Abstract
Neural stem cells (NSCs) of the ventricular-subventricular zone (V-SVZ) generate diverse cell types including striatal glia during the neonatal period. NSC progeny uncouple stem cell-related mRNA transcripts from being translated during differentiation. We previously demonstrated that Tsc2 inactivation, which occurs in the neurodevelopmental disorder Tuberous Sclerosis Complex (TSC), prevents this from happening. Loss of Tsc2 causes hyperactivation of the protein kinase mechanistic target of rapamycin complex 1 (mTORC1), altered translation, retention of stemness in striatal glia, and the production of misplaced cytomegalic neurons having hypertrophic dendrite arbors. These phenotypes model characteristics of TSC hamartomas called subependymal giant cell astrocytomas (SEGAs). mTORC1 inhibitors called rapamycin analogs (rapalogs) are currently used to treat TSC and to assess the role of mTORC1 in regulating TSC-related phenotypes. Rapalogs are useful for treating SEGAs. However, they require lifelong application, have untoward side effects, and resistance may occur. They also incompletely inhibit mTORC1 and have limited efficacy. Rapalink-1 is a bitopic inhibitor that links rapamycin to a second-generation mTOR ATP competitive inhibitor, MLN0128. Here we explored the effect of Rapalink-1 on a TSC hamartoma model. The model is created by neonatal electroporation of mice having conditional Tsc2 genes. Prolonged Rapalink-1 treatment could be achieved with 1.5 or 3.0 mg/Kg injected intraperitoneally every five days. Rapalink-1 inhibited the mTORC1 pathway, decreased cell size, reduced neuron dendrite arbors, and reduced hamartoma size. In conclusion, these results demonstrate that cellular phenotypes in a TSC SEGA model are reversed by Rapalink-1 which may be useful to resolve TSC brain hamartomas.
Similar content being viewed by others
Introduction
Tuberous Sclerosis Complex (TSC) is a multisystem genetic disorder affecting ~ 0.0166% of the population1. TSC is caused by loss of function mutations in TSC1 or TSC22,3. TSC1 and TSC2 encode for the proteins hamartin and tuberin which inhibit Rheb-mTORC1 signaling4. TSC1/2 loss of function mutations activate the mTORC1 pathway5,6,7,8. mTORC1 is a protein kinase complex that promotes cell growth and proliferation9. TSC patients have too much mTORC1 activity and enlarged cells that create tumors called hamartomas within the heart, kidney, lung, skin, and brain10,11. Identifying the mechanisms that cause hamartomas to form is important for understanding the many disorders whose genetic mutations affect molecular pathways that intersect with mTORC1 and for developing therapeutic strategies to treat TSC patients.
TSC patients have brain hamartomas called subependymal nodules (SENs) that invade the striatum and subependymal zone (SEZ)12,13. SENs are commonly detected during childhood3,13. Approximately a quarter of SENs are categorized as subependymal giant cell astrocytomas (SEGAs)12,13,14. SENs may transition into SEGAs15. SENs and SEGAs share all histopathological features16. Although there is no consensus, the criterion of SEGA diagnosis is > 0.5–1.0 cm in size or serial growth17. While SEGAs occur throughout the ventricular system, cerebrospinal fluid (CSF) circulation blockade along the caudothalamic groove can cause obstructive hydrocephalus associated with migraines, seizures, and death. Unexplained changes in neurological status or TSC-associated neuropsychiatric disorders (TANDs) can also be a sign of SEGA growth18. The median age of SEGA diagnosis is 1 year. Only ~ 2.4% of SEGAs are identified after age 4019. SEGAs can bleed when being removed leading to non-obstructive hydrocephalus, tissue damage, and mortality20. Because SEGAs occur in young children and are located deep within the brain and because surgery comes with a risk, pharmacological intervention is warranted.
mTORC1 inhibitors including rapamycin analogs (rapalogs) are now the standard of care except for cases of acute hydrocephalus21. ~57% of SEGAs are reduced by 50% volume within two years and maintenance doses are not typically associated with changed SEGA volume18,19,22. Thus, SEGAs do not always respond to rapalogs. Children may poorly tolerate rapalogs, and if treatments stop, SEGAs grow back23. Even surgical removal of SEGAs is followed by regrowth in nearly 40% of patients. The mechanisms that account for SEGA regrowth are unclear but may be related to the fact that mTORC1 allosteric inhibitors incompletely inhibit mTORC1 phosphorylation of select substrates24,25. Alternatively, resistance to rapalog binding may occur26. A novel bisteric inhibitor linking MLN0128 to rapamycin called Rapalink-1 was generated26. Rapalink-1 simultaneously inhibits mTORC1 through the rapamycin moiety by targeting FK506-binding protein 12 (FKBP12) and the FKBP12 rapamycin binding ___domain of mTOR as well as inhibiting both mTORCs through the ATP competitive inhibitor moiety, MLN012826.
We created a mouse model of TSC SEGAs by electroporating CRE recombinase into NSCs of mice having conditional Tsc2 genes to provide mechanistic insight into SEGA pathogenesis27,28. Mice developed hamartomas with SEN-like lesions that develop into SEGA-like hamartomas. We previously found that TSC mutant NSC translational programs were altered and prevented differentiation leading to the aberrant production of neurons in the striatum. These lesions were associated with ensembles of cytomegalic neurons and giant cells. We performed single nuclei RNA sequencing (snRNA-Seq) of these mice and discovered altered NSC transitional states caused by loss of Tsc2. Moreover, neurons were a core feature in this model and had altered transcriptomes. The extent to which mTOR activity might cause these phenotypes was not assessed.
What follows are the results of a study that utilizes the bisteric inhibitor Rapalink-1 on a TSC model of striatal hamartomas representing SEGA-like lesions.
Results
Rapalink-1 treatment of a TSC mouse model
Tsc2wt/wt and Tsc2f/f x RFP neonatal mice were electroporated with Cre recombinase and GFP encoding DNA plasmids (Fig. 1A). Electroporation allows for the targeting of lateral V-SVZ NSCs that generate striatal glia (Fig. 1B). This causes recombination leading to deletion of exons 2–4 of Tsc2 and red fluorescence along the lateral ventricles that appear as SEGA-like hamartomas in postnatal day (P) 30 Tsc2f/f mice (Fig. 1C, D). Additionally, V-SVZ NSCs generate granule cell neurons that migrate into the olfactory bulb (OB) and are fully mature 30 days later. We previously reported hamartomas at P30 having elevated mTORC1 activity assessed by p4E-BP in glial cells27. We also reported increased mTORC1 as assessed by pS6 staining in neurons produced from V-SVZ NSCs29. We further reported measurements of P60 hamartoma and OB neuron growth. Thus, these time points helped to guide the design of our experiments. Indeed, in comparison to Tsc2wt/wt NSCs (Fig. 1E-G) that produced mostly glia, there were numerous Tsc2mut/mut pS6 positive neurons and giant cells near the striatal border with the V-SVZ (Fig. 1H-M).
Rapalink-1 Treatment in a TSC Mouse Model. (A) Schematic diagram of conditional Tsc2 and inducible RFP genes. CRE recombinase causes Tsc2 exons 2–4 to recombine, and RFP to be expressed. (B) Schematic diagram of CAG-CRE and CAG-GFP (Green) plasmid electroporation. Plasmid is injected intraventricularly into P0-1 mice. Electrodes placed on the head of the pup subsequently cause plasmids to be taken up by NSCs lining the lateral ventricles when an electrical field is applied. C, D) Macroscopic images of coronal sections of P60 mice following electroporation. Black indicates RFP expression whereas blue indicates RFP saturation. E-G) Images of 20× Tsc2wt/wt coronal section demonstrating induction of RFP (red, E), pS6 staining (green, F), and composite with GFP expression (blue, G). H-J) Images of 20× Tsc2mut/mut coronal section demonstrating induction of RFP (red, H), pS6 staining (green, I), and composite with GFP expression (blue, J). K-L) 4× digital zoom of H-I showing cells with neuronal morphology and high pS6. Chevrons indicate macroscopic neurons, and arrowhead indicates a giant cell. E-J) Scale bar = 75 μm. K-M) Scale bar = 75 μm.
As confirmation of Tsc2 recombination, there was a mean 23.3% increase in pS6 per mutant RFP positive neuron (Tsc2wt/wt 1.000 ± 0.03042, n = 110 vs. Tsc2mut/mut 1.233 ± 0.04392, n = 110) (Supplemental Fig. 1A). CRE electroporated V-SVZs from mice having conditional Tsc2 were collected and NSCs were cultured to assess recombination as indicated by RFP (Supplemental Fig. 1B). We further verified high rates of Tsc2 recombination in RFP but not in non-RFP positive NSCs (Supplemental Fig. 1C, D). We verified that phosphorylation of the mTORC1 substrate Ulk1 was resistant to 100 nM Rapamycin but sensitive to 100 nM ATP-competitive mTORC inhibitors (Supplemental Fig. 1E, F). These results further confirm that CRE-mediated recombination occurs in the TSC model and that some mTORC1 substrates are resistant to rapamycin.
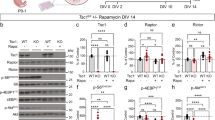
Tsc2f/f x RFP neonatal mice were subsequently randomized, assigned a unique identification number, and treated with Rapalink-1 or DMSO (vehicle) for 30 days or until sacrificed (Fig. 2A). Animals were euthanized by intraperitoneal injection of euthasol or CO2 inhalation followed by swift decapitation. No mice in the control group died and no significant changes in behavior or signs of distress were noted (Fig. 2B). Cohorts of mice were given 1.5 mg/kg or 3.0 mg/kg Rapalink-1 once every five days. 3.0 mg/kg Raplink-1 treated mice survived on average 88.2 days (N = 8) (Fig. 2B). Likewise, 1.5 mg/kg Rapalink-1 was well tolerated (N = 5) with all mice surviving 90 days. These doses were generally tolerated with death occasionally occurring at 3.0 mg/kg and having slightly lower weights at the end of treatment (Fig. 2C).
Rapalink-1 inhibits mTORC1 activity
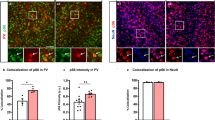
mTORC1 activity and substrate phosphorylation in the brain is cell type dependent29,30,31. We noted that pS6 levels were high within RFP positive Tsc2 null cells having neuron-like morphologies (Fig. 3A-F)27. pS6 was analyzed in vehicle, Rapalink-1 (1.5 mg/kg), or Rapalink-1 (3.0 mg/kg) Tsc2 mutant neurons (Fig. 3G-L). 1.5 mg/kg Rapalink-1 reduced pS6 (p < 0.05) in comparison to vehicle treated mice. 3.0 mg/kg Rapalink-1 also significantly reduced pS6 (p < 0.0001) compared to vehicle conditions (Vehicle = 1.066 ± 0.029, n = 857 vs. Rapalink-1 1.5 mg/kg = 0.9127 ± 0.065, n = 218 vs. Rapalink-1 3.0 mg/kg = 0.835 ± 0.029 n = 452) (Fig. 3M).
Rapalink-1 Inhibits mTORC1 Activity. A-C) 20× images of RFP (red, A), pS6 (green, B), and composite with GFP (blue, C) in a coronal section of a Tsc2mut/mut P90 vehicle treated mouse brain. Rectangle denotes magnified region in D-F. D-F) 4× digital zoomed in images of A-C demonstrating neuronal morphology. G-I) 20× images of RFP (red, G), pS6 (green, H), and composite with GFP (blue, I) in a coronal section of a brain from a Tsc2mut/mut P90 Rapalink-1 (3 mg/kg) treated mouse. J-L) 4× digital zoomed in images of G-I demonstrating neuronal morphology. M) Quantification of pS6 in RFP positive cells. *=p < 0.05, ****=p < 0.0001. Data is represented as mean ± SEM. A-C and G-I scale bar = 75 μm. D-F and J-L scale bar = 18.75 μm.
We confirmed the extent that mTORC1 signaling was reduced by using acute daily Rapalink-1 treatment for five days. Rapalink-1 has been used at higher doses for acute in vivo experiments32. We therefore tested acute efficacy of Rapalink-1 at a higher dose (6 mg/kg) but less than 10 mg/kg, which is the dose of rapamycin used to compare the two drugs26. Rapalink-1 reduced mTORC1 signaling as detected by examining p4EBP and pS6 staining in the brain and by immunoblotting for pS6 (Vehicle = 1.000 ± 0.04189, N = 3 vs. Rapalink-1 = 0.7707 ± 0.02518, N = 3; P = 0.0094) (Supplemental Fig. 2). Rapalink-1 inhibition of mTORC2 was confirmed by a reduction in pAKT as previously reported (Supplemental Fig. 2)32. These results confirm that Rapalink-1 can indeed reduce mTOR signaling in vivo.
Rapalink-1 reduces Tsc2 mutant neuronal soma size
mTOR regulates OB granule cell soma growth33. Loss of mTOR and inhibition of mTORC1 with rapamycin decreases granule cell soma size33. Conversely, loss of Tsc2 increases granule cell and striatal neuron soma size27,29. We wondered to what extent soma size might be decreased in Tsc2 mutant neurons following Rapalink-1 treatment. Rapalink-1 low dose (1.5 mg/kg) and Rapalink-1 high dose (3.0 mg/kg) decreased the average soma size of mutant Tsc2 neurons (Vehicle = 1.00 ± 0.016 vs. Rapalink-1 1.5 mg/kg = 0.7463 ± 0.01 vs. Rapalink-1 3.0 mg/kg = 0.689 ± 0.015, p < 0.0001) (Fig. 4A-H, K). We documented a population of cells appearing as giant cells having high mTORC1 activity even after 3.0 mg/kg Rapalink-1 treatment (Fig. 4I, J). However, the relative proportion of cells that were classified as giant cells in relation to the total cell number per section, was reduced in the 3.0 mg/kg Rapalink-1 condition (Vehicle = 0.0451 ± 0.0044 vs. Rapalink-1 1.5 mg/kg = 0.0415 ± 0.0125 vs. Rapalink-1 3.0 mg/kg = 0.0274 ± 0.03623, p < 0.01). Taken together, Rapalink-1 appears to decrease the average size of Tsc2 mutant neurons.
Rapalink-1 Reduces Cell Size. A-D) 20× images of GFP (blue, A), RFP (red, B), pS6 (green, C), and composite (D) in Tsc2mut/mut P90 control brains. E-H) 20× images of GFP (blue, E), RFP (red, F), pS6 (green, G), and composite (H) in Tsc2mut/mut P90 Rapalink-1 (3 mg/kg) brains. I, J) Images from D and H magnified 400% showing examples of giant cells. K) Quantification of neuron soma size relative to controls. Data is represented as mean ± SEM. Scale bar = 75 μm. ****=p < 0.0001.
Rapalink-1 treatment decreases neuron dendrite arbors
We previously demonstrated that loss of Tsc1, Tsc2, or increasing Rheb, increased dendrite arbors of OB granule cell neurons produced from V-SVZ NSCs29,34,35. This is likely because mTOR and mTOR complex components raptor and rictor regulate granule cell dendrite arbors33. Neurons present in the striatum of the SEGA model also have more dendrites than wild type or Tsc2 mutant OB granule cell neurons27. These mutant neurons are also larger and have greater dendrite complexity in comparison to any control wild-type neurons found in the striatum.
Gross reductions in dendrite arbors caused by Rapalink-1 were noted at low magnifications (Fig. 5A, B). To confirm and quantify this observation, neurons from control and Rapalink-1 treated mice were traced (Fig. 5C, D). Rapalink-1 substantially reduced the length and complexity of Tsc2mut/mut neuron dendrites. Rapalink-1 (3.0 mg/kg) significantly (p < 0.01, ANOVA with Tukey’s multiple comparisons test) reduced the total number of dendrite crossings in comparison to vehicle treated conditions (Vehicle = 713.0 ± 74.53, n = 24 vs. Rapalink-1 1.5 mg/kg = 563.8 ± 46.64, n = 24 vs. Rapalink-1 3.0 mg/kg 465.5 ± 33.85, n = 50) (Fig. 5E, F). Thus, long-term Rapalink-1 treatment significantly reduces dendrite arbors in Tsc2 mutant neurons.
Rapalink-1 Reduces Dendrite Arbors in a TSC Model. (A) 20× image of RFP from a vehicle treated Tsc2mut/mut P90 mouse brain. (B) 20× image of RFP from a Tsc2mut/mut P90 3.0 mg/kg Rapalink-1 treated mouse brain. (C) Zoomed in image with a neuron in square of A subjected to tracing (green highlight). (D) Zoomed in image with a neuron in square of B subjected to tracing (green highlight). (E) Sholl Analysis demonstrating the effect of Rapalink-1 on dendrite arbors. (F) The total number of dendrite crossings in each neuron of control and Rapalink-1 treated mice. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. Data is represented as mean ± SEM. Scale bar = 75 μm (A, B) or 37.5 μm (C, D). **=p < 0.01.
Efficacy of rapalink-1 for the treatment of TSC hamartomas
The final determinant of the efficacy of Rapalink-1 was the average lesion size at P90. Striatal hamartomas were measured by tracing lesions as we previously described27. We noted that z-projected cross sectional area of hamartomas continue to increase notably at P90 with some hamartomas appearing aggressive and invading different regions reaching 10 times the size of those measured at P60 (Figs. 1 and 6). In comparison, 1.5 mg/kg Rapalink-1 treated mice had hamartomas that were considerably (~ 68.6%) smaller calculated using z projected cross sectional area and 3.0 mg/kg Rapalink-1 significantly (p = 0.0363) reduced hamartoma sizes by ~ half in relation to control conditions (Vehicle = 40096 ± 8461, n = 38 vs. Rapalink-1 1.5 mg/kg = 27520 ± 2243, n = 58 vs. Rapalink-1 3.0 mg/kg 19636 ± 3266, n = 33) (Fig. 6I).
Rapalink-1 Reduces Hamartoma Size. A-D) 20× images of GFP (blue), pS6 (green), and RFP (red) in Tsc2mut/mut P90 brains treated with vehicle or E-H) 20× images of GFP (blue), pS6 (green), and RFP (red) in Tsc2mut/mut P90 3.0 mg/kg Rapalink-1 treated brains. I) Quantification of lesion area. J) Schematic diagram of molecular pathways regulated by Tsc1/Tsc2 in TSC model in control and Rapalink-1 treated brains. Tsc1/Tsc2 turn off mTORC1 pathway under normal conditions. However, in the TSC SEGA model (left), Tsc1/Tsc2 encoded protein products cannot turn mTORC1 off leading to ectopic/cytomegalic neurons with excessive mTORC1 activity, hypertrophic dendrites, giant cells, and SEGA-like lesions (i.e. striatal hamartomas). Rapalink-1 appears to partially rescue these changes with mTORC1 inhibited, neuron size decreased, and reduced SEGA-like lesions. Data are represented as mean ± SEM. Scale bar = 150 μm (A, B) or 37.5 μm (C, D). *=p < 0.05.
Discussion
SEGAs are a significant cause of morbidity in TSC patients. Rapalogs are a front-line treatment for SEGAs36,37,38,39. Here we demonstrate that likewise, Rapalink-1 is useful for reversing neural phenotypes in a TSC mouse model of SEGA-like hamartomas (Fig. 6J). Although, ~ 57% of TSC SEGAs are reduced by 50% within two years, continued treatment does not further reduce SEGA volume18,19,22. Protracted use of Rapalogs is also necessary because stopping treatment is associated with SEGA regrowth. New therapeutics should be developed based on the limited efficacy of Rapalogs and their side effects23. Studies have also demonstrated the development of resistance to rapamycin through changes in the FKB12 rapamycin binding ___domain of mTOR26. Finally, rapamycin does not fully inhibit mTORC1 phosphorylation of several substrates25. There is an urgent need to test new therapies that may overcome these issues. Rapalink-1 is an excellent candidate because it fulfills the need to completely inhibit mTORC1 phosphorylation of rapamycin-resistant substrates and simultaneously inhibits mTORC2 and overcomes issues with resistance26. It is therefore conceivable that Rapalink-1 might be useful to treat TSC. To fulfill the goal of examining additional TSC therapies, we tested Rapalink-1 in a mouse model of TSC SEGA-like hamartomas.
The mechanisms that account for SEGA growth are unclear in patients and this may be related to the fact that we have little understanding of the cellular composition, regional differences in SEGAs, and whether molecular mechanisms involved in the different regions and timing of their appearance may differ. The fact that the composition of SEGAs has been debated for nearly a century is evidence that experiments must carefully examine the effect drugs have not just on SEGA size, but also on the different cell types within SEGAs40,41. SEGAs also appear to change over time. Imaging and RNA sequencing experiments suggest a stepwise change in composition with SEGAs eventually containing neurons and cytomegalic giant cells42,43,44,45,46,47,48. Gliotic scarring, calcification, and immune cell invasion also occur48. This is similar to events in TSC patient cortical tubers. Although SEGAs do not frequently directly cause seizures, the presence of mutant neurons and giant cells warrants closer inspection as does determining their contributions to patient presentation.
Loss of Tsc2 in V-SVZ neuroprogenitors results in the aberrant transition from quiescent to active states and during differentiation30,31,49,50. Loss of Tsc2 prevents the further downregulation of mTORC1 activity in a subset of cells within the striatum leading to growths including nodular hamartomas containing cells with heterogenous morphologies. The hamartomas are characterized by abnormal heterotopic clusters of morphologically heterogenous cells which include neurons both within and outside of the growths. Thus, the TSC SEGA-like hamartoma mouse model allowed us to examine the utility of Rapalink-1 treatment in controlling neural phenotypes and hamartoma size. Male and female mice were used, and no differences were noted between sexes, but the studies here were not designed and statistically powered to explore this variable. This biological variable could be relevant to examine in future studies. Rapalink-1 intermittent dosing (1.5 mg/kg and 3.0 mg/kg every five days) was generally tolerated during the study, but preliminary experiments demonstrated acute toxicity during daily treatment (data not shown). As predicted, Rapalink-1 reduced mTORC1 activity. However, it was reduced by only ~ 15–23% as measured using pS6 as a readout and pS6 remained apparent throughout the brain. Thus, these doses might incompletely inhibit mTORC1. Indeed, a higher dose (6 mg/kg) was also tested based on efficacy in glioma and appeared more effective by immunohistochemistry and western blot but was associated with toxicity32. It is unclear whether extended treatments of 1.5 mg/kg and 3.0 mg/kg Rapalink-1 for more than 30 days would be accompanied by additional side effects. Additionally, 1.5 and 3.0 mg/kg Rapalink-1 treated mice were sacrificed 5 days after the last injection of Rapalink-1. Thus, mTORC1 activity could recover and our data may underestimate how effective Rapalink-1 is. Nevertheless, the reduction in pS6 served as an indicator that Rapalink-1 inhibited mTORC1.
We reasoned that the prolonged inhibition of mTORC1 would decrease the well-documented mTORC1-regulated phenomenon, of cell growth51. Previous work from our laboratory and our colleagues have demonstrated that neonatal V-SVZ NSC generated granule cells grow when Tsc1 or Tsc2 are removed29,34. Moreover, ectopic expression of wild-type or mutant Rheb can drive cell growth in granule cells35,52. Growth of granule cells is dependent on mTOR since CRE electroporation of conditional mTOR reduces soma size33. Moreover, rapamycin treatment reduces granule cell soma size supporting the importance of mTORC1 in this process33. As expected, Rapalink-1 at both doses reduced Tsc2 mutant neuron cell size by ~ 25–31%. These results further support that Rapalink-1 is likely inhibiting mTORC1-dependent cellular events.
While the role of striatal neurons in the hamartoma SEGA-like model is unclear, ectopic neurons can affect a wide range of other cell types. Cortical tubers and focal malformations of cortical development appear to undergo analogous changes including astrogliosis and microglia activation11. And this is directly linked to neuron hyperexcitability. Thus, a major goal for TSC and related disorder research has been to reduce dendrite growth in the hopes of modulating neuronal activity. mTOR, through mTORC1 and mTORC2 regulates OB granule cell dendrite arbors33. While cytoskeleton regulation is most often attributed to mTORC2, rapamycin and mTORC1 have been carefully studied in relation to dendrite growth. For example, like cell growth, loss of Tsc1 and Tsc2 as well as increased Rheb activity promote dendrite growth29,34,35,52. We found that Rapalink-1 similarly reduced the dendrites of striatal Tsc2 mutant neurons. Thus, Rapalink-1, could be advantageous in that it binds both arms of mTOR signaling32.
This is particularly important as several groups have posited that additional pathways may regulate SEGA growth. For example, one group simultaneously removed Pten and Tsc1 in postnatal V-SVZ NSCs16. This resulted in the generation of SEGAs that recapitulated most aspects of those in patients. Pten/Tsc1 mutant NSCs were subsequently injected subcutaneously and generated tumors. The Pten/Tsc1 mutant NSCs had altered Erk and Akt activity too. Knockdown of the mTORC2 component rictor or combined rapamycin and PI3K-mTOR inhibition reduced tumor growth16. Thus, inhibitors that act on both mTORC1 and mTORC2 such as Rapalink-1 have several advantages. In line with these results, we found that Rapalink-1 produced a moderate but significant effect on the average size of SEGAs. The effect of Rapalink-1 was disproportionate to that seen on cell size or dendrites. Whether the effectiveness of Rapalink-1 is due to the effect on neuronal activity or on other cell types is unclear and will require additional future experiments. A limitation of inhibiting mTORC2 is that this could lead to additional side effects not seen with rapalogs. Future studies should directly compare the efficacy of rapamycin or rapalogs and Rapalink-1.
Taken together, this study provides in vivo evidence for the utility of Rapalink-1 to control mTOR, cell size, dendrite hypertrophy, and SEGA-like hamartoma growth which may be of clinical importance.
Methods
Experimental model and subject details
Animals
All experiments were approved by the Clemson University Institutional Animal Care and Use Committee and the Animal Care and Use Review Office (ACURO), a component of the USAMRDC Office of Research Protections (ORP) within the Department of Defense (DoD). All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines. Red fluorescent protein (RFP+/−,+/+) (B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J) (Strain #007909, RRID: IMSR_JAX:007909), and Tsc2tm1.1Mjg/J (Strain #027458, RRID: IMSR_JAX:027458) were acquired from Jackson Laboratories53. Pharmacological treatment of cultured NSCs is on CD1 mice. Sentinel mice were free of pathogens throughout the study. Samples/subjects were allocated randomly to experimental group. Experimental manipulations were performed on mouse pups that were not involved in previous procedures and sacrificed accordingly. Both sexes were used, and ages are as indicated in figures. Mice were housed under standard pathogen-free conditions in cages on racks within isolated cubicles with a 12-h light/dark cycle and fed ad libitum. Mice were injected intraperitoneally using the indicated doses and schedule. Drugs were prepared as previously described in DMSO, PEG-300, and PBS32. Injections were performed in biological safety cabinets. Mice were weighed 5–7 days each week and weights recorded. Animals were euthanized by intraperitoneal injection of euthasol or CO2 inhalation followed by swift decapitation.
Electroporation
B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J) x Tsc2tm1.1Mjg/J mouse pups were electroporated as previously described54,55. Mice were injected with equal concentrations and volumes of DNA plasmids diluted in phosphate buffered saline (PBS) with 0.1% fast green. CAG-CRE (Plasmid #13775, Addgene) and CAG-GFP (Plasmid #11150, Addgene) plasmids were used56,57. A borosilicate glass micropipette generated from pulled capillary tubes was loaded with DNA and injected into the lateral ventricles. Square pulse generation was performed using a pulse generator (ECM830; BTX) and tweezer electrodes (model 520; BTX) with five, 100-volt square pulses of 50 ms duration with 950-ms intervals.
Polymerase chain reaction (PCR)
Toe or tail snips were subject to modified hotshot DNA extraction and genotyped by PCR using Invitrogen™ Platinum™ Taq polymerase, mixed with nuclease-free water, magnesium free PCR buffer, MgCl2, dNTP mix with primers and DNA according to the manufacturers’ protocol (Invitrogen). Primers for Tsc2 were 5’-ACAATGGGAGGCACATTACC-3’ and 5-AAGCAGCAGGTCTGCAGTG-3’ and for Tomato (RFP) 5’-AAGGGAGCTGCAGTGGAGTA-3’ and 5’-CCGAAAATCTGTGGGAAGTC-3’ and 5’-GGCATTAAAGCAGCGTATCC-3’ and 5’-CTGTTCCTGTACGGCATGG-3’. Amplicons were loaded onto agarose gels with 1X Blue Juice and ran at 100 V for 20–30 min and visualized on a BioRad Chemidoc MP.
Immunohistochemistry
Brains were removed in room temperature PBS, transferred to 4% paraformaldehyde in PBS, and incubated overnight at 4 °C. Brains were rinsed in PBS and mounted in 3% agarose. A Leica VTS 1000 vibratome was used to section brains coronally. Sections were blocked in 0.1% Triton X-100, 0.1% Tween-20 and 2% BSA in PBS for 1 h at room temperature. Sections were washed in 0.1% Tween-20 in PBS. Sections were incubated in primary antibody, anti-pS6 (1:500; Cell Signaling Technology; Ser 240/244, 61H9, #4838), in 0.1% Tween-20 and 2% BSA in PBS overnight at 4 °C. Sections were subjected to three additional washes in PBS containing 0.1% Tween-20. Sections were incubated with the appropriate secondary antibody (Alexa Fluor series; 1:500; Invitrogen) in 0.1% Tween-20 and 2% BSA in PBS overnight at 4 °C. Sections were mounted in ProLong Antifade Mountant (ThermoFisher). Images were acquired on a spectral confocal microscope (Leica SPE) with a ×20 dry objective (N.A. 0.75). Low-magnification images were acquired with ×10 dry or a ×5 dry (N.A. 0.15) objective.
Image analysis
Images (×20) of RFP positive cells were uploaded to FIJI (ImageJ 1.5 g) and analyzed as described elsewhere27. The freehand selection tool was used to trace electroporated and non-electroporated cell somas in the same Z section and mean gray values for pS6 were quantified. Ratios of electroporated and non-electroporated cells were compared for RFP positive cells in Tsc2wt/wt and Tsc2mut/mut conditions to account for immunohistochemical variation. Soma size was simultaneously recorded for traced cells. The RFP positive hamartoma perimeter was outlined in each Z section by hand by scrolling through individual Z sections and hamartomas were subsequently traced in (×20) images. The freehand selection tool was used to trace RFP positive lesions. Images (×20) were used to measure dendrite morphology. Dendrites were traced using the simple neurite tracer plug-in. Sholl analysis was performed at 1 μm intervals to quantify dendrite arborization using the Sholl plug-in. The total number of dendritic crossings was calculated by taking the sum of crossings at all intervals for each traced neuron and averaging the total number of crossings per neuron in each condition.
Western blot
Tissue (0.1 gram) was harvested and finely minced in 2% SDS, Protease Inhibitor Cocktail (Pierce) and Phosphatase Inhibitor Cocktail, in RIPA buffer (25 mM Tris HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS). Samples were briefly sonicated at maximum settings (100 Amplitude, QSonica) for 10 s three times with 30 s resting intervals. Lysate was transferred on ice to a fresh reaction tube and centrifuged at 15,000 rpm for 15 min in a tabletop Eppendorf 5415 centrifuge at 4 °C. Protein concentration was quantified using the Pierce MicroBCA assay. Equal protein amounts were brought up to equal volumes with lysis buffer as described above and Laemmli buffer and heated to 95 °C for 5 min. Proteins were resolved by 10% polyacrylamide precast mini-Protean gels (BioRad) and transferred to polyvinylidene difluoride (PVDF) membranes. PVDF membranes were rinsed in Tris-buffered saline (TBS-T, 0.1% Tween 20) for 5 min at room temperature and blocked in 5% blotting grade block (BioRad) in TBS-T for 1 h at room temperature. Membranes were incubated for 1 h at room temperature or overnight at 4 °C with the following antibodies from Cell Signaling Technology at a 1:1,000 dilution: phospho-RPS6 (D68F8, Cat# 5364), RPS6 (5G10, Cat# 2217), phospho-Ulk (D706U, Cat# 14202), Ulk (D8H5, Cat# 8054), phospho-AKT (D9E, Cat# 4060), and AKT (Cat# 9272). Membranes were rinsed three times each for 10 min in TBS-T. Membranes were incubated for 1 h at room temperature with donkey or goat anti-rabbit antibodies in blocking buffer. Membranes were washed for 15 min in TBS-T and visualized using a Bio-Rad Chemidoc MP imaging system using enhanced chemiluminescence reagent (Pierce). PVDF membranes were stripped for at room temperature using Restore Western Blot Stripping Buffer according to manufacturer’s recommendations (Cat# 21059, Thermo Fisher Scientific).
Primary cell culture
Briefly, P0-1 CD1 mice were anesthetized on ice and decapitated and brains were removed and placed in 4 °C Neurobasal A media on pre-chilled petri dishes. Micro-dissected V-SVZs were placed into 0.05% trypsin with 0.02% EDTA in Neurobasal A for 7 min in a 37 °C incubator. Equal volumes of defined trypsin inhibitor (1X; Life Technologies; Lot# 1837475) were added. The samples were centrifuged at 300 x g for 5 min. The pellet was re-suspended with 1 mL Neurobasal A. The pellet was triturated with three pasture pipettes having sequentially decreased bore size to dissociate tissue. Cells were centrifuged at 300 x g for 5 min and resuspended in 200 µL Neurobasal A complete Media (1X Glutamax, 50 units/mL Penicillin/streptomycin, 20 ng/mL EGF, 20 ng/mL FGF-2 and 2% B27 Supplement). Cells were placed on laminin-coated coverslips in 24 well plates in Neurobasal A complete media for pharmacological experiments performed 24 h later or placed into 6 well plates for recombination analysis as previously described27,29. Samples were harvested in 2% SDS, Protease Inhibitor Cocktail (Pierce) and Phosphatase Inhibitor Cocktail, in RIPA buffer for western blot. Alternatively, samples were subjected to DNA isolation and long range PCR for Tsc2 recombination products as previously described27,29.
Pharmacological treatments
NSCs were treated with 100 nM Rapamycin (#9904s, Cell Signaling Technology) or 100 nM ATP-competitive inhibitor, Torin1 (#14379, Cell Signaling Technology). Torin1 from a 1mM stock was re-constituted in DMSO (MP Biomedicals). Rapamycin was re-constituted in DMSO for a 100 mM stock. Equi-molar DMSO was used for all conditions. For the primary cell starvation experiment, NSC were placed into PBS for 2 h prior to drug treatment or placed back into complete media to activate mTORC1 for the duration of drug treatment (2 h).
Quantification and statistical analysis
Measurements were graphed and statistical analysis was performed with GraphPad Prism software (Version 8.2.0, GraphPad Software Inc.). Statistical significance was determined using One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test or Student’s T-test. N (number of mice) and n (number of cells or hamartomas) are listed where applicable. Figure 3 (pS6) and Fig. 4 (cell size) analysis were performed on vehicle (N = 6), Rapalink-1 1.5 mg/kg (N = 4), and Rapalink-1 3.0 mg/kg (N = 7) mice. Sholl and hamartoma analysis are reported for vehicle (N = 4), Rapalink-1 1.5 mg/kg (N = 4), and Rapalink-1 3.0 mg/kg (N = 6) mice in Figs. 5 and 6. Supplemental data were performed on N = 3 mice for all immunohistochemistry analysis and western blot conditions. Error bars are reported as the standard error mean.
Key resources table
REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
Antibodies | ||
Rabbit Monoclonal pS6 Ser 240/244 | Cell Signaling Technology | Cat#4838; RRID: AB_659977; Clone: 61H9 |
Rabbit Monoclonal p4EBP Thr 37/46 | Cell Signaling Technology | Cat#2855; RRID: AB_560835; Clone: 236B4 |
Rabbit Monoclonal RPS6 | Cell Signaling Technology | Cat#2217; RRID: AB_331355; Clone: 5G10 |
Chemicals, Peptides, and Recombinant Proteins | ||
Experimental Models: Organisms/Strains | ||
Mouse: RFP+/−, RFP+/+: B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J | Jackson Laboratories | Cat#007909; RRID: IMSR_JAX:007909 |
Mouse: Tsc2wt/wt, Tsc2mut/mut: Tsc2tm1.1Mjg/J | Jackson Laboratories | Cat#027458; RRID: IMSR_JAX:027458 |
Oligonucleotides | ||
Primers: Tsc2: 5’-ACAATGGGAGGCACATTACC-3’, 5- AAGCAGCAGGTCTGCAGTG-3’ | Integrated DNA Technologies | N/A |
Primers: RFP: 5’-AAGGGAGCTGCAGTGGAG TA-3’, 5’-CCGAAAATCTGTGGGAAG TC-3’, 5’- GGCATTAAAGCAGCGTATCC-3’, 5’-CTGTTCCTGTACGGCATGG-3’ | Integrated DNA Technologies | N/A |
Primers: For detecting Tsc2 recombination 5’-AAGATTCCGGCTTGAAGGAG-3’, 5’-CACTA-GTCTAGCCTGACTCT-3’, and 5’-GAGGACAAGCCAACATCCAT-3’ | Integrated DNA Technologies | N/A |
Recombinant DNA | ||
Plasmid: CAG-CRE | 57 | Cat#13,775; RRID: Addgene_13775 |
Plasmid: CAG-GFP | 58 | Cat#11,150; RRID: Addgene_11150 |
Software and Algorithms | ||
FIJI (ImageJ 1.5 g) | ||
GraphPad Prism (v. 8.2.0) | GraphPad Software Inc | |
Data availability
The datasets generated and/or analysed during the current study are available in the Mendeley Data and NeuroMorpho repository. Western blot data is deposited as Feliciano, David (2025), “A Bitopic mTORC Inhibitor Reverses Phenotypes in a Tuberous Sclerosis Complex Model Westerns”, Mendeley Data, V1, doi: 10.17632/hjt9m462d3.1 (https://data.mendeley.com/datasets/hjt9m462d3/1) to Mendeley Data. Neuron traces are available at NeuroMorpho.org under “A Bitopic mTORC Inhibitor Reverses Phenotypes in a Tuberous Sclerosis Complex Model” or navigating to neuromorpho.org/dableFiles/mukherjee_feliciano/Supplementary/Mukherjee_Feliciano.zip.
References
Northrup, H. et al. Updated international tuberous sclerosis complex diagnostic criteria and surveillance and management recommendations. Pediatr. Neurol. 123 https://doi.org/10.1016/j.pediatrneurol.2021.07.011 (2021).
The European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75, 1305–1315. https://doi.org/10.1016/0092-8674(93)90618-Z (1993).
Van Slegtenhorst, M. et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277, 805–808. https://doi.org/10.1126/science.277.5327.805 (1997).
Feliciano, D. M. Modeling genetic mosaicism of the mammalian target of rapamycin pathway in the cerebral cortex. Front. Mammal Sci. 2. (2023). https://doi.org/10.3389/fmamm.2023.1231778
Inoki, K., Li, Y., Xu, T. & Guan, K. L. Rheb GTpase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17, 1829–1834. https://doi.org/10.1101/gad.1110003 (2003).
Beugnet, A., Tee, A. R., Taylor, P. M. & Proud, C. G. Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochem. J. 372, 555–566. https://doi.org/10.1042/BJ20021266 (2003).
Garami, A. et al. Insulin activation of rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell. 11, 1457–1466. https://doi.org/10.1016/S1097-2765(03)00220-X (2003).
Zhang, Y. et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell. Biol. 5, 578–581. https://doi.org/10.1038/ncb999 (2003).
Saxton, R. A. & Sabatini, D. M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976. (2017). https://doi.org/10.1016/j.cell.2017.02.004
Feliciano, D. M. et al. A circuitry and biochemical basis for tuberous sclerosis symptoms: from epilepsy to neurocognitive deficits, (2013). https://doi.org/10.1016/j.ijdevneu.2013.02.008
Feliciano, D. M. The Neurodevelopmental Pathogenesis of Tuberous Sclerosis Complex (TSC) at Frontiers, (2020). https://doi.org/10.3389/fnana.2020.00039
Northrup, H. et al. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr. Neurol. 49, 243–254. https://doi.org/10.1016/j.pediatrneurol.2013.08.001 (2013).
Chan, D. L., Calder, T., Lawson, J. A., Mowat, D. & Kennedy, S. E. The natural history of subependymal giant cell Astrocytomas in tuberous sclerosis complex: A review. Rev. Neurosci. 29, 295–301. https://doi.org/10.1515/revneuro-2017-0027 (2018).
Adriaensen, M. E. A. P. M. et al. Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur. J. Neurol. 16, 691–696. https://doi.org/10.1111/j.1468-1331.2009.02567.x (2009).
Fujiwara, S., Takaki, T., Hikita, T. & Nishio, S. Subependymal giant-cell astrocytoma associated with tuberous sclerosis - Do subependymal nodules grow? Child’s Nerv. Syst. 5. (1989). https://doi.org/10.1007/BF00706748
Zordan, P. et al. Tuberous sclerosis complex-associated CNS abnormalities depend on hyperactivation of mTORC1 and Akt. J. Clin. Invest. 128, 1688–1706. https://doi.org/10.1172/JCI96342 (2018).
Jóźwiak, S., Nabbout, R. & Curatolo, P. Management of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC): Clinical recommendations. Eur. J. Paediatr. Neurol. 17. (2013). https://doi.org/10.1016/j.ejpn.2012.12.008
Jansen, A. C. et al. Clinical characteristics of subependymal giant cell Astrocytoma in tuberous sclerosis complex. Front. Neurol. https://doi.org/10.3389/fneur.2019.00705 (2019).
Jansen, A. C. et al. Newly diagnosed and growing subependymal giant cell Astrocytoma in adults with tuberous sclerosis complex: results from the international TOSCA study. Front. Neurol. 10 https://doi.org/10.3389/fneur.2019.00821 (2019).
Ryoo, J. S. et al. Trends in survival and treatment of SEGA: National Cancer Database Analysis. Neuro-Oncology Pract. 8. (2021). https://doi.org/10.1093/nop/npaa060
Franz, D. N. et al. Efficacy and safety of everolimus for subependymal giant cell Astrocytomas associated with tuberous sclerosis complex (EXIST-1): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 381, 125–132. https://doi.org/10.1016/S0140-6736(12)61134-9 (2013).
Kotulska, K. et al. Long-term effect of everolimus on epilepsy and growth in children under 3 years of age treated for subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Eur. J. Paediatr. Neurol. 17. (2013). https://doi.org/10.1016/j.ejpn.2013.03.002
Franz, D. N. & Capal, J. K. MTOR inhibitors in the Pharmacologic management of tuberous sclerosis complex and their potential role in other rare neurodevelopmental disorders, (2017). https://doi.org/10.1186/s13023-017-0596-2
Thoreen, C. C. et al. An ATP-competitive mammalian target of Rapamycin inhibitor reveals Rapamycin-resistant functions of mTORC1. J. Biol. Chem. 284, 8023–8032. https://doi.org/10.1074/jbc.M900301200 (2009).
Kang, S. A. et al. mTORC1 phosphorylation sites encode their sensitivity to starvation and Rapamycin. Science 341, 364–373. https://doi.org/10.1126/science.1236566 (2013).
Rodrik-Outmezguine, V. S. et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature 534 https://doi.org/10.1038/nature17963 (2016).
Riley, V. A. et al. Tsc2 coordinates neuroprogenitor differentiation. iScience 26, 108442. (2023). https://doi.org/10.1016/j.isci.2023.108442
Holmberg, J., Riley, V., Sokolov, A., Mukherjee, S. & Feliciano, D. Protocol for Electroporating and Isolating Murine (Sub-) Ventricular Zone Cells for Single Nuclei Omics (Star Protoc, 2024).
Riley, V. A., Holmberg, J. C., Sokolov, A. M. & Feliciano, D. M. Tsc2 shapes olfactory bulb granule cell molecular and morphological characteristics. Front. Mol. Neurosci. 15, 19 (2022).
Paliouras, G. N. et al. Mammalian target of Rapamycin signaling is a key regulator of the transit-amplifying progenitor pool in the adult and aging forebrain. J. Neurosci. 32, 15012–15026. https://doi.org/10.1523/JNEUROSCI.2248-12.2012 (2012).
Hartman, N. W. et al. MTORC1 targets the translational repressor 4E-BP2, but not S6 kinase 1/2, to regulate neural stem cell Self-Renewal invivo. Cell. Rep. 5, 433–444. https://doi.org/10.1016/j.celrep.2013.09.017 (2013).
Fan, Q. W. et al. A kinase inhibitor targeted to mTORC1 drives regression in glioblastoma. Cancer Cell. 31, 424–435. https://doi.org/10.1016/j.ccell.2017.01.014 (2017).
Skalecka, A. et al. mTOR kinase is needed for the development and stabilization of dendritic arbors in newly born olfactory bulb neurons. Dev. Neurobiol. 76, 1308–1327. https://doi.org/10.1002/dneu.22392 (2016).
Feliciano, D. M., Quon, J. L., Su, T., Taylor, M. M. & Bordey, A. Postnatal neurogenesis generates heterotopias, olfactory micronodules and cortical infiltration following single-cell TSC1 deletion. Hum. Mol. Genet. 21, 799–810. https://doi.org/10.1093/hmg/ddr511 (2012).
Sokolov, A. M., Holmberg, J. C. & Feliciano, D. M. The amino acid transporter Slc7a5 regulates the mTOR pathway and is required for granule cell development. Hum. Mol. Genet. https://doi.org/10.1093/hmg/ddaa186 (2020).
Franz, D. N. et al. Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann. Neurol. 78, 929–938. https://doi.org/10.1002/ana.24523 (2015).
Krueger, D. A. et al. Everolimus long-term safety and efficacy in subependymal giant cell Astrocytoma. Neurology 80, 574–580. https://doi.org/10.1212/WNL.0b013e3182815428 (2013).
Bobeff, K. et al. Maintenance Therapy With Everolimus for Subependymal Giant Cell Astrocytoma in Patients With Tuberous Sclerosis – Final Results From the EMINENTS Study. Front. Neurol. 12. (2021). https://doi.org/10.3389/fneur.2021.581102
Sasongko, T. H., Ismail, N. F. D. & Zabidi-Hussin, Z. A. M. H. Rapamycin and Rapalogs for tuberous sclerosis complex, (2016). https://doi.org/10.1002/14651858.CD011272.pub2
Crithchley, M. & Earl, C. J. C. Tuberose sclerosis and allied conditions. Brain 55. (1932). https://doi.org/10.1093/brain/55.3.311
Buccoliero, A. M. et al. Subependymal giant cell astrocytoma (SEGA): Is it an astrocytoma? Morphological, immunohistochemical and ultrastructural study. Neuropathology 29. (2009). https://doi.org/10.1111/j.1440-1789.2008.00934.x
Gelot, A. B. & Represa, A. Progression of Fetal Brain Lesions in Tuberous Sclerosis Complex. Front. Neurosci. 14. (2020). https://doi.org/10.3389/fnins.2020.00899
Eichmüller, O. L. et al. Amplification of human interneuron progenitors promotes brain tumors and neurological defects. Science 375 https://doi.org/10.1126/science.abf5546 (2022).
Stefansson, K. & Wollmann, R. Distribution of glial fibrillary acidic protein in central nervous system lesions of tuberous sclerosis. Acta Neuropathol. 52 https://doi.org/10.1007/BF00688011 (1980).
Stefansson, K. & Wollmann, R. Distribution of the neuronal specific protein, 14-3-2, in central nervous system lesions of tuberous sclerosis. Acta Neuropathol. 53 https://doi.org/10.1007/BF00689991 (1981).
Tobo, M., Mitsuyama, Y. & Kaku, T. Glial fibrillary acidic protein in tuberous sclerosis. Psychiatry Clin. Neurosci. 37, 95–100. https://doi.org/10.1111/j.1440-1819.1983.tb00307.x (1983).
Mizuguchi, M. Abnormal giant cells in the cerebral lesions of tuberous sclerosis complex. Congenit Anom. (Kyoto). 47, 2–8. https://doi.org/10.1111/j.1741-4520.2006.00134.x (2007).
Martin, K. R. et al. The genomic landscape of tuberous sclerosis complex. Nat. Commun. 8, 1–13. https://doi.org/10.1038/ncomms15816 (2017).
Blair, J. D., Hockemeyer, D., Doudna, J. A., Bateup, H. S. & Floor, S. N. Widespread translational remodeling during human neuronal differentiation. Cell. Rep. 21, 2005–2016. https://doi.org/10.1016/j.celrep.2017.10.095 (2017).
Baser, A. et al. Onset of differentiation is post-transcriptionally controlled in adult neural stem cells. Nature 566, 100–104. https://doi.org/10.1038/s41586-019-0888-x (2019).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth. Metabolism Disease. https://doi.org/10.1016/j.cell.2017.02.004 (2017).
Lafourcade, C. A. et al. Rheb activation in subventricular zone progenitors leads to heterotopia, ectopic neuronal differentiation, and rapamycin-sensitive olfactory micronodules and dendrite hypertrophy of newborn neurons. J. Neurosci. 33, 2419–2431. https://doi.org/10.1523/JNEUROSCI.1840-12.2013 (2013).
Hernandez, O., Way, S., McKenna, J. & Gambello, M. J. Generation of a conditional disruption of the Tsc2 gene. Genesis 45, 101–106. https://doi.org/10.1002/dvg.20271 (2007).
Feliciano, D. M., Lafourcade, C. A. & Bordey, A. Neonatal subventricular zone electroporation. J. Vis. Exp. https://doi.org/10.3791/50197 (2013).
Holmberg, J. C., Riley, V. A., Sokolov, A. M., Mukherjee, S. & Feliciano, D. M. Protocol for electroporating and isolating murine (sub)ventricular zone cells for single-nuclei omics. STAR. Protoc. 5, 103095. https://doi.org/10.1016/j.xpro.2024.103095 (2024).
Matsuda, T. & Cepko, C. L. Controlled expression of transgenes introduced by in vivo electroporation. Proc. Natl. Acad. Sci. U. S. A. 104. (2007). https://doi.org/10.1073/pnas.0610155104
Matsuda, T. & Cepko, C. L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. U. S. A. 101. (2004). https://doi.org/10.1073/pnas.2235688100
Acknowledgements
This work was supported by the United States of America Department of Defense U.S. Army Medical Research Activity Award Congressionally Directed Medical Research Program Tuberous Sclerosis Complex Research Program W81XWH2010447.
Funding
DMF is supported by United States of America Department of Defense U.S. Army Medical Research Activity Award Congressionally Directed Medical Research Program Tuberous Sclerosis Complex Research Program W81XWH2010447.
Author information
Authors and Affiliations
Contributions
Conceptualization, DF; Methodology, SM, DF, MW, MS, AS, VR; Validation, DF, VR, MW, MS, AS; Formal Analysis, SM, MW, MS, AS, DF; Investigation, DF, SM, VR, MW, MS, AS; Resources, DF; Data Curation, DF, MW, MS; Writing-Original Draft, DF; Writing-Reviewing and Editing; DF, SM, VR, MW, MS, AS; Visualization, SM, DF; Supervision, DF; Project Administration, DF; Funding Acquisition, DF.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that the researchers have no competing financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mukherjee, S., Wolan, M.J., Scott, M.K. et al. A bitopic mTORC inhibitor reverses phenotypes in a tuberous sclerosis complex model. Sci Rep 15, 20367 (2025). https://doi.org/10.1038/s41598-025-08345-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08345-z