Abstract
The main objective of this study is to clarify the effect of bottom ash (BA) powder replacement rate, water-cement ratio, and sand-cement ratio on alkali-activated slag mortar (AASM) by comprehensively investigating the following parameters: fluidity, consistency, setting time, pH, compressive strength, and flexural strength, as well as observing the microstructure by XRD and SEM. Finally, the relationship between the parameters was explored by means of correlation coefficient heatmaps in concert with scatter plots (including quadratic polynomial linear fitting). The results show that it is feasible to use up to 60% of BA powder to replace slag for AASM. Meanwhile, it is suggested that the AASM with a water-cement ratio of 0.44 and a sand-cement ratio of 2.6 can obtain better workability, mechanical properties, and a denser microstructure. The incorporation of BA powder produces the unique hydration product Magadiite. In addition, the mechanical models of compressive strength and flexural strength of AASM were proposed \(y = 0.23 \cdot x^{{0.82}} ,\)\(\:{R}^{2}=0.86\). This study provides a reference for the application of BA powder in the alkali-activated system, which is beneficial for resource recycling.
Similar content being viewed by others
Introduction
Amidst growing concerns over global climate change, the construction industry faces significant challenges in reducing CO2 emissions. The traditional silicate cement production process releases a substantial amount of CO2, with each ton of cement produced emitting approximately 0.8 tons of CO21. To address this issue, researchers have proposed alkali-activated slag as an alternative material, which demonstrates many superior properties compared to cement-based materials. These properties include high early strength2, chemical resistance3,4, weatherability5, high temperature resistance6, and tighter interfacial transition zones (ITZs) at the matrix interface7, while also reducing CO2 emissions by 30–50%8.
However, the performance of alkali-activated slag mortar (AASM) is affected by various factors, among which supplementary cementitious materials, water-cement ratio, and sand-cement ratio are key parameters. Supplementary cementitious materials include fly ash, metakaolin, red mud, and bottom ash (BA) powder. For instance, Luo L et al. (2023)9 investigated the autogenous shrinkage, workability, and microstructure of alkali-activated slag/fly ash as a precursor. The results showed that with increasing fly ash content, the slump increased, the setting time was prolonged, the strength slightly decreased, and the autogenous shrinkage was significantly reduced. Fu C et al. (2024)10 used slag and metakaolin as alumino-silicate precursors, with dolomite powder as a filler to make AASM, and concluded that AASM performs better in chemical erosion environments when metakaolin replacement is 40%, with metakaolin replacement being more effective than dolomite replacement. Tian K et al. (2022)11 investigated the effect of red mud on the fluidity, setting time, compressive strength, and microstructure of AASM, and found that red mud reduced fluidity and prolonged the setting time. Additionally, an appropriate amount of red mud can promote the alkali activation reaction of slag, which is beneficial to the development of compressive strength. Nevertheless, considering the environmental, economic, and ecological benefits of supplementary cementitious materials12, the application of materials such as fly ash, metakaolin, and red mud in AASM is not as favorable as that of BA powder. Meanwhile, the study of BA powder in AASM is limited and requires further research.
The water-cement ratio is a crucial factor in the study and application of mortar or concrete. A high water-cement ratio leads to an increase in free water in the slurry and a weakening of the adhesion between the hydration products and aggregates, which reduces the strength of the mortar or concrete13. Additionally, a high water-cement ratio increases the porosity of mortar or concrete14, which reduces its compactness and durability15. A low water-cement ratio significantly reduces the fluidity of mortar or concrete, making it less workable16, which leads to difficulties in the uniform distribution of internal particles and a reduction in densification. Although a low water-cement ratio increases the early strength of mortar or concrete, it leads to increased drying shrinkage thereof, increasing the risk of cracking17. Therefore, in the design and application of AASMs, the choice of water-cement ratio should be fully considered to ensure that the overall performance of the mortar meets the desired objectives, including better mechanical properties18, workability18, and durability19. Moreover, the factor of sand-cement ratio in AASM is an issue that has received little attention in previous studies. An appropriate sand-cement ratio not only helps to improve the mechanical properties, workability, and durability of mortar or concrete, but also brings significant economic benefits20. Therefore, the study of sand-cement ratio can provide a more scientific basis for engineering design and help to achieve higher material properties and economic benefits in practical applications.
In summary, this paper investigates the effects of BA powder replacement rate, water-cement ratio and sand-cement ratio on the mechanical (compressive and flexural strengths), workability (fluidity, consistency, and setting time), pH and microstructure (X-ray diffraction (XRD)) and scanning electron microscope (SEM)) of AASM, and finally explores the relationships between the parameters by using a combination of correlation coefficient heatmaps and scatter plots (including quadratic polynomial linear fitting).
Materials and methods
Raw materials
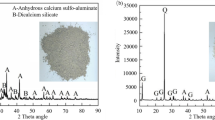
The slag type is S95 grade, specific surface area is 429 m2/kg, density is 3100 m2/kg, and the specific surface area of BA powder is 398 m2/kg. Figure 1 shows the XRD diagram of BA and slag, and it can be seen from the diagram that the physical phase of BA and slag is relatively similar, especially note that the BA contains aluminum metal, which will produce H2 under alkaline environment and increase the porosity of mortar. Meanwhile, it can be found that the chemical compositions of both BA and slag are dominated by CaO, SiO2 and Al2O3 (Fig. 2). The chemical composition of BA and slag was tested by X-ray fluorescence spectroscopy (XRF) (Fig. 4).
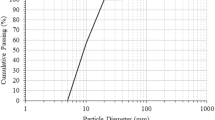
Figure 3 shows the particle size distribution of BA powder and slag, from which it can be seen that the particle size distribution of BA powder is more homogeneous compared to slag, with a distribution range of roughly 0.7–30 μm, while the particle size of slag is concentrated around 20 μm.
The fine aggregate used in this experiment is ISO standard sand. The main components of the water glass (Na2SiO3 solution) were 27.06% SiO2 and 8.57% Na2O with a modulus of 3.25 and a total solid content of 60.74%. Water glass is added in the form of a gel. The purity of NaOH was not less than 96.0%. The water used was industrial deionized water. The NaOH solution consisted of 400 g of NaOH solid mixed with 1 L of deionized water and left to stand for more than 24 h.
There are three main variables in this experiment, which are BA powder replacement rate, water-cement ratio and sand-cement ratio. BA powder and slag were used as alkali-activated solid precursors in this study. Water glass was mixed with NaOH solution in the ratio of 2:1. The BA powder replaced slag at the rate of 20%, 40%, 60% and 80%. The water-cement ratios were 0.38, 0.41, 0.44 and 0.47, and the sand-cement ratios were 2.2, 2.6, 3.0 and 3.4. A control group with 0% bottom ash replacement was also set up and named Group N. The selection of this test mix was based on extensive pre-experimentation by the subject group (unpublished). The cementitious material was 450 g/m3. The ratio of alkali activator (NaOH solution and water glass) to cementitious material (BA powder and slag) was 0.4. The specific ratios are shown in Table 1, and the preparation process of the specimens is shown in Fig. 4.
Experimental methods
Fluidity test
According to “Test method for fluidity of cement mortar” (GB/T 2419 − 2005) for the determination of the mortar fluidity (Fig. 4). The testing time of fluidity was controlled within 6 min, and the average value of the two test results was taken as the fluidity of mortar, and the result was taken as an integer (mm).
Consistency test
The consistency test is conducted in accordance with the code “Standard for Test Method of Basic Properties of Construction Mortar” (JGJ/T 70-2009) (Fig. 4). Before starting the test, lubricate the slide bar to ensure it can move freely. The mortar should be loaded into the container in a single step, ensuring its surface is approximately 10 mm below the top edge of the test container. After loading, vibrate the container to level the mortar surface. After 10 s, record the sinking data of the test cone, with the result accurate to 1 mm.
Setting time test
The setting time of the mortar was determined in accordance with the code “Test Methods for Water Consumption, Setting Time and Stability of Cement Standard Consistency” (GB/T1346-2011). The setting time instrument is shown in Fig. 4.
The initial setting time is defined as the duration from the beginning of mixing the slurry with an aqueous solution to its initial solidification state. Prior to the test, the Vickers instrument should be wiped with a damp cloth, moistened, and calibrated. During determination, the test needle is immersed in the pulp. When the needle sinks to 4 mm ± 1 mm from the bottom plate, the cement is considered to have reached its initial solidification state. If this condition is not met, determinations are made at 5-minute intervals (or shorter if necessary).
The final setting time is defined as the duration from the beginning of mixing the slurry with an aqueous solution to its final solidification state. The final set needle is installed, and after the initial set of samples is maintained, if the test needle sinks into the sample by 0.5 mm or the ring attachment leaves no trace on the specimen, the slurry is considered to have reached its final state. If these conditions are not met, determinations are made at 15-minute intervals (or shorter if necessary).
pH test
The acid-base test was conducted using the solid-liquid extraction method of New C and Sykes J (1987). When the sample reaches the appropriate age, the crushed sample is ground into powder. According to a water-solid ratio of 10:1, 5 g of the sample is mixed with 50mL of deionized water, mixed thoroughly, and shaken every 5 min. The mixture is allowed to stand for 2 h, and then filtered after 24 h. The pH value of the solution is determined using a PHS-25-type Precision pH meter (Fig. 4). Each sample is measured three times, and the difference between the maximum and minimum values must be less than 0.3 from the median. If any measurement exceeds 0.3, the intermediate value is taken. If all are greater than 0.3, the test is repeated with a new sample. Additionally, the average of the three measurements is taken, accurate to 0.1.
Compressive/flexural strength test
Mortar strength test in accordance with the “Test method of cement mortar strength (ISO method)” GB/T 17,671 − 2021 for determination (Fig. 4), to reach the specified age of maintenance for compressive and flexural strength test, the test results are accurate to 0.1 MPa.
Particle size test
The laser particle size meter is mainly used to measure the particle size distribution of BA and slag (Fig. 4). This test determines the particle size distribution of the test material which is not limited by the physico-chemical properties of the particles.
XRF test
XRF tests were performed using an X-ray fluorescence spectrometer (XRF-1800) to analyze the chemical composition of BA and slag (Fig. 4). Firstly, the waste incineration BA specimens were finely ground into a powder and passed through a 1.5 mm sieve, and then placed in an oven to dry to a constant weight, and then the test specimens were pressed into shape by using boric acid to test the specimens.
XRD test
XRD tests were performed using a DX-2700 X-ray diffractometer (Fig. 4), which was mainly used to determine the physical composition of BA, slag and mortar (28 days). The diffraction angles ranged from 5◦ to 80◦. The measurement time is 0.1 s and the step width angle is 0.06°. XRD data were analysed using Jade software.
SEM test
SEM tests were performed using a HTTACHIS-3000 N scanning electron microscope (Fig. 4), which focuses on observing the internal micro-morphology of the mortar (28 days) and the generation of hydration products, among other things.
Results and discussion
Fluidity analysis
Figure 5 shows the effect of BA powder replacement rate, water-cement ratio and sand-cement ratio on the fluidity of AASM. The code “Common portland cement” (GB175-2007) and “Specification for mix proportion design of masonry mortar” (JGJ 98-2000) require that the fluidity of the mortar is not less than 180 mm.
From Fig. 5a, it is observed that the fluidity of AASM initially increases and then decreases with the increase in the replacement rate of BA powder (20-80%). The maximum fluidity of 224 mm is achieved at a 60% replacement rate of BA powder. This enhancement in fluidity can be attributed to the addition of a specific proportion of BA powder, which optimizes the particle gradation of the AASM, reducing the inter-particle void ratio and thus achieving an optimal fluidity state for the slurry. In addition, the fluidity (211 mm) of group N was between groups B2 and B3, which may be due to the absence of BA with high water absorption characteristics and the increase of water in the unit volume of AASM, so that the fluidity of AASM did not decrease significantly but was higher than that of groups B1, B2 and B4.
Furthermore, in the alkali-activated slag system, the increase in BA powder content dilutes the concentration of the active component, weakening the particle bonds and enhancing fluidity. Even at an 80% replacement rate of BA powder, the fluidity reaches 183 mm, thereby satisfying the code requirement of a minimum of 180 mm.
From Fig. 5b, it is evident that the fluidity of AASM demonstrates an increasing trend (61 mm to 224 mm) with an increasing water-cement ratio (0.38 to 0.47), consistent with the findings of Dong J et al. (2024)21. An increased water-cement ratio indicates a higher amount of water per unit of cementitious material, directly increasing the amount of free water in the AASM and consequently enhancing its fluidity. Additionally, water acts as a lubricant in the alkali-activated mortar, reducing friction and adhesion between particles, thereby promoting the fluidity of the mortar. Particularly noteworthy is the significant increase in fluidity between the S1 and S2 groups, where the fluidity of the S2 group is approximately three times higher than that of the S1 group. This indicates that the increase in free water volume is especially significant for enhancing the fluidity of AASM when the water-cement ratio is relatively low. Specifically, when the water-cement ratio is 0.38 and 0.41, the fluidity of the mortar is 61 mm and 171 mm, respectively, both of which are below the code of 180 mm. Conversely, when the water-cement ratio is 0.44 and 0.47, the fluidity meets the code requirements, registering at 195 mm and 224 mm, respectively.
From Fig. 5c, it is observed that the fluidity of AASC exhibits a decreasing trend (276 mm to 139 mm) as the sand-cement ratio increases (2.2 to 3.4). An increased sand-cement ratio indicates a higher proportion of sand per unit volume and a corresponding decrease in the proportion of cementitious material. This results in a reduced degree of slurry coating on each sand particle, which subsequently increases the mutual contact area between sand particles in the AASM. This increased contact area leads to higher internal frictional resistance, thereby reducing the fluidity of the mortar22. In particular, the cementitious material will play a lubricating role in the mortar23, so the increase of the sand-cement ratio will lead to the reduction of the ‘lubricant’ in the AASM, which will further reduce the fluidity of AASM. Specifically, when the sand-cement ratio is 2.2, 2.6, and 3.0, the fluidity measures 276 mm, 239 mm, and 199 mm, respectively, all of which satisfy the code requirements. However, at a sand-cement ratio of 3.4, the fluidity drops to 139 mm, falling short of the code requirement of 180 mm.
Consistency analysis
Figure 6 shows the effects of BA powder replacement rate, water-cement ratio and sand-cement ratio on the consistency of AASM. From the figure, it can be found that the trend of AASM consistency is similar to that of fluidity. The code “Common portland cement” (GB175-2007) and “Specification for mix proportion design of masonry mortar” (JGJ 98-2000) require that the consistency of mortar should be in the range of 70 to 100 mm.
From Fig. 6a, it can be observed that the consistency of the B3 group is the highest, measuring 75 mm. As discussed in Sect. 3.1, the inclusion of BA powder in a specific proportion results in optimal gradation with the AASM, leading to a denser structure and peak consistency. Additionally, compared to slag, BA powder exhibits a higher water absorption rate24. As the replacement rate of BA powder increases, the amount of free water within the AASM decreases, thereby increasing its consistency. However, when the BA powder replacement rate becomes excessively high (Group B4), it introduces additional voids within the AASM and significantly reduces the amount of free water. This results in the mortar becoming too dry and loose, consequently decreasing its consistency. Similar to fluidity changes, the increase in free water volume is notable when the water-cement ratio is relatively low, significantly impacting the consistency of the AASM. The consistency of the AASM (75 mm) meets the code requirements only when the BA powder replacement rate is 60%.
From Fig. 6b, it is observed that as the water-cement ratio increases from 0.38 to 0.47, the consistency also increases from 18 mm to 85 mm. This increase in the water-cement ratio primarily results in a dilution effect on the cementitious materials in the AASM. Specifically, the concentration of cementitious materials per unit volume decreases, thereby weakening the bonding ability and leading to an increase in consistency. When the water-cement ratio is 0.38 and 0.41, the consistency of the AASM is 18 mm and 68 mm, respectively, both of which fall short of the code requirements. Conversely, when the water-cement ratio is 0.44 and 0.47, the consistency of the mortar is 75 mm and 85 mm, respectively, meeting the code requirements.
From Fig. 6c, it is observed that the consistency of AASM decreases as the sand-cement ratio increases, with the most significant decrease noted in group G4, which is 44 mm less compared to group G3. The cementitious material is the primary component providing the bonding force in the mortar. Due to the relatively large and smooth surface of the sand grains, the friction and cohesion between them are considerably lower. An increase in the sand-cement ratio indicates a decrease in the bond, friction, and cohesion within the AASM, leading to a reduction in consistency. The consistency of AASM with a sand-cement ratio of 2.6 is 84 mm, which satisfies the code requirements. However, at sand-cement ratios of 2.2, 3.0, and 3.4, the consistencies are 105 mm, 54 mm, and 10 mm, respectively, with the latter two values falling short of the code threshold.
3.3 Setting time analysis
Figure 7 shows the effect of BA powder replacement rate, water-cement ratio and sand-cement ratio on the setting time of AASM. From the figure, it can be seen that the initial setting time and final setting time of AASM are on the rise with the increase of the replacement rate of BA powder, water-cement ratio and sand-cement ratio. According to “Common portland cement” (GB175-2007), the initial setting time is ≥ 45 min, and the final setting time is ≤ 6.5 h. The final setting time of AASM with different BA powder replacement rate, water-cement ratio and sand-cement ratio meets the code requirements, and part of the initial setting time exceeds the threshold value.
Since the activity of BA powder is not as good as slag, and the water absorption rate of BA powder is higher than that of slag, the hydration reaction process of AASM is slowed as the replacement rate of BA powder increases, thus prolonging the initial and final setting times. It is worth noting that the presence of metallic aluminum in the BA may have an inhibitory effect on the formation of hydration products25. In addition, it can be noted from Fig. 7a that the increase in setting time is most pronounced when the BA powder replacement rate changes from group B2 (40%) to group B3 (60%), with growth rates of up to 133% for both initial and final setting times. When the BA powder replacement rate is 20%, the initial setting time of AASM is 32 min, which is lower than the code requirement. Of particular note, the initial setting time of group N was higher than the other groups and the final setting time was between groups B3 and B4. A replacement rate of 0% for BA leads to an increase in the viscosity of the mortar because BA particles are coarser and more absorbent than slag particles, which may affect water distribution and reaction rates. Higher viscosity mortar systems may reduce the effective contact on the reactive surface area, slowing down the chemical reaction process and therefore prolonging the setting time.
The increase in the water-cement ratio implies the presence of more free water in the AASM, which dilutes the alkali-activated and cementitious material reactants, thereby slowing down the hydration reaction and prolonging the setting time. In addition, it is noted from Fig. 7b that the variation in the water-cement ratio from group S3 (0.44) to group S4 (0.47) shows the largest increase in setting time, with growth rates of 73% and 36% for initial and final setting times, respectively. When the water-cement ratio is 0.38, the initial setting time of AASM is 43 min, which does not meet the code requirements.
Figure 7c shows a relatively slow increase in the setting time for AASM as the sand-cement ratio increases. The cementitious material is the primary component involved in the hydration reaction. An increase in the sand-cement ratio implies a decrease in the amount of cementitious material per unit volume, as well as an increase in sand content, which reduces the overall activity of the AASM, thereby slowing its setting process. It was also found that the slow increase in setting time of AASMs as the sand-cement ratio increased may be due to the fact that alkali-activated slag is generally more reactive, and the slag reacts faster with the alkaline solution, allowing for a more rapid production of gelation products. Even if the sand-cement ratio is increased, the reactivity of the slag may still be high enough so that the reaction continues and the setting time is not significantly prolonged. When the sand-cement ratios were 2.2 and 2.6, the initial setting times of AASM were 40 min and 44 min, respectively, which did not meet the code requirements.
3.4 pH analysis
Figure 8 shows the effect of BA powder replacement rate, water-cement ratio and sand-cement ratio on pH of AASM. pH is a key determinant of the health of concrete as it affects corrosion of steel reinforcement26. The effect of pH on the workability is reflected in its regulation of the chemical reaction rate and setting time of the material. pH changes can affect the fluidity and consistency of the material, which indirectly affects the workability of the alkali-activated mortar.
From Fig. 8a, it can be seen that, in general, the pH of AASM tends to increase and then decrease with the increase in the replacement rate of BA powder, reaching a peak at a 40% replacement rate (Group B2). BA powder has a higher water absorption rate than slag. When the BA powder replacement rate is less than 40%, the BA absorbs part of the free water, contributing to the increase of OH− ion concentration per unit volume and thus increasing the pH. However, when the replacement rate of BA powder is too high, the BA powder with high water absorption characteristics reduces a large amount of free water inside the AASM, resulting in an increase in the concentration of OH− ions per unit volume, which is manifested as an increase in pH. Simultaneously, the slag and BA powder undergo alkali activation reactions and consume some of the OH− ions. The OH− ions consumed by the BA powder (low activity, high replacement rate) and the slag have little effect on the pH value. In addition, the BA contains metallic aluminium, which releases OH− ions in an alkaline environment, further enhancing the pH. As the age of maintenance increases, the pH of AASM peaks at 56 days. The change in pH, and the difference in the peak with the reduction of slag content, show that as the hydration reaction progresses inside the AASM, the active ingredients in the BA powder and slag are fully activated, producing more hydration products. At this time, the OH− ion concentration in the system also reaches a peak at 56 days, resulting in a higher pH value. On the contrary, the pH of group N decreased with the increase in the curing age, which was attributed to the high concentration of slag admixture, which underwent alkali activation reaction and consumed a large amount of OH− ions, resulting in a decrease in pH.
It is well known that the increase in the water-cement ratio dilutes the OH− ion concentration in AASM27. From Fig. 8b, it can be seen that with the increase in the water-cement ratio of AASM, the pH at 7 and 28 days showed a tendency to increase and then decrease slowly. This was mainly due to the rapid progression of the alkali-activated reaction at the initial stage, releasing a large number of OH− ions and increasing the pH. As the reaction equilibrated, the pH began to decrease slowly. The pH of AASM at 56 and 120 days showed a slow decreasing trend. This was mainly due to the gradual stabilization of the reaction, the solidification of hydration products, and the continuation of side reactions, which resulted in the gradual decrease of OH− ions. The high water-cement ratio accelerated the reaction at the initial stage, but in the long term, it affected the continuous supply and retention of OH− ions, resulting in a slow decrease in pH. In addition, when the water-cement ratio of AASM was 0.38–0.44, the pH showed a tendency to decrease, then increase, and finally decrease with the increase in the age of maintenance. In contrast, the AASM with a water-cement ratio of 0.47 showed a tendency of decreasing pH with the increase in the age of maintenance. This may be related to the hydration reactions within the AASM.
From Fig. 8c, it can be seen that the pH showed a trend of decreasing, then increasing, and finally decreasing with the increase in the curing age, regardless of the sand-cement ratio. The pH of group G2 was the highest overall, which may be attributed to the proper sand-cement ratio enhancing the mixing of AASM. This allows the alkali-activated reaction to reach maximum efficiency, resulting in the peak release of OH− ions and, consequently, the peak pH.
Mechanical properties analysis
Effect of BA powder replacement rate
Effect of BA powder replacement rate on mechanical properties of AASM (Note: The compressive strengths of group N at 7, 28, 56 and 120 days were 32.3 MPa, 41.3 MPa, 46.5 MPa and 48.5 MPa, respectively. The flexural strength of group N at 7, 28, 56 and 120 days was 4.2 MPa, 6.8 MPa, 7.5 MPa and 7.8 MPa, respectively.)
From Fig. 9a, it can be seen that the compressive strength of AASM shows a decreasing trend with the increase in the BA powder replacement rate. Notably, the compressive strength of AASM decreases less when the replacement rate of BA powder increases from 20 to 60% (B1→B3) but decreases abruptly when the replacement rate increases from 60 to 80%, with a decrease in compressive strength ranging from 46 to 57%. This can be mainly attributed to the following reasons: Although the activity of BA powder is not as high as slag, it can be mixed with other particles to form a good filling effect, thereby increasing the compactness of the AASM. However, a high dosage of BA powder brings significant negative effects, including the reduction of active material, weakening of the filling effect, and reduction of free water volume.
Figure 9c shows that the flexural strength of AASM follows a decreasing trend with the increase in the BA powder replacement rate, consistent with the trend observed in Fig. 9a. Additionally, the 120-day flexural strength of AASM still reaches 5.2 MPa when the BA powder replacement rate is 60%.
The effects of curing age on the mechanical properties of AASM with different replacement rates of BA powder are shown and fitted using quadratic polynomial fitting in Fig. 9b and d. It can be seen from the figure that the compressive and flexural strengths of AASM increased rapidly in the early stage (7–28 days) and then leveled off in the later stage (56–120 days) as the curing age increased. It was also found that with a 20% replacement of BA powder, the compressive strength of group B1 at 120 days decreased by 3.1% compared to the compressive strength at 56 days. At a 40% replacement of BA powder, the 120-day flexural strength of group B2 decreased by 2.9% compared to the 56-day flexural strength. This may be attributed to the drying cracks that develop in the later stages of AASM.
Notably, the mechanical properties of AASM with a 0% BA replacement rate were not significantly superior to those of the other groups, and were even observed to be lower. This may be attributed to a higher effective water-cement ratio in the AASM at 0% BA replacement, which can inhibit strength development.
In conclusion, it appears feasible to use AASM with a 60% replacement rate of BA powder in practical engineering applications.
Effect of water-cement ratio
Figure 10a and c show the effect of the water-cement ratio on the strength of AASM. It can be seen from the figures that, at 7 days, the strength of AASM decreases with increasing water-cement ratio. In the later stages (56 and 128 days), the strength of AASM showed an increasing and then decreasing trend with the water-cement ratio. The compressive strength peaked at a water-cement ratio of 0.44 (S3 group), while the flexural strength peaked at 0.41 (S2 group). Notably, the late strengths of AASM at water-cement ratios of 0.41 and 0.44 differed by only 3-3.4 MPa (compressive strength) and 0.3–0.7 MPa (flexural strength), respectively.
The trend of strength of AASM for each water-cement ratio with curing age is shown in Fig. 10b and d, and the data are fitted using a quadratic polynomial. As seen from the figures, the strength of AASM with a water-cement ratio of 0.38 (S1 group) showed more moderate growth from 7 days to 120 days. The strength at 7 days was significantly higher than that of the other groups, attributed to the fact that a lower water-cement ratio enhances the proximity of cementitious material particles, which increases the reaction rate and rapidly boosts early strength28. However, the lack of free water resulted in a slower overall strength increase in the S1 group. In contrast, the increased free water in the AASM accelerated the strength growth rate of groups S2, S3, and S4 between 7 and 56 days, with the growth rate slowing down significantly after 56 days.
Effect of sand-cement ratio
Figure 11a and c show the effect of the sand-cement ratio on the strength of AASM. It can be seen from the figures that, with an increasing sand-cement ratio, the compressive and flexural strengths of AASM increase and then decrease, reaching a peak at a sand-cement ratio of 2.6 (Group G2). This is mainly because an appropriate sand-cement ratio optimizes the ITZ between sand particles and cementitious material in AASM, making the structure more uniform and denser, thereby improving overall strength (Fig. 15b).
Figure 11b and d show the trend of strength with maintenance age for each sand-cement ratio. From the figures, it can be seen that the strength of AASM increased rapidly from 7 to 56 days and then slowed down from 56 to 120 days, except for Group G1. In Group G1, the increase in cementitious materials per unit volume meant that there was insufficient water to fully hydrate all the materials, resulting in slower strength growth and lower overall strength. Additionally, the lack of aggregate support in AASM at a sand-cement ratio of 2.2 also resulted in lower strength.
XRD analysis
Based on the XRD image of Fig. 12 and referring to the literature29] and [30, the main hydration products in AASM are albite (N-A-S-H), Calcium silicate hydrate (C-S-H), gehlenite (C-A-S-H), and CaCO3. The diffraction peak intensities of AASM fluctuated to varying degrees with changes in the BA replacement rate, water-cement ratio, and sand-cement ratio. The anomalous variation in the diffraction peaks of SiO2 may be due to variations in the sands present in the slurry at the time of sampling and will not be analyzed further.
As seen in Fig. 12a, the CaCO3 diffraction peak intensities follow the order B2 > B1 > B3 ≈ B4. Typically, as the BA powder replacement rate increases, the pores inside AASM increase, allowing more CO2 from the air to penetrate and produce more CaCO329. However, a significant discrepancy is observed here, which may be due to the higher replacement rates of BA powder (especially 60% and 80%) reducing the amount of reactive material, limiting the reaction points available for CO2. Additionally, the high replacement rate of BA powder led to a continuous and stable increase in the diffraction peak of Magadiite, a hydration product specific to BA powder incorporation. Notably, there is a slight increase in the diffraction peaks of C-S-H and C-A-S-H in group B4, with only 20% slag content. This may be due to the high replacement rate of BA powder, which absorbs large amounts of water and increases the OH− concentration per unit volume, reinforcing the alkaline environment, stimulating the activity of additional BA powder, and promoting the formation of C-S-H and C-A-S-H. Simultaneously, additional aluminum ions are produced from metallic aluminum.
As seen in Fig. 12b, the diffraction peak intensities of AASM were enhanced to varying degrees as the water-cement ratio increased, particularly in the C-S-H and CaCO3 peaks. This may be due to the higher water-cement ratio providing sufficient water for hydration, although the increase in water-cement ratio also weakens the alkaline nature of the slag mortar, thereby reducing the activation reaction of the mortar. At a low water-cement ratio, the cementitious material lacks sufficient water, inhibiting the hydration reaction. This may explain the weakening or disappearance of the N-A-S-H and C-A-S-H diffraction peaks in groups S1 and S4.
As seen in Fig. 12c, the diffraction peak intensity of C-S-H is significantly enhanced in the G2 group, aligning with its mechanical property performance. In contrast, the diffraction peak intensities of the hydration products were not significantly increased in group G1. Adequate water inside the mortar is essential for proper reaction of the cementitious material. A lower sand-cement ratio means more cementitious material per unit volume, but insufficient moisture can lead to inadequate internal hydration. It is noteworthy that the peak of the C-S-H fraction in Fig. 12c is shifted compared to Fig. 12a and b, which may be related to factors such as C-S-H structure, chemical composition, or pH during hydration.
SEM analysis
Effect of BA powder replacement rate
Figure 13 illustrates the effect of the BA powder replacement rate on the microscopic appearance of AASM. The figure shows that as the BA powder replacement rate increases, the microscopic morphology of AASM changes significantly, mainly in terms of compactness, cracks, and holes in the slurry. At a 20% replacement rate of BA powder (Group B1), the AASM shows a large area of C-A-S-H gel, with a small amount of ettringite (AFt) forming in the pores, which helps reduce pore size and increase slurry densification. However, gels like C-A-S-H exhibit fine drying cracks.
At a 40% replacement rate (Group B2), the AASM exhibited noticeable cracks, with the dense ITZ being significantly longer than the loose ITZ. This may be related to the formation of gels like C-A-S-H. At a 60% replacement rate (Group B3), incompletely reacted BA powder particles were clearly visible within the AASM. Some of the BA powder was observed in stacked forms, but overall, the microstructure remained relatively dense. Additionally, long microcracks in the ITZ affected the strength of the AASM (Fig. 13c). At an 80% replacement rate (Group B4), a large amount of BA powder accumulates within the AASM, creating a relatively loose microstructure. The lack of bonding between hydration products in the ITZ leads to noticeable microcracks and a significant decrease in strength.
Effect of water-cement ratio
Figure 14 illustrates the effect of the water-cement ratio on the microscopic appearance of AASM. The figure shows a stark contrast between the loose microstructure of groups S1 and S3 and the dense microstructure of groups S3 and S4. At lower water-cement ratios (0.38 and 0.41), a significant amount of unreacted cementitious material is present in the AASM (Fig. 14a, b), resulting in a loose microstructure. This insufficient reaction of cementitious material, coupled with the lack of bonding of hydration products between aggregate and mortar, leads to more microcracks around the ITZ (Fig. 14a). Additionally, Fig. 14b shows that the high percentage of cementitious material may lead to uneven distribution of free water, causing stress concentrations and the formation of fine cracks and holes within the slurry. When the water-cement ratio reaches 0.44, the ITZs are relatively dense (Fig. 14, ITZ1), with only a few microcracks (Fig. 14, ITZ2), resulting in fewer cracks and higher mechanical properties. At a water-cement ratio of 0.47, the number of cracks within the AASM increases significantly, leading to a notable decrease in strength. This is attributed to accelerated water dissipation at higher water-cement ratios, causing volumetric shrinkage and microcracking when the shrinkage exceeds the load-bearing capacity of the mortar. Additionally, a small amount of acicular AFt is observed within the slurry at this ratio, but its low content has minimal impact on strength.
Effect of sand-cement ratio
Figure 15 illustrates the effect of the sand-cement ratio on the microscopic appearance of AASM. When the sand-cement ratio is low (2.2 and 2.6), the ITZ is relatively dense, and the fine aggregate does not exhibit significant damage (Fig. 15a, b). At a sand-cement ratio of 2.2, the slurry was almost completely coated with aggregate, and microcracks were present in part of the slurry (Fig. 15a). These microcracks can be attributed to the substantial reduction in fine aggregate combined with a significant increase in cementitious material, which led to increased drying shrinkage of the AASM and, consequently, more microcracks31. At a sand-cement ratio of 2.6, the slurry was denser, and no obvious microcracks were observed; the slurry on the aggregate surface was thicker (Fig. 15b), corresponding to its improved mechanical properties. With sand-cement ratios of 3.0 and 3.4, the coverage area and thickness of the slurry on the aggregate surface were markedly reduced. Although most ITZs remained relatively tight, the bond between aggregates was weaker due to reduced cementitious material and increased sand, resulting in lower strength (Fig. 15c, d). Increased sand-cement ratios led to insufficient cementitious material buffering during mixing and molding, causing some coarse aggregates to develop microcracks (Fig. 15c, d), thereby diminishing the strength of the AASM.
Relationships between test parameters for AASM
To clarify the relationship between the tested parameters of AASM, a combination of correlation coefficient heat maps and scatter plots was employed for mutual corroboration.
-
1)
Principle.
The basic principle of a correlation coefficient heat map is to generate a Correlation Matrix by calculating the correlation coefficients between pairs of variables and then visualising the matrix using colour coding. Each cell in the matrix represents the value of the correlation coefficient between two variables. A common correlation coefficient is the Pearson’s correlation coefficient, which is used to measure the linear relationship between two variables.
-
2)
Calculation steps.
-
(i)
Calculating the Pearson correlation coefficient.
First, calculate the correlation coefficient between each pair of variables. Suppose we have n samples and p variables, the variables are denoted as X1, X2, …, Xp.
Pearson’s correlation coefficient is calculated as follows,
where \(\:{X}_{i,k}\) and \(\:{X}_{j,k}\) denote the value of each of the kth samples on the variables \(\:{X}_{i}\) and \(\:{X}_{j}\), respectively. \(\:{\stackrel{-}{X}}_{i}\) and \(\:{\stackrel{-}{X}}_{j}\) are the mean values of the variables \(\:{X}_{i}\) and \(\:{X}_{j}\), respectively.
-
(ii)
Constructing the correlation coefficient matrix.
By calculating the correlation coefficients between each pair of variables, a p × p matrix of correlation coefficients is obtained, representing the correlation between all variables. The diagonal elements of the matrix are usually 1 (each variable has a correlation coefficient of 1 with itself), while the other elements are the correlation coefficients between the variables.
-
(iii)
Generate heat maps.
The calculated matrix of correlation coefficients is visualised in the form of a heat map.
In particular, it is noted that the correlation matrix shows only a linear relationship between the variables, not a causal relationship.
The correlation coefficients are of Pearson type with ten factors as variables: BA powder replacement rate, water-cement ratio, sand-cement ratio, fluidity, consistency, initial setting time, final setting time, pH (28 days), compressive strength (28 days), and flexural strength (28 days). The heat map of correlation coefficient is shown in Fig. 12. The size of the circle in the graph represents the size of the absolute value of the correlation coefficient, the closer it is to 1 (red) or -1 (blue), the larger the circle is, and vice versa, the smaller it is. The data in the figure are the correlation coefficients.
Figure 16 illustrates that the replacement rate of BA powder is negatively correlated with compressive strength, flexural strength, and pH at 28 days, with correlation coefficients of -0.88, -0.75, and − 0.78, respectively. It is positively correlated with initial and final setting times, with correlation coefficients of 0.71 and 0.87, respectively. The water-cement ratio exhibits a weaker correlation with fluidity, with a correlation coefficient of 0.66. The sand-cement ratio also shows a weak correlation with fluidity, with correlation coefficients of -0.62 and − 0.74. These correlations are further detailed in the previous sections. It was also found that the p-values of values with correlation coefficients greater than 0.6 were less than 0.05, indicating that the correlation coefficients heatmap has some reliability.
Significant correlations were observed among several tested parameters: fluidity, consistency, initial setting time, final setting time, compressive strength at 28 days, flexural strength at 28 days, and pH at 28 days. The correlation coefficient between fluidity and consistency was 0.89, indicating a strong correlation. Setting time exhibited a negative correlation with compressive strength and flexural strength at 28 days, while initial setting time was positively correlated with final setting time, with a correlation coefficient of 0.88. The pH at 28 days was positively correlated with compressive strength and flexural strength at 28 days, with correlation coefficients of 0.76 and 0.62, respectively. Compressive strength was positively correlated with flexural strength, with a correlation coefficient of 0.90.
Pearson correlation coefficients are sensitive to outliers, which may introduce errors affecting the accuracy of the test results. Therefore, scatter plots and linear fitting, supported by the heat map of correlation coefficients, were employed to verify the accuracy of the test results. The subsequent scatter plots and linear fittings focus on seven test parameters: fluidity, consistency, initial setting time, final setting time, pH (28 days), compressive strength (28 days), and flexural strength (28 days). Quadratic polynomial curve fitting was used for linear fitting.
Figure 17 illustrates the scatter plots and their linear fits for the tested parameters. Figure 17a and b show a clear linear correlation between the setting time of AASM and both compressive and flexural strengths, with R2 values ranging from 0.70 to 0.82. The initial setting time also has a significant linear correlation with final setting time, with an R2 value of 0.81. However, pH shows no clear correlation with setting time, compressive strength, or flexural strength (Fig. 17a and c), which differs from the correlation coefficient heat map. The R2 value for the fitted curve of fluidity versus consistency is 0.82, and for compressive strength (28 days) versus flexural strength (28 days) is 0.84. Overall, the correlation coefficients presented in the heat map of AASM demonstrate good accuracy.
Previous studies have established very few relational equations between compressive strength and flexural strength of AASM. Therefore, this paper develops relational equations for these strengths. The relational equation of the form \(y = a \cdot x^{b}\) (where y is the flexural strength, \(\:x\) is the compressive strength, and \(\:a\) and \(\:b\) are constants), as referenced from Beeby and Narayanan (1995)32, is fitted as shown in Fig. 18. The fitting results show that the curves for Groups B, S, and G are closely aligned, with R2 values all exceeding 0.75:\(y = 0.16 \cdot x^{{0.90}} ,\:R^{2} = 0.96;\:y = 0.15 \cdot x^{{0.95}} ,\:R^{2} = 0.75;\:y = 0.37 \cdot x^{{0.71}} ,\:R^{2} = 0.76\)). Thus, combining the compressive and flexural strengths of Groups B, S, and G, the overall relationship was fitted as \(\:y=0.23\cdot{x}^{0.82}\), with \(\:{R}^{2}=0.86\).
In addition, the setting time is closely related to the early strength of AASM. A quadratic polynomial fit was performed to examine the relationship between the compressive strength at 7 days and both the initial and final setting times, as shown in Fig. 19. The goodness-of-fit measures, with R2 values of 0.95 and 0.91 respectively, indicate a better fit compared to the quadratic polynomial fit of the compressive strength at 28 days to the setting times.
Conclusion
In this study, the effects of BA powder replacement rate, water-cement ratio, and sand-cement ratio on the working performance, mechanical properties, and microstructure of AASM were investigated. Using a combination of correlation coefficient heatmaps and scatter plots (including linear fitting), the following conclusions were drawn.
-
(1)
AASM has the maximum fluidity (224 mm) and the consistency meets the code requirements when the BA powder replacement rate is 60%. When the water-cement ratio is 0.44 and 0.47, the fluidity and consistency meet the code requirements. When the sand-cement ratio is 2.6, the fluidity and consistency meet the code requirements simultaneously. In addition, the setting time of AASM tended to increase with increasing replacement rate of BA powder (20-80%), water-cement ratio and sand-cement ratio.
-
(2)
An increase in the BA powder replacement rate from 60 to 80% resulted in a significant reduction in compressive strength, with a decrease ranging from 46 to 57%. Nevertheless, the flexural strength at 120 days remained at 5.2 MPa. Therefore, a BA powder replacement rate of 60% is recommended for practical applications. Compressive strength was optimal at a water-cement ratio of 0.44, whereas flexural strength peaked at a water-cement ratio of 0.41. The sand-cement ratio of 2.6 yielded the best overall mechanical properties.
-
(3)
The intensity of the CaCO₃ diffraction peak increased when the BA powder replacement rate was 40%. Furthermore, the diffraction peaks of C-S-H and CaCO₃ were enhanced with increasing water-cement ratio. Additionally, the diffraction peak of C-S-H was more pronounced at a sand-cement ratio of 2.6.
-
(4)
At a BA powder replacement rate of 60%, the AASM maintains a relatively dense microstructure, although microcracks are present in the ITZ. With a water-cement ratio of 0.44, the hydration reaction is adequate, resulting in fewer cracks. A sand-cement ratio of 2.6 produces a denser microstructure with no significant microcracks.
-
(5)
Fluidity and consistency of AASM are positively correlated. The setting time is negatively correlated with both compressive and flexural strengths, while positively correlated with the final setting time. Compressive and flexural strengths exhibit a positive correlation.
-
(6)
The mechanical model for predicting the compressive and flexural strengths of AASM is expressed as \(\:=0.23\cdot{x}^{0.82},\)\(\:{R}^{2}=0.86\).
In this study, the resource utilisation of waste incineration BA in alkali-activated materials has been provided, but due to the fact that the characteristics of waste incineration BA may show different properties with regional differences. Therefore, a set of rational recycling scheme33 is needed to achieve the standardised application of waste incineration BA.
Data availability
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author at a reasonable request.
References
Gartner, E. Industrially interesting approaches to low-CO2 cements. Cem. Concr. Res. 34 (9), 1489–1498 (2004).
Coppola, L. et al. The durability of one-part alkali-activated slag-based mortars in different environments. Sustainability 12 (9), 3561 (2020).
Li, Q. et al. The long-term failure mechanisms of alkali-activated slag mortar exposed to wet-dry cycles of sodium sulphate. Cem. Concr. Compos. 116, 103893 (2021).
Bernal, S. A. et al. Performance of alkali-activated slag mortars exposed to acids. J. Sustainable Cement-Based Mater. 1 (3), 138–151 (2012).
Ju, C. et al. Effect of alkali cation on performance of alkali-activated slag mortar in cold environments. Separations 9 (12), 450 (2022).
Huang, W. et al. Experimental study of high-temperature resistance of alkali-activated slag crushed aggregate mortar. J. Mater. Res. Technol. 23, 3961–3973 (2023).
Brough, A. R. & Atkinson, A. Sodium silicate-based, alkali-activated slag mortars: part I. Strength, hydration and microstructure. Cem. Concr. Res. 32 (6), 865–879 (2002).
McLellan, B. C. et al. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 19 (9–10), 1080–1090 (2011).
Luo, L. et al. Workability, autogenous shrinkage and microstructure of alkali-activated slag/fly ash slurries: Effect of precursor composition and sodium silicate modulus. J. Building Eng. 73, 106712 (2023).
Fu, C. et al. Synergistic effects of metakaolin and dolomite on alkali-activated slag exposed to chloride and sulfate solutions. Constr. Build. Mater. 420, 135590 (2024).
Tian, K. et al. Engineering and micro-properties of alkali-activated slag pastes with Bayer red mud. Constr. Build. Mater. 351, 128869 (2022).
Heidrich, C., Feuerborn, H. J. & Weir, A. Coal combustion products: A global perspective[C]//World of coal ash conference. 22: 25. (2013).
Aitcin, P. C. & Neville, A. How the water-cement ratio affects concrete strength. Concr. Int. 25 (8), 51–58 (2003).
Liu, Z. et al. Effect of water-cement ratio on pore structure and strength of foam concrete. Adv. Mater. Sci. Eng. 2016 (1), 9520294 (2016).
Gao, D. et al. Durability of steel fibre-reinforced recycled coarse aggregate concrete. Constr. Build. Mater. 232, 117119 (2020).
Jumadi, N., Razali, N. & Razali, N. Preliminary study on response of different water-cement ratio and curing conditions on concrete Properties. Int. J. (BEAM). 2 (2), 8–14 (2023).
Shen, D. et al. Effect of water-to-cement ratio on cracking resistance of concrete at early age in restrained ring specimens. Can. J. Civ. Eng. 49 (5), 706–715 (2022).
Zidan, I., Khalaf, M. A. & Helmy A I I. properties of alkali-activated slag mortar and prediction of its compressive strength. Ain Shams Eng. J. 14 (11), 102536 (2023).
Chi, M., Chang, J. & Huang, R. Strength and drying shrinkage of Alkali-activated slag paste and Mortar. Adv. Civil Eng. 2012 (1), 579732 (2012).
Kosmatka, S. H., Kerkhoff, B. & Panarese, W. C. Design and control of concrete mixtures[R]. (2003).
Dong, J. et al. Investigation into the alkali-activation of lithium slag: A sustainable alternative to conventional cement with optimized mechanical properties. Constr. Build. Mater. 416, 135022 (2024).
Zhang, Z. et al. The effect of mortar film thickness on the fluidity of concrete: Experiment and simulation. Constr. Build. Mater. 447, 138096 (2024).
Wang, M. et al. Unlocking cementitious performance: nano-lubrication via polycarboxylate superplasticizers. J. Am. Ceram. Soc. 107 (5), 3055–3067 (2024).
Hannan, N. I. R. R. et al. A comprehensive review on the properties of coal bottom ash in concrete as sound absorption material[C]//MATEC Web of conferences. EDP Sciences, 103: 01005. (2017).
Chen, J., Jia, J. & Zhu, M. Understanding the effect of alkali content on hydration, hardening, and performance of Portland cement-a comprehensive review. Mater. Today Commun., : 109728. (2024).
Natkunarajah, K. et al. Analysis of the trend of pH changes of concrete pore solution during the hydration by various analytical methods. Cem. Concr. Res. 156, 106780 (2022).
Yang, J. et al. The water absorption-release of superabsorbent polymers in fresh cement paste: An NMR study. J. Adv. Concr. Technol. 18 (3), 139–145 (2020).
Yu, J. et al. Study on the performance of low water-binder ratio cement mortar with excavated soil exposed to NaCl freeze-thaw environment. Mater. Res. Express. 8 (9), 095511 (2021).
Wang, C. et al. Effects of alkali equivalent and polypropylene fibres on performance of alkali-activated municipal waste incineration bottom ash-slag mortar. J. Building Eng. 84, 108496 (2024).
Türker, H. T. et al. Microstructural alteration of alkali activated slag mortars depend on exposed high temperature level. Constr. Build. Mater. 104, 169–180 (2016).
Zhang, D., Zhang, S. & Yang, Q. Effect of replacing fine aggregate with fly Ash on the performance of Mortar. Materials 16, 4292 (2023).
Beeby, A. W. & Narayanan, R. S. Designers’ handbook to Eurocode 2: Design of concrete structures[M] (Thomas Telford, 1995).
Wang, C. et al. Study of waste incineration bottom ash as fine aggregate applied to green alkali-activated bottom ash-slag concrete: mechanical properties, microstructure, durability. Constr. Build. Mater. 449, 138484 (2024).
Acknowledgements
The research presented in this manuscript is funded by the State Key Laboratory of Geohazard Prevention and Geoenvironment Protection Independent Research Project (SKLGP2022Z001).
Author information
Authors and Affiliations
Contributions
Cheng Wang: Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Conceptualization. Xiao Zhao: Writing – review & editing, Supervision, Funding acquisition, Data curation. Jianjun Zhao: Writing – review & editing, Supervision, Funding acquisition, Data curation. Xiyu Zhang: Validation, Supervision, Investigation, Formal analysis, Data curation, Conceptualization. Xuejin Ying: Validation, Investigation. Xiangde Xu: Validation, Investigation. jin.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, C., Zhao, X., Zhao, J. et al. Effect of bottom ash powder replacement rate, water cement ratio and sand cement ratio on alkali activated slag mortar. Sci Rep 15, 4061 (2025). https://doi.org/10.1038/s41598-025-87552-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87552-0