Abstract
Micropterus salmoides, adaptable and fast-growing, is a major farmed fish in China. Intensive farming causes multiple diseases, especially hard-to-treat nocardiosis. Vaccines are an effective and safe prevention and control strategy against fish diseases. In this experiment, the highly antigenic HRP1 gene of Nocardia seriolae (N. seriolae) was displayed on the surface of Bacillus subtilis (B. subtilis), and an oral vaccine (HRP1-cotC-B. subtilis) was successfully developed. Immune responses in inoculated fish were tested intermittently over 5 weeks post-administration. Immune protection was evaluated via challenge test. Serological parameters testing showed that the activity of lysozyme and Glutathione Peroxidase (GSH-Px) were significantly increased at the 7th and 21st day post-immunization, and the activity of Alkaline Phosphatase was extremely increased compared to the control. qRT-PCR detection found that oral vaccine could significantly boost the IgM expression of spleen and head-kidney. The expression of spleen major histocompatibility complex (MHC) was enhanced, MHCII at the 7th, 21st day and MHCI at 35th day post-immunization respectively. MHCI has an increasing trend in the head-kidney. CD8 increased in both spleen and head-kidney at different stages of immunization. The inflammatory cytokine il-1β and anti-inflammatory cytokine TGF-β significantly increased in head-kidney at 35th day after immunization. The live bacterial vaccine altered the composition of the intestinal flora, demonstrated a decline in Firmicutes and an increase in Fusobacteria, and a significant decrease in Clostridium replaced by Cetobacterium at the genus level. Largemouth bass immunized with HRP1-cotC-B. subtilis spores exhibited a 18.18% relative survival rate after N. seriolae infection. In conclusion, this study developed a novel oral vaccine against N. seriolae in largemouth bass using B. subtilis spore surface display technology. Oral vaccination improves nonspecific immunity, induces innate and cellular immunity, strengthens bacterial resistance, and increases survival after pathogen infection. This offers an effective strategy for controlling fish nocardiosis in aquaculture.
Similar content being viewed by others
Introduction
According to statistics, the production of largemouth bass (Micropterus salmoides) has reached over 800,000 tons, and emerging as a significant commercial fish in aquaculture1. With intensive farming, largemouth bass are susceptible to invasion by various pathogens, nocardiosis is a difficult-treated bacterial disease in fish, resulting in serious economic losses2. Four nocardia spp., including Nocardia salmonicida, Nocardia asteroids, Nocardia crassostreae, Nocardia seriolae are mainly responsible for nocardiosis3,4,5,6. N. seriolae is one of the most frequently species associated with this disease. It is a gram-positive branching filamentous bacteria and intracellular parasitism. This property allows them penetrates and multiplies inside the phagocytes and other host cells, which may contribute to proliferate in the host to cause long-term chronic infections7. Fishes that are infected primarily through the gills, anus, lateral line, or surface lesions, and lead to numerous white granuloma and injury especially in the liver, kidney, spleen, intestine, and muscle8. Numerous epidemics of nocardiosis with significant mortality rates up to 100% have been documented due to the inability to see symptoms and treat them for a long time after infection9. Currently, the most effective treatment for N. serioale infection is antibiotic therapy, including sulfamonomehizol, sulfisizole, oxytetracycline (OTC)10,11. Although these antibiotics are valuable for the control of nocardiosis, there are some concerns about the increasing emergence of antibiotic-resistant N. seriolae isolates and serious environmental pollution12. It has been previously reported that the utilization of heated- or formalin-inactivated N. seriolae strain (low virulence) could induce high antibody titres and generated immunological responses, but it cannot protect fish against N. seriolae infection13. DNA vaccines and recombinant protein vaccines have been developed with a sensible and immune protection effect to combat N. seriolae14,15. However, the drawbacks of these strategies include deficiency of low virulent strains, need numerous booster immunizations, complex preparation procedures, infection risk and high costs making them difficult in practical aquaculture industry. To control nocardiosis in fish, a live vaccine with a convenient, efficient, and safe as preventative strategy is critically needed.
N. seriolae UTF1 have completed whole genome sequencing with a circular chromosome of 8,121,733 bp that encodes 7697 predicted proteins. The genome database has discovered orthologs of virulence components that are involved in host cell invasion, phagocyte function modification, and macrophage survival16,17. The release of genomic data will aid to screen the useful antigen genes for the development of vaccines against fish nocardiosis. Kato et al.18 found the N. seriolae antigen 85-like (Ag85L) gene provided Seriola dumerili with protective effectiveness and less bacterial count in the spleen. Heat shock proteins DnaK and GroEL are capable of could induce memory T cell response as strong immune stimulators19. Two immunodominant antigens, phage shock protein A (PspA) and tellurium resistance protein D (TerD) were identified from N. seriolae genome, the related vaccines were able to boost humoral and cell-mediated immune responses of hybrid snakehead (Channa Argus♂ × Channa Maculate♀)20. According to predictions, hypoxic response protein 1 (HRP1) has two cystathionine-β-synthase (CBS) domains, which is a possible antigen with utility in diagnosis and vaccine development of intracellular bacteria. It may play an important role in bacterial response to cellular stress and function well as an antigenic marker for persistent bacteria. Previous research utilized the HRP1 of Mycobacterium tuberculosis to create recombinant vaccine and demonstrated HRP1 could offer improved immunogenicity and protection against tuberculosis21. Hoang et al.22 produced recombinant TRX-tagged HRP1 protein (rHRP1) of N. seriolae, and proved it could provide better immunogenicity and protection. However, no study has been conducted on the use of the HRP1 gene to create an oral live vaccination.
Probiotics displaying functional antigens on their surfaces can serve as an attractive strategy for the development of oral live vaccines, which is easier administration and application, lower costs and save labor and time. Additionally, probiotics could potentially enhance the systemic and mucosal immune response by interacting with the host microbiota, making them a better suitable carrier for oral vaccines23. Live vaccines are increasingly being developed and used to prevent and control aquatic infections. The hirame novirhabdovirus (HIRRV) oral vaccine based on surface display produced high level of specific IgM and offering 60.7% protection against HIRRV infection24. Zhao et al.25 developed an oral yeast vaccine displayed the glycoprotein (G) of infectious hematopoietic necrosis virus (IHNV) strain, it could induce both innate and adaptive immune response of rainbow trout (Oncorhynchus mykiss). Bacillus subtilis, a gram-positive probiotic, has been widely used in aquaculture with probiotic effects such as promoting growth, improving water quality, resisting diseases, and regulating intestinal microorganisms26. Most importantly, B. subtilis could form spores in harsh environments makes it used as a classic delivery vehicle in vaccine development. Therefore, oral vaccines based on B. subtilis can avoid the disruption of activity and stability during feed preparation. Oral vaccines using B. subtilis spore-display technology have commonly explored against grass carp reovirus, vibrio in seabass, red-spotted grouper nervous necrosis virus (RGNNV) in juvenile grouper (Epinephelus coioides)27,28,29. The oral vaccine made based on the surface display method of probiotics can utilize the characteristics of probiotics or be directly added to feed. Probiotic display systems seem to be the preferable method and are increasingly being used in the development of aquatic vaccines.
Therefore, this study aims to prepare a B. subtilis spore-based vaccine, which displays the antigen HRP1 of N. seriolae on the surface of bacteria. The immune activation potential was determined by detecting serum non-specific immune indicators, as well as the expression level of immune related factors in the spleen, head-kidney at different immunization stages. 16S rRNA sequencing to investigate the interaction of vaccine and gut microbiota. Most importantly, N. seriolae challenge test was used to estimate the vaccine protective efficiency. This study provides a strategy for the prevention and treatment of nocardiosis in largemouth bass.
Materials and methods
Bacterial strains and growth conditions
B. subtilis 168 was used as the original strain for displaying the antigen and obtaining recombinant oral vaccine. Escherichia coli DH5α was used for the preparation and extraction of recombinant plasmids. And B. subtilis and E. coli DH5 was generally cultured in Luria–Bertani (LB) medium at 200 rpm overnight. The cultivation of B. subtilis competent cells and the induction of spore production was described in detail below. The original plasmid pDG364-N-4108 was used as a template for constructing the surface display recombinant plasmid in this experiment. The N. seriolae strain (NK20211208) was used as a pathogenic bacterium to test the effectiveness of the oral vaccine (preservation in our laboratory by Lecturer Fen Dong of Zhejiang Ocean University). Firstly, the bacteria were activated by brain–heart immersion solid medium (BHI) for 3–5 days at 25 °C, and single clone was selected for liquid expansion culture. The cultivation conditions were shaking at 25 °C, 180 rpm for 48 h, followed by plate colony counting. According to the counting results, the bacteria was collected and diluted with sterile PBS to a concentration of 2.5 × 105 cfu/ml for the challenge test.
Bioinformatic analysis of HRP1 and construction of recombinant plasmid
Antigenic HRP1 gene was selected from the genome DNA of N. seriolae (GenBank: AP017900.1). The secondary structure of HRP1 antigen gene was predicted using SMART online tool (SMART: embl-heidelberg.de), and the antigenic epitope was predicted using BepiPred-2.0 (BepiPred 2.0-DTU Health Tech-Bioinformatic Services). Prediction of tertiary structure was performed using Phyer2 software (PHYRE2 Protein Fold Recognition Server (ic.ac.uk), visualized in pymol and labeled epitopes site. The HRP1 antigen fragment was amplified using the template of extracted genome DNA of N. seriolae, and the his-tag sequence was added at its N-terminals. The primers for amplifying homologous arms are shown in Table 2. The genomic DNA of N. seriolae and the original plasmid pDG364-N-4108 were used as templates to amplify the homologous arms of the HRP1 gene and the vector respectively. Subsequently, these two homologous DNA fragments were purified and then connected by NEBuilder HiFi DNA Assembly Master Mix (E2621L, New England Biolabs, Inc.). The connected gene product has been transformed into E. coli DH5α, and the correct recombinant plasmid was determined through PCR identification and sequencing, names as HRP1-cotC-pDG364.
Preparation of oral vaccine
Preparation and transformation of competent cells of B. subtilis
B. subtilis monoclone was picked up and rinsed in 3 mL SPI (0.2% (NH4)2SO4, 1.4% K2HPO4·3H2O, 0.6% KH2PO4, 0.1% sodium citrate, 0.02% MgSO4·7H2O 0.02% peptone, 0.1% yeast powder, 0.5% glucose) with 600 μl 20% Tween 80 to incubate at 37 °C overnight. Take 200 μl culture to 10 mL fresh SPI medium, subsequently it was cultured at 37 °C, 200 rpm for 4.5 h until the logarithmic growth phase. Then the bacteria was added to fresh SPII medium (adding 0.5 mmol/l CaCl2 and 25 mmol/l MgCl2 on the basis of SPI medium) at a 10% ratio for another 1.5 h (37 °C, 100 rpm). And 10 mmol/l of EGTA (pH8.0) was supplemented to increase the permeability of the cell membrane, the competent cells of B. subtilis were successfully prepared after 10 min cultivation at 37 °C, 100 rpm.
The competent cells should be transformed immediately after preparation. The process of transformation was briefly described as follows: gently mix the HRP1-cotC-pDG364 plasmid (5–7 ug) with the competent cells, incubating for 30 min. Then the complex was cultured at 250 rpm for 1 h. Disperse the culture on LB plate (1% starch) with a concentration of 5 g/mL chloramphenicol to screen suspected positive clones. Chloramphenicol-resistant clones were further identified by PCR and sequencing to determine the correct connection and precisely insertion of HRP1-cotC.
Induction of B. subtilis spores and identification of fusion protein on the surface
HRP1-cotC-B. subtilis and native B. subtilis were cultured in Difco-Sporulation medium (DSM) for 48 h to induce spores production. The culture of spores was collected by high-speed centrifugation. Then the culture was diluted with sterile PBS buffer and subjected to plate counting for feed addition. Meanwhile, spores are washed and used for identification of fusion protein expression. Spores were first treated with decoating buffer (0.1 mol/L NaCl, 0.1 mol/L NaOH, 1% SDS, 0.1 mol/L DTT) at 70 °C for 1 h, during which they were oscillated continuously 4 times. By high-speed centrifugation (4 °C, 12,000 rpm, 10 min), spore coat proteins were collected from the supernatant, which was further performed the SDS-PAGE gel electrophoresis. Protein bands was transferred to the PVDF membrane and then fusion protein identified by western blotting using the first antibody of Anti-His Antibody (1:1000 dilution in PBS containing 5% skim milk, AB102-02, TIANGEN, Beijing, China) and second antibody of horseradish peroxidase (HRP) Conjugated Goat Anti-Mouse IgG (1:500 dilution in PBS containing 5% skim milk, CW0102S, CWBIO, China). This membrane was washed three times in TBST buffer for 10 min each time. Finally, the enhanced chemiluminescence (ECL) (Proteintech, America) was used for coloration and visualized by a ChemiDoc MP Imaging System (Bio-Rad, America).
Oral immunization and sample collection
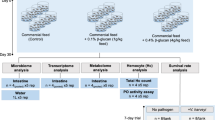
All the experiments were approved by the Ethics Committee of Zhejiang Ocean University (Approval number was 2023023). All methods were carried out in accordance with the relevant guidelines and regulations. This study adheres to the requirements of the ARRIVE guidelines in its reporting, the details are described as follows. The largemouth bass (5 ± 2 g) used in this experiment was provided by Zhengda Aquatic Products Co., Ltd (Huzhou, Zhejiang, China), and was maintained in aquarium tanks for two weeks at 28 °C before the official experiment. The experiment was divided into three groups: namely control group, B. subtilis group, HRP1-B. subtilis group. The basic feed formula for control group was shown in Table 1. The latter two groups were orally immunized with spores of B. subtilis and HRP1-cotC-B. subtilis respectively (spores at a concentration of 108 cfu/g basal diet). Each group was allocated a total of 120 largemouth bass to a 500 L aquarium tanks. The largemouth bass was satiety feeding twice a day, at 9:00 am and 16:00 pm. In the constructed circulating water system, the dissolved oxygen (> 7.0 mg/L) was provided by aeration and water flow. The water temperature was 25 ± 2 °C, and the ammonia nitrogen content was 0.23 ± 0.06 mg/L. During the rearing period, there was 12 h of light and 12 h of darkness. Blood, spleen, head-kidney sample were collected at week 1, week 3, week 5. Intestinal contents was obtained from 5 fish each group at week 5. The remaining fishes of each group were conducted the N. seriolae challenge test after a one week interval. Figure 1 depicted the immunization regimen and sampling management for oral vaccine.
Vaccination schedule and sampling management of oral vaccine. The image was drawn with the online software FIGDRAW 2.0. (https://www.figdraw.com/static/index.html#/).
Detection of serum immune-related enzyme activity
Blood samples was extracted from the caudal vein of fish using a sterile syringe, and put it at room temperature for 1 h. The serum sample was collected subsequently by centrifuging at 4 °C, 5000 rpm, for 10 min. The alkaline phosphatase (AKP), acid phosphatase (ACP), Glutathione peroxidase (GSH-Px) and lysozyme (LZM) activity of serum was measured according to the manufacturer’s instructions (Nanjing Jiancheng Institute, China).
Relative expression of immune-related gene detected by qRT-PCR
Total RNA of spleen and head-kidney of largemouth bass were isolated using Trizol reagent (CW0580S, Jiangsu Cowin Biotech Co., Ltd). The brief steps are as follows: the samples were homogenized for 5–10 s in 1 ml Trizol reagent, and centrifuged at 4 °C, 12,000 rpm, for 10 min. The supernatant was transferred to new RNA-free EP tubes, and 200 μl chloroform was added. Subsequently, it was shaken vigorously, the layered supernatant was precipitated with isopropanol of equal volume. 1 mL 75% absolute ethanol was to purify RNA for two times. The concentration of RNA was measured using an Ultra micro spectrophotometer (NanoDrop 2000, Thermo). And the corresponding cDNA samples were obtained through reverse transcription (TIANGEN, China).
The relative expression levels of innate immune-related interleukin 1β (il-1β), interleukin 10 (il-10), transforming growth factor-β (TGF-β), tumor necrosis factor α (TNF-α), histocompatibility complex class II (MHCII), histocompatibility complex class I (MHCI), T-box expressed in T cell (T-bet) and adaptive immune-related immunoglobulin M (IgM), cluster of differentiation 4 (CD4), cluster of differentiation 8(CD8) were evaluated using qRT-PCR. The primers used in qRT-PCR were shown in Table 2. The three-step reaction was carried out using SYBR Green SuperReal PreMix Plus kit (TIANGEN, China), The PCR condition was as follows: 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s, 57 °C for 30 s, annealing temperature adjusted slightly. After amplification, the CT values was exported, and relative expression level was analyzed using 2−ΔΔCT method with actin as the reference gene.
16S rRNA high-throughput sequencing of gut microbiota
At the 5th week after oral vaccine immunization, intestinal contents were extracted from all groups for 16S rRNA high-throughput sequencing (Shanghai Majorbio Bio-pharm Technology Co., Ltd). The genomic DNA was extracted using bacterial genomic DNA extraction kit (TIANGEN, China). The V3-V4 region of 16S rRNA was amplified using U338F(ACTCCTACGGGAGGCAGCAG) and U806R(GGACTACHVGGGTWTCTAAT). The sequenced data was concatenated and optimized used DADA2. And then the Naive Bayes classifier in Qiime2 was applied to perform taxonomic analysis on Amplicon Sequence Variants (ASVs) representative sequences, and the community species composition of each sample was statistically analyzed. LEfSe based on the non parametric Kruskal Wallis (KW) sum rank test was used to detect significantly different species from the phylum level to the genus level, and LDA linear discriminant analysis was estimated the impact of these different species on inter group differences. Data analysis and plotting were conducted on the majorbio Cloud platform.
N. seriolae challenge
After 5 weeks of oral administration, fish vaccinated with HRP1-B. subtilis spores, B. subtilis spores, PBS (20 largemouth bass of each tank, 3 repetitions per group) was selected for intraperitoneal (IP) injection with 100 μl of N. seriolae (with a concentration of 2.5 × 105 cfu/ml dissolved in PBS). Each group of injected fish was randomly assigned to three repeating tanks, reared at 25 °C for 14 days. Furthermore, the mortality of largemouth bass was monitored daily in control and vaccinated group. The immune effect of the vaccine was evaluated by RPS, the RPS was calculated with the following formula as: [1 − (vaccine group mortality %/control group mortality%)] × 100. The cumulative mortality rate and RPS of each experimental group and control group was calculated and compared using the mean value of three duplicates.
Statistic analysis
The data from three independent experiments were represented as mean ± standard error. Serological testing, qRT-PCR data analysis were all conducted using Student’s t-test to compare the differences between the two groups. Data related gut microbiota was utilized one-way ANOVA by Duncan’s test (Except for the LEfSe analysis). The Kaplan–Meier survival curve of post-challenge was statistically analyzed by log-rank (Mantel Cox) test using GraphPad Prism version 8.0.2 (GraphPad Software Inc., USA). p < 0.05 regarded as statistically significant, p < 0.01 and p < 0.001 regarded as extremely significant.
Results
Construction of recombinant plasmid and identification of HRP1-cotC expression on spore surface
The secondary structure of the HRP1 antigen and construction of plasmid and flowchart displayed on the surface was shown in Fig. 2. The HRP1 gene was cloned from the chromosomal DNA of N. seriolae. Then it was fused with linker and cotC sequence. The fusion fragment inserted into the plasmid according to the surface display design using the methods of homologous recombination. The precise insertion of HRP1 was determined through PCR and sequencing, and the recombinant plasmid HRP1-cotC-pDG364 was obtained.
Principles of plasmid construction and flow chart of oral vaccine preparation. The image was drawn with the online software FIGDRAW 2.0. (https://www.figdraw.com/static/index.html#/).
The screening of positive clones and expression identification of fusion protein were shown in Fig. 3. Firstly, the design of the surface display expression cassette was shown in Fig. 3A. After the plasmid was transformed into B. subtilis, the bacteria were spread on a starch plate containing iodine solution. And it was found that some colonies had transparent circles around them, while others did not. Due to the insertion sequence disrupting amylase gene of B. subtilis, the bacteria without amylase activity were considered positive clones. The positive clones were further identified by PCR amplification (Fig. 3B). Meanwhile, we analyzed the structure of the HRP1 protein and found that the amino acid sequences of the two CBS domains were located at positions 11–59 and 77–124. Moreover, the three antigenic epitopes are: D6-A10, Q63-V68, and V128-G134, which are wrapped with white cross hatch in the 3D structure (Fig. 3C). The recombinant bacteria was induced to spores after 48 h, and the spore coat protein of HRP1-cotC-B. subtilis and native B. subtilis was extracted. A 26 kDa protein band was identified in HRP1-cotC-B. subtilis rather than the B. subtilis (Fig. 3D). The results showed that the HRP1 protein was undoubtedly displayed on the spore surface of B. subtilis fused with cotC protein. The HRP1-cotC-B. subtilis was extensively fermented for 48 h and then subjected to nutritional starvation to induction the spores, which was used as an oral vaccine to immunize largemouth bass.
Successful construction of HRP1-cotC-pDG364 recombinant plasmid and identification of CotC-HRP1 fused protein of recombinant spores by Western blotting. (A) Schematic diagram of HRP1-cotC-pDG364 recombinant plasmid construction. (B) Confirmation of positive clones by PCR after recombinant plasmid transformation. Lane M: DNA marker, Lane 1–2: randomly selected negative clones, Lane 3–4: randomly selected positive clones. (C) The 3D structure of the HRP1 antigen protein and the ___location of the three main epitopes site. (D) The expression of cotC-HRP1fusion protein of recombinant spores by Western blotting. Lane M: protein marker, Lane 1: protein of B. subtilis spores, Lane 2: protein of recombinant HRP1-cotC-B. subtilis spores, Lane 3: negative control.
Detection of serum non-specific immune parameters
The effect of oral vaccine on the serum non-specific immune parameters of largemouth bass was shown in Fig. 4. Oral vaccine has no effect on the activity of serum ACP activity throughout the entire immune process (Fig. 4A). However, the HRP1- B. subtilis group caused a significant increase of AKP activity on the 35st day after oral immunization (p < 0.001, Fig. 4B). On the 7th day after vaccination, the serum lysozyme content was extreme significantly elevated in HRP1-B. subtilis group compared to control group, while B. subtilis group did not (p < 0.001, Fig. 4C). Serum GSH-Px activity in HRP1-B. subtilis group were significant higher than the control group on the 7th day after immunized, which lasted until the 21st day (p < 0.01, p < 0.05; Fig. 4D).
The effect of oral vaccine immunization on serum non-specific immune indicators. ACP activity (A), AKP activity (B), Lysozyme content (C), GSH-Px activity (D) was measured at week1, week 3, week 5 post-immunization. The mean value (SEM) (n = 5–6) was used to represent the data, *p < 0.05, **p < 0.01 and ***p < 0.0001 comparsion to the control group.
Expression of immune-related genes of spleen and head-kidney
The spleen and head-kidney are important immune organs in fish. On the 7th, 21st, and 35th day after oral immunization, the qRT-PCR experiment was used to detect the immune and inflammatory responses of them. Spleen IgM showed significant increase on the 7th and 21st day after immunization, but a significant increase was observed in the head-kidney on final phase of immunization (p < 0.05; Figs. 5A and 6A). Spleen MHCII showed a significant increasing trend compared to the control group at the 7th and 21st day (p < 0.05, p = 0.09; Fig. 5B), However, MHCI reach the peak at 35th day after immunization (p < 0.05; Fig. 5C). There was a tendency of increased MHCI during the immune process, while there was no significant difference in MHCII in the head-kidney (Fig. 6B,C). There was no significant differences of CD4 throughout the immune process in both the spleen and head-kidney (Figs. 5D and 6D). Spleen CD8 was significantly higher than the control group 21st and 35th day (p < 0.05; Fig. 5E), while this elevated change appeared in the head-kidney on 7th and 21st days (p < 0.05; Fig. 6E). In contrast, the TNF-α levels of head-kidney was significantly rised in the head-kidney but not in the spleen (Figs. 5F and 6F). The inflammatory cytokine il-1β in the spleen decreased 35th days after immunization, while there was no obvious change in head-kidney (Figs. 5G and 6G). The anti-inflammatory cytokine TGF-β showed a decreasing trend after 21 days of spleen (p = 0.08; Fig. 5H), while there was a remarkably higher than control group in the head-kidney on 35th day after immunization (p < 0.01; Fig. 6H). Il-10 showed no significant changes in both the spleen and head-kidney after oral immunization (Figs. 5I and 6I). Our detection found that expression of T-bet in the head-kidney was about four-fold higher in HRP1-B. subtilis group than that in control group on the 35th day after immunization (Fig. 6J). The results demonstrated that HRP1-B. subtilis spores triggered innate and adaptive immunity against N. seriolae.
Oral vaccine alters the structure of gut microbiota
Therefore, intestinal contents were collected after 5 weeks of immunization to perform 16S rRNA high-throughput sequencing. Illumina PE300 has completed sequencing of control and vaccinated fish, and 843,768 high-quality sequences with an average sequence length of 421 bp were obtained through Qiime2 optimization. The paired-end sequences for all samples have been deposited in the NCBI SRA (accession number PRJNA1253184). The α-diversity analysis revealed no significant differences in the evenness and richness of gut microbiota (Fig. 7A, Table 3). β-diversity analysis based on Bray–Curtis PCoA showed that significant separation on the PC1 axis, indicating that oral vaccine significantly altered the structure of microbial community (Fig. 7B). The predominant gut bacterial of largemouth bass at phyla level was Firmicutes, Fusobacteria, and Proteobacteria feeding with control, B. subtilis spores, HRP1-B. subtilis spores (Table 4, Fig. 7C). At the genus level, it is manifested as Staphylococcus, Clostridium, Cetobacterium, Clostridiaceae was the core microorganism (Table 5, Fig. 7D). Compared to the control group, feeding with HRP1-B. subtilis spores significantly reduced the relative abundance of Firmicutes (56.42% vs. 88.13%, p < 0.05) and increased the abundance of Fusobacteria (5.7% vs. 40.97%, p < 0.05). At the genus level, the relative abundance of Staphylococcus significantly decreased in HRP1-B. subtilis group compared to control group (12.08% vs. 86.87%, p < 0.001). On the contrary, the relative abundance of Clostridium (0.12% vs. 25.43%, p < 0.05) and Cetobacterium (5.7% vs. 40.98%, p < 0.05) in HRP1-B. subtilis group was higher than that in the control group. LEfSe analysis employed linear discriminant analysis (LDA) was shown in Fig. 7E, suggesting that the genus Staphylococeales and Clostridiaceae, Peptostreptococcaceae and are likely associated with control and HRP1-B. subtilis.
The effect of live recombinant vaccine on the gut microbiota structure of largemouth bass. (A) α-diversity analysis (ACE, Chao, Shannon, Simpson, Coverage) demonstrated the impact of oral vaccine on the richness and diversity of gut microbiota. (B) Using the Bray Curtis index for principal coordinate analysis (PCoA), evaluate β-diversity of intestine microbiota among the three groups. (C,D) Community composition analysis of gut microbiota at the phylum and genus levels among three groups. (E) LEfSe based on linear discriminant analysis (LDA) of microbial communities to analyze the impact of different species on grouping.
Immune protection efficiency of oral vaccine
After 5 consecutive weeks of oral immunization, the three groups were vaccinated with HRP1-B. subtilis spores or B. Subtilis spores or PBS fish are challenged with N. seriolae. The mortality rate is mainly concentrated in the 3–8 days after infection, and the survival rate of the control group sharply decreased from 59.7 to 8.1%. However, the survival rate of the oral vaccine group only decreased from 70% on day 3 to 31.7% on day 8. The cumulative mortality rate of the HRP1-B. subtilis group (75 ± 13.23%) was significantly lower than the B. subtilis group (90 ± 13.23%) and the control group (91.67 ± 5.77%) at the end of the 14th day. Therefore, the corresponding RPS of HRP1-B. subtilis spores or B. Subtilis spores compared to the control was 18.18% and 1.82%, respectively. Furthermore, combining the survival curve shown in Fig. 8, we could conclude that oral vaccine significantly increased the resistance of largemouth bass to N. seriolae.
Cumulative survival rate of largemouth bass after challenged with N. seriolae. The number of deaths of largemouth bass after challenging was monitored daily for 14 days. The survival rate was analyzed by Kaplan–Meier survival curve with log-rank (Mantel Cox) test. The p-value is an significant differences analysis between the immunised and the control group using log-rank test (*p < 0.05, **p < 0.01).
Discussion
Nocardiosis is a greatest cause of high morbidity and mortality in a wide range of fish. It has been documented that approximately 42 different species of freshwater and marine fish infected by N. seriolae30. Nocardia species grow slowly, and the induced diseases may not show any symptoms at all31. In addition, N. seriolae inhibits the production of reactive oxygen species and releases large amounts of nitric oxide, and inhibition of apoptosis to survive inside the host macrophages32. Therefore, treatment and prevention of it are extremely challenging because of diagnosis difficulties and immune escape. Currently, there are no proven anti-nocardiosis treatments available in the market, despite the fact that nocardiosis generates significant financial losses for the global aquaculture industry. Oral vaccine can generate powerful and long-lasting humoral and cellular immunity, as well as provide benefits in terms of safety, environmental friendliness and long-term effectiveness protection, making them appealing in vaccine development. The preparation of an oral nocardia vaccine is expected to be a promising approach to combat nocardia infection.
B. subtilis is a generally considered safe (GRAS) bacteria, and its spores have extreme resistance to dry heat, acidic environment of the gastrointestinal tract, and a variety of toxic chemicals33. This characteristics enable it to maintain activity passage through feed preparation and gastrointestinal digestion, making it become a promising delivery carrier for different molecules by surface display technology34. Spores can also be addition directly to animal diet with a simple production technique and achieve a long and robust shelf-life without refrigeration, which is very attractive for industrial applications35. In addition, it can exert a vaccine adjuvant effect and then promote antibody production after co-administration with antigens35. Most importantly, oral administration incorporated with feed seems to be the preferable method and a number of vaccines based B. subtilis spores displayed system has gradually increased in recent years28,36,37,38. And several of the outer coat proteins (e.g. cotC, cotG, oxdD, cotZ) from B. subtilis spores are frequently selected for surface display systems as anchors fused to the N-terminus or C-terminus of the antigen39. CotC, 12 kDa, due to its relatively high abundance in the out coat, can achieve efficient expression fused with exogenous protein40. These facts make cotC of B. subtilis the best choice for a vaccine delivery system. In this study, we intends to use the anchoring protein cotC as a carrier to display the antigen protein HRP1 on the surface of B. subtilis spores. Through verification of fused protein expression, the oral vaccine HRP1-cotC-B. subtilis was successfully developed by spore delivery system. And it was then added to the diet of largemouth bass for consecutive 5 weeks, and the protective effectiveness post-immunization and preliminary mechanism of immune response were evaluated. This study suggested that HRP1-cotC-B. subtilis can be used as a potential candidate vaccine to achieve green, safe and convenient prevention and control of nocardiosis in fish.
The innate immune system was the first defense line of host, and teleost fish rely on innate immunity response coping with the invasion of pathogens under different conditions41. It has been demonstrated that activation of the innate immune system are essential to the function of acquired immunity42. The innate immune parameters in fish have been widely investigated, and their components are typically classified as physical, cellular, and humoral variables43. Lysozyme, as an important humorous parameter for immune defense, has bactericidal capacity through hydrolyze the β-linked glycoside bonds of peptidoglycans on bacteria cell walls. And it can activate the complement system and phagocytes, determining phagocytes’ ability to successfully eliminate phagocytic pathogens44. This study found that the level of serum lysozyme have reached its peak after 7 days of oral vaccine immunization. The increase of serum lysozyme may contribute to bacterial removal in immunized fish at early stage of N. seriolae infection. This result was also consistent with other nocardia vaccines, especially when HRP1 was used as a antigenic gene45,46. AKP and ACP are two important enzymes that can serve as physiological indicators stress and immune response of fish. A large number of studies have shown that its activity was closely related to cellular and humoral immune activity. And it can improve the immune capacity of animals and promote the elimination of bacteria and viruses47,48. They can help fish resist infections by neutralizing and clearing pathogenic microorganisms and their produced toxins. They are also the major components of the lysosome system, participated in inflammatory response during infection by eliminating and hydrolyzing microbes49. In present study, we found the oral vaccine caused a significant increase of AKP. The elevation of these indicators was beneficial for mitigation and prevention of tissue damage, consistent with the results of other studies47,50. The serological immune response results indicate that oral vaccine may eliminate subsequent bacteria infection by activating innate immunity.
In teleost fish, the spleen and head-kidney are the largest peripheral lymphoid and immunocompetent organs51. The anterior kidney is mostly composed of macrophages, lymphoid cells, which are predominantly exist as Ig+ cells (B cells)52. Fish spleen contains collections of T lymphocytes, B lymphocytes, macrophages and dendritic cells, that plays an active role in phagocytosis and capture of antigens53. B lymphocytes play a pivotal role in producing immunoglobulin molecules, such as IgM, IgD, IgT in teleost. IgM of largemouth bass was thought to be an important component of specific humoral immunity, and its concentration has proven a valid marker for assessing fish immunotoxicity54,55. In present study, the peak of IgM expression in the spleen was received at 21th day post-immunisation, and in the head-kidney at 35th day. This result indicates that oral vaccine effectively could stimulate humoral immunity of spleen and head-kidney. This is similar to the effect of increased IgM expression caused by inactivated vaccines of N. seriolae56. There is no doubt that humoral immunity can block microbial adherence or invasion, thereby preventing disease. In addition, cell-mediated immunity (CMI) that protects against intracellular and facultative intracellular type of pathogens is more important. CMI eliminates viral, bacterial, or parasitic infections through coordinated interactions between different T cell subsets and their signature cytokines57. In particularly, cytotoxic T lymphocytes (CTLs) play an important role in eliminating intracellular bacteria. CD8 CTLs are vital in the elimination of germs because they kill cells that contain bacteria in their cytoplasm. Meanwhile, CD4 T cells can activate macrophages and stimulate the activity of natural killer cells and cytotoxic T cells to eliminate ingested bacteria that can survive phagocytosis58. In this study, we found that spore-based oral vaccine could increase the expression of CD8 instead of CD4. This result indicates that the oral vaccine clears intracellular bacteria by inducing CD8 CTLs in the head-kidney and spleen to activate CMI. A previous study also demonstrated that the number of IgM and CD8a T cells was significantly upregulated while CD4 T cells remained unchanged after infection of N. seriolae56. MHCIα, a molecule responsible for displaying peptide fragments degraded by intracellular pathogens to generate cytotoxic CD8 T cells, while MHC-II-specific signals generate cytotoxic CD4 T cells59. In this experiment, we found that the increase in MCHI of the spleen can be attributed to the production of CD8 cells, while the elevation of MHCII in the spleen failed to activate CD4 cells. Upregulation expression of MHCIα confirmed oral vaccine excite CMI once again. Microbial infections often induce chemokine and cytokine cascades. These cytokine and chemokine secreted by immune cells control and coordinate innate and adaptive immune responses in protection against bacterial and viral infections60. TNF-α is mainly secreted by macrophages and lymphocytes, regulating the inflammatory response and cellular immune response. In our investigation, we found that the expression level peaked in head-kidney instead of the spleen at 35th days after vaccination. Il-1β-expressing cells are recruited to specific tissues following bacterial infection. It has been demonstrated that il-1β was induced to be highly expressed in Paralichthys olivaceus and N. seriolae infection61. In our study, oral vaccine did not cause significant changes in expression of il-1β. TGF-β is an anti-inflammatory cytokine that regulates inflammation through controlling interleukins62. TGF-β was highly expressed in the head-kidney of immunized-fish at 35th day, which may help maintain immune balance in fish after oral immunized. The elevated expression of TNF-α and TGF-β suggests that immunized fish may maintain a high immune response without causing inflammation. All these results indicated that the protective humoral immune response and CMI were induced by immunization with HRP1-cotC- B. subtilis.
The gut microbiota was a complex ecosystem that affects the intestinal homeostasis of host. Especially, the gut microbiota as an important component of mucosal immunity can shape the intestinal immune system63. The gut microbiota trigger innate immunity and adaptive immunity through interactions with macrophages, dendritic cells (DCs), neutrophils, and T, B lymphocytes respectively64. For example, Clostridia species can promote naive CD4 T cells differentiate into activated Treg cells to induce the adaptive immunity. And microbial metabolites of Clostridia can also stimulate the secretion cytokine TGF-β, which promote Treg cell differentiation65. Butyric acid, a metabolite of Clostridium butyricum, can regulate gene expression and function of CD8 T lymphocytes66. The latest research indicated that the expression of MHCII on Tim4+ macrophages was controlled by the microbiota, and microbiota dysbiosis leads to downregulation of MHCII expression in the colon67. Research has shown that the composition and function of gut microbiota played an important role in modulating vaccine immunogenicity68. Antibiotic-induced changes in the structure of the intestinal flora lead to impaired antibody responses to vaccination69. Immunizing germ-free mice with Streptococcus dramatically reduced B-1 cell clonotypes and specific blood antibodies, which were restored after bacterial colonization70. This may be due to the fact that microbial antigens determine the clonality of the mature B-1 cell repertoire and the subsequent antibody response. The study demonstrate that the antigen conserved in gram-negative symbiotic bacteria can drive microbiota-specific protective IgG, providing essential defense against systemic Salmonella infection71. Considering the important role of microbiota and its regulation of vaccine-induced immune response, this study conducted gut microbiota sequencing after oral immunization. β-diversity analysis showed that the intestinal flora of the HRP1-B. subtilis and B. subtilis group were significantly different from the control group. The community composition at the phylum level showed a decrease in the abundance of Firmicutes and an increase of Fusobacteria. one of the dominant bacterial phyla present across vertebrate classes, fluctuates between promoting host resistance to infection or inducing more severe inflammatory damage, which depends on the host’s health status72. Recent studies have shown that Fusobacteria can serve as potential probiotics, with their intestinal abundance significantly decreasing during infection. Consistently, after N. seriolae infection, the immunity of the oral vaccine significantly increased their abundance73. At the genus level, the decrease in Staphylococcus was filled by Clostridium and Cetobacterium, especially in HRP1-B. subtilis group. Staphylococcus has been reported as a pathogenic bacterium, which can cause high mortality rates in fish after infection74. Existing studies have shown that Cetobacterium genus, as a potential intestinal probiotic, has anti-disease effects75. Increased Clostridium may enhance the cellular immune response of vaccines by promoting the maturation and differentiation of T cells and the release of cytokines. In short, oral vaccination may boost vaccine efficacy by altering the gut microbiota composition and raising the abundance of relevant disease-resistant genus.
In this experiment, the survival rate after 5 weeks immunization is 100%, indicating that long-term oral administration of B. subtilis spores (data not shown) without any visible harmful effect. There was almost no difference in survival rate between the native spore-vaccinated and control group after N. seriolae challenge. But what makes this work meaningful is more importantly the cumulative mortality rate of recombinant spores-vaccinated fish 75% was significantly lower than the native spore-vaccinated 90% and the PBS-vaccinated 91.67%. This indicated that the recombinant spores provide protection against N. seriolae infection. This may be explained by the activation of humoral and cellular immunity of oral vaccine. Meanwhile, the increase in the abundance of disease-resistant bacteria may contribute to the vaccine efficiency. These factors together contributed to the immune protection of the vaccine after N. seriolae challenge. It has been reported that the anti-nocardia DNA vaccines has the RPS of 53.01–83.14% in hybrid snakehead16,76,77. Three recombinant proteins NGL6936 (CBP), NGL3372 (LLE), and rHRP1were injected at 0.1 mg/fish for 3 times every 6 weeks, resulting in relatively high RPS of 50.00%, and 44.45%, 73.33% respectively22,78. This low immune protection performance of this oral vaccine may also be due to high pathogenicity and lethality of the N. seriolae used in this study. Of course, it may also owing to the fact that oral administration does not directly contact the fish’s immune system compared to injection method. Additionally, the amount of recombinant protein used for injection is much higher than that for oral delivery, and experimental fish is much larger than in this study. As orally delivered vaccines, have traditionally not featured well compared to the subunit vaccines. But oral vaccines could avoid complex protein purification, immune procedures, mechanical damage, and the inability of juvenile fish to use them. Consequently, the display of antigens by B. subtilis spores remains a powerful oral vaccine delivery platform. Oral vaccines could achieve better results in future aquaculture by fusing other antigen proteins, increasing the dosage, extended immunity time or combining immunization in the later stage.
Conclusion
The HRP1 antigen gene fused with anchoring protein cotC was successfully displayed on the surface of B. subtilis to develop an recombinant oral vaccine based-spores: HRP1-cotC-B. subtilis. It can successfully improve serum nonspecific immunity after the continuous immunization for 5 weeks. qRT-PCR detection of immune-related gene expression revealed that the vaccine can activate humoral immunity and cellular immunity of vaccined fish. 16S rRNA sequencing showed that the structure of intestinal flora changed dramatically, and the relative abundance of disease-resistant Cetobacterium increased. Importantly, it can significantly trigger the immune protection rate after N. seriolae invasion. Further considering its low price, convenience and safety, the genetically engineered-recombinant vaccine can be regarded as a candidate vaccine to prevent nocardiosis.
Data availability
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/search/all/?term=PRJNA1253184 And this Sequence Read Archive (SRA) submission will be released on 2026–05-01 or upon publication, whichever is first. And further enquires can be made to the corresponding author.
References
According to National Bureau of Statistics of China. China Fisheries Statistical Yearbook (2023).
Maekawa, S. et al. Current knowledge of nocardiosis in teleost fish. J. Fish Dis. 41(3), 413. https://doi.org/10.1111/jfd.12782 (2018).
Isik, K. et al. Nocardia salmonicida nom. Rev., a fish pathogen. Int. J. Syst. Evol. Microbiol. 49(2), 833–837. https://doi.org/10.1099/00207713-49-2-833 (1999).
Baradkar, V. P. et al. Sporotrichoid pattern of cutaneous nocardiasis due to Nocardia asteroids. Indian J. Pathol. Microbiol. 51(3), 432–434. https://doi.org/10.4103/0377-4929.42553 (2008).
Engelsma, M. Y., Roozenburg, I. & Joly, J. P. First isolation of Nocardia crassostreae from pacific oyster Crassostrea gigas in Europe. Dis. Aquat. Org. 80(3), 229. https://doi.org/10.3354/dao01938 (2008).
Shimahara, Y. et al. Genetic and phenotypic comparison of Nocardia seriolae isolated from fish in Japan. J. Fish Dis. 31(7), 481–488. https://doi.org/10.1111/j.1365-2761.2008.00920.x (2010).
Hou, S. et al. Identification of a cell-wall peptidase (NlpC/P60) from Nocardia seriolae which in duces apoptosis in fathead minnow cells. J. Fish Dis. 43(5), 571–581. https://doi.org/10.1111/jfd.13154 (2020).
Hyun, J. et al. Genomic characterization of Nocardia seriolae strains isolated from diseased fish. MicrobiologyOpen 8(3), e00656. https://doi.org/10.1002/mbo3.656 (2018).
Wang, F. et al. Nocardia seriolae infection in cultured jade perch, Scortum bar coo. Aquac. Int. 25(6), 2201–2212. https://doi.org/10.1007/s10499-017-0184-4 (2017).
Ismail, T. F. et al. Modified resazurin microtiter assay for in vitro and in vivo assessment of sulfamonomethoxine activity against the fish pathogen Nocardia seriolae. Fish. Sci. 78(2), 351–357. https://doi.org/10.1007/s12562-011-0450-8 (2012).
Imajoh, M. et al. Draft genome sequence of erythromycin- and oxytetracycline-sensitive Nocardia seriolae strain U-1 (NBRC110359). Genome Announc. 4(1), 10–128. https://doi.org/10.1128/genomeA.01606-15 (2016).
Akiyama, K., Hirazawa, N. & Hatanaka, A. The absorption enhancing effect of citric acid on oral oxytetracycline treatment against Nocardia seriolae infection in yellowtail, Seriola quinqueradiata. Aquaculture 500, 464–468. https://doi.org/10.1016/j.aquaculture.2018.10.047 (2019).
Itano, T. et al. Live vaccine trials against nocardiosis in yellowtail Seriola quinqueradiata. Aquaculture 261(4), 1175–1180. https://doi.org/10.1016/j.aquaculture.2006.09.006 (2006).
Nayak, S. K., Shibasaki, Y. & Nakanishi, T. Immune responses to live and inactivated Nocardia seriolae and protective effect of recombinant interferon gamma (rIFN γ) against nocardiosis in ginbuna crucian carp, Carassius auratus langsdorfii. Fish Shellfish Immunol. 39(2), 354–364. https://doi.org/10.1016/j.fsi.2014.05.015 (2014).
Shao, P. et al. A comparative study on the effect of two inactivated Nocardia seriolae vaccine immunization methods in pearl gentian grouper. Res. Sq. https://doi.org/10.21203/rs.3.rs-3089977/V1 (2023).
Yasuike, M. et al. Analysis of the complete genome sequence of Nocardia seriolae UTF1, the causative agent of fish nocardiosis: The first reference genome sequence of the fish pathogenic Nocardia species. PLoS ONE 12(3), e0173198. https://doi.org/10.1371/journal.pone.0173198 (2017).
Hashemi-Shahraki, A. et al. Genetic diversity and antimicrobial susceptibility of Nocardia species among patients with nocardiosis. Sci. Rep. 7(5), 17862. https://doi.org/10.1038/srep17862 (2015).
Kato, G. et al. Development of DNA vaccines against Nocardia seriolae infection in fish. Fish Pathol. 49(4), 165–172. https://doi.org/10.3147/jsfp.49.165 (2014).
Chen, J. et al. Identification of a mitochondrial-targeting secretory protein from Nocardia seriolae which induces apoptosis in fathead min now cells. J. Fish Dis. 42(11), 1493–1507. https://doi.org/10.1111/jfd.13062 (2019).
Chen, J. et al. Immunogenicity and efficacy of two DNA vaccines encoding antigenic PspA and TerD against Nocardia seriolae in hybrid snakehead. Fish Shellfish Immunol. 106, 742–754. https://doi.org/10.1016/j.fsi.2020.08.013 (2020).
Sharpe, M. L. et al. The structure and unusual protein chemistry of hypoxic response protein 1, a latency antigen and highly expressed member of the DosR regulon in Mycobacterium tuberculosis. J. Mol. Biol. 383(4), 822–836. https://doi.org/10.1016/j.jmb.2008.07.001 (2008).
Hoang, H. H., Wang, P. C. & Chen, S. C. The protective efficacy of recombinant hypoxic response protein 1 of Nocardia seriolae in largemouth bass (Micropterus salmoides). Vaccine 38(14), 2925–2936. https://doi.org/10.1016/j.vaccine.2020.02.062 (2020).
Yao, Y. Y. et al. Surface display system for probiotics and its application in aquaculture. Rev. Aquac. 12(4), 2333–2350. https://doi.org/10.1111/raq.12437 (2020).
Zhao, L. et al. Surface display of hirame novirhabdovirus (HIRRV) G protein in Lactococcus lactis and its immune protection in flounder (Paralichthys olivaceus). BioMed Central https://doi.org/10.1186/S12934-019-1195-9 (2019).
Zhao, J. Z. et al. Preliminary study of an oral vaccine against infectious hematopoietic necrosis virus using improved yeast surface display technology. Mol. Immunol. 85, 196–204. https://doi.org/10.1016/j.molimm.2017.03.001 (2017).
Elshaghabee Fouad, M. F. et al. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 8, 1490. https://doi.org/10.3389/fmicb.2017.01490 (2017).
Chen, D. D. et al. Comparative study of the immunoprotective effect of two grass carp-sourced Bacillus subtilis spore-based vaccines against grass carp reovirus. Aquaculture 504, 88–95. https://doi.org/10.1016/j.aquaculture.2019.01.055 (2019).
Gonçalves, G. et al. Oral vaccination of fish against vibriosis using spore-display technology. Front. Immunol. 13, 1012301. https://doi.org/10.3389/fimmu.2022.1012301 (2022).
Mai, W., Yan, B. & Xin, J. Oral immunizations with Bacillus subtilis spores expressing MCP protein provide protection against red-spotted grouper nervous necrosis virus (RGNNV) infection in juvenile grouper, Epinephelus coioides. Aquaculture 552, 738008. https://doi.org/10.1016/j.aquaculture.2022.738008 (2022).
Chen, G. et al. Function and characterization of an alanine dehydrogenase homolog from Nocardia seriolae. Front. Vet. Sci. 8, 801990. https://doi.org/10.3389/fvets.2021.801990 (2022).
Mehta, H. H. & Shamoo, Y. Pathogenic nocardia: A diverse genus of emerging pathogens or just poorly recognized?. PLoS Pathog. 16(3), e1008280. https://doi.org/10.1371/journal.ppat.1008280 (2020).
Liu, W. et al. Intracellular behavior of Nocardia seriolae and its apoptotic effect on RAW264.7 macrophages. Front. Cell. Infect. Microbiol. 28(13), 1138422. https://doi.org/10.3389/fcimb.2023.1138422 (2023).
Setlow, P. Spore resistance properties. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec (2014).
Sticato, R. & Ricca, E. Spore surface display. Microbiol. Spectr. 2(5), 0011–2012. https://doi.org/10.1128/microbiolspec.TBS-0011-2012 (2014).
De Souza, R. D. et al. Bacillus subtilis spores as vaccine adjuvants: Further insights into the mechanisms of action. PLoS ONE 9(1), e87454. https://doi.org/10.1371/journal.pone.0087454 (2014).
Copland, A. et al. Mucosal delivery of fusion proteins with Bacillus subtilis spores enhances protection against tuberculosis by Bacillus Calmette-Guérin. Front. Immunol. 9, 346. https://doi.org/10.3389/fimmu.2018.00346 (2018).
Oh, Y. et al. Bacillus subtilis spore vaccines displaying protective antigen induce functional antibodies and protective potency. BMC Vet. Res. 16(1), 259. https://doi.org/10.1186/s12917-020-02468-3 (2020).
Gao, Y. et al. Oral administration of Bacillus subtilis subunit vaccine significantly enhances the immune protection of grass carp against GCRV-II infection. Viruses 14(1), 30. https://doi.org/10.3390/v14010030 (2021).
Lin, P. et al. Progress in research and application development of surface display technology using Bacillus subtilis spores. Appl. Microbiol. Biotechnol. 104(6), 2319–2331. https://doi.org/10.1007/s00253-020-10348-x (2020).
Isticato, R. & Ricca, E. Spore surface display. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.TBS-0011-2012 (2014).
Ellis, A. E. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 25(8–9), 827–839. https://doi.org/10.1016/s0145-305x(01)00038-6 (2001).
Iwasaki, A. & Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16, 343–353. https://doi.org/10.1038/ni.3123 (2015).
Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 20(2), 137–151. https://doi.org/10.1016/j.fsi.2004.09.006 (2006).
Zhang, Q. et al. Growth performance, antioxidant and immunity capacity were significantly affected by feeding fermented soybean meal in Juvenile Coho Salmon (Oncorhynchus kisutch). Animals 13, 945. https://doi.org/10.3390/ani13050945 (2023).
Nguyen, P. T. D. et al. An Integrated in silico and in vivo study of nucleic acid vaccine against Nocardia seriolae infection in orange-spotted grouper Epinephelus coioides. Fish Shellfish Immunol. 143, 109202. https://doi.org/10.1016/j.fsi.2023.109202 (2023).
Hoang, H. H., Wang, P. C. & Chen, S. C. Recombinant resuscitation-promoting factor protein of Nocardia seriolae, a promissing vaccine candidate for largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 111, 127–139. https://doi.org/10.1016/j.fsi.2021.01.015 (2021).
Munang’andu, H. M., Paul, J. & Evensen, Ø. An overview of vaccination strategies and antigen delivery systems for Streptococcus agalactiae vaccines in Nile tilapia (Oreochromis niloticus). Vaccines (Basel) 4(4), 48. https://doi.org/10.3390/vaccines4040048 (2016).
Wei, J. J. et al. Effects of dietary folic acid on growth, antioxidant capacity, non-specific immune response and disease resistance of juvenile Chinese mitten crab Eriocheir sinensis (Milne-Edwards, 1853). Aquac. Nutr. 22(3), 567–574. https://doi.org/10.1111/anu.12275 (2016).
Yin, X. L. et al. Effect of guava leaves on growth and the non-specific immune response of Penaeus monodon. Fish Shellfish Immunol. 40(1), 190–196. https://doi.org/10.1016/j.fsi.2014.07.001 (2014).
Wu, X. et al. Protective cellular and humoral immune responses to Edwardsiella tarda in flounder (Paralichthys olivaceus) immunized by an inactivated vaccine. Mol. Immunol. 149, 77–86. https://doi.org/10.1016/j.molimm.2022.06.008 (2022).
Zapata, A. et al. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 20(2), 126–136. https://doi.org/10.1016/j.fsi.2004.09.005 (2006).
Uribe, C. et al. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 56(10), 486–503. https://doi.org/10.17221/3294-VETMED (2011).
Zapata, A. G. The fish spleen. Fish Shellfish Immunol. 144, 109280. https://doi.org/10.1016/j.fsi.2023.109280 (2024).
Shun, Y. et al. Comparison of the roles of IgM in systemic and mucosal immunity via tissue distribution analysis in largemouth bass (Micropterus salmoides). Aquaculture https://doi.org/10.1016/j.aquaculture.2020.735488 (2020).
Ye, J. et al. The teleost humoral immune response. Fish Shellfish Immunol. 35(6), 1719–1728. https://doi.org/10.1016/j.fsi.2013.10.015 (2013).
Nayak, S. K., Shibasaki, Y. & Nakanishi, T. Immune responses to live and inactivated Nocardia seriolae and protective effect of recombinant interferon gamma (rIFNγ) against nocardiosis in ginbuna crucian carp, Carassius auratus langsdorfii. Fish Shellfish Immunol. 39(2), 354–364. https://doi.org/10.1016/j.fsi.2014.05.015 (2014).
Garg, A. D. & Agostinis, P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol. Rev. 280(1), 126–148. https://doi.org/10.1111/imr.12574 (2017).
Tian, L. et al. CTLs: Killers of intracellular bacteria. Front. Cell. Infect. Microbiol. 12, 967679. https://doi.org/10.3389/fcimb.2022.967679 (2022).
Tang, X. et al. Recombinant NADP-dependent isocitrate dehydrogenase of Edwardsiella tarda induces both Th1 and Th2 type immune responses and evokes protective efficacy against edwardsiellosis. Vaccine 36(17), 2337–2345. https://doi.org/10.1016/j.vaccine.2018.03.022 (2018).
Gandhi, J., Dave, V. P. & Joseph, J. Cytokine profiling plays a crucial role in activating immune system in fungal endophthalmitis. Int. J. Infect. Dis. 101, 240. https://doi.org/10.1016/j.ijid.2020.11.062 (2020).
Tanekhy, M. et al. Expression of cytokine genes in head kidney and spleen cells of Japanese flounder (Paralichthys olivaceus) infected with Nocardia seriolae. Vet. Immunol. Immunopathol. 134, 178–183. https://doi.org/10.1016/j.vetimm.2009.09.001 (2009).
Jung-Schroers, V. et al. Response of the intestinal mucosal barrier of carp (Cyprinus carpio) to a bacterial challenge by Aeromonas hydrophila intubation after feeding with β-1, 3/1, 6-glucan. J. Fish Dis. 41, 1077–1092. https://doi.org/10.1111/jfd.12799 (2018).
Perez-Lopez, A. et al. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 16(3), 135–148. https://doi.org/10.1038/nri.2015.17 (2016).
Wang, J., He, M., Yang, M. & Ai, X. Gut microbiota as a key regulator of intestinal mucosal immunity. Life Sci. 345, 122612. https://doi.org/10.1016/j.lfs.2024.122612 (2024).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500(7461), 232–236. https://doi.org/10.1038/nature12331 (2013).
Luu, M. et al. Regulation of the effector function of CD8+ T cells by gut microbiota-derived metabolite butyrate. Sci. Rep. 8(1), 14430. https://doi.org/10.1038/s41598-018-32860-x (2018).
Guillaume, J. et al. MHCII expression on gut macrophages supports T cell homeostasis and is regulated by microbiota and ontogeny. Sci. Rep. 13(1), 1509. https://doi.org/10.1038/s41598-023-28554-8 (2023).
Lynn, D. J. et al. Modulation of immune responses to vaccination by the microbiota: Implications and potential mechanisms. Nat. Rev. Immunol. 22(1), 33–46. https://doi.org/10.1038/s41577-021-00554-7 (2022).
Hagan, T. et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell 178(6), 1313-1328.e13. https://doi.org/10.1016/j.cell.2019.08.010 (2019).
New, J. S. et al. Neonatal exposure to commensal-bacteria-derived antigens directs polysaccharide-specific B-1 B cell repertoire development. Immunity 53(1), 172-186.e6. https://doi.org/10.1016/j.immuni.2020.06.006 (2020).
Zeng, M. et al. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 44(3), 647–658. https://doi.org/10.1016/j.immuni.2016.02.006 (2016).
Jordan, C. K. I. et al. Symbiotic Firmicutes establish mutualism with the host via innate tolerance and resistance to control systemic immunity. Cell Host Microbe. 31(9), 1433-1449.e9. https://doi.org/10.1016/j.chom.2023.07.008 (2023).
Meng, K. F. et al. Interactions between commensal microbiota and mucosal immunity in teleost fish during viral infection with SVCV. Front. Immunol. 7(12), 654758. https://doi.org/10.3389/fimmu.2021.654758 (2021).
Plumet, L. et al. The zebrafish embryo model: Unveiling its potential for investigating phage therapy against methicillin-resistant Staphylococcus aureus infection. Antimicrob. Agents Chemother. 68(7), e0056124. https://doi.org/10.1128/aac.00561-24 (2024).
Xie, M. X. et al. Stabilized fermentation product of Cetobacterium somerae improves gut and liver health and antiviral immunity of zebrafish. Fish Shellfish Immunol. 120, 56–66. https://doi.org/10.1016/j.fsi.2021.11.017 (2022).
Chen, J. et al. Identification of a mitochondrial-targeting secretory protein from Nocardia seriolae which induces apoptosis in fathead minnow cells. J. Fish Dis. 42(11), 1493–1507. https://doi.org/10.1111/jfd.13062 (2019).
Chen, J. et al. Development of DNA vaccines encoding ribosomal proteins (RplL and RpsA) against Nocardia seriolae infection in fish. Fish Shellfish Immunol. 96, 201–212. https://doi.org/10.1016/j.fsi.2019.12.014 (2020).
Ho, P. Y. et al. Efficacy of recombinant protein vaccines for protection against Nocardia seriolae infection in the largemouth bass Micropterus salmoides. Fish Shellfish Immunol. 78, 35–41. https://doi.org/10.1016/j.fsi.2018.04.024 (2018).
Acknowledgements
We very much appreciated the contributions of Sai-xiang Feng (South China Agricultural University) for B. subtilis 168.
Funding
This work was supported by the Zhoushan Science and Technology Project (No: 2023C41023), the Natural Science Foundation of Zhejiang Province (No: TGD24C190001) and Talent Introduction Research Fund of Zhejiang Ocean University (JX6311032023).
Author information
Authors and Affiliations
Contributions
Fengli zhang conducted investigation, experimental operation, data acquisition and analysis, methodology, project administration, supervision, funding acquisition, and original manuscript preparation. Jitong Li, Tengyuan Zhang, Yuanyuan Yao conducted the investigation, experimental operation, data acquisition and analysis. Xiao Wang and Zhigang Zhou are responsible for supervision, review and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, F., Li, JT., Zhang, T. et al. Surface display of Nocardia seriolae HRP1 on Bacillus subtilis and its application as live vaccine for largemouth bass. Sci Rep 15, 23666 (2025). https://doi.org/10.1038/s41598-025-08150-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08150-8