Abstract
Bacillus velezensis strain DMB07, isolated from the traditional fermented Korean soybean meju, exhibits resistance to streptomycin [minimum inhibitory concentration (MIC) 128 mg/L]. To shed light on the genetic background behind this phenotype, this study determined the complete genome sequence of strain DMB07 and compared it with the genomes of two B. velezensis strains that are sensitive to streptomycin. Compared with the streptomycin-sensitive strains, in strain DMB07 there was a mutation of a nucleotide (C58T) of the 16 S rRNA (guanine527-N7)-methyltransferase gene (gidB) that leads to a change in the amino acid sequence of the protein (Arg20Cys). This sequence of gidB gene was previously linked with streptomycin resistance. To test the hypothesis that this change in the gidB gene sequence of strain DMB07 confers streptomycin resistance, a temperature-sensitive plasmid, pIMAY-tgidBT58C, was constructed for site-directed mutation (from thymine to cytosine) of nucleotide 58 of gidB in strain DMB07. The resulting strain, DMB07gidBT58C, showed the decreased MIC value (32 mg/L) against streptomycin. Furthermore, introduction of the wild-type gidB gene into strain DMB07gidBT58C resulted in recovery of the MIC for streptomycin to 128 mg/L. Thus, a single mutation of the nucleotide sequence of the gidB gene can confer resistance to streptomycin.
Similar content being viewed by others
Introduction
Bacillus velezensis has been identified in various habitats, including air, soil, and water, but, predominantly, in several types of fermented food1,3,4,5,6,6. B. velezensis produces exoenzymes that contribute to the sensory properties of fermented foods, such as proteases, amylases, β-glucanases, and γ-glutamyltransferases7,8,9. B. velezensis contributes to the enhancement of the sensory and nutritional properties of fermented food9,10,11. Thus, scientists have continued to select B. velezensis as a starter culture for fermented foods12,13,14,15. We recently determined the bacterial migration from meju, which is fermented soybean brick and the major ingredient of high-salt fermented doenjang, into doenjang using culture-dependent and -independent analyses6. B. velezensis was found to be dominant in both meju and doenjang. The safety and enzyme abilities of 171 B. velezensis isolates from fermented soybean were assessed to identify safe and efficient starter culture candidates13. Strain DMB07 was one of the preferable starter candidates because of properties such as its protease activity. However, this strain was resistant to the antibiotic streptomycin [minimum inhibitory concentration (MIC) 128 mg/L], and thus there was concern about its safety13.
Antibiotic resistance has become one of the biggest threats to global health16. In 2013, the Centers for Disease Control and Prevention of the United States published a report warning about the spread of antibiotic resistance genes into bacteria in different niches, such as from food bacteria into human intestinal bacteria17. In 2017, the World Health Organization identified priority pathogens to research for antibiotic resistance, with the goal of preventing horizontal transfer of antibiotic resistance genes18. The European Food Safety Authority (EFSA) presented guidelines for antibiotic susceptibility and used the term “acquired resistance” for horizontal transfer of antibiotic resistance genes19. According to these guidelines, resistance resulting from mutation of the genome is generally acceptable in microorganisms used for food or feed20.
Strains as starter candidates carrying acquired antibiotic resistance genes, especially for fermented foods, may facilitate the transfer of antibiotic resistance genes between species or genera during the fermentation process20,21. Therefore, when selecting starter cultures, strains that do not carry horizontally transferable antibiotic resistance genes should be prioritized18,19. Moreover, even if acquired antibiotic resistance genes are present, research on the environmental factors that induce horizontal transfer is still insufficient.
During the antibiotic sensitivity assessment for selecting starter cultures, the B. velezensis DMB07 strain, which showed resistance to streptomycin, was isolated in a previous study13. Although the DMB07 strain exhibited protease activity as a starter candidate for fermented foods, it remained unclear whether its resistance to streptomycin was an acquired trait capable of horizontal transfer, raising concerns about its suitability for food use. Therefore, in this study, to shed light on the genetic determinant of streptomycin resistance, we performed complete genome sequencing of B. velezensis DMB07 and showed by comparative genomic analysis that the chromosomal gene gidB was related to the streptomycin resistance. We then experimentally confirmed the contribution of mutated gidB to resistance to this antibiotic.
Materials and methods
Bacterial strains plasmids and culture conditions
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli and B. velezensis were grown in Luria-Bertani broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and tryptic soy broth (TSB; Becton, Dickinson and Company), respectively. Antibiotics were added to the growth media at the following concentrations when necessary: ampicillin 100 µg/mL, erythromycin 10 µg/mL, kanamycin 10 µg/mL, and chloramphenicol 5 µg/mL.
Antibiotic susceptibility
MICs for antibiotic susceptibility were determined by the broth microdilution method23,24. Streptomycin was prepared by twofold serial dilution in deionized water, and 20 µL was added to each well of a 96-microwell plate to achieve final streptomycin concentrations ranging from 0.5 to 512 mg/L. Bacteria were sub-cultured in TSB and matched to a 0.5 McFarland turbidity standard (BioMérieux, Marcy-l’Étoile, France). Each suspension was diluted 1:100 with cation-adjusted Mueller-Hinton broth (Becton, Dickinson and Company) according to the Clinical and Laboratory Standards Institute guideline for antibiotic susceptibility to produce inoculum24. The final inoculum density was 5 × 105 colony-forming units/mL, of which 200 µL was added to each well of the 96-microwell plate. The MIC of streptomycin was recorded as the lowest concentration at which no bacterial growth was observed after incubation at 37 °C for 18 h, and the MIC results were confirmed by at least three independent tests. Strains with an MIC higher than 8 mg/L, the cut-off value for streptomycin in Bacillus species as suggested by EFSA, was considered resistant19.
Genome sequencing
Whole-genome sequencing of B. velezensis strain DMB07 was performed at CJ Bioscience, Inc. (Seoul, Korea), using the PacBio_10K sequencing system (Pacific Bioscience, Menlo Park, CA, USA). Flye assembler (v2.8.3) for Single-Molecule Real-Time Link (PacBio, Menlo Park, CA, USA) obtained one contig from a total of 61,190 reads (797× coverage). Gene annotation was performed using the National Center for Biotechnology Information (NCBI) Prokaryotic Genome Annotation Pipeline (v4.6)25. Function of genes was predicted using CLgenomics™ (v1.55, CJ Bioscience, Inc.) software based on the Clusters of Orthologous Groups (COG) database of the COGNITOR program26 and the SEED database (https://rast.nmpdr.org/rast.cgi, accessed on September 30, 2021) in the RAST server27.
Comparative genome analysis
Genome sequence data for B. velezensis strains DMB06 (GenBank accession no. NZ_CP083763) and KMU01 (GenBank accession no. NZ_CP063768) were retrieved from the NCBI database (http://ncbi.nlm.nih.gov/genomes) for comparative genomic analysis. The average nucleotide identity (ANI), which provides a robust measurement of genetic distance among the conserved genes of bacterial genomes, was used for comparative analysis28. To determine rearrangements in each genome, a progressive alignment algorithm implemented in Mauve was used29. Core-genome and pan-genome analyses for comparative analyses at the protein level were performed using the Efficient Database framework for comparative Genome Analyses using BLASTP score Ratios (EDGAR)30. The genome of strain DMB06 was used as a reference genome for Venn diagram construction. Comparative analyses at the predicted protein level were conducted using an all-against-all comparison of the annotated genomes. BLASTP was used as the algorithm, and results were normalized according to the best score31. The score ratio value, indicating the quality of the hit, was calculated by dividing the scores of subsequent hits by the best hit32. Two genes were considered orthologous if they exhibited a bidirectional best BLAST hit with a score ratio threshold of at least 32% for orthology estimation. Protein tertiary structures were predicted using AlphaFold33. The presence of antibiotic resistance genes was predicted using two antibiotic resistance prediction programs, the Comprehensive Antibiotic Resistance Database (CARD) algorithms34 and ResFinder35,36. The G + C content and GC skew of the DMB07 was performed at CJ Bioscience, Inc. using simple a custom Python script. The nucleotide sequence of genes presumed to contribute to streptomycin resistance and the deduced amino acid sequence were analyzed for homology between the nucleotide sequence and the amino acid sequence using the Jotun Hein method and ClustalV method of the MegAlign software (SeqMan NGen®. Version 5.0 DNASTAR, Inc., Madison, WI, USA), respectively. The DMB07 gene was used as the reference sequence.
DNA manipulation and PCR
Restriction enzymes KpnI and EcoRV were purchased from New England BioLabs (Ipswich, MA, USA). Plasmids and genomic DNA were extracted with an Inclone™ Plasmid Mini Prep Kit (Inclone Biotech Co., Jeonju, Korea) and PureHelix™ Genomic DNA Prep Kit (Nanohelix Co., Jeonju, Korea), respectively, according to the manufacturers’ instructions. Constructed plasmid DNA was introduced into E. coli DH5α by the method of Hanahan and Meselson37, and into B. velezensis DMB07 by electroporation38 with a gene pulser (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
PCR amplifications were performed using a T3000 thermocycler (Analytik Jena, Jena, Germany) and an Inclone™ Taq Polymerase Kit (Inclone Biotech Co.) according to the manual. Except for asymmetric PCR, PCRs were performed with 30 cycles of denaturation at 95 °C for 1 min, annealing at 60 °C for 2 min, and elongation at 72 °C for 1 min. Asymmetric PCR was conducted using 10 cycles without primer sets, with denaturation at 95 °C for 30 s, annealing at 60 °C for 10 min, and elongation at 72 °C for 5 min. Splicing by overlap extension (SOE) by PCR was performed using the asymmetric PCR amplicon as the template.
Site-directed mutagenesis of gidB in B. velezensis strain DMB07
The construction of tgidBT58C using SOE by PCR, which replaced the thymine at the 58th position of gidB in strain DMB07 with cytosine, involved two separate reactions according to previous approaches with minor modification39 (Fig. 1A). PCR primers were designed based on the gidB gene and nearby sequence of B. velezensis strain DMB07 (Table 2). In brief, regions A1 and A2 (Fig. 1) were amplified using primer sets A1-F/A1-R and A2-F/A2-R, respectively. Primers A2-F and A1-R contained complementary sequence and the T58C mutation of the gidB gene. Asymmetric PCR without primers was performed using amplified fragments A1 and A2, and then the A1–A2 amplicon was obtained using primers A1-F and A2-R. In this way, an amplified fragment consisting of A1–A2–C was obtained, and then inserted into the KpnI and EcoRV restriction enzyme sites of pIMAY, a thermally sensitive plasmid22. The resulting plasmid was named pIMAY-tgidBT58C.
Method for exchanging the thymine at the 58th nucleotide position of the gidB gene of B. velezensis DMB07 into cytosine using pIMAY. (A) Schematic of allelic exchange using pIMAY via splicing overlap extension-PCR. (B) PCR profiles of DMB07gidB. The names and locations of the primers are shown in panel A. Lanes: M, molecular size markers (NICSROgene 100 bp DNA Ladder, BIONICS, Seoul, Korea); 1, strain DMB07; 2, DMB07::pIMAY-tgidBT58C; 3, DMB07gidBT58C.
To integrate pIMAY-tgidBT58C into chromosomal DNA, the plasmid was introduced into B. velezensis strain DMB07, and chloramphenicol-resistant colonies were obtained after incubation at 30 °C for 18 h. B. velezensis DMB07 containing pIMAY-tgidBT58C was incubated in TSB containing chloramphenicol at 30 °C for 90 min, and then it was incubated at a non-permissive temperature of pIMAY-derivatives, 37 °C, overnight. Temperature-shift-treated transformants were spread on TSA containing anhydrotetracycline and chloramphenicol, and the big colonies were selected. The resulting transformants included pIMAY-tgidBT58C integrated into B. velezensis by single-crossover integration. Then, B. velezensis DMB07::pIMAY-tgidBT58C was incubated on TSA without chloramphenicol but containing anhydrotetracycline (1 µg/mL) at 28 °C overnight. Anhydrotetracycline induces the expression of anti-secY, which inhibits the expression of SecY, an essential membrane protein for growth, thereby suppressing colony growth22,40. Consequently, isolates with pIMAY-tgidBT58C and single cross-over transformants carrying anti-secY fail to grow, while only double cross-over transformants can survive. Among the survival transformants, colonies showing susceptibility in media including chloramphenicol were selected and the curing was checked by PCR using primer sets A2-F/pIMAY-R, pIMAY-F/A1-R, and M-F/N-R (Fig. 1B). Finally, the constructed strain DMB07gidBT58C was confirmed by DNA sequencing at Macrogen Inc. (Seoul, Korea).
Construction of complementation plasmid
The wild-type gidB gene from B. velezensis DMB07 was amplified with primers gidB-BamHI-F/gidB-SalI-R containing BamHI and SalI restriction sites, respectively (Table 2). The amplicon was digested with BamHI and SalI, and inserted into the same sites of pLipSM; the resulting vector was named pLipSM-gidB. This plasmid was introduced into strain DMB07gidBT58C by electroporation with a gene pulser. The DMB07gidBT58C containing pLipSM-gidB was confirmed by survival on TSA containing kanamycinand PCR using primer sets pLipSM-F/pLipSM-R (Table 2).
Genome and strain deposit numbers
The complete genome sequence of B. velezensis strain DMB07 (previous strain number: 12SSM01) was deposited in the DDBJ/ENA/GenBank under accession number NZ_CP083764, and the strain was deposited in the Korean Culture Center of Microorganisms with accession number KCCM 90490.
Results and discussion
Genome properties of B. velezensis DMB07
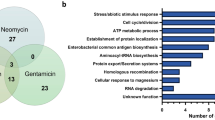
Among 171 B. velezensis strains tested in a previous study, only strain DMB07 showed streptomycin resistance13, and thus the genome of strain DMB07 was sequenced in the current study to identify the genetic background of the streptomycin resistance. The complete genome of B. velezensis strain DMB07 was a circular chromosome of 4,183,042 bp, with no plasmids detected. The G + C content was 45.6%. Ninety-two tRNA genes and 32 rRNA genes were identified in the genome. Genomic analysis predicted 4,267 open reading frames (ORFs), and 4,155 genes were functionally assigned to categories based on the COG and 1,699 genes were functionally assigned to categories based on SEED databases. The most abundant COG category was amino acid transport and metabolism (296 genes, 8.0%), followed by transcription (268 genes, 7.2%) and carbohydrate transport and metabolism (246 genes, 6.8%). Using the SEED subsystem, 305 genes (18.38%) were related to amino acids and their derivatives (Fig. 2). The next most abundant subsystem category was carbohydrates (221 genes, 13.2%), followed by protein metabolism (212 genes, 12.7%).
Comparative analysis of B. velezensis genomes
To find clues about the origin of the streptomycin resistance of strain DMB07 (MIC 128 mg/L), its genome sequence was compared with the complete genome sequences of B. velezensis strains KMU01 (MIC 16 mg/L) and DMB06 (MIC 4 mg/L), which are sensitive to streptomycin (Supplementary Fig. 1)12,13. The sizes of the genomes of the latter were 3.93 and 4.16 Mbp, with a G + C content of 46.20% and 46.50%, respectively (Table 3). The genomes contained a single chromosome with no plasmid(s).
The ANI of the genome of B. velezensis strain DMB07 was 99.28% and 97.99% with those of B. velezensis strains DMB06 and KMU01, respectively. Alignment of the three genomes using the software Mauve identified two locally collinear blocks—regions where specific chromosomal segments align— without significant differences in the homologous backbone sequences (Supplementary Fig. 2), and Mauve analysis showed great similarity across B. velezensis strains DMB07, DMB06, and KMU01. Additionally, whole genome comparison of the three strains showed that the genomes are highly homologous in terms of functional categories based on COG and SEED (Fig. 2).
Aiming to identify the specific streptomycin resistance gene(s) in B. velezensis DMB07, the shared gene pools were analyzed using the EDGAR and depicted in a Venn diagram (Fig. 3). The three strains shared 3,295 coding sequences (CDSs) in their core genome, corresponding to 78.7–87.1% of their ORFs. Many of the CDSs in the core genome were assigned via COG annotation to functions relating to metabolism and the transport of amino acids and carbohydrates. The proportions of unique CDSs in the genomes of strains DMB07, DMB06, and KMU01 were 7.3%, 6.1%, and 4.9%, respectively. The majority of singleton-specific genes were associated with hypothetical proteins (Supplementary Table 1). Among these strains, only strain DMB07 showed streptomycin resistance, and thus we hypothesized that the genome of strain DMB07 possesses a strain-specific gene that results in the resistance to streptomycin. However, no putative streptomycin resistance gene could be identified among the unique CDSs of strain DMB07 (Supplementary Table 1).
Insights into streptomycin resistance
Streptomycin, an aminoglycoside antibiotic, inhibits protein synthesis by binding to the 16 S rRNA and ribosomal protein S12 in the 30 S ribosomal subunit41,42. Bacteria can survive challenge by streptomycin via streptomycin modification and/or mutation of the streptomycin binding site43. Some enzymes, such as acetyltransferase AadA, modify streptomycin44,45, and thus we searched for genes encoding potential streptomycin-modifying enzymes in the strain DMB07 genome. Consequently, genes encoding aminoglycoside 6-adenylyltransferase (aadK) and an aminoglycoside phosphotransferase family protein (aph3-II) were identified as a core genome.
A previous study suggested that the aadK gene conferred weak resistance to streptomycin44, but confirmatory experimental results were lacking. Moreover, the aadK gene was commonly possessed by B. licheniformis and B. paralicheniformis strains, exhibiting high homology between strains46. Streptomycin sensitivity varies by species, and deletions or insertions in specific amino acid sequences of AadK have been suggested to increase the MIC for streptomycin to 4–64 mg/L in B. licheniformis and B. paralicheniformis46. In the present work, the aadK gene was present in both the streptomycin-resistant strain DMB07 and the sensitive strains DMB06 and KMU01. The putative amino acid sequence of AadK in strain DMB07 showed 100% homology with that in strain DMB06 (Supplementary Fig. 3A). These results suggest that the aadK gene does not confer resistance to streptomycin in strain DMB07.
The aph3-II gene was proposed to contribute to streptomycin resistance through modification of aminoglycoside antibiotics47, but direct evidence indicating its involvement in streptomycin resistance was scarce. Additionally, the sequence homology of the aph3-II gene between streptomycin-sensitive strains DMB06 and KMU01 and streptomycin-resistant strain DMB07 was 100% and 97.9%, respectively, at the nucleotide level (Data not shown). The putative amino acid sequence of Aph3-II in strain DMB07 showed 100% homology with that in strain DMB06 (Supplementary Fig. 3B). These results also suggest that the aph3-II gene is unlikely to contribute to streptomycin resistance in strain DMB07.
On the basis of these results, we concluded that the aadK and aph3-II genes, which potentially induce modifications to streptomycin, did not contribute to the streptomycin resistance of strain DMB07. Furthermore, using two search engines, CARD and ResFinder, to predict antibiotic resistance genes from the genome, no antibiotic resistance genes were detected. Therefore, we next hypothesized that the streptomycin resistance of strain DMB07 may have been conferred by alterations in the binding site of streptomycin, inhibiting its binding. Thus, we investigated genes related to mutation of streptomycin binding sites, the 16 S rRNA (rrs) gene and ribosomal protein S12 (rpsL) gene48,49,50.
Mutation in the 530 loop (A514C; C517T) and the 912 loop (A906G; A917T) of rrs has been reported to cause streptomycin resistance by impairing the binding of streptomycin to the bacterial ribosome48. Although six different nucleotide sequences out of 1,550 sequences were identified among three strains, no mutations were found in the 530 loop and 912 loop sequences, which are known to contribute to streptomycin resistance (Supplementary Fig. 4). Comparing the sequence of the rrs gene of strain DMB07 with that of strain KMU01, which shows sensitivity to streptomycin, showed that two bases were different (179T→G and 1250G→A) (Supplementary Fig. 4). In addition, comparison of the rrs gene sequences of strain DMB06 revealed that one base was different (126 A→G) (Supplementary Fig. 4). However, there have been no reports that these sequences contribute to streptomycin. No mutations were observed among the three strains in the rpsL gene. These results indicate that rrs and rpsL do not contribute to the resistance of strain DMB07 to streptomycin.
Jagielski et al.48 reported that substitution mutations of the 16 S rRNA (guanine527-N7)-methyltransferase gene, also called gidB, were involved in streptomycin resistance, and that this gene was highly polymorphic48. Fifteen polymorphic sites were identified in this gene between the three B. velezensis strains analyzed in this study (Fig. 4). Twelve of these 15 nucleotide sequence differences did not result in any amino acid change in the encoded protein. The differences in two nucleotide sequences (259 A or G; 502G or A) resulted in amino acid changes (amino acid 87 Ile or Val; 168 Val or Ile). However, these amino acids possessed similar properties due to their hydrophobic side chains. Furthermore, a previous study showed that these sequences did not contribute to streptomycin resistance48. Therefore, it was presumed that the changes at the 87th and 168th amino acids did not contribute to streptomycin resistance. The final difference in nucleotide sequences was at position 58, which was thymine (T) in strain DMB07 and cytosine (C) in strains DMB06 and KMU06. Mutation 58T→C of the gidB gene changes amino acid at position 20 in GidB from Arg (in strains DMB06 and KMU01) to Cys (in strain DMB07) (Fig. 4A). The GidB structure predicted using AlphaFold did not show changes in its secondary or tertiary structure, but it revealed a difference in the direction of amino acid residues at the N-terminal regions (Fig. 4B). Specifically, in the case of the 20th amino acid, which showed a change in the nucleotide sequence, the orientation of the functional group has changed (Fig. 4B). A previous study reported that resistance to streptomycin decreased when this arginine was substituted49. gidB gene mutations confer low-level streptomycin resistance due to the secondary structure of the resulting 16 S rRNA49,51. GidB is known to methylate the 527th guanosine of 16 S rRNA, and mutations in GidB were reported to weaken methylation, leading to resistance to streptomycin50,52,53. Therefore, we hypothesized that the mutation in GidB of strain DMB07 affected the methylation of 16 S rRNA, resulting in reduced binding with streptomycin and thereby causing resistance.
Nucleotide and amino acid sequences of gidB in three B. velezensis strains (A) and three-dimensional structure of putative GidB (B). In (A), red letters indicate non-identical base sequences. The amino acids produced by the sequences are boxed. In (B), The blue color in the box represents the 20th amino acid of the GidB.
gidB mutation for streptomycin resistance
Plasmid pIMAY is a temperature-sensitive integration plasmid and E. coli/staphylococcal vector22. However, the replicon of pIMAY-derived plasmid pE194 also replicates in other Gram-positive bacteria such as Lacotococcus and Bacillus54,55. Therefore, it is reasonable to suspect that pIMAY can be replicated in B. velezensis, and we used pIMAY as the vector for point mutation of gidB. pIMAY-tgidBT58C replicated in B. velezensis and, as previously reported22,40, the antisense RNA of secY, an essential gene for bacterial growth, worked well as an auxiliary screening marker in B. velezensis. Furthermore, the thymine located at the 58th nucleotide position of the gidB gene in the chromosome of B. velezensis strain DMB07 was changed to cytosine by doble cross-over using pIMAY-tgidBT58C, as found at that position in strains DMB06 and KMU01 (Figs. 1 and 4). The modified strain was named DMB07gidBT58C. Unlike strain DMB07, it did not grow on tryptic soy agar (TSA) containing 64 mg/L streptomycin (Fig. 5). Strain DMB07gidBT58C showed low-level streptomycin resistance (MIC 32 mg/L) compared with strain DMB07 (MIC 128 mg/L). These results show that the replacement of one nucleotide in the gidB gene, which results in a single amino acid sequence change in the corresponding protein, in turn affects the resistance of the bacterium to streptomycin.
To confirm the effect of the gidB gene, the gidB gene derived from strain DMB07, which has thymine at the 58th nucleotide position, was introduced into strain DMB07gidBT58C. The gidB gene from strain DMB07 was then inserted into pLipSM22, an E. coli–Bacillus shuttle vector, and subsequently introduced into strain DMB07gidBT58C. B. velezensis DMB07gidBT58C (pLipSM-gidB) exhibited a MIC value for streptomycin of 128 mg/L, the same as that of the parental strain DMB07 (Fig. 5). These results suggest that the gidB gene was involved in the streptomycin resistance of B. velezensis strain DMB07. However, the mechanism of streptomycin resistance caused by mutations in the gidB gene remains unclear, requiring further research. Above all, the substitution of T58C in the gidB gene was expected to result in streptomycin MIC values similar to those of the control strains DMB06 and KMU01. However, the MIC of DMB07gidBT58C was 32 mg/L, which was lower than that of DMB07 but still 4-fold and 2-fold higher than those of DMB06 (4 mg/L) and KMU01 (16 mg/L), respectively. This suggests that factors beyond the gidB gene may influence streptomycin resistance.
The above experiments confirmed for the first time that the gidB gene, located on the chromosome, plays an important role in antibiotic susceptibility and bacterial growth under streptomycin in B. velezensis. Additionally, no transposons were found near the gidB gene, nor were there phage elements necessary for transduction (Data not shown). Additionally, when a gene is acquired through horizontal transfer, its G + C content and GC skew tend to show different patterns compared to surrounding genes. However, upon examining the G + C contents and GC skew of the gidB gene and its surrounding genes, it was found to be similar. (Supplementary Fig. 5). Therefore, it was suggested that the gidB gene was likely transferred vertically rather than through gene exchange (horizontal transmission). In other words, the mutation in the gidB gene is not species trait but strain-specific, it does not represent an acquired antibiotic resistance gene capable of horizontal transfer. This suggests that strain DMB07, despite exhibiting resistance to streptomycin, is suitable for industrial use, as its resistance results from a mutation in a chromosomal gene, aligning with the Qualified Presumption of Safety antibiotic resistance guidelines of EFSA19. Selecting strains with antibiotic susceptibility for use as starter cultures in fermented foods is important to prevent the spread of antibiotic resistance genes among microorganisms present in the food during fermentation. Additionally, consuming fermented foods containing strains that exhibit antibiotic resistance raises concerns about the potential transfer of these resistance genes to the gut microbiota of the host. However, we propose that strains showing antibiotic resistance, yet incapable of horizontal transfer of antibiotic resistance genes, may pose reduced risks. In a previous experiment, we demonstrated that strains carrying plasmids with the lincomycin resistance gene were capable of horizontally transferring antibiotic resistance genes during fermentation when exposed to antibiotics21. However, given the low likelihood of antibiotic exposure during food fermentation, the risk of horizontal transfer of antibiotic resistance genes in fermented foods is considered minimal.
Consequently, the resistance of B. velezensis strain DMB07 to streptomycin was suggested to be due to a change in the GidB protein sequence caused by a nucleotide alteration in the gidB gene. The GidB mutation was specific to the strain rather than the species. Additionally, the gidB gene was located on the chromosome, not on mobile genetic elements like plasmids. Therefore, we assumed that the mutated gidB gene will be inherited vertically into daughter cells, rather than horizontally transferred to other strains. According to the EFSA guidelines for antibiotic resistance19, strains showing antibiotic resistance due to genomic mutation are generally acceptable for feed use. The potential streptomycin resistance-related genes analyzed in this study—aadK, aph3-II, gidB, rpsL, and rrs—are all located on the chromosome. These are not horizontally transferable genes specific to individual strains, but rather genes commonly found in a species-specific manner. Although the parent strain is antibiotic-sensitive, spontaneous mutations can lead to the emergence of antibiotic-resistant strains. In other words, for B. velezensis, it is challenging to confirm streptomycin resistance based solely on the presence or absence of genes. It is necessary to verify either changes in the nucleotide sequences of genes or phenotypic characteristics. Consequently, if the mutation in the gidB gene of B. velezensis confers resistance to streptomycin, it is suggested that the strain could be applied industrially, as the antibiotic resistance gene would not undergo horizontal transfer.
Data availability
The complete genome sequence of B. velezensis strain DMB07 (7SSD30) was deposited in the DDBJ/ENA/GenBank under accession number CP083764, and the strain was deposited in the Korean Culture Center of Microorganisms with accession number KCCM 90490. All relevant data are within the manuscript and its supporting information files.
References
Cho, M. S. et al. Understanding the ontogeny and succession of Bacillus velezensis and B. subtilis subsp. subtilis by focusing on kimchi fermentation. Sci. Rep. 8, 7045 (2018).
Ha, G. et al. Complete genome sequence of Bacillus velezensis SRCM102755, a high menaquinone-7 producer, isolated from Doenjang. Korean J. Microbiol. 56, 74–75 (2020).
Honjo, M. et al. Cloning and expression of the gene for neutral protease of Bacillus amyloliquefaciens in Bacillus subtilis. J. Biotechnol. 1, 165–277 (1984).
Jang, M., Jeong, D. W. & Lee, J. H. Identification of the predominant Bacillus, Enterococcus, and Staphylococcus species in meju, a spontaneously fermented soybean product. Microbiol. Biotechnol. Lett. 47, 359–363 (2019).
Lee, H. J. et al. Complete genome sequence of Bacillus velezensis YJ11-1-4, a strain with broad-spectrum antimicrobial activity, isolated from traditional Korean fermented soybean paste. Genome Announc. 5, e01352 (2017).
Lee, J. M. et al. Culture-dependent and -independent investigations of bacterial migration into doenjang from its components meju and solar salt. PLoS ONE. 15, e0239971 (2020).
Jang, M. et al. Genetic background behind the amino acid profiles of fermented soybeans produced by four Bacillus spp. J. Microbiol. Biotechnol. 31, 447–455 (2021).
Khalid, A. et al. Production of β-glucanase and protease from Bacillus velezensis strain isolated from the manure of piglets. Prep. Biochem. Biotechnol. 51, 497–510 (2021).
Moon, S. et al. Isolation and characterization of Bacillus velezensis SS360-1 from seed soy sauce. Korean J. Commun. Living Sci. 29, 49–58 (2018).
Gil, N. Y. et al. Comparative evaluation of quality and metabolite profiles in meju using starter cultures of Bacillus velezensis and aspergillus oryzae. Foods 11, 68 (2021).
Liu, H. et al. Novel green soybean shuidouchi fermented by Bacillus velezensis with multibioactivities. Food Sci. Nutr. 9, 6538–6547 (2021).
Heo, S. et al. Functional annotation genome unravels potential probiotic Bacillus velezensis strain KMU01 from traditional Korean fermented kimchi. Foods 10, 563 (2021).
Na, H. E. et al. The safety and technological properties of Bacillus velezensis DMB06 used as a starter candidate were evaluated by genome analysis. LWT Food Sci. Technol. 161, 113398 (2022).
Wang, H. et al. Engineering of a Bacillus amyloliquefaciens strain with high neutral protease producing capacity and optimization of its fermentation conditions. PLoS ONE. 11, e0146373 (2016).
Yao, Z., Kim, J. A. & Kim, J. H. Characterization of a fibrinolytic enzyme secreted by Bacillus velezensis BS2 isolated from sea squirt jeotgal. J. Microbiol. Biotechnol. 29, 347–356 (2019).
Huttner, A. et al. Antimicrobial resistance: A global view from the 2013 World Healthcare-Associated infections Forum. Antimicrob. Resist. Infect. Control. 2, 1–13 (2013).
CDC, Antibiotic resistance threats in the United States. (U.S. Department of Health and Human Services, CDC, Atlanta, Georgia, 2013).
Tacconelli, E. & Magrini, N. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. (World Health Organ., 2017).
EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on theassessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 10, 2740–2749 (2012).
Lee, J. H. et al. Transfer of a mobile Staphylococcus saprophyticus plasmid isolated from fermented seafood that confers tetracycline resistance. PLoS ONE. 14, e0213289 (2019).
Heo, S. et al. Transfer of a lincomycin-resistant plasmid between coagulase-negative staphylococci during soybean fermentation and mouse intestine passage. FEMS Microbiol. Lett. 366, fnz113 (2019).
Monk, I. R. et al. Transforming the untransformable: Application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio. 3, e00277 (2012).
Lee, M. H. et al. High-level expression and secretion of Bacillus pumilus lipase B26 in Bacillus subtilis Chungkookjang. J. Microbiol. Biotechnol. 13, 892–896 (2003).
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing, 30th ed. CLSI supplement M100 CLSI (2020).
Tatusova, T. et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 44, 6614–6624 (2016).
Tatusov, R. L., Galperin, M. Y., Natale, D. A. & Koonin, E. V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 28, 33–36 (2000).
Overbeek, R. et al. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res. 42, D206–D214 (2014).
Goris, J. et al. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 57, 81–91 (2007).
Darling, A. C. et al. Mauve: Multiple alignment of conserved genomic sequence with reaerrangements. Genome Res. 14, 1394–1403 (2004).
Dieckmann, M. A. et al. EDGAR3.0: Comparative genomics and phylogenomics on a scalable infrastructure. Nucleic Acids Res. 49, W189–W192 (2021).
Blom, J. et al. EDGAR 2.0: An enhanced software platform for comparative gene content analyses. Nucleic Acids Res. 44, W22–28 (2016).
Lerat, E., Daubin, V. & Moran, N. A. From gene trees to organismal phylogeny in prokaryotes: The case of the gamma-Proteobacteria. PLoS Biol. 1, E19 (2003).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Alcock, B. et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the comprehensive antibiotic resistance database. Nucleic Acid Res. 51, D690–699 (2020).
Bortolaia, V. et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 75, 3491–3500 (2020).
Clausen, P. T. L. C., Aarestrup, F. M. & Lund, O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinform. 19, 307 (2018).
Hanahan, D. & Meselson, M. Plasmid screening at high colony density. Methods Enzymol. 100, 333–342 (1983).
Kraemer, G. R. & Iandolo, J. J. High-frequency transformation of Staphylococcus aureus by electroporation. Curr. Microbiol. 21, 373–376 (1990).
Jeong, D. W., Lee, J. H. & Lee, H. J. Construction of recombinant Lactobacillus casei strains using splicing by overlap extension. J. Microbiol. Biotechnol. 18, 1953–1957 (2008).
Bae, T. & Schneewind, O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55, 58–63 (2006).
Carr, J. F. et al. Effects of streptomycin resistance mutations on posttranslational modification of ribosomal protein S12. J. Bacteriol. 188, 2020–2023 (2006).
Kalapala, S. K. et al. Mutation K42R in ribosomal protein S12 does not affect susceptibility of Mycobacterium smegmatis 16S rRNA A-site mutants to 2-deoxystreptamines. PLoS ONE. 5, e11960 (2010).
Springer, B. et al. Mechanisms of streptomycin resistance: Selection of mutations in the 16S rRNA gene conferring resistance. Antimicrob. Agents Chemother. 45, 2877–2884 (2001).
Noguchi, N., Sasatsu, M. & Kono, M. Genetic mapping in Bacillus subtilis 168 of the aadK gene which encodes aminoglycoside 6-adenylyltransferase. FEMS Microbiol. Lett. 114, 47–52 (1993).
Clark, N. C. et al. Detection of a streptomycin/spectinomycin adenylyltransferase gene (aadA) in Enterococcus faecalis. Antimicrob. Agents Chemother. 43, 157–160 (1999).
Agersø, Y. et al. Putative antibiotic resistance genes present in extant Bacillus licheniformis and Bacillus paralicheniformis strains are probably intrinsic and part of the ancient resistome. PLoS ONE. 14, e0210363 (2019).
Mazodier, P., Giraud, E. & Gasser, F. Genetic analysis of the streptomycin resistance encoded by Tn5. Mol. Gen. Genet. 192, 155–162 (1983).
Jagielski, T. et al. Screening for streptomycin resistance-conferring mutations in Mycobacterium tuberculosis clinical isolates from Poland. PLoS ONE. 9, e100078 (2014).
Wang, Y. et al. The roles of rpsL, rrs, and gidB mutations in predicting streptomycin-resistant drugs used on clinical Mycobacterium tuberculosis isolates from Hebei Province, China. Int. J. Clin. Exp. Pathol. 12, 2713–2721 (2019).
Howarth, R. E. et al. Three genes controlling streptomycin susceptibility in Agrobacterium Fabrum. J. Bacteriol. 205, e0016523 (2023).
Wong, S. Y. et al. Mutations in gidB confer low-level streptomycin resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 55, 2515–2522 (2011).
Okamoto, S. et al. Loss of a conserved 7-methylguanosine modification in 16S rRNA confers low-level streptomycin resistance in bacteria. Mol. Microbiol. 63, 1096–1106 (2007).
Nishimura, K. et al. Identification of the RsmG methyltransferase target as 16S rRNA nucleotide G527 and characterization of Bacillus subtilis rsmG mutants. J. Bacteriol. 189, 6068–6073 (2007).
Gryczan, T. J. & Dubnau, D. Direct selection of recombinant plasmids in Bacillus subtilis. Gene 20, 459–469 (1982).
Chopin, M. C. et al. Insertion and amplification of foreign genes in the Lactococcus lactis subsp. lactis chromosome. Appl. Environ. Microbiol. 55, 1769–1774 (1989).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) [NRF-RS-2024-00334769]. We thank Edanz (www.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
H.E.N. Data curation, Formal analysis, Investigation, Methodology, Writing-original draft, Writing - review & editing. S.H. Data curation, Formal analysis, Investigation, Methodology, Writing-original draft, Writing - review & editing; S.L. Formal analysis, Validation; G.L. Investigation, Methodology; J.H.L. Conceptualization, Investigation, Writing - original draft, and Writing - review & editing; D.W.J. Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing - original draft, and Writing - review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Na, HE., Heo, S., Lee, S. et al. Mutation of the gidB gene causes intrinsic streptomycin resistance in Bacillus velezensis. Sci Rep 15, 5565 (2025). https://doi.org/10.1038/s41598-025-90258-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90258-y