Abstract
The best conditioning for hematopoietic stem cell transplantation (HSCT) in acute lymphoblastic leukemia is debated. We analyzed 178 adults undergoing HSCT with total body irradiation (TBI) or chemotherapy conditioning regimens for B-cell leukemia in complete remission 1 (CR1). Both TBI and chemotherapy regimens showed comparable overall survival (OS) and leukemia-free survival (LFS). However, TBI had a trend of reduced relapse (HR 0.56, P = 0.064) but higher non-relapse mortality (NRM, HR 3.23, P = 0.072) due to increased grade 3 to 4 graft-versus-host disease (22.8% vs. 8.8%, P = 0.023). The key factor affecting survival was minimal residual disease (MRD) after three chemotherapy cycles (OS P = 0.004, LFS P = 0.006). In MRD-negative patients, both regimens gave similar LFS and cumulative incidence of relapse (CIR) irrespective of whether the transplantation was allogeneic (allo-HSCT) or autologous (auto-HSCT), but the allo-HSCT group had a lower OS due to higher NRM (5.3% vs. 20.6%, P = 0.020). For MRD-positive patients, TBI was superior in LFS (71.9% vs. 43.9%, P = 0.017) and relapse rate (18.5% vs. 48.7%, P = 0.006). Our research indicates the choice between TBI and non-TBI should be based on MRD after three chemotherapy cycles.
Similar content being viewed by others
Introduction
Acute lymphoblastic leukemia (ALL) is a prevalent form of acute leukemia in adults, comprising approximately 20–30% of all cases of adult acute leukemia. The integration of hematopoietic stem cell transplantation (HSCT) into the standard care protocol for ALL has been well-established in the literature[1, 2]. Total body irradiation (TBI)-based conditioning regimens have traditionally served as the cornerstone for HSCT protocols, principally due to their non-cross-resistance with chemotherapeutic agents and their potent immunosuppressive and anti-neoplastic capabilities, which are effective even across physiological barriers that usually protect neoplastic cells[3]. While fractionated irradiation strategies have been developed to preserve anti-tumor efficacy and minimize organ toxicity[4], myeloablative doses of TBI continue to be linked with acute and long-term toxicities, elevated treatment-related mortality, and an increased risk of secondary malignancies. Consequently, this has spurred the advancement of alternative chemotherapy-based conditioning regimens[5–8].
A multi-center, open-label, randomized Phase III clinical trial conducted across 13 hospitals in China found that the busulfan (BU)/ cyclophosphamide (CY) regimen exhibited non-inferior efficacy and safety compared to TBI-Cy (4.5 Gy×2) for adult patients with standard-risk B-cell ALL in CR1 undergoing HLA-matched allogeneic HSCT[9]. Numerous registry studies and retrospective analyses have echoed these findings, showing largely comparable outcomes between TBI-based and chemotherapy-based regimens[10, 11]. Nonetheless, some studies have presented evidence of superior leukemia-free survival, overall survival, and reduced relapse rates in patients undergoing TBI-based conditioning[3, 12]. As a result, the optimal conditioning regimen for HSCT in ALL remains a contentious issue within the medical community.
Moreover, advances in flow cytometry, molecular biology, and cytogenetic testing have substantially improved the precision of minimal residual disease (MRD) evaluation, thereby potentially influencing treatment planning. Friend et al. advocated that the selection between TBI and non-TBI conditioning regimens should be contingent upon next-generation sequencing-minimal residual disease (NGS-MRD) status. Specifically, they recommended that TBI-based regimens should be reserved for patients unable to achieve NGS-MRD negativity prior to undergoing allogeneic HSCT[13].
In the present study, we retrospectively compared the outcomes of TBI-based and chemotherapy-based conditioning regimens, taking into account variations in donor type and MRD status. The objective of this analysis is to furnish a robust empirical foundation to aid clinicians in the more discerning selection of conditioning regimens for ALL patients undergoing HSCT.
Materials and methods
Study design and data collection
Clinical data for this retrospective analysis were harvested from our institutional medical records database, spanning a period from January 2019 to December 2021. The study population consisted of adult patients, ranging in age from 15 to 60 years, who were diagnosed with B-cell acute lymphoblastic leukemia (B-ALL) and underwent their first HSCT while in complete remission 1 (CR1). Ethical approval for this investigation was granted by the institutional ethics committee in accordance with the tenets of the Declaration of Helsinki. Furthermore, written informed consent was procured from each participating patient in compliance with federal and institutional guidelines.
Procedures of transplantation
The procedural aspects of the transplantation, including pre-transplant chemotherapy, donor selection, graft preparation, prevention of graft-versus-host disease (GVHD), and maintenance therapy, adhered to the protocols delineated in our previous publications[14]. GVHD prophylaxis for MSD-HSCT patients was cyclosporine A (1 mg/kg/d) or tacrolimus (0.03 mg/kg/d) starting on day − 1 and methotrexate (MTX, 15 mg/m2 on day + 1; and 10 mg/m2 on days + 3 and + 6). For haplo-HSCT and URD-HSCT recipients, cyclosporine A or tacrolimus was started on day − 9 and 1 dose of methotrexate was additionally administered on day + 11. Mycophenolate mofetil (MMF, 15 mg/kg/d from day − 9) and antithymocyte globulin (ATG) (rabbit, 2.5 mg/kg/d, days − 5, −4, −3 and − 2) were also given as GVHD prophylaxis. MMF was discontinued on day 60 in the absence of GVHD.
When WBC numbers reached 3 × 109/L and platelets 50 × 109/L after auto-HSCT, all atients were given maintenance therapy, which may need to be continued for 1–2 years. The maintenance therapy generally was based on a VP/VDP/VIP regimen (vincristine 1.4 mg/m2, day 1, day 8; daunorubicin 20–30 mg/m2 day 1, day 8 or idamycin 6 mg/m2 day 1, day 8; prednisone 1 mg/kg, days 1 to14) or MM regimen (MTX 15–20 mg/m2, day 1 and day 8; 6-mercaptopurine 50–75 mg/m2, days 1 to 14) or venetoclax 400 mg days 1 to7 alternatively. In addition, TKIs were given to Ph + ALL patients. Allo-HSCT patients who were at high risk of relapse as determined by: (1) primary induction failure requiring more than 1 line of therapy, (2) positive MRD before allo-HSCT and/or (3) high-risk cytogenetic or molecular profile were also given maintenance therapy as auto-HSCT patients.
Conditioning regimens
In the TBI cohort (n = 117), the predominant conditioning regimens were TBI/CY or TBI/melphalan (Mel), often supplemented with fludarabine (Flu), cladribine (Clad), cytarabine (Ara-C), or etoposide (VP-16). Detailed dosages are provided: TBI (10 Gy), CY (80 mg/kg), Mel (100-150mg/m2), Flu (90mg/m2), Clad (15mg/m2), Ara-C (6 g/m2), VP-16 (50 mg/kg). A subset of 61 patients received non-TBI conditioning regimens, primarily comprising intravenous busulfan (BU)/CY (n = 47), BU/Mel (n = 3), Mel/Clad (n = 7), or VP-16/Clad (n = 4), with BU dosed at 9.6 mg/kg. Specific regimens are tabulated in Table 1.
Supplementary Table 1. The specific conditioning regimens for patients in CR1 B-ALL.
Definitions
Criteria for neutrophil and platelet engraftment, acute GVHD (aGVHD), and chronic GVHD (cGVHD) were aligned with previously established guidelines. Overall survival (OS) was calculated from the date of stem cell transplantation to the time of death from any cause or the date of last follow-up. Leukemia-Free Survival (LFS) was ascertained from the date of transplantation until relapse, death, or the last follow-up date. Non-Relapse Mortality (NRM) was defined as death not attributed to leukemic relapse. Relapse was characterized by the resurgence of lymphoblasts in bone marrow (> 5%), any presence of lymphoblasts in peripheral blood (hematological relapse), or molecular relapse.
Statistical analysis
Comparisons between patients’ characteristics were made using the chi-squared test for categorical variables and the Kruskal-Wallis test for continuous variables. Cumulative incidences of GVHD were ascertained through Gray’s test, considering death and relapse as competing events. The probabilities of OS and LFS were estimated via the Kaplan-Meier method, with subgroup differences evaluated using the log-rank test. Relapse and NRM rates were calculated through cumulative incidence analyses and contrasted using Gray’s test. Univariate and multivariate risk factor analyses for survival were conducted employing Cox proportional regression and competing risk models. Variables manifesting a P-value < 0.15 in the univariate analysis were included in the multivariate assessment. All statistical tests were two-sided, with a P-value < 0.05 deemed statistically significant. Statistical computations were executed using SPSS version 22.0 (IBM, Armonk, NY, USA) and R 4.1.1 (R Development Core Team, Vienna, Austria) software packages.
Results
Patient characteristics
A total of 61 and 117 patients were enrolled in the chemotherapy-based and TBI-based groups, respectively. A comprehensive overview of the patients’ clinical attributes can be found in Table 1. Notably, the two cohorts exhibited considerable comparability in various parameters, including gender distribution, age distribution, white blood cell count, incidence of central nervous system leukemia, ALL subtype, donor origin, risk stratification, MRD status post 3 cycles of chemotherapy, duration of maintenance therapy, frequency of lumbar punctures, and total number of chemotherapy cycles administered. However, the chemotherapy-based group received lower median doses of infused mononuclear (10 × 108/kg vs. 11.59 × 108/kg, P = 0.037) and CD34 + cells (2.53 × 106/kg vs. 3.07 × 106/kg, P = 0.040). Supplementary Tables 2 and 3 further detailed the characteristics of patients with TBI-based and chemotherapy-based conditioning regimens in both auto-HSCT and allo-HSCT.
Hematopoietic reconstitution, GVHD and infectious complications
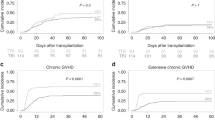
All patients achieved neutrophil reconstitution, although five failed in platelet recovery and succumbed to aGVHD. The median time to neutrophil engraftment was 13 (9–23) days and the median time to platelet recovery was 17 (10–193) days. In the allo-HSCT group, the incidence of grade II-IV acute GVHD within 3 months was 26.7% [95% confidence interval (CI): 15.8–43.2%] and 35.0% (95% CI: 27.9–47.9%) in the chemotherapy-based and TBI-based groups, respectively (P = 0.232). The incidence of grade III-IV acute GVHD within 3 months was 8.8% (95% CI: 2.2–21.4%) and 22.8% (95% CI: 16.7–34.8%) in the chemotherapy-based and TBI-based groups, respectively (P = 0.023). The incidence of cGVHD within 2 years was 30.8% (95% CI: 23.1–50.8%) and 44.3% (95% CI: 36.5–55.9%), respectively (P = 0.148). The incidence of II-III cGVHD within 2 years was 19.8% (95% CI: 10.7–27.5%) and 20.4% (95% CI: 11.7–36.4%), respectively (P = 0.889).
Out of the entire cohort, CMV reactivation was observed in 96 individuals (24 in non-TBI-based and 72 in TBI-based group, P = 0.005). Thirteen patients experienced reactivation of Epstein-Barr virus (EBV) (6 in non-TBI-based and 7 in TBI-based group, P = 0.348). Furthermore, four patients developed EBV-associated post-transplant lymphoproliferative disorder (PTLD), with an equal distribution of 2 cases in both the non-TBI-based and TBI-based groups (P = 0.608). It is noteworthy that all affected patients responded positively to antiviral treatments and rituximab therapy, leading to successful recovery.
Long-term survival
The median duration of patient follow-up extended to 23 (1.5–48) months subsequent to transplantation. Until the last follow up, a total of 35 patients experienced mortality, which can be further classified into 14 cases attributed to disease recurrence, 10 cases resulting from infections, 10 cases stemming from GVHD, and 1 case linked to pulmonary hemorrhage. The OS and LFS rates 3 years post-transplantation were 75.5% (95% CI: 68.1–83.7%) and 60.9% (95% CI: 53.0–69.9%], respectively. The 3-year estimated OS [81.4% (95% CI: 72.0–92.0%) vs. 65.3% (95% CI: 50.4–84.6%), P = 0.190] and LFS [62.1% (95% CI: 51.0–75.6%) vs. 51.4% (95% CI: 35.3–74.9%), P = 0.974] did not differ significantly between non-TBI and TBI group. The outcomes of univariate and multivariate analyses uniformly indicated a reduction in the risk of mortality and recurrence with the implementation of maintenance therapy post transplantation (Tables 2 and 3).
Relapse and NRM
At the end of the follow-up, 41 of 178 patients had relapsed at a median time of 6 months (range, 1–33 months) after transplantation. One important concern is the control of sanctuary sites using a non-TBI regimen. We noted one case in chemotherapy-based and nine in TBI-based group with a pre-existing history of central nervous system (CNS) involvement exhibited no instances of recurrence. In the TBI group, a solitary patient underwent CNS relapse, despite the absence of CNS involvement prior to transplantation. However, due to the limited size of the population, definitive conclusions regarding this matter remain constrained. The cumulative incidence of relapse (CIR) and NRM rates were 25.8% (95% CI: 18.8–33.4%) and 13.3% (95% CI: 8.2–19.7%) across the entire patient cohort. A distinct advantage emerged from the chemotherapy-based conditioning regimens in terms of NRM [(4.9%, 95% CI: 1.3–12.5%) vs. (22.7%, 95% CI: 9.3–39.5%), P = 0.020], while concurrently revealing a trend towards a relatively higher CIR [(33.0%, 95% CI: 21.5–45.0%) vs. (25.9%, 95% CI: 11.9–42.5%), (P = 0.078)]. Nevertheless, subsequent multivariate analyses did not substantiate any conditioning regimen-related influence on CIR (Table 3).
Comparable outcomes of TBI-based and chemotherapy-based conditioning regimens
To assess treatment efficacy, an evaluation was conducted based on distinct donor types and MRD status post 3 cycles of chemotherapy, encompassing auto-HSCT with negative MRD after 3 chemotherapy cycles, allo-HSCT with negative MRD post 3 chemotherapy cycles, and allo-HSCT with positive MRD following 3 chemotherapy cycles, of which interactions were tested.
All patients who underwent auto-HSCT achieved negative MRD post 3 chemotherapy cycles and subsequently received maintenance therapy. Within the auto-HSCT subgroup, a comparative analysis revealed no significant differences between chemotherapy-based and TBI-based groups in terms of the 3-year LFS [80.0% (95% CI: 62.1–100.0%) vs. 43.8% (95% CI: 18.0–100.0%), P = 0.203], CIR [20.0% (95% CI: 4.5–43.3%) vs. 56.2% (95% CI: 9.0–87.2%), P = 0.203], and NRM rates [0% vs. 0%, P = 1.0]. However, a noticeable disparity emerged in OS [100.0% vs. 68.6% (95% CI: 40.3–100.0%), P = 0.024], favoring chemotherapy-based regimens (15 patients with chemotherapy-based regimens, 16 with TBI-based regimens) (Fig. 1).
Among the subset of 67 patients (19 with chemotherapy-based regimens, 48 with TBI-based regimens) who exhibited negative MRD status and underwent allo-HSCT, statistically significant advantages were observed in the 3-year OS rates with chemotherapy-based regimens [89.5% (95% CI: 76.7–100.0%) vs. 41.8% (95% CI: 19.9–88.1%), P = 0.017], attributed to lower NRM [5.3% (95% CI: 0.3–22.1%) vs. 20.6% (95% CI: 9.2–78.2%), P = 0.020]. However, no substantial disparities were identified in terms of LFS [73.7% (95% CI: 56.3–96.4%) vs. 39.9% (95% CI: 17.3–92.3%), P = 0.118] and CIR [21.1% (95% CI: 6.2–41.7%) vs. 13.6% (95% CI: 5.3–25.7%), P = 0.644] rates between chemotherapy-based and TBI-based regimens (Fig. 2).
In the subset of the remaining 80 cases (27 with chemotherapy-based regimens and 53 with TBI-based regimens) who underwent allo-HSCT and exhibited positive MRD status after completing 3 cycles of chemotherapy, a noticeable discrepancy emerged as patients within the chemotherapy-based group demonstrated a considerably higher relapse rate [48.7% (95% CI: 28.5–66.3%) vs. 18.5% (95% CI: 8.8–31.0%), P = 0.006] in comparison to the TBI group. Consequently, this escalation in relapse was accompanied by a reduction in the 3-year LFS [43.9% (95% CI: 28.5–67.5%) vs. 71.9% (95% CI: 60.1–85.9%), P = 0.017] and overall survival (OS) [64.7% (95% CI: 48.3–86.6%) vs. 86.5% (95% CI: 77.7–96.3%), P = 0.109]. Nevertheless, no significant disparities were noted in NRM [7.4% (95% CI: 12.3–21.5%) vs. 9.7% (95% CI: 3.5–19.6%), P = 0.755] between the two groups (Fig. 3).
The possibility of early and swift MRD clearance emerges as a potential determinant for identifying a subgroup of patients characterized by low relapse risk, thus designating them as “good-risk” individuals who could potentially benefit from chemotherapy-based regimens. Consequently, we conducted an interaction test to evaluate the interplay between MRD status after 3 chemotherapy cycles and the conditioning regimens employed. This analysis yielded significant findings for both OS (P = 0.004) and LFS (P = 0.006) (Table 4).
Discussion
In ALL, relapse serves as the principal cause of treatment failure following transplantation[15]. Introduced in the 1970s, TBI has subsequently been established as the standard conditioning regimen for ALL patients in many medical centers. Its adoption is attributed to its tolerable toxicity profile and efficacy in eliminating leukemic cells in sanctuary sites[16]. Recent investigations have explored non-TBI regimens as alternatives to circumvent the documented adverse effects of TBI, such as secondary malignancies and cognitive impairments, while offering easier administration and broader availability, particularly in centers lacking irradiation facilities[17]. Nonetheless, the optimal dosage for TBI remains elusive. Initial clinical experiences employed a single 10 Gy dose of TBI. Comparative studies between single-dose and fractionated TBI (fTBI) have demonstrated that fTBI significantly reduces the incidence of interstitial pneumonia and improves short-term survival rates. The adoption of a dose-fractionated strategy, elevating the total TBI dose to 12 Gy or beyond, demonstrated amplified extramedullary toxicity while not resulting in corresponding enhancements in survival, despite lowered relapse rates [18]. In our study, a 10 Gy fTBI regimen was selected to uphold therapeutic effectiveness while concurrently minimizing adverse effects.
Previous literature provides conflicting data on outcomes between TBI-based and chemotherapy-based conditioning regimens. While some studies indicate worse survival and higher relapse rates for chemotherapy-based approaches, others report comparable outcomes[6, 19, 20, 21]. These findings predominantly stem from retrospective analyses. Recently, in a multinational, randomized phase III investigation, a notable enhancement in OS coupled with a reduced relapse risk was identified in cases of allogeneic HSCT involving the combination of TBI and etoposide, in contrast to cases employing chemo-conditioning. This led to the endorsement of TBI plus etoposide for individuals over the age of 4 years diagnosed with high-risk childhood ALL [22]. While a distinct open-label, randomized phase III trial conducted in China unveiled the noninferior efficiency and safety profiles exhibited by the TBI-Cy and BuCy regimens for patients with standard-risk B cell-ALL in CR1 undergoing HLA-matched allo-HSCT [9]. The divergent outcomes gleaned from these two randomized controlled trials (RCTs) can be reasonably attributed to the distinct target populations and conditioning strategies under investigation. In our investigation, TBI and chemotherapy-based conditioning yielded comparable OS and LFS. Nonetheless, a trend towards a reduction in relapse rates (HR 0.555, 95% CI 0.30–1.03, P = 0.064) and a corresponding elevation in NRM (HR 3.230, 95% CI 0.90–11.60, P = 0.072) were discernible within the TBI group, a trend that mirrors observations from prior studies. This result could, in part, be attributed to a heightened incidence of grade 3 to 4 aGVHD associated with TBI-based conditioning. The amplification in GVHD incidence subsequent to TBI has been consistently documented in various studies, aligning with our own study results [3, 21, 23]. Lower relapse relates are related to a GVL effect, which has been elucidated in the previous studies. The observed predilection for GVHD among recipients of TBI-based regimens may be explicable by a heightened release of inflammatory cytokines and resultant endothelial damage [24].
Furthermore, the MRD status preceding transplantation exerts a pivotal influence on the selection of conditioning regimens. Preceding investigations have consistently underscored the prognostic significance of pretransplant MRD positivity in relation to subsequent post-transplant relapse risk [25–28]. Friend et al.‘s work particularly underscores the significance of employing the most sensitive method available for MRD assessment, such as NGS-MRD. The study demonstrated that individuals exhibiting NGS-MRD negativity immediately prior to transplantation exhibited no instance of relapse, irrespective of whether a TBI or non-TBI regimen was administered. In contrast, patients with unknown MRD or NGS-MRD positivity faced elevated relapse risk following non-TBI-based regimens (57% vs. 24%, P = 0.03), resulting in a notable discrepancy in the three-year event-free survival (EFS) (69% vs. 25%, P = 0.01) [13]. In congruence with these findings, our own analysis supports the rationale that the selection between TBI and non-TBI regimens in B-ALL should hinge upon MRD status following three chemotherapy cycles. Our interaction tests highlight MRD status after three chemotherapy cycles as the solitary determinant influencing the choice of conditioning regimen concerning both OS (P = 0.004) and LFS (P = 0.006). Notably, LFS and CIR were similar with TBI-based and chemotherapy-based conditioning regimens both in allo- and auto-HSCT patients who achieved negative MRD after 3 chemotherapies while the inferior OS was attributed to higher NRM in allo-HSCT. For patients with positive MRD, the TBI-based regimen exhibited pronounced superiority over the non-TBI regimen in terms of both LFS and CIR. These outcomes highlight that patients with a lower risk of relapse might accrue benefits from the avoidance of aGVHD, while concurrently sidestepping the toxicities associated with irradiation. Conversely, for patients harboring a higher risk of tumor burden, the greater GVL effect induced by irradiation is indispensable. In recent years, the emergence and rapid development of novel targeted therapies in B-ALL, including monoclonal antibodies against specific tumor antigens (inotuzumab, blinatumomab, etc.) and chimeric antigen receptor T (CART) cells characterized by a high response rate and acceptable tolerance, have been witnessed[29–31]. Currently, researches from Handgretinger and Lang has suggested that beyond the mere incorporation of novel sensitive methods for detection of MRD, the development of novel immunotherapies in combination with non-TBI-based conditioning regimens has improved the survival outcomes and may lead to comparable results compared to TBI-based standard approaches[32].
Our study had several limitations given its single-center, retrospective nature and low case numbers. Secondly, a variety of conditioning regimens increased the heterogeneity and the results need validation in further prospective clinical trials. Thirdly, whether non-TBI-based versus TBI-based regimens have comparable outcomes in patients with CNS infiltration is difficult to address as the population is too small. Finally, the median follow-up period for this trial was relatively short, thus long-term quality of life measures following use of different regimens should be studied in order to better understand late effects further.
Conclusions
In summary, for both auto-HSCT and allo-HSCT, chemotherapy-based pre-treatment regimens may serve as viable alternatives for B-ALL patients in CR1, particularly for those who achieve MRD negativity after three chemotherapy cycles. Conversely, for patients displaying MRD positivity, the adoption of TBI-based pretreatment regimens emerges as a more promising and effective course of action.
Data availability
The datasets generated and/or analyzed during the course of this study are available from the corresponding author upon reasonable request.
References
1. Hematology Oncology Committee, C.A.-C.A., Leukemia, and C.S.o.H.C.M.A. Lymphoma Group, [Chinese guidelines for diagnosis and treatment of adult acute lymphoblastic leukemia (2021)]. Zhonghua Xue Ye Xue Za Zhi, 2021. 42(9): p. 705–716.
2. Gökbuget, N., et al., Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood, 2012. 120(10): p. 2032-41.
3. Khimani, F., et al., Impact of Total Body Irradiation-Based Myeloablative Conditioning Regimens in Patients with Acute Lymphoblastic Leukemia Undergoing Allogeneic Hematopoietic Stem Cell Transplantation: Systematic Review and Meta-Analysis. Transplant Cell Ther, 2021. 27(7): p. 620.e1-620.e9.
4. Girinsky, T., et al., Prospective randomized comparison of single-dose versus hyperfractionated total-body irradiation in patients with hematologic malignancies. J Clin Oncol, 2000. 18(5): p. 981-6.
5. Banet, A., et al., Thiotepa, busulfan and fludarabine conditioning-regimen is a promising approach for older adult patients with acute lymphoblastic leukemia treated with allogeneic stem cell transplantation. Bone Marrow Transplant, 2023. 58(1): p. 61–67.
6. Mitsuhashi, K., et al., Comparison of Cyclophosphamide Combined with Total Body Irradiation, Oral Busulfan, or Intravenous Busulfan for Allogeneic Hematopoietic Cell Transplantation in Adults with Acute Lymphoblastic Leukemia. Biol Blood Marrow Transplant, 2016. 22(12): p. 2194–2200.
7. Kebriaei, P., et al., Intravenous BU plus Mel: an effective, chemotherapy-only transplant conditioning regimen in patients with ALL. Bone Marrow Transplant, 2013. 48(1): p. 26–31.
8. Gooptu, M., et al., A Comparison of the Myeloablative Conditioning Regimen Fludarabine/Busulfan with Cyclophosphamide/Total Body Irradiation, for Allogeneic Stem Cell Transplantation in the Modern Era: A Cohort Analysis. Biol Blood Marrow Transplant, 2018. 24(8): p. 1733–1740.
9. Zhang, H., et al., Busulfan Plus Cyclophosphamide Versus Total Body Irradiation Plus Cyclophosphamide for Adults Acute B Lymphoblastic Leukemia: An Open-Label, Multicenter, Phase III Trial. J Clin Oncol, 2023. 41(2): p. 343–353.
10. Sakellari, I., et al., Long-term outcomes of total body irradiation plus cyclophosphamide versus busulfan plus cyclophosphamide as conditioning regimen for acute lymphoblastic leukemia: a comparative study. Ann Hematol, 2018. 97(10): p. 1987–1994.
11. Wang, Y.H., et al., Busulfan-containing conditioning regimens in allogeneic hematopoietic stem cell transplantation for acute lymphoblastic leukemia: A Taiwan observational study. Cancer Rep (Hoboken), 2022. 5(3): p. e1488.
12. Rehman, M.E.U., et al., Total Body Irradiation Versus Chemotherapy Conditioning in Pediatric Acute Lymphoblastic Leukemia Patients Undergoing Hematopoietic Stem Cell Transplant: A Systematic Review and Meta-Analysis. Clin Lymphoma Myeloma Leuk, 2023. 23(4): p. 249–258.
13. Friend, B.D., et al., The impact of total body irradiation-based regimens on outcomes in children and young adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer, 2020. 67(2): p. e28079.
14. Lv, M., et al., Outcomes of allogeneic or autologous stem cell transplantation followed by maintenance chemotherapy in adult patient with B-ALL in CR1 with no detectable minimal residual disease. Br J Haematol, 2023. 202(2): p. 369–378.
15. Bazarbachi, A., et al., 20-Year Steady Increase in Survival of Adult Patients with Relapsed Philadelphia-Positive Acute Lymphoblastic Leukemia Post Allogeneic Hematopoietic Cell Transplantation. Clin Cancer Res, 2022. 28(5): p. 1004–1012.
16. Ben Hassine, K., et al., Total Body Irradiation Forever? Optimising Chemotherapeutic Options for Irradiation-Free Conditioning for Paediatric Acute Lymphoblastic Leukaemia. Front Pediatr, 2021. 9: p. 775485.
17. Mardani, M., et al., Total body irradiation-free haploidentical peripheral blood stem cell transplantation compared to related and unrelated donor transplantation in pediatrics with acute lymphoblastic leukemia. Pediatr Blood Cancer, 2023. 70(5): p. e30255.
18. Niu, J., et al., Total Body Irradiation-Based Conditioning Regimen Improved the Survival of Adult Patients With T-Cell Lymphoblastic Lymphoma After Allogeneic Peripheral Blood Stem Cell Transplantation. Cell Transplant, 2022. 31: p. 9636897221108890.
19. Cahu, X., et al., Impact of conditioning with TBI in adult patients with T-cell ALL who receive a myeloablative allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Bone Marrow Transplant, 2016. 51(3): p. 351-7.
20. Dholaria, B., et al., Improved Outcomes of Haploidentical Hematopoietic Cell Transplantation with Total Body Irradiation-Based Myeloablative Conditioning in Acute Lymphoblastic Leukemia. Transplant Cell Ther, 2021. 27(2): p. 171.e1-171.e8.
21. Kebriaei, P., et al., Intravenous Busulfan Compared with Total Body Irradiation Pretransplant Conditioning for Adults with Acute Lymphoblastic Leukemia. Biol Blood Marrow Transplant, 2018. 24(4): p. 726–733.
22. Peters, C., et al., Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J Clin Oncol, 2021. 39(4): p. 295–307.
23. McDonald, A.M., et al., Total Body Irradiation and Risk of Breast Cancer After Blood or Marrow Transplantation: A Blood or Marrow Transplantation Survivor Study Report. J Clin Oncol, 2020. 38(25): p. 2872–2882.
24. Andersen, J., et al., Differential effect of conditioning regimens on cytokine responses during allogeneic stem cell transplantation. Bone Marrow Transplant, 2006. 37(7): p. 635 − 40.
25. Czyz, A. and A. Nagler, The Role of Measurable Residual Disease (MRD) in Hematopoietic Stem Cell Transplantation for Hematological Malignancies Focusing on Acute Leukemia. Int J Mol Sci, 2019. 20(21).
26. Bar, M., et al., Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treatment, 2014. 2014: p. 421723.
27. Shen, Z., et al., Influence of pre-transplant minimal residual disease on prognosis after Allo-SCT for patients with acute lymphoblastic leukemia: systematic review and meta-analysis. BMC Cancer, 2018. 18(1): p. 755.
28. Zhao, X.S., et al., Minimal residual disease status determined by multiparametric flow cytometry pretransplantation predicts the outcome of patients with ALL receiving unmanipulated haploidentical allografts. Am J Hematol, 2019. 94(5): p. 512–521.
29. Shi, Z., et al., Monoclonal antibodies: new chance in the management of B-cell acute lymphoblastic leukemia. Hematology, 2022. 27(1): p. 642–652.
30. Deak, D., et al., Let’s Talk About BiTEs and Other Drugs in the Real-Life Setting for B-Cell Acute Lymphoblastic Leukemia. Front Immunol, 2019. 10: p. 2856.
31. Sun, W. and X. Huang, Role of allogeneic haematopoietic stem cell transplantation in the treatment of adult acute lymphoblastic leukaemia in the era of immunotherapy. Chin Med J (Engl), 2022. 135(8): p. 890–900.
32. Handgretinger, R. and P. Lang, Could (should) we abandon total body irradiation for conditioning in children with leukemia. Blood Rev, 2022. 56: p. 100966.
Acknowledgements
We extend our heartfelt gratitude to all the patients who graciously consented to the use of their samples for this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JEL and ZRL conceptualized and designed the study, carried out data analysis, and were principal contributors in writing the manuscript. LMN played an integral role in data collection and analysis and also participated in drafting the manuscript. FSZ and HMZ undertook the task of editing the manuscript. HY, YDL, MQL, PAM, ZWH, WJL, HY, CX, and ZGX were responsible for data collection. All authors have reviewed and given their approval to the final version of the manuscript submitted for publication.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethics approval statement
The study was approved by the ethics committees of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College according to the guidelines of the Declaration of Helsinki.
Patient consent statement
Written informed consent was obtained for each patients according to federal and institutional guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lv, M., He, Y., Yang, D. et al. Total body irradiation versus chemotherapy myeloablative conditioning in B-cell acute lymphoblastic leukaemia patients with first complete remission. Sci Rep 15, 10079 (2025). https://doi.org/10.1038/s41598-025-94556-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94556-3