Abstract
Epithelial‒mesenchymal transition (EMT) is a process in which polar epithelial cells transform into active mesenchymal cells and acquire invasion and migration capabilities (Cano CE, Motoo Y, Iovanna JL in Sci World J 10, 2010). EMT is involved in multiple physiological and pathological processes in the human body. The occurrence of EMT involves many signal transduction pathways and complex molecular mechanisms related to calnexin, growth factors, transcription factors, and the microenvironment. EMT is closely related to the invasion and metastasis of tumour cells. The MYH9 gene is closely associated with tumour progression. However, the function and regulatory mechanism of MYH9 in tumour occurrence and development remain unclear. Herein, we revealed for the first time that the migration, invasion, and metastatic abilities of glioma cells decreased significantly after MYH9 expression was downregulated. Moreover, our results suggested that MYH9 regulated the epithelial‒mesenchymal transition (EMT) process in glioma cells via β-catenin. In this mechanism, MYH9 was shown to bind to β-catenin and to increase its protein level by recruiting the deubiquitinase USP2 to form a complex. This complex suppressed β-catenin protein degradation, ultimately enhancing EMT signal transduction. This MYH9/β-catenin/EMT pathway represents a new molecular mechanism involved in the invasion promotion in gliomas. Our findings also demonstrated that MYH9 was crucial for inducing glioma cell migration. In summary, we suggest that MYH9 is a potential molecular marker for predicting tumour progression and prognosis and highlight its role in a new molecular mechanism of tumour metastasis.
Similar content being viewed by others
Introduction
Gliomas are primary malignant tumours of the brain1. Glioma occurrence and development can be attributed to numerous factors, including cellular origin, hereditary factors, biochemistry, and exposure to ionizing radiation. These factors often lead to genetic and epigenetic changes, resulting in the dysregulation of gene expression and the induction of tumour development and progression2,3,4,5,6,7. Although chemotherapy, targeted therapy, and immunotherapy for glioma treatment have advanced, the 5-year prognosis of patients with gliomas remains poor, which is primarily due to the susceptibility to metastasis. Therefore, studies are urgently needed to elucidate the molecular mechanism of epithelial‒mesenchymal transition (EMT) and stemness maintenance in glioma cells and to identify biomarkers and targets that can further improve glioma prognosis.
Myosin heavy chain 9 (MYH9), a member of the myosin family, is vital for cell adhesion, polarity, motility, migration, and other biological activities8. MYH9 is a cytoskeletal protein that is closely linked to numerous human diseases9,10,11,12,13. This cytoskeletal protein was initially considered a tumour suppressor in head and neck squamous cell carcinoma; however, recent research has recognized MYH9 as an oncogene in several types of cancer, including non-small cell lung cancer14, colorectal cancer15, prostate cancer16, diffuse large B-cell lymphoma17, renal cell carcinoma18, and liver cancer19. Additionally, our research group previously reported that MYH9 could bind to NAP1L1, which could in turn regulate c-Myc expression, ultimately affecting glioma proliferation20,21. However, the exact role and molecular mechanism of MYH9 in modulating glioma metastasis remain unsolved.
Herein, our study revealed that MYH9 was highly expressed in gliomas and significantly promoted the migration, invasion, and metastasis abilities of glioma cells. Moreover, MYH9 was shown to bind to β-catenin and increase its protein level by recruiting the deubiquitinase USP2 for complex formation. This process led to the suppression of β-catenin protein degradation, eventually enhancing EMT signalling to promote glioma cell metastasis. Thus, our study revealed that MYH9 was a crucial molecule that might be involved in cancer progression and increased the migration, invasion, and metastasis abilities of tumour cells, which highlights MYH9 potential as a new drug target for glioma treatment.
Results
Differential expression of MYH9 in cancers
We analysed the expression of MYH9 in several cancers via the GEPIA database. The results revealed that MYH9 was upregulated in bladder, liver, stomach, cholangial, colorectal, pancreatic, oesophageal, brain, and kidney cancer tissues compared with homologous normal tissues (Fig. 1A). In addition, MYH9 was downregulated in adrenocortical carcinoma, invasive breast carcinoma, lung cancer, thyroid carcinoma, prostate adenocarcinoma, and uterine carcinosarcoma. To further verify the expression of MYH9 in different cancers, pancancer analysis data were derived from the CPTAC resource by accessing the UALCAN database. The results revealed that MYH9 was upregulated in colon cancer, clear cell RCC, PAAD, head and neck cancer, glioblastoma, and liver cancer (Fig. 1B). Moreover, MYH9 was downregulated in breast cancer, ovarian cancer, UCEC, and lung cancer. The combined results from the two databases revealed that MYH9 was upregulated in a variety of cancers, including liver, pancreatic, colorectal, and brain cancers.
MYH9 is highly expressed in human gliomas
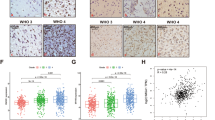
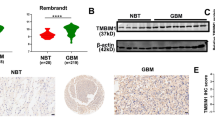
To further explore the possible function of MYH9 in glioma, we obtained MYH9 mRNA expression data via GEPIA. We found that MYH9 mRNA expression was significantly upregulated in patients with gliomas in the TCGA and GTEx databases (Fig. 2A). Furthermore, we searched the Chinese Glioma Genome Atlas (CGGA) database and obtained MYH9 mRNA expression data from glioma samples to clarify its specific role. With an increasing glioma grade, the mRNA expression of MYH9 increased significantly (Fig. 2B). Moreover, MYH9 was more highly expressed in IDH-wildtype gliomas (Fig. 2C). To determine the possible involvement of MYH9 in gliomas, we first analysed the mRNA and protein levels of MYH9 in gliomas and normal brain (NB) tissues. Immunohistochemical (IHC) staining results revealed that MYH9 expression in gliomas was significantly higher than that in NB tissues (Fig. 2D). This upregulation of MYH9 mRNA and protein expression was further confirmed by RT‒qPCR and WB analysis of human gliomas and neuroglia cell lines (Fig. 2E, F). Moreover, β-catenin and N-cadherin expression was positively correlated with MYH9 expression, whereas E-cadherin expression was negatively correlated with MYH9 expression from the GEPIA database (Fig. 2G-I). Collectively, these results suggest that the expression of MYH9 may be associated with the malignant progression of glioma.
MYH9 was highly expressed in human gliomas. (A) MYH9 mRNA expression in glioma tissues and para-tumor tissues among the glioma patients obtained from the TCGA database. (B) MYH9 mRNA level in gliomas from the CGGA database. (C) MYH9 had higher expression in IDH-wildtype gliomas. (D) The expression of MYH9 in tumor and adjacent samples were monitored by immunohistochemistry. (E,F) MYH9 mRNA and protein levels in glioma cell lines. (G–I) β-catenin and N-cadherin expression was positively correlated with MYH9 expression, whereas E-cadherin expression was negatively correlated with MYH9 expression from the GEPIA database. *P < 0.05, **P < 0.01, ***P < 0.001.
Upregulation of MYH9 is correlated with a poor prognosis of glioma patients
After showing that MYH9 was expressed in gliomas, we were interested in how MYH9 affects the clinical outcomes of glioma patients. We queried CGGA datasets and found that MYH9 expression was associated with a poor prognosis in WHO grade II, III, and IV glioma patients. Moreover, the upregulation of MYH9 in both primary and recurrent gliomas affected the prognosis of patients (Fig. 3A, B). The results from the UALCAN database suggested that patients with low MYH9 expression had longer survival times than patients with high MYH9 expression did (Fig. 3C). Moreover, patients with low MYH9 expression and low WHO grade gliomas experienced significantly longer survival than did those with high MYH9 expression and high WHO grade gliomas (Fig. 3D). Therefore, abnormal MYH9 protein levels are significantly associated with the prognosis of survival of glioma patients.
The upregulation of MYH9 correlated with poor prognosis in gliomas. (A,B) Survival analysis from CGGA database suggested that mRNA expression level of MYH9 had a negaitive relationship with glioma patients’ survival time. (C,D) The results of UALCAN database suggested that patients with low expression of MYH9 had a longer survival time compared to patients with high expression. Data are presented as the mean ± SD for three independent experiments.
MYH9 promotes epithelial‒mesenchymal transition signaling
To study the potential role of MYH9 in glioma progression, we transfected U87 and U251 cells with lentiviral constructs expressing a short hairpin RNA (shRNA) targeting MYH9 and the corresponding negative control (shNC). Moreover, small interfering RNAs (siRNAs) were used to silence MYH9 in U87 and U251 cells, which were then compared with negative control (siNC) cells. Next, we analysed MYH9 protein levels in U87 and U251 cells via WB analysis (Fig. 4A, B). After confirming efficient MYH9 gene silencing in the U87 cell line with shMYH9 and the siRNA, the same fragment in the U251 cell line was analysed via RT‒PCR and WB and compared with that in the shNC and siNC groups. To explore the biological role of MYH9, we silenced MYH9 and performed Transwell experiments. Our results revealed that the downregulation of MYH9 expression in gliomas significantly inhibited tumour cell migration (Fig. 4C) and invasion (Fig. 4D, E). Moreover, WB analysis demonstrated β-catenin and N-cadherin downregulation and E-cadherin upregulation after MYH9 knockdown (Fig. 4F-H). Therefore, we suggest that MYH9 regulates glioma invasion and metastasis via EMT modulation.
MYH9 promotes epithelial-mesenchymal transition signaling. (A,B) MYH9 protein level was measured by western blot in U87 and U251 cells transfected with siMYH9. GAPDH was used as a loading control. (C–E) MYH9 knockdown reduced cell migration and invasion identified using Transwell and Boyden assays in U87 and U251 cells. (F–H) MYH9 knockdown reduced the expression of β-catenin and EMT signals in U87 and U251 cells. Data are presented as the mean ± SD for three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
MYH9 interacts with β-catenin
To further investigate the induction of glioma metastasis by MYH9, we performed coimmunoprecipitation (Co-IP) and immunofluorescence assays to identify proteins that bind to MYH9. In a previous study on gastric cancer, our research team demonstrated the interaction of β-catenin with MYH922. In the present study, β-catenin was considered a candidate protein for the interaction with MYH9 in U87 cells based on our assessment of the BIOGRID database results and the Co-IP findings. Therefore, we examined whether β-catenin binding to MYH9 regulates glioma cell metastasis. Exogenous and endogenous Co-IP experiments confirmed that MYH9 interacted with β-catenin in glioma cells (Fig. 5A–C). Moreover, the results of the immunofluorescence tests revealed that the β-catenin and MYH9 proteins were predominantly colocalized in the cytoplasm (Fig. 5D). Additionally, we utilized InterPro (http://www.ebi.ac.uk/interpro/) to predict the structure of the three MYH9 domains, namely, the muscle ball tail, muscle ball head movement, and muscle N domains (Fig. 5E). Structural analysis revealed that the myosin tail ___domain was bound to β-catenin (Fig. 5F). These results suggest that MYH9 interacts with β-catenin.
MYH9 interacts with β-catenin. (A–C) The interaction of MYH9 and β-catenin in glioma cells was confirmed by exogenous and endogenous Co-IP experiments in U87 cells. (D) Representative immunofluorescence staining of MYH9 and β-catenin protein in U87 cells. (E) A schematic diagram of the Myosin tail, Myosin head motor, and Myosin N in MYH9 is shown. (F) Co-IP analysis was performed to examine the interaction between MYH9 domains and β-catenin in U87 cells.
MYH9 recruits the deubiquitinase USP2 to stabilize the β-catenin protein
Prior studies have shown that MYH9 activates the Wnt pathway by increasing β-catenin transcription23 or inducing GSK-3β ubiquitination24,25. To investigate the mechanism by which MYH9 modulates the Wnt pathway in gliomas, we suppressed MYH9 expression and evaluated the expression levels of β-catenin, a key molecule in the Wnt pathway. As depicted in this study, MYH9 inhibition reduced β-catenin protein levels but did not affect β-catenin mRNA expression. Therefore, MYH9 is unlikely to regulate β-catenin at the transcriptional level. Furthermore, we conducted a cycloheximide (CHX) tracing assay and an MG132 treatment experiment to determine whether MYH9 modulates β-catenin levels posttranslationally. Our results revealed that the half-life of the β-catenin protein was significantly shortened and that CHX and MG132 treatments led to significant accumulation of the protein in MYH9-knockdown cells (Fig. 6A–C), suggesting that MYH9 is involved in regulating β-catenin protein stability.
MYH9 recruits deubiquitinase USP2 to stabilize β-catenin protein. (A–C) WB was used to detect the effects of DMSO or MG132 treatment and CHX treatment for different duration on the stability of β-catenin protein in the control and MYH9 knockdown groups. (D,E) Co-IP assays were performed to detect the interaction of MYH9 and β-catenin with USP2. (F) Co-IP detected the effects of MYH9 knockdown on protein stability of β-catenin in U87cells. (G) WB experiment was used to detect the level of β-catenin in USP2-silenced glioma cells.
Considering that the ubiquitin proteasome pathway is a common protein degradation mechanism26, we next explored whether MYH9 inhibits β-catenin degradation in glioma cells by inducing β-catenin deubiquitination. In line with this assumption, our Co-IP assay revealed that MYH9 recruited the deubiquitinase USP2 and β-catenin to form a complex (Fig. 6D, E). Furthermore, we employed an anti-ubiquitin antibody to assess the β-catenin ubiquitination status. Accordingly, MYH9 knockdown significantly increased β-catenin ubiquitination levels (Fig. 6F), whereas USP2 inhibition suppressed β-catenin in MYH9-overexpressing glioma cells (Fig. 6G). These observations suggest that MYH9 may aggregate the deubiquitinase USP2 to stabilize the β-catenin protein, thereby promoting EMT signalling.
β-catenin overexpression reverses the effects of MYH9 knockdown on glioma EMT
To investigate whether β-catenin overexpression can reverse the effects of MYH9 knockdown in gliomas, we first determined β-catenin expression in MYH9-silenced glioma cells via WB (Fig. 7A). We found that glioma cells transfected with ov-β-catenin presented a significantly greater number of migrated cells than did those in the siMYH9 group (Fig. 7B, C). Similarly, invasion experiments using Matrigel-coated membranes revealed that increasing β-catenin expression in MYH9-silenced glioma cells increased cell invasion (Fig. 7D, E). These results suggest that MYH9 depends on β-catenin for its ability to promote glioma cell invasion and migration. Given that EMT is a vital process in cancer metastasis, we next applied WB analysis to examine the effect of β-catenin overexpression on the recovery of siMYH9-induced EMT signal suppression. WB revealed that β-catenin overexpression markedly decreased the level of the epithelial marker E-cadherin, whereas that of N-cadherin was elevated (Fig. 7F). Thus, increasing β-catenin expression in MYH9-silenced glioma cells restores the expression patterns of EMT-related proteins. Collectively, these results suggest that β-catenin overexpression reverses the effects of MYH9 silencing on glioma cell metastasis and EMT.
β-catenin overexpression reverses the effect of MYH9 knockdown on metastasis and EMT of glioma cells. (A) Protein levels of β-catenin that changed following MYH9 knockdown in glioma cell lines overexpressing β-catenin. GAPDH was used as a loading control. (B–E) overexpressing β-catenin increased cell migration and invasion identified using Transwell and Boyden assays in glioma cell lines with knockdown MYH9. (F) Protein levels of EMT markers that changed following MYH9 knockdown in glioma cell lines overexpressing β-catenin. GAPDH was used as a loading control. Data are presented as the mean ± SD for three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Epithelial‒mesenchymal transition (EMT) is a process in which polar epithelial cells transform into active mesenchymal cells and acquire invasion and migration capabilities (1). EMT is involved in multiple physiological and pathological processes in the human body. The occurrence of EMT involves many signal transduction pathways and complex molecular mechanisms related to calnexin, growth factors, transcription factors, and the microenvironment. EMT is closely related to the invasion and metastasis of tumour cells. The migration, invasion, and metastasis of glioma cells are great challenges in the treatment of glioma. Although chemotherapy, targeted therapy, and immunotherapy have advanced in recent years, the 5-year prognosis of patients with gliomas continues to be poor because of the susceptibility to metastasis. Hence, studies are urgently needed to elucidate the molecular mechanism of epithelial‒mesenchymal transition (EMT) and stemness maintenance in glioma cells and to identify biomarkers and targets that can further improve glioma prognosis.
MYH9, a myosin family protein, is involved in cell adhesion, polarity, motility, migration, and other biological activities8. Moreover, MYH9, which functions as a cytoskeletal protein, has been reported to be a critical component in numerous human diseases9,10,11,12,13. Furthermore, an increasing number of studies over the last few years have reported increased MYH9 levels in many cancers. However, the function and regulatory mechanism of MYH9 in tumour occurrence and development remain unclear. The current study provides strong evidence for the oncogenic role of MYH9 in gliomas. Specifically, we demonstrated that MYH9 knockdown significantly inhibited glioma migration and invasion. Furthermore, increased MYH9 expression was associated with a poor prognosis in patients with gliomas. Therefore, our study results suggest that MYH9 is a potential glioma oncogene.
The results of the present study revealed that MYH9 significantly regulated glioma cell migration and invasion, thus revealing a new mechanism involved in the rapid progression of glioma. Our discovery of the association of MYH9 with EMT represents a breakthrough in understanding glioma metastasis because EMT is a known mechanism involved in diffuse metastasis of these tumours27,28,29. In our study, MYH9 knockdown led to a significant increase in E-cadherin levels in vitro, whereas N-cadherin and β-catenin levels were decreased. This alteration pattern indicates that MYH9 inhibition results in a more epithelial phenotype. Consistent with previous findings, we detected that MYH9 knockdown significantly affected N-cadherin levels. These results can be attributed to N-cadherin being a relatively reliable connexin indicator in the glioma EMT process30,31,32. Therefore, our findings, which are supported by the levels of several EMT markers, including N-cadherin, suggest that MYH9 is a vital effector in the induction of glioma EMT.
Prior studies have demonstrated that β-catenin is translocated into the nucleus, where it induces the transcriptional activation of target genes and directly and indirectly promotes EMT in conjunction with T-lymphocyte cytokine factors (including cadherins) and other key TFs (such as Snail and Slug)33,34,35. As illustrated in the schematic diagram in Fig. 8, we propose a novel molecular mechanism to elucidate the role of MYH9 in glioma metastasis. In this mechanism, MYH9 induces EMT by stabilizing β-catenin levels and activating the Wnt/β-catenin pathway. GSK-3β is known to phosphorylate the Ser33, Ser37 and Thr41 residues of β-catenin in the absence of WNT signalling. These phosphorylated residues are then recognized by E3 ubiquitin ligases and are labelled for ubiquitination, ultimately leading to proteasomal degradation of β-catenin36. The present study revealed that MYH9 prevented β-catenin degradation and enhanced its nuclear activity by recruiting the deubiquitinase USP2. Moreover, MYH9-mediated Wnt production has been demonstrated in breast cancer studies37. Whether there is a similar mechanism involving MYH9 and WNT in gliomas requires further exploration.
In summary, our study describes a regulatory mechanism of MYH9 in gliomas, which is presented as an overview in Fig. 8. We revealed that MYH9 played a crucial role in glioma cells via β-catenin-mediated EMT. In this process, MYH9 binds to β-catenin and increases its protein level by recruiting the deubiquitinase USP2 to form a complex. These changes ultimately lead to the inhibition of β-catenin protein degradation and the induction of EMT signalling. The MYH9/β-catenin/EMT pathway is a novel molecular mechanism for promoting glioma invasion. Furthermore, our study data suggest that MYH9 is a critical tumour-promoting factor that facilitates glioma migration, invasion, and metastasis. Moreover, increased expression of MYH9 leads to a shorter survival time for patients with gliomas. Therefore, the MYH9 expression level may have particular application value in the pathogenesis and clinical management of gliomas. Finally, future studies targeting MYH9 may yield new therapeutic strategies to prevent the rapid progression of diffuse invasive gliomas.
Schematic of MYH9 promoting glioma development. MYH9 bound to β-catenin and increased its protein expression by recruiting deubiquitinase USP2 to form a complex. Therefore, the degradation of β-catenin protein is inhibited and EMT signaling is finally promoted. MYH9/β-catenin/EMT is a newly discovered molecular mechanism for promoting glioma invasion.
Materials and methods
Experimental cell lines and study patients
The U87 and U251 human glioma cell lines were provided by the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). These cell lines were cultured in DMEM (Gibco) supplemented with 10% foetal bovine serum (HyClone) at 37 °C and 5% CO2. Additionally, gliomas and the corresponding adjacent tissues were obtained from patients who underwent a surgical procedure at the Affiliated Haikou Hospital of the Xiangya Medical School. Ethics approval from the Ethics Committee of the Affiliated Haikou Hospital of the Xiangya Medical School and patient consent were obtained for this study.
Lentivirus production and infection
The lentiviruses carrying the MYH9 shRNA vector (shMYH9) and the negative control (shNC) were constructed by GeneChem (Shanghai, China). The glioma cells were transfected with shMYH9 or shNC, as described in a previous study36. The MYH9-targeting shRNA sequence is presented in Supplementary Table 1. RT‒qPCR and WB analyses were used to assess MYH9 expression.
Plasmid or small interfering RNA (siRNA) transfection
β-Catenin plasmids were obtained from GeneChem, and siRNAs for MYH9 and USP2 were designed and synthesized by IGE Biotechnology, Ltd. (Guangzhou, China). According to the manufacturer’s instructions, when the confluence of U87 and U251 cells reached 70%, they were transfected with the plasmids and siRNAs by using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific). The medium was subsequently changed after 8 h, and the cells were collected at 36 and 60 h after transfection.
Reverse transcription‒quantitative polymerase chain reaction (RT‒qPCR)
RNA was extracted from the transfected cells 36 h posttransfection with QIAzol (Qiagen, Shanghai, China). Complementary DNA (cDNA) was then synthesized from 1 µg of the extracted RNA with random primers and the Maxima first-strand cDNA synthesis kit (Takara Bio, Inc., Otsu, Japan) on a Bio-Rad CFX96 system. Next, a SYBR® Green master mix was used for qPCR on a Bio-Rad T100 detection system. All the primers used in this study are listed in Supplementary Table 2.
Cycloheximide (CHX) chase assay
First, CHX (Selleck, Cambridge, UK) was dissolved in DMSO (200 mL) and stored at 20 °C. The cells were seeded into 6-well plates in 2 mL of culture media. Once attached, the cells were incubated with 50 µg/mL CHX at 37 °C and 5% CO2 for various durations. Finally, the cells were collected and further analysed via WB.
Coimmunoprecipitation (Co-IP) assay
The plasmids carrying Flag in the functional MYH9 ___domain were transfected into U87 and U251 cells. Next, IP was performed according to the Thermo Fisher Co-IP kit instructions38. WB analysis was then performed to detect the interacting proteins, including β-catenin and USP2.
Cell migration and invasion assays
The cell migration and invasion experiments were conducted by using Transwell plates (24-well inserts with 8-µm pores; Corning, Inc., Lowell, MA, USA), as described in a previous study38.
Western blot (WB) analysis
Total proteins were extracted with RIPA lysis buffer (Beyotime Institute of Biotechnology) containing PMSF (Bio-Rad Laboratories) and phosphatase inhibitors (Bio-Rad Laboratories) at a 100:1:1 ratio, as previously described19. The primary antibodies used, including those against MYH9, E-cadherin, N-cadherin, and β-catenin, are presented in Supplementary Table 3. The WB tests were repeated at least three times.
Ubiquitination assay
Cells were harvested and lysated in a buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 2% SDS. Before immunoprecipitation, the lysates were boiled at 95℃ for 5 min. Immunoprecipitation and western blot analysis were conducted as described above.
Statistical analysis
All the experiments were conducted at least three times. Statistical analyses were performed by using the SPSS 21.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA) software. The data are presented as the means ± standard deviations. Statistical significance is indicated for each graph (NS: p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- MYH9:
-
Myosin heavy chain 9
- CGGA:
-
Chinese glioma genome atlas database
- CHX:
-
Cycloheximide
- IHC:
-
Immunohistochemical
- RT-qPCR:
-
Real time quantitative polymerase chain reaction
- PAAD:
-
Pancreatic adenocarcinoma
- UCEC:
-
Uterine corpus endometrial carcinoma
- Clear cell RCC:
-
Clear cell renal cell carcinoma
References
Schaff LR. & Mellinghoff IK. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 329(7), 574-587 (2023)
Andrews, J. P., Coleman, C., Hastings, C. & Sun, P. P. Oncogenic NTRK fusion in congenital spinal cord glioblastoma: sequencing directs treatment. Lancet 398, 10317 (2021).
El Hout, M., Cosialls, E., Mehrpour, M. & Hamaï, A. Crosstalk between autophagy and metabolic regulation of cancer stem cells. Mol. Cancer 19(1) (2020).
Mohile, N. A. et al. Therapy for diffuse astrocytic and oligodendroglial tumors in adults: ASCO-SNO guideline. J. Clin. Oncol. 40(4) (2022).
Wang, Q. et al. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nat. Commun. 9 (1), 559 (2018).
Izquierdo, E. et al. DIPG harbors alterations targetable by MEK inhibitors, with acquired resistance mechanisms overcome by combinatorial inhibition. Cancer Discov. (2021).
Molinaro, A. M., Taylor, J. W., Wiencke, J. K. & Wrensch, M. R. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 15 (7), 405–417 (2019).
Vicente-Manzanares, M., Ma, X., Adelstein, R. S. & Horwitz, A. R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell. Biol. 10, 778–790 (2009).
Pecci, A., Ma, X., Savoia, A. & Adelstein, R. S. MYH9: structure, functions and role of nonmuscle myosin IIA in human disease. Gene 664, 152–167 (2018).
Baumann, J. et al. MYH9 reduced platelet forces underlie impaired hemostasis in mouse models of -related disease. Sci. Adv. 8(20) (2022).
Sadaf, A. & Ware, R. E. Microscope diagnosis of MYH9-related thrombocytopenia. Blood 138(11) (2021).
Pal, K. et al. Megakaryocyte migration defects due to nonmuscle myosin IIA mutations underlie thrombocytopenia in MYH9-related disease. Blood 135(21) (2020).
An, Q. et al. Myh9 plays an essential role in the survival and maintenance of hematopoietic Stem/Progenitor cells. Cells 11(12) (2022).
Chen, M. et al. MYH9 is crucial for stem cell-like properties in non-small cell lung cancer by activating mTOR signaling. Cell. Death Discov. 7(1) (2021).
Zhong, Y. et al. MYH9-dependent polarization of ATG9B promotes colorectal cancer metastasis by accelerating focal adhesion assembly. Cell. Death Differ. 28(12) (2021).
Gao, S. et al. TUBB4A interacts with MYH9 to protect the nucleus during cell migration and promotes prostate cancer via GSK3β/β-catenin signalling. Nat. Commun. 13(1) (2022).
Hu, S. et al. Glycoprotein PTGDS promotes tumorigenesis of diffuse large B-cell lymphoma by MYH9-mediated regulation of Wnt-β-catenin-STAT3 signaling. Cell. Death Differ. 29(3) (2022).
Xu, Z. et al. Single-cell RNA-sequencing analysis reveals MYH9 promotes renal cell carcinoma development and sunitinib resistance via AKT signaling pathway. Cell. Death Discov 8(1) (2022).
Lin, X. et al. Silencing MYH9 blocks HBx-induced GSK3β ubiquitination and degradation to inhibit tumor stemness in hepatocellular carcinoma. Signal. Transduct. Target. Ther. 5, 13 (2020).
Chen, Z. et al. Targeting MYH9 represses USP14-mediated NAP1L1 deubiquitination and cell proliferation in glioma. Cancer Cell. Int. 23(1) (2023).
Liu, Q. et al. MYH9: A key protein involved in tumor progression and virus-related diseases. Biomed Pharmacother. 171, 116118 (2024)
Liu, J. et al. ENKUR recruits FBXW7 to ubiquitinate and degrade MYH9 and further suppress MYH9-induced deubiquitination of β-catenin to block gastric cancer metastasis. MedComm. (2020) 2022 Dec;3(4):e185
Ye, G. et al. Nuclear MYH9-induced CTNNB1 transcription, targeted by staurosporin, promotes gastric cancer cell anoikis resistance and metastasis. Theranostics 10, 7545–7560 (2020).
Li, Y. et al. Chemical compound cinobufotalin potently induces FOXO1-stimulated cisplatin sensitivity by antagonizing its binding partner MYH9. Signal. Transduct. Target. Ther. 4, 48 (2019).
Liu, Y. et al. Cinobufotalin powerfully reversed EBV-miR-BART22-induced cisplatin resistance via stimulating MAP2K4 to antagonize non-muscle myosin heavy chain IIA/glycogen synthase 3beta/beta-catenin signaling pathway. EBioMedicine 48, 386–404 (2019).
Suber, T. et al. SCFFBXO17 E3 ligase modulates inflammation by regulating proteasomal degradation of glycogen synthase kinase-3beta in lung epithelia. J. Biol. Chem. 292, 7452–7461 (2017).
Depner, C. et al. EphrinB2 repression through ZEB2 mediates tumour invasion and anti-angiogenic resistance. Nat. Commun. 7, 12329 (2016).
Zhang, J. et al. LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/beta-catenin pathway and predicts poor survival of glioma patients. J. Exp. Clin. Cancer Res. 37, 225 (2018).
Mader, L. et al. Pericytes/vessel-associated mural cells (VAMCs) are the major source of key epithelial-mesenchymal transition (EMT) factors SLUG and TWIST in human glioma. Oncotarget 9, 24041–24053 (2018).
Camand, E., Peglion, F., Osmani, N., Sanson, M. & Etienne-Manneville S. N cadherin expression level modulates integrin-mediated polarity and strongly impacts on the speed and directionality of glial cell migration. J. Cell. Sci. 125, 844–857 (2012).
Appolloni, I. et al. A cadherin switch underlies malignancy in high-grade gliomas. Oncogene 34, 1991–2002 (2015).
Catalano, M. et al. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol. Oncol. 9, 1612–1625 (2015).
Yuan, K. et al. TXNDC12 promotes EMT and metastasis of hepatocellular carcinoma cells via activation of beta-catenin. Cell. Death Differ. 27, 1355–1368 (2020).
Das, V., Bhattacharya, S., Chikkaputtaiah, C., Hazra, S. & Pal, M. The basics of epithelial-mesenchymal transition (EMT): a study from a structure, dynamics, and functional perspective. J. Cell. Physiol. https://doi.org/10.1002/jcp.28160 (2019).
Basu, S., Cheriyamundath, S. & Ben-Ze’ev, A. Cell-cell adhesion: linking Wnt/ beta-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Research 7. https://doi.org/10.12688/f1000research.15782.1 (2018).
Nusse, R. & Clevers, H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Vadnais, C. et al. Autocrine activation of the Wnt/beta-catenin pathway by CUX1 and GLIS1 in breast cancers. Biol. Open 3, 937–946 (2014).
Hou, R. et al. ENKUR expression induced by chemically synthesized cinobufotalin suppresses malignant activities of hepatocellular carcinoma by modulating β-catenin/c-Jun/MYH9/USP7/c-Myc axis. Int. J. Biol. Sci. 18(6) (2022).
Acknowledgements
We express our sincere thanks to all authors of this study.
Funding
This work was supported by Hainan Province Clinical Medical Research Center (grant no. LCYX202410, LCYX202309) and Hainan Province Clinical Medical Center (grant no. 0202068); Natural Science Foundation of Hunan Province (grant no. 2024JJ9290); Changsha Natural Science Foundation (grant no. kq2403145).
Author information
Authors and Affiliations
Contributions
Z.G.C designed and supervised the study; Z.G.C, C.L, W.Z, Z.L.L and Y.H.T performed experiments and analyzed the data. Y.X and Z.G.C designed the research, interpreted data, and contributed analytical tools; J.P, D.D.Z and C.F.M provided advice and technical assistance. Z.G.C, J.P, D.D.Z, C.F.M and Y.X wrote the manuscript and edited the article. All of the authors have read the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The experiments and experimental protocols in this paper were approved by the Ethics Committee of the Affiliated Haikou Hospital of Xiangya Medical School. All procedures performed in this study were according with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was provided and signed by all patients prior to sample collection.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Z., Liu, C., Zheng, W. et al. MYH9 promotes malignant progression of glioma cells through regulating β-catenin stability via epithelial-mesenchymal transition signaling pathway. Sci Rep 15, 4618 (2025). https://doi.org/10.1038/s41598-025-87151-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87151-z