Abstract
Human leukocyte concentrates recovered from leukocyte reduction system (LRS) chambers of the Trima Accel automated blood collection device are a by-product of platelet apheresis that is often used in research. The Trima Accel software was obligatorily updated in 2023 from version 6 to 7. Here, we investigated software-dependent differences in the cellular composition of LRS concentrates when performing apheresis with either plasma or platelet additive solution. When using plasma as suspension medium, the software update to Trima 7 led to higher fraction of B cells as revealed by flow cytometry. Compared to platelets-in-plasma collection, the total recovered volume and leukocyte density was significantly reduced when running Trima 7 software with platelet additive solution including a plasma rinseback. Moreover, the proportion of lymphocytes and monocytes in these products was lower, whereas the proportion of neutrophils and eosinophils was higher. Researchers working with leukocytes isolated from LRS chambers should be aware that performing apheresis with platelet additive solution alters the composition of their starting material, which could affect the interpretation of some experiments.
Similar content being viewed by others
Introduction
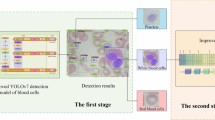
Freshly isolated human peripheral blood mononuclear cells (PBMC) are a limited resource, but are in high demand for hematological and immunological research1,2,3. The Trima Accel apheresis device (Terumo BCT, Inc., Lakewood, USA) for collecting platelets incorporates a leukocyte reduction system (LRS) to minimize contamination of thrombocyte products with leukocytes (Fig. 1a). PBMC captured in LRS chambers as a by-product of platelet donation have long been exploited in research3,4,5 as a source of cells from healthy donors because of their convenience, cell quality and pathogen-free origin (Fig. 1b). From 2023 onwards, the device manufacturer mandated an update of the running software from Trima Accel version 6 to version 7. According to the manufacturer, this update implemented an improved algorithm for platelet collection and LRS management with interface regulation to achieve a shorter processing time and higher platelet yields. The main advantage of the new software version is to increase the proportion of donors who are qualified for double platelet collection due to higher productivity6. In general, the manufacturer recommends the use of platelet additive solution (PAS) instead of autologous plasma as a suspension medium to minimize plasma mediated risks like platelet transfusion reactions7 (Fig. 1c). PAS like Intersol and T-PAS are nutritive solutions containing citrate, acetates, phosphates and salts. PAS is added after the collection procedure to the platelet concentrates for stabilization during storage, as well as avoiding transfusion of large plasma volumes to patients and improving photochemical inactivation of pathogens in platelet concentrates8. The effects of PAS on platelets has been investigated in several in vitro and in vivo studies9. Platelet concentrates collected with Trima 7 platelet-in-PAS showed less residual leukocytes than those collected in plasma10. Long-term donation using apheresis devices with LRS chambers has been associated with T cell lymphopenia11,12,13 that some studies, but not others14, associate an increased risk of infections15. To mitigate loss of lymphocytes, a so-called “rinseback” procedure can be inserted at the end of the apheresis process to flush cells back to the donor16.
LRS chamber products were obtained from platelet apheresis using Trima Accel software version 6 and 7. In-house platelet apheresis were run using the Trima Accel automated blood collection system including the Trima Accel LRS Platelet Plasma Set (a). The LRS chamber (b) as a source of highly concentrated leukocytes was collected after apheresis and the composition of the cell product was analyzed. Currently, a software update from Trima Accel version 6 (Trima 6) to version 7 (Trima 7) was available, both using plasma for platelet collection (c). LRS chamber products generated during apheresis with software version 7 and platelet additive solution (PAS) instead of plasma (Trima 7 PAS) were obtained from external suppliers.
The modifications introduced by Trima Accel version 7 potentially impacted researchers who relied upon cells collected using version 6 for their research. Hence, the aim of our study was to document software-dependent differences in cellular composition of LRS chamber concentrates, and to investigate the influence of using PAS instead of plasma.
Results
LRS chamber concentrates differ between Trima 6 and 7
LRS chambers were collected after platelet apheresis running with Trima Accel software version 6 using plasma (Trima 6), Trima Accel software version 7 using plasma (Trima 7) and Trima 7 using platelet additive solution (Trima 7 PAS). The volume of concentrate was recorded and its cellular composition was determined using an automated Sysmex cell counter. Trima 7 PAS yielded only 3.4 ± 1.9 mL concentrate, compared to Trima 6 (8.1 ± 0.5 mL, p < 0.0001, d > 0.8) and Trima 7 (8.3 ± 0.5 mL, p < 0.0001, d > 0.8) (Fig. 2a). White blood cell (WBC) concentrations were also decreased after apheresis with Trima 7 PAS (71.0 ± 27.8 × 103/µL) compared to Trima 6 (125.8 ± 43.4 × 103/µL, p < 0.001, d > 0.8) and Trima 7 (161.4 ± 55.8 × 103/µL, p < 0.0001, d > 0.8) (Fig. 2b). Of note, Trima 7 PAS products contained significantly lower concentrations of lymphocytes (Fig. 2c) and monocytes (Fig. 2d) than Trima 6 or Trima 7 apheresates. In contrast, using PAS resulted in more erythrocytes (Fig. 2e), neutrophils (Fig. 2f) and eosinophils (Fig. 2g). Basophil concentrations differed between Trima 7 and Trima 7 PAS (Fig. 2h). Platelet concentrations were comparable for all three conditions (Fig. 2i). Overall, the yield of PBMC (lymphocytes, monocytes and basophils) from Trima 7 PAS LRS chambers was significantly lower in absolute (Table S1) and relative terms (Fig. S1), especially after adjusting for recovered concentrate volumes (Fig. S2).
After platelet apheresis with Trima 7 and platelet additive solution substantial differences in cellular composition of LRS chamber products were observed. Leukocyte product was recovered from LRS chamber and total volume was determined (a). The cellular composition was analyzed, including white blood cell (b), lymphocytes (c), monocytes (d), red blood cells (e), neutrophils (f), eosinophils (g), basophils (h) and platelets (i) (n = 16 for Trima 6, n = 12 for Trima 7 and n = 20 for Trima 7 PAS; for (a) it is n = 13 for Trima 7 and n = 14 for Trima 7 PAS; statistical analyses were carried out using one-way ANOVA with Tukey test for multiple comparisons; effect size is reported as Cohen’s d).
T cell subset distribution is affected by Trima 7
LRS chamber concentrates were characterized by flow cytometry and leukocyte subset frequencies were extracted by manual gating (Fig. S3). Compared to Trima 6 (69.9 ± 10.7%, p < 0.05, d > 0.8) and Trima 7 PAS (70.8 ± 7.7%, p < 0.05, d > 0.8) the frequency of CD4+ T cells was significantly lower in Trima 7 concentrates (59.6 ± 10.8%) (Fig. 3a). Frequencies of CD8+ T cells were correspondingly increased in Trima 7 (32.0 ± 11.7%) compared to Trima 6 (22.1 ± 8.6%, p < 0.05, d > 0.8) and Trima 7 PAS (18.7 ± 5.4%, p < 0.005, d > 0.8) (Fig. 3b). No other significant differences in frequencies of manually gated populations were found (Fig. 3c–h; Table S2).
Flow cytometry analysis of LRS chamber cell products revealed differences in CD4+ and CD8+ T cell frequencies. Leukocyte product was recovered from LRS chamber and stained for flow cytometry analysis. Frequencies of cell populations were determined by manual gating, including CD3+ CD4+ T cells (a), CD3+ CD8+ T cells (b), CD45+ leukocytes (c), CD3+ T cells (d), CD19+ B cells (e), CD56+ NK cells (f), CD56high NK cells (g) and CD14+ monocytes (h) (n = 15 for Trima 6, n = 9 for Trima 7 and n = 14 for Trima 7 PAS; statistical analyses were carried out using one-way ANOVA with Tukey test for multiple comparison; effect size is reported as Cohen’s d).
CITRUS is a machine learning algorithm for analyzing flow cytometry data17,18. CITRUS defined 38 cell clusters and 12 metaclusters showing biological signatures between Trima 6, Trima 7 and Trima 7 PAS samples (Fig. S4). Exploring the phenotype of these cell subsets revealed that 9 of 12 metaclusters represented T cell subpopulations, including eight CD4+ T cell subsets and one CD8+ T cell subset (Fig. S5). The remaining three metaclusters lacked T cell markers, but expressed CD16, CD19 or CD14. To explore differences between sample groups, we compared absolute numbers of events per cluster, confirming differences in manually gated data — namely, a Trima 7-dependent increase in CD8+ T cells (Fig. S6a) and a corresponding decrease in CD4+ T cells (Fig. S6b). Furthermore, CITRUS was able to find additional apheresis-dependent differences in CD4+ cell clusters that were not identified by manual gating (Fig. S6 c-i). The three non-T cell metaclusters comprised CD19+ B cells (Fig. 4a), CD14+ monocytes (Fig. 4b) and CD16+ neutrophils (Fig. 4c).
CITRUS analysis of flow cytometry data revealed non-T cell apheresis-dependent differences. Flow cytometry data were fully recompensated and uploaded into Cytobank. CITRUS analysis was performed using PAMR as predictive model. Absolute numbers of events were determined in the three non-T cell metaclusters (a) # 379956, (b) # 379974 and (c) # 379962. (n = 15 for Trima 6, n = 9 for Trima 7 and n = 14 for Trima 7 PAS; statistical analyses were carried out using one-way ANOVA with Tukey test for multiple comparisons; effect size is reported as Cohen’s d). Heatmaps were generated displaying MFI per channel to phenotypically characterize the selected clusters (one representative sample per condition is shown as an example).
Discussion
Here, we report that upgrading from Trima 6 to Trima 7 software only slightly affects the composition of leukocyte concentrates from Trima Accel LRS chambers when plasma was used as suspension medium. In contrast, recovered volume and cell yield were greatly reduced when using PAS and a rinseback with plasma at the end of the apheresis process. Researchers using PBMC from LRS chambers should be aware that changes to the cell collection protocol impact the cells present in their starting material and also possibly influence their function.
Using an automated cell counter to analyze the cellular composition of LRS chambers produced with Trima 6 or 7, we found higher numbers of all leucocytes compared to some previously published data3,19. Such variability likely reflects local variations in clinical protocols and instrument settings; for instance, differences in anticoagulation and flow management. No gross differences were found in concentrates produced with Trima 6 or 7 using plasma as the suspension medium using the Sysmex counter. However, flow cytometry did suggest software-dependent differences between Trima 6 and 7. Whereas proportion of total CD3+ cells remained unchanged, differences in the composition of CD4+ and CD8+ T cell subpopulations were observed. We attribute this difference to a donor sampling effect in our small cohort so that differences are due to donor-to-donor variation, and not to the mechanics of cell collection. Irradiating platelet products prevents proliferation of CD8+ T cells, so reduces GvHD risk20. If collection conditions reduced the number of residual CD8+ T cells in platelet products, we might expect a clinical benefit in lowering GvHD risk. Further work is necessary to assess whether CD8+ T cells numbers are consistently reduced in PAS versus plasma products.
When running apheresis with the updated software version 7 and PAS as suspension medium we found lower numbers of lymphocytes and monocytes. This may result from implementing a rinseback with plasma at the end of the collection process with Trima 7 PAS. This additional step intends to mitigate lymphodepletion of the donor avoiding a higher susceptibility for infections. Additionally, the software programmed for collection with PAS may also influence the numbers of lymphocytes and monocytes in the LRS chamber as reducing them possibly during the centrifugation step of apheresis or during the rinseback due to their lower density compared to granulocytes21.
Flow cytometry is a powerful tool for analyzing a wide range of biological markers within one single blood sample but the downstream analysis is limited by the restriction of manual gating in particular when working with high dimensional data. Machine learning algorithms like CITRUS can be a promising approach for the analysis of complex single cell datasets by identification of stratifying biological signatures between different treatment groups17,18. Here, CITRUS analysis of LRS chamber flow cytometry data not only confirmed results obtained by automated cell counting or manual gating but also revealed new software-dependent differences in B cell populations not discovered by manual gating so far. Furthermore, CITRUS was capable to identify additional CD4+ T cell clusters showing apheresis-dependent biological signatures that were not be detected by manual gating. Computational approaches like CITRUS are known to be superior to manual gating in terms of efficiency, reproducibility and reduction of human bias22. In contrast to manual gating, the CITRUS algorithm combines all samples into one aggregate dataset before identifying cell populations by hierarchical clustering of phenotypically similar cells. Cells are then assigned back to the individual samples and cluster characteristics are calculated23.
In research routine, PBMC isolated from LRS chamber product serve as source for the isolation of monocytes24,25,26 that are often further differentiated into macrophages or dendritic cells4,5,27,28. So far, our data focusses on cellular composition of concentrates and does not allow any statement to be made about activation status or functionality of recovered cells. These important aspects need to be addressed in further investigations.
Here, we report for the first time that apheresis procedure with PAS and plasma rinseback influences the composition of leukocytes that are enriched in the leukocyte reduction system. Potential confounders due to different donor characteristics as age, sex and individual peripheral blood cell distribution may influence the composition of LRS products. However, we attribute the observed differences to the technical aspects of the collection process when running a platelet-in-PAS apheresis.
In conclusion, we found only subtle differences between concentrates obtained with Trima 6 versus Trima 7 software when using plasma as the suspension medium. However, using PAS instead of plasma led to a much reduced cell yield and grossly altered leukocyte subset distributions. Thus, researchers have to be aware of the LRS chamber origin and have to take in account whether apheresis was run with plasma or PAS. We highlight this issue so others can assess the impact of software update and suspension medium in their own experimental systems.
Methods
Apheresis procedure and sampling protocol
Apheresis from healthy platelet donors were performed by the Department of Transfusion Medicine at University Hospital Regensburg using the Trima Accel automated blood collection system (Terumo BCT, Inc., Lakewood, USA). The device ran either Trima Accel software version 6 or version 7 with plasma for platelet collection. Platelet apheresis was run with an ACDA ratio of 1:10 and 3.5–4.5 L processed blood volume. Per donor a maximum of 26 plateletpheresis per year is allowed14. LRS chamber products generated during apheresis with software version 7 and PAS (Intersol or T-PAS +) were obtained from external suppliers (Blutspendedienst BRK GmbH, Regensburg, Germany or ITM Suhl gGmbH, Suhl, Germany). Here, a rinseback with plasma was inserted at the end of the apheresis process. This study was authorized by the local ethics committee (approval 22–2780-101) and all participants gave full, informed written consent. Samples were collected from APR-2023 until MAY-2024.
Automated analysis of LRS chamber products
Leukocyte concentrates were recovered from LRS chambers and total volume was determined using a serological pipette. Concentrates were diluted with PBS (1:5) and their cellular composition was analyzed using an automated cell counter (XN-550, Sysmex, Norderstedt, Germany) returning values for white blood cells (WBC), red blood cells (RBC), platelets (PLT), lymphocytes (LYMPH), monocytes (MONO), neutrophils (NEUT), eosinophils (EO) and basophils (BASO).
Clinical flow cytometry
Cell concentrates were stained for flow cytometry using the DURAClone IM Phenotyping Basic Tube (B53328, Beckman Coulter, Krefeld, Germany) according to standard operating procedures29,30,31. Data were recorded with a Navios cytometer running Cytometry List Mode Data Acquisition and Analysis Software version 1.3 (Beckman Coulter). Analyses were performed using Kaluza software version 2.2 (Beckman Coulter).
Statistics and visualisation
Data visualisation and statistical analyses were performed using GraphPad Prism version 10.3.1 (GraphPad Software, Inc., Boston, USA). Statistics were calculated using one-way ANOVA with Tukey test for multiple comparison. P-values were given in categories as p < 0.05, p < 0.01, p < 0.005, p < 0.001, p < 0.0005 and p < 0.0001. Effect size was calculated in SPSS version 29 (IBM SPSS Statistics, New York, USA). Cohen’s d was reported in categories (d > 0.2, d > 0.5 and d > 0.8).
CITRUS analysis
For the identification of stratifying biological signatures in a complex single cell dataset, multi-parameter flow cytometry data of LRS chamber products were analyzed using CITRUS algorithm available on Cytobank Premium platform (Beckman Coulter Life Sciences, Indianapolis, USA). Data were recompensated and analyzed in Kaluza (software version 2.2, Beckman Coulter) according to the described gating strategy (Fig. S3). Compensated events were exported through the CD45+ leukocyte gate and uploaded into Cytobank. After scaling and assigning the data to the corresponding treatment groups (Trima 6, Trima 7 and Trima 7 PAS), a CITRUS analysis was run using PAMR as predictive model with a minimum cluster size of 0.5%, a tenfold cross validation and a false discovery rate of 1.0%. Event counts were exported for all metaclusters and statistically analyzed and visualized using GraphPad Prism (software version 10.3.1).
Data availability
Data are available from the corresponding author upon reasonable request.
References
Boudreau, G. et al. Leukoreduction system chambers are a reliable cellular source for the manufacturing of T-cell therapeutics. Transfusion 59, 1300–1311 (2019).
Rocha, F. A. et al. Development of a highly cytotoxic, clinical-grade virus-specific T cell product for adoptive T cell therapy. Cell Immunol. 395–396, 104795 (2024).
Néron, S. et al. Characterization of mononuclear cells remaining in the leukoreduction system chambers of apheresis instruments after routine platelet collection: A new source of viable human blood cells. Transfusion 47, 1042–1049 (2007).
Tiscornia, I., Sánchez-Martins, V., Hernández, A. & Bollati-Fogolín, M. Human monocyte-derived dendritic cells from leukoreduction system chambers after plateletpheresis are functional in an in vitro co-culture assay with intestinal epithelial cells. J. Immunol. Methods. 384, 164–170 (2012).
Pfeiffer, I. A. et al. Leukoreduction system chambers are an efficient, valid, and economic source of functional monocyte-derived dendritic cells and lymphocytes. Immunobiology 218, 1392–1401 (2013).
Terumo, B. C. T. Trima Accel® Version 7 Automated Blood Collection System. Terumo BCT (2024).
Leitner, G. C. et al. Additive solutions differentially affect metabolic and functional parameters of platelet concentrates. Vox Sang. 110, 20–26 (2016).
Gulliksson, H. Platelet storage media. Vox Sang. 107, 205–212 (2014).
van der Meer, P. F. & de Korte, D. Platelet additive solutions: A review of the latest developments and their clinical implications. Transfus. Med. Hemother. 45, 98–102 (2018).
Thomas, K. A. et al. Comparison of platelet quality and function across apheresis collection platforms. Transfusion 63(Suppl 3), S146–S158 (2023).
Gansner, J. M. et al. Plateletpheresis-associated lymphopenia in frequent platelet donors. Blood 133, 605–614 (2019).
Rahmani, M. et al. CD4+ T-cell lymphopenia in frequent platelet donors who have ceased platelet donation for at least 1 year. Transfusion 59, 1644–1647 (2019).
Gansner, J. M. et al. Severe CD4+ T-cell lymphopenia is not observed in frequent plateletpheresis donors collected on the Fenwal Amicus. Transfusion 59, 2783–2787 (2019).
Thuer, L. et al. Total platelet donation count and donation frequency are determinants of plateletpheresis-associated lymphopenia. Transfusion 61, 3161–3173 (2021).
Zhao, J. et al. Frequent platelet donation is associated with lymphopenia and risk of infections: A nationwide cohort study. Transfusion 61, 464–473 (2021).
Kaufman, R. M. et al. Risk factors for T-cell lymphopenia in frequent platelet donors: The BEST collaborative study. Transfusion 63, 2072–2082 (2023).
Bruggner, R. V., Bodenmiller, B., Dill, D. L., Tibshirani, R. J. & Nolan, G. P. Automated identification of stratifying signatures in cellular subpopulations. Proc. Natl. Acad. Sci. USA 111, E2770–E2777 (2014).
Hengst, J. et al. High-resolution determination of human immune cell signatures from fine-needle liver aspirates. Eur. J. Immunol. 45, 2154–2157 (2015).
Strasser EF, Weidinger T, Zimmermann R, Ringwald J, Eckstein R. Recovery of white blood cells and platelets from leukoreduction system chambers of Trima Accel and COBE Spectra plateletpheresis devices. Transfusion. ;47:1943–4 2007.
Naghinezhad, J. et al. Designing a linear accelerator-based “X irradiation system” for platelet products: An efficient, safe, accessible and cost-effective alternative for conventional X- or gamma irradiators. Sci Rep. 14, 28363 (2024).
Zipursky, A., Bow, E., Seshadri, R. S. & Brown, E. J. Leukocyte density and volume in normal subjects and in patients with acute lymphoblastic leukemia. Blood 48, 361–371 (1976).
Lee, A. J. et al. DAFi: A directed recursive data filtering and clustering approach for improving and interpreting data clustering identification of cell populations from polychromatic flow cytometry data. Cytometry A. 93, 597–610 (2018).
Mair, F. et al. The end of gating? An introduction to automated analysis of high dimensional cytometry data. Eur. J. Immunol. 46, 34–43 (2016).
Tremblay, M. M. & Houtman, J. C. TCR-mediated functions are enhanced in activated peripheral blood T cells isolated from leucocyte reduction systems. J. Immunol. Methods 416, 137–145 (2015).
Dietz, A. B. et al. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion 46, 2083–2089 (2006).
Strasser, E. F. & Eckstein, R. Optimization of leukocyte collection and monocyte isolation for dendritic cell culture. Transfus. Med. Rev. 24, 130–139 (2010).
Riquelme, P. et al. TIGIT(+) iTregs elicited by human regulatory macrophages control T cell immunity. Nat. Commun. 9, 2858 (2018).
Ebner, S. et al. Generation of large numbers of human dendritic cells from whole blood passaged through leukocyte removal filters: an alternative to standard buffy coats. J. Immunol. Methods 252, 93–104 (2001).
Kronenberg, K., Riquelme, P. & Hutchinson, J. A. Standard protocols for immune profiling of peripheral blood leucocyte subsets by flow cytometry using DuraClone IM reagents. Protocol Exchange (2019).
Hutchinson, J. A. et al. Virus-specific memory T cell responses unmasked by immune checkpoint blockade cause hepatitis. Nat. Commun. 12, 1439 (2021).
Glehr, G. et al. Restricting datasets to classifiable samples augments discovery of immune disease biomarkers. Nat. Commun. 15, 5417 (2024).
Acknowledgements
The authors are grateful to Blutspendedienst BRK GmbH and ITM Suhl gGmbH to provide LRS chamber products. The authors thank Erika Ostermeier, Christine Wagner-Bock, Anna-Luise Schmidl and Norbert Pichlmaier for their outstanding technical support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
This work was supported by the European Union as part of the Horizon EU IMMUTOL project (grant number 101080562).
Author information
Authors and Affiliations
Contributions
KK, JAH and VH designed experiments, analyzed data and wrote the manuscript. FD revised the manuscript. AB and IP provided clinical samples. RO provided expert opinion in Laboratory Medicine. EKG, JAH and RB provided infrastructural support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The studies involving human blood donors were reviewed and approved by Ethics Committee University of Regensburg (22-2780-101). All experiments were performed in accordance with relevant guidelines and regulations and in accordance with the Declaration of Helsinki. All participants gave full informed, written consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kronenberg, K., Dormann, F., Brosig, A. et al. Platelet apheresis with additive solution and plasma rinseback affects the cellular composition of LRS chamber products. Sci Rep 15, 19923 (2025). https://doi.org/10.1038/s41598-025-04350-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04350-4