status14. In a study of critically ill patients with chronic HF, Zhou et al. found a positive correlation between the TyG index and patients’ mortality. Notably, compared to TyG-BMI and TG/HDL-C ratio, the TyG index is the most promising indicator for prevention and risk stratification. The study showed that each unit increase in TyG index is associated with an increased 5-year mortality risk with a HR of 1.56 (95% CI, 1.29–1.9). In contrast, TyG-BMI and TG/HDL-C have weaker associations with 5-year mortality, with HRs of 1.002 (95% CI, 1.000-1.003) and 1.01 (95% CI, 1.00-1.03), respectively13. However, another study of critically ill patients with HF reported an inverse association between the TyG-BMI index and mortality11. Correspondingly, a cross-sectional study utilizing the UK Biobank database had yielded no significant association between the TyG index and HF outcomes23. In our study, we identified a linearly positive association between the TyG index and short-term mortality in critically ill patients with HF, but no significant association with one-year mortality in long-term. This discrepancy may be attributed to both the IR status and the acute stress. In the early acute stages, IR is associated with hyperinsulinemia, and elevated insulin levels can stimulate sympathetic nerve excitation and induce vasoconstriction24. This could lead to a poorer prognosis in HF patients in the short-term. However, the situation may be more complex and sophisticated in terms of long-term prospects. Those who have successfully survived the acute period should have greater resilience and gained more advantages for long-term survival. In addition, complications, medication and control of risk factor also exert important effects on the prognosis of HF patients. Novack and colleagues had retrospectively assessed the association between routine blood glucose levels and 30-day or 1-year mortality in patients admitted for decompensated HF. Similarly, no association was found between glucose levels and 1-year mortality in non-diabetic patients25. It should be noted that in our study patients were suffered from more severe illness and required ICU admission, complicated multiple underlying diseases and complex conditions which may affect their long-term mortality.
Subgroup analyses indicated a more prominent association between the TyG index and 30-day mortality in patients ≤ 65 years and in women. In patients > 65 years, the predictive value of the TyG index for short-term outcomes might be partly masked by a higher burden of chronic comorbidities and disease severity scores (Table S3). In women of postmenopausal, the loss of estrogen-dependent cardiometabolic protection may worsen IR and dyslipidemia26,27, and their typically higher fat-to-lean mass ratio may amplify metabolic risks via proinflammatory and oxidative stress pathways28,29. Moreover, HFpEF which is more common in women and closely related to IR and metabolic dysfunction30,31,32, also enhances the prognostic value of the TyG index in this population. In addition, our findings indicate that higher TyG indices are associated with longer stays in ICU, which undoubtedly puts a heavy economic burden on patients. Greater attention should be paid to the prognosis of critical patients with HF, together with the identification of possible risk factors contributing to this residual mortality.
Strengths and limitations
Our study have some strengths. Prior studies utilizing the MIMIC-IV database typically used the non-fasting or first-recorded glucose values upon ICU admission to calculate the TyG index, rather than fasting blood glucose11,33. It’s because timely obtaining fasting blood from patients admitted into ICU is usually impractical. Our study here used the average glucose level during the ICU stay for calculation of TyG index. it is better to reduce extreme fluctuations from single measurements, such as stress—induced hyperglycemia or iatrogenic hypoglycemia, and provide a more stable reflection of the overall IR status. And it has been demonstrated that glycemic variability, rather than single glucose values, is an independent predictor of mortality risk in ICU patients34, suggesting that averaged glucose measurements may be a better surrogate measurement rather than the first record glucose level. Moreover, LVEF is a crucial predictor of prognosis in HF patients, and is typically neglected in similar studies. We manually extracted the LVEF measurements from the MIMIC-IV database and incorporated them into a fully adjusted regression model. LVEF levels were found to be higher in the highest quintiles of TyG values compared to the lowest quintiles, suggesting that IR is more prevalent in HF patients with preserved EF.
Our study also has some limitations. Firstly, it is a single-center retrospective analysis, which limits the ability to establish causation. Although we had integrated multiple variables and sensitivity analysis, it is important to note that the impact of confounding factors cannot be completely ruled out. Second, our study was limited to the evaluation of the prognostic significance of baseline TyG index in HF patients, without monitoring the dynamic alterations of TyG index during hospitalization and follow-up. Third, it’s difficult to verify whether the triglyceride and glucose measurements have been performed in fasting patients, which could potentially bias our findings. In addition, we did not incorporate medication in the regression analysis, and future studies are warranted to further investigate the association between the TyG index and mortality in patients with HF.
Conclusion
The TyG index is a highly reliable independent predictor of short-term mortality in patients with severe heart failure. In addition, there is a significant and linear correlation between the TyG index and the short-term risk of all-cause mortality in critically ill patients with heart failure. Therefore, measuring the TyG index is useful for stratifying risk and predicting future outcomes in this population.
Data availability
The datasets generated and analyzed during this study are available from the corresponding author upon reasonable request.
References
Savarese, G. et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc. Res. 118, 3272–3287 (2023).
Wang, H. et al. Prevalence and incidence of heart failure among urban patients in China: A National population-based analysis. Circulation Heart Fail. 14, e008406 (2021).
Zhang, Y. et al. High triglyceride-glucose index is associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Nutr. Metab. Cardiovasc. Dis. 30, 2351–2362 (2020).
Lopaschuk, G. D., Karwi, Q. G., Tian, R., Wende, A. R. & Abel, E. D. Cardiac energy metabolism in heart failure. Circ. Res. 128, 1487–1513 (2021).
Marsico, F., Gargiulo, P., Marra, A. M., Parente, A. & Paolillo, S. Glucose metabolism abnormalities in heart failure patients: insights and prognostic relevance. Heart Fail. Clin. 15, 333–340 (2019).
Wamil, M., Coleman, R. L., Adler, A. I., McMurray, J. J. V. & Holman, R. R. Increased risk of incident heart failure and death is associated with insulin resistance in people with newly diagnosed type 2 diabetes: UKPDS 89. Diabetes Care. 44, 1877–1884 (2021).
Yang, C. D. et al. Insulin resistance and dysglycemia are associated with left ventricular remodeling after myocardial infarction in non-diabetic patients. Cardiovasc. Diabetol. 18, 100 (2019).
Jian, S., Su-Mei, N., Xue, C., Jie, Z. & Xue-Sen, W. Association and interaction between triglyceride-glucose index and obesity on risk of hypertension in middle-aged and elderly adults. Clin. Exp. Hypertens. 39, 732–739 (2017).
Lambrinoudaki, I. et al. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ. 27, 716–724 (2018).
Jiang, Y. et al. Association of triglyceride glucose index with stroke: from two large cohort studies and Mendelian randomization analysis. Int. J. Surg. https://doi.org/10.1097/JS9.0000000000001795 (2024).
Dou, J. et al. Association between triglyceride glucose-body mass and one-year all-cause mortality of patients with heart failure: a retrospective study utilizing the MIMIC-IV database. Cardiovasc. Diabetol. 22, 309 (2023).
Zhou, Q. et al. High triglyceride-glucose (TyG) index is associated with poor prognosis of heart failure with preserved ejection fraction. Cardiovasc. Diabetol. 22, 263 (2023).
Zhou, Z. et al. Comparative study on the predictive value of TG/HDL-C, TyG and TyG-BMI indices for 5-year mortality in critically ill patients with chronic heart failure: a retrospective study. Cardiovasc. Diabetol. 23, 213 (2024).
Huang, R. et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc. Diabetol. 21, 88 (2022).
Erqou, S., Adler, A. I., Challa, A. A., Fonarow, G. C. & Echouffo-Tcheugui, J. B. Insulin resistance and incident heart failure: a meta‐analysis. Eur. J. Heart Fail. 24, 1139–1141 (2022).
Nielsen, R. et al. Heart failure patients with prediabetes and newly diagnosed diabetes display abnormalities in myocardial metabolism. J. Nuclear Cardiol. 25, 169–176 (2018).
Tao, L. C., Xu, J. N., Wang, T. T., Hua, F. & Li, J. J. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc. Diabetol. 21, 68 (2022).
Aroor, A. R., Mandavia, C. H. & Sowers, J. R. Insulin resistance and heart failure: molecular mechanisms. Heart Fail. Clin. 8, 609–617 (2012).
Hill, M. A. et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism Clin. Experimental. 119, 1 (2021).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351 (2010).
Triglyceride–glucose index. Associates with incident heart failure: A cohort study. Diabetes Metab. 48, 101365 (2022).
Li, X. et al. Triglyceride-glucose index and the risk of heart failure: evidence from two large cohorts and a Mendelian randomization analysis. Cardiovasc. Diabetol. 21, 229 (2022).
Si, S. et al. Causal effect of the triglyceride-glucose index and the joint exposure of higher glucose and triglyceride with extensive cardio-cerebrovascular metabolic outcomes in the UK biobank: A Mendelian randomization study. Front. Cardiovasc. Med. 7 (2021).
Hausberg, M., Mark, A. L., Hoffman, R. P., Sinkey, C. A. & Anderson, E. A. Dissociation of sympathoexcitatory and vasodilator actions of modestly elevated plasma insulin levels. J. Hypertens. 13, 1015–1021 (1995).
Novack, V. et al. Routine laboratory results and Thirty day and one-year mortality risk following hospitalization with acute decompensated heart failure. PLoS ONE. 5, e12184 (2010).
Knowlton, A. A. & Lee, A. R. Estrogen and the cardiovascular system. Pharmacol. Ther. 135, 54–70 (2012).
Janssen, I., Powell, L. H., Crawford, S., Lasley, B. & Sutton-Tyrrell, K. Menopause and the metabolic syndrome: the study of women’s health across the Nation. Arch. Intern. Med. 168, 1568–1575 (2008).
Bredella, M. A. Sex differences in body composition. Adv. Exp. Med. Biol. 1043, 9–27 (2017).
El Meouchy, P. et al. Hypertension related to obesity: pathogenesis, characteristics and factors for control. Int. J. Mol. Sci. 23, 12305 (2022).
Lam, C. S. P. et al. Sex differences in heart failure. Eur. Heart J. 40, 3859–3868c (2019).
Abudureyimu, M. et al. Heart failure with preserved ejection fraction (HFpEF) in type 2 diabetes mellitus: from pathophysiology to therapeutics. J. Mol. Cell. Biol. 14, 028 (2022).
Hahn, V. S. et al. Myocardial metabolomics of human heart failure with preserved ejection fraction. Circulation 147, 1147–1161 (2023).
Shao, Y. et al. Correlation of the triglyceride-glucose index and heart rate with 28-day all-cause mortality in severely ill patients: analysis of the MIMIC-IV database. Lipids Health Dis. 23, 387 (2024).
Krinsley, J. S. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit. Care Med. 36, 3008–3013 (2008).
Funding
This research was funded by National Natural Science Foundation of China (82100359), Xi’an Science and Technology Plan Project (24YXYJ0148), and Scientific Research Fund Youth Project of the Second Affiliated Hospital of Xi’an Jiaotong University (YJ(QN)202325).
Author information
Authors and Affiliations
Contributions
TH, CYZ, JD, and YZ were responsible for study conception and data collection. YZ, CYZ, FYC, and JD designed the study. Data extraction was performed by HG and FYC. Data analysis and intellectual guidance were provided by TH, YL, JX, and FXD. The initial manuscript draft was written by TH, YL, and JX. WJ, CXW, CYZ, JD, and YZ revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, J., Li, Y., Gao, X. et al. Association between triglyceride-glucose index and all cause mortality in critically ill patients with heart failure. Sci Rep 15, 16157 (2025). https://doi.org/10.1038/s41598-025-00129-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00129-9
Keywords
This article is cited by
-
Evaluating the impact of metabolic indicators and scores on cardiovascular events using machine learning
Diabetology & Metabolic Syndrome (2025)