Abstract
Sepsis is a severe disorder that is always accompanied by brain injury and dysfunction. This study aimed to evaluate the effects of remimazolam, a new ultra-short-acting sedative, on LPS-induced neuronal injury, and the role of Nrf2 signaling pathway involved. LPS was administered to Sprague-Dawley rats in the presence or absence of remimazolam. Then the behavior analysis was performed by using the Morris Water Maze and Open Field Test. The levels of the Superoxide Dismutase (SOD) and Malondialdehyde (MDA), the neuronal apoptosis, and the expression of Nrf2, HO-1, and Bcl-2 were detected in the hippocampus. In vitro, primary hippocampal neurons were exposed to LPS with or without remimazolam administration. Then the cell viability, apoptosis, mitochondrial membrane potential (MMP), and intracellular ROS were measured to assess oxidative stress and neuron injury. The expression of Nrf2, and HO-1 was also determined by Western blotting. LPS triggered neuroapoptosis, evoked oxidative stress, and inhibited the expression of Nrf2, and HO-1 in rat hippocampus, which were attenuated by remimazolam treatment. Additionally, remimazolam alleviated LPS-induced cognitive dysfunction and anxiety‑like behaviors in rats. In vitro, remimazolam could ameliorate neuronal damage, decrease the production of ROS, and increase the MMP of neurons exposed to LPS, which was accompanied by an increase in the expression of Nrf2 and HO-1. However, ML385 (an Nrf2 inhibitor) reversed the beneficial effects of remimazolam on primary hippocampal neurons. These findings suggest that remimazolam exerted protective effects on LPS-induced hippocampal neuronal injury in vivo and in vitro, which was associated with activation of Nrf2 signaling. Further experiments are needed to fully explore the exact molecular mechanism of Nrf2 upstream and downstream of remimazolam and its effects on distinct brain regions, which will help to better understand the neural effects of remimazolam.
Similar content being viewed by others
Introduction
Sepsis is a severe disorder that represents a dysregulated host response to infection. It triggers cell damage and organ failure, leading to high morbidity and mortality in the emergency surgery and intensive care unit (ICU)1. The progression of sepsis is always accompanied by complications of multiple systems, such as the cardiovascular, respiratory, urinary, and nervous systems, which cause considerable economic and social consequences1,2. The central nervous system (CNS) is very vulnerable to sepsis3. It is reported that many sepsis survivors suffer from significant, persistent cognitive dysfunction or psychological disorders, including memory and attention impairment, depression, and anxiety, probably due to brain injury induced by sepsis4,5,6.
During inflammatory responses, cell damage is always associated with excess oxidative stress, mainly characterized by an imbalance between high levels of reactive oxygen species (ROS) and the capacity of the antioxidant defense system7,8. Oxidative stress served as one of the major events that participated in neuronal injury and neuroinflammation8. ROS is a normal metabolic product of redox reactions. The excess production of ROS would damage the normal morphology and physiological process of cells, leading to metabolic disturbance and dysfunction of the cells via peroxidation of lipids, proteins, mitochondria, and DNA9,10. Therefore, modulation of oxidative stress is considered a key point of inflammatory responses.
Nuclear factor erythroid-2 related factor 2 (Nrf2) is a key regulator of antioxidant response and acute/chronic inflammation process, and emerged as a potential therapeutic target for oxidative stress-related inflammatory disorders11,12,13. Nrf2 regulates the expression of antioxidant enzymes such as heme oxygenase-1 (HO-1) under oxidative stress14. Increased HO-1 expression provides a protective effect against high levels of ROS, thus influencing the course of cell injury15. It is reported that activation of the Nrf2/HO-1 signaling pathway could attenuate oxidative stress-related neuronal death and brain injury after intracerebral hemorrhage in rats16. Another study demonstrated that Nrf2/HO-1 exerted neuroprotective effects against cerebral ischemia damage may be achieved by the instigation of antioxidant mechanisms17. Therefore, activation of the Nrf2/HO-1 signaling pathway was a benefit for counteracting the intracellular production of reactive oxygen species (ROS) and conferring neuroprotection15,18,19. The antioxidant effect of Nrf2 can further regulate the polarization of microglia, that is, inhibit the pro-inflammatory M1 polarization and promote the anti-inflammatory M2 polarization20, thereby inhibiting neuroinflammatory response and improving brain dysfunction21,22. Furthermore, the activation of the Nrf2 signaling pathway has been demonstrated to reverse the reduction of tight junction protein expression induced by LPS, leading to an improvement in the blood-brain barrier’s high permeability23.
Septic patients in the perioperative period or in the ICU who receive multiple treatments, including mechanical ventilation, often require sedation. It is of great significance to choose sedative drugs that do not compromise respiratory and circulatory functions, exacerbate neurological damage in septic patients, or even confer potential benefits on brain function. Benzodiazepines are commonly used for sedation in clinical practice. Midazolam, one of the classic benzodiazepines, is converted to the active metabolite that extends the duration of its effects. The prolonged recovery period after midazolam infusion in ICU patients may be due to the accumulation of active metabolites or alterations in kinetics24. Thus, its effects are often unpredictable, and it may necessitate increased caution with prolonged use. Moreover, the use of midazolam for induction of anaesthesia remains restricted in patients exhibiting impaired haemodynamics25. Remimazolam is a new ultra-short-acting benzodiazepine, currently under development for intravenous use in procedural sedation and general anesthesia. In contrast to midazolam, the metabolite of remimazolam is inactive, and higher doses or long-term infusion is unlikely to result in accumulated and prolonged effects26. Meanwhile, remimazolam provides faster onset and offset of sedation than midazolam27 and has obvious advantages in low liability for cardiovascular depression, respiratory depression, and injection pain, which facilitates its use as a sedative agent in the intensive care unit and in the perioperative period.
Fang et al.28demonstrated that remimazolam could suppress the inflammatory response and attenuate sepsis-associated acute liver injury and pathological changes. Other study results show that remimazolam decreased LPS-induced lung and liver damage, and increased the survival rate of endotoxemia mice by inhibiting the release of pro-inflammatory cytokines29. Therefore, it seems that remimazolam is more applicable for septic patients applied in general anesthesia and ICU sedation, and has a benefit for septic-induced organ damage. LPS is known as a pro-inflammatory component of Gram-negative bacteria, promoting various forms of neuronal injury and death in vitro and in vivo in rodent models30,31,32,33. A recent study showed that remimazolam activated the Nrf2 pathway, thereby attenuating oxidative stress and apoptosis to protect against brain I/R injury34, which has a similar pathological process to the brain damage caused by sepsis. Additionally, it showed that midazolam, another benzodiazepine, could ameliorate liver oxidative stress by activating the Nrf2 pathway35. Therefore, we hypothesised that the neuroprotective effects of remimazolam might be related to the Nrf2 pathway.
The CNS is susceptible to sepsis damage, leading to memory and cognitive impairment. The effects and mechanisms of remimazolam on sepsis-induced brain damage need to be further explored. In this study, we explore the effects of remimazolam on LPS-induced neuronal injury in vitro and in vivo, and whether Nrf2 signaling pathways is involved in the neuroprotection of remimazolam, to provide data to support the rational use of remimazolam in septic patients and to offer new options for optimizing sedation protocols in the perioperative period and ICU.
Methods
Animals and drug treatment
Male Sprague-Dawley rats (aged 4–5 weeks, weighing 120∼160 g) were obtained from the laboratory animal center of Guangxi Medical University. All rats were allowed to acclimate for 1 week before experiments (22–25 °C, relative humidity 55–60%, 12/12 light/dark), with free water and diet. All procedures were approved by the Animal Use and Care Committee of Guangxi Medical University (No.202109015) and were performed in accordance with The Guide for Care and Use of Laboratory Animals released by the National Institutes of Health and were reported in accordance with ARRIVE guidelines.
The experiments in vivo consisted of two parts as shown in Fig. 1.
Experiment procedure in vivo. In experiment 1, the rats were injected intraperitoneally with vehicle saline,1, 2, or 5 mg/kg LPS, respectively. In experiment 2, the rats were administrated with LPS, followed by intraperitoneal injection with or without 5, 10, or 20 mg/kg remimazolam. After 24 h of LPS administration, Morris Water Maze (MWM) consisted of swimming training phases and a probe trial were performed. Subsequently, the Open Field Test (OFT) was conducted. Then the rats were sacrificed and the brain was immediately isolated for Tunel, SOD and MDA, and Western Blot analysis.
Experiment 1: The rats were injected intraperitoneally with 1, 2, 5 mg/kg LPS (Sigma, USA, dissolved in saline)36,37,38 or normal saline, and divided into LPS1, LPS2, LPS5 and Control groups, to observe the effects of different doses of LPS on brain function in rats and to determine the appropriate dose of LPS that caused brain damage in our study. After 24 h of LPS administration, the rats were used for behavioral analyses. And then, the rats were euthanized by deep anesthesia with isoflurane followed by cervical dislocation. Based on the results of behavioral analyses, the LPS dose causing significant brain damage was selected for Experiment 2.
Experiment 2: The dose of LPS used in Experiment 2 was 5 mg/kg. The rats were administrated with LPS, followed by intraperitoneal injection with or without 5, 10, or 20 mg/kg remimazolam (HengRui Medicine Co., Ltd., China, dissolved in saline), respectively, or normal saline, and then divided into LPS, LR5, LR10, LR20, and Control groups. The dose gradients of 5, 10, and 20 mg/kg were selected according to the ED50 of remimazolam in rats39 and other studies on the organ-protective effects of remimazolam40. After 24 h of LPS and remimazolam administration, the rats were used for behavioral analyses. When the behavioral analyses were completed, the rats were euthanized by deep anesthesia with isoflurane followed by cervical dislocation. Rat brains were then immediately isolated for further analysis. It is noticed that an escalation in dosage results in a concomitant increase in both the sedative effect and respiratory depression of remimazolam. Consequently, the consciousness and respiration of rats throughout the experimental process were meticulously monitored. Before conducting behavioral experiments, the rats were ensured to be conscious, on a normal diet, and free of cyanosis.
Behavioral analyses
Animal behavior analysis was performed by using Morris Water Maze (MWM) and Open Field Test (OFT). The assessors were blinded to the group allocation of the animals throughout the experiment. Before the behavior test, the rats were placed in the behavior test room for a week for environmental acclimation, and environmental factors not involved in the test, like temperature, noise, and light intensity, were controlled. The MWM was conducted 24 h after LPS administration. The water temperature was set to 22–24℃. A quantity of 100 g of black edible dye was added to the water, after which no reflected light was evident on the water’s surface, thus ensuring optimal conditions for capturing the track route of the white rats by a camera positioned above the water maze equipment. MWM consisted of three phases (training with a visible platform, training with a hidden platform, and a probe trial). During training with a visible platform, the water maze equipment should be filled with water and the platform was kept above water. The rats were trained four times (90 s each time) with a visible platform in a circular tank of water for 1 day. This was followed by the hidden platform training period, during which the rats were required to navigate in the circular tank and then locate and climb onto a hidden platform. Rats were trained for 4 days with 4 trials/day (90 s/trial) and the training trial was completed when the rat mounted and remained on the platform for 2 s or spent 90 s in the pool. Next, the probe test was performed. In the probe trial, the platform in the target quadrant was removed, and the rat was allowed to swim freely for 90 s. The escape latency (seconds), the time spent in the platform quadrant (seconds), and the number of platform quadrant crossings were analyzed to assess spatial learning and memory.
The OFT was used to investigate effect of LPS and remimazolam on anxiety-like behaviors. The OFT was conducted in a black box (100 × 100 × 40 cm) to evaluate the spontaneous and exploration abilities of the rats. Each rat was placed in the center of the box. The rats’ activity(movements) was automatically recorded using a video tracking system. Each test lasted 5 min. The total distance (cm), frequency of crossing and rearing, and the time spent (s) in the central area of each rat were recorded. After the completion of the experiment by each rat, the black box was meticulously cleansed with 75% alcohol, and then permitted to dry before its subsequent utilisation for the evaluation of the next rat.
Tunel assay
Terminal Deoxynucleotidyl Transferase‑Mediated dUTP‑Biotin Nick End Labeling (TUNEL) Assay was used to label the cut ends of DNA fragments in apoptotic nuclei. The effect of remimazolam on LPS-induced neuronal apoptosis was evaluated by TUNEL assay, using an In Situ Cell Death Detection Kit (Roche, Switzerland) according to the manufacturer’s instructions. The nuclei of all cells were stained with DAPI. Fluorescence microscopy was employed to identify neuronal apoptosis of hippocampal CA1 and DG regions in the brain section.
Measurement of superoxide dismutase (SOD) and malondialdehyde (MDA)
The rat hippocampus was suspended in PBS to prepare a tissue homogenate and then centrifuged at 12,000×g for 15 min at 4 ◦C. The supernatant was collected directly. SOD activity and MDA level in tissue homogenate of the hippocampus were measured by using the SOD and MDA assay kit (Nanjing Jiancheng, China) according to the manufacturer’s procedure. The absorbance of each sample at 450 nm (SOD) or 532 nm (MDA) was measured using a microplate reader. Total SOD activity of tissue homogenate (U/mgprot) = (control OD value -measured OD value)/control OD value ×2× total volume of reaction solution (ml)/sample volume (ml)/protein content at the same homogenate concentration (mgprot/ml). MDA content in tissue homogenate (nmol/mgprot) = (measured OD value - control OD value)/(standard OD value - blank OD value) × concentration of standard substance (10nmol/ml) /concentration of protein in the sample to be tested (mgprot/ml).
Primary neuronal culture and drug treatments
Primary neuronal culture was performed according to previous studies with some modifications41 Neonatal 1-day-old SD rats were euthanized by deep anesthesia with isoflurane followed by decapitation. The rat brain was removed, and then the hippocampus was quickly isolated and transferred to Dulbecco ‘s modified eagle ‘s medium (DMEM) (Invitrogen, USA). The tissues were mechanically dissociated, and then digested with 0.25% trypsin (Invitrogen, USA) for 25 min at 37°C. After termination of digestion, the mixture was gently stirred with a pipette to obtain a single-cell suspension. The suspension was then filtered through a 400 mesh cell filter and centrifuged for 5 min. Single cells were resuspended in DMEM containing 10% fetal bovine serum (FBS), 2 g/l HEPES, penicillin g (100 U/ml), and 100 g/ml streptomycin (Invitrogen, USA) and plated on polylysine-coated plates. After 24 h, the medium was replaced with a maintenance medium containing 96% neurobasal medium supplemented with 2% B27 (50×, Gibco®; Thermo Fisher Scientific Inc. USA), 1% 200 mM glutamine (100×, Sigma-Aldrich Chemical Co. USA), and 1% penicillin/streptomycin (100×, Beijing Solarbio Science & Technology Co. China), and half of the maintenance medium was replaced twice every 7 days. All experiments were performed at 7 days in vitro (DIV 7). All procedures were approved by the Animal Use and Care Committee of Guangxi Medical University (No.202109015) and were performed in accordance with The Guide for Care and Use of Laboratory Animals released by the National Institutes of Health and were reported in accordance with ARRIVE guidelines.
The experiments in vitro consisted of three parts as shown in Fig. 2A.
Experiment procedure in vitro and primary hippocampal neurons identification. (A) Experimental design is illustrated schematically. (B) Primary hippocampal neurons at 7 days in vitro (DIV 7) were subjected to identification and purity analyses using immunofluorescent staining with MAP2 (green), NeuN (red), and DAPI (blue). The scale bar represents 100 μm.
Experiment 1: The cells were incubated with 0.1, 1, 10, or 100 µM LPS (Sigma, USA, dissolved in PBS) for 24 h, respectively (LPS0.1, LPS1, LPS10, and LPS100 group), or fresh maintenance medium (Control group). Then the cells were harvested and used for the CCK-8 assay and Flow cytometry analysis, to determine the optimal concentration of LPS in this study.
Experiment 2: To explore the effects of remimazolam on neuronal injury induced by LPS, the cells were exposed to 10 µM LPS for 24 h (LPS group), or alternatively for 0.5 h, followed by incubation with or without 1, 5, 10, 20, 50 µM remimazolam (HengRui Medicine Co., Ltd., China, dissolved in saline) for 24 h (LR1, LR5, LR10, LR20, and LR50 group), or with fresh maintenance medium for 24 h (Control group). The concentration gradients of remimazolam were selected according to the studies on the protective effects and pharmacology of remimazolam in vitro42,43. Then the cells were harvested and used for further analysis.
Experiment 3: The Nrf2 inhibitor ML385 (Selleck, USA) was dissolved in 5 µM DMSO (Sigma, USA). The cells were exposed to 5 µM ML385 for 0.5 h, after which 10 µM LPS was administered, followed by 20 µM remimazolam for 24 h (LRM group). The cells were either exposed to remimazolam in the presence of LPS (LR group) or were given only remimazolam (R group), LPS (LPS group), DMSO (DMSO group) or fresh maintenance medium (Control group) for 24 h, respectively. The establishment of a DMSO group was driven by the necessity to eliminate any potential effects that DMSO might have on cells. Then the cells were harvested and used for further analysis to investigate whether the Nrf2 pathway was involved in the neuroprotective effects of remimazolam.
Hippocampal neuron identification
Cells were plated on poly-lysine-coated coverslips and fixed with 4% paraformaldehyde (Beijing Solarbio Science & Technology Co., China) for 40 min at room temperature. Then the cells were permeabilized with 0.1% Triton X-100 (Beijing Solar Science & Technology Co., China) and blocked with 5% goat serum (Beijing Solar Science & Technology Co., China) for 30 min, followed by incubation at 4 °C overnight with the primary antibody against microtubule-associated protein 2 (MAP2), neuron-specific nuclear protein (NeuN) (dilution: 1:200). After incubation with FITC/TRITC-conjugated secondary antibodies for 2 h at room temperature, the cell was visualized under a fluorescence microscope. Additionally, 2(4amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) was also applied for nuclear staining.
Cell viability assay
Cell viability was measured using Cell Counting Kit-8 (CCK-8, Beyotime, China), according to the manufacturer’s instructions. In brief, neurons were inoculated with 96-well plates. The medium was removed and then 10 µL/100 µL medium CCK-8 assay reagents were added to each well. Incubate at 37 °C in a 5% CO2 incubator for 1.5 h. Optical density (OD) was measured at 450 nm under a microplate spectrophotometer (Thermo Fisher Scientific, USA) for calculating the relative cellular activity.
Flow cytometry analysis
Cell apoptosis was quantified by flow cytometry using the Annexin V-APC/7‐AAD double‐staining. The neurons were harvested, washed with cold phosphate‐buffered saline (PBS), centrifuged, and resuspended with 1 × binding buffer. The neurons were added with 5 µL Annexin V‐APC and 10 µL7‐AAD (MultiSciences Biotech, China) staining solution and incubated for 10 min at room temperature in the dark, before being analyzed by flow cytometry (BD Biosciences, USA). Cells with Annexin V‐APC+/7‐AAD − staining were identified as cells in early‐stage apoptosis, whereas cells with Annexin V‐APC+/7‐AAD + staining were identified as cells in late‐stage apoptosis or necrosis. The apoptosis rate was determined by the ratio of V‐APC+/7-AAD + cells and V‐APC +/ 7-AAD − cells to the total number of cells.
ROS assay
2,7-Dichlorofluorescein-diacetate (DCFH-DA) assay was used to analyze intracellular ROS levels. DCFH-DA assay is based on the ROS-dependent oxidation of DCFH to the highly fluorescent compound 20,70-dichlorofluorescein (DCF). The primary hippocampal neurons were incubated with DCFH-DA (10 µM) (Beyotime Institute Biotechnology, China) at 37 ◦C in the dark for 30 min. The neurons were washed three times with a serum-free cell culture medium to sufficiently remove the DCFH-DA, followed by being measured by flow cytometry (BD Biosciences, USA). ROS level was evaluated by detecting the fluorescence amount of FITC channel.
Mitochondrial membrane potential measurement
The mitochondrial membrane potential (MMP) of cells was measured using a JC-1 mitochondrial membrane potential assay kit (Beyotime Institute Biotechnology, China) according to the manufacturer’s instructions. JC-1 is a dual-emission membrane potential-sensitive probe that exists as a green fluorescent monomer at a low MMP and forms aggregates with red/orange fluorescence at a high MMP. The neurons were seeded in a 24-well plate and stained with JC‐1 via incubation in the working solution for 30 min. The change in fluorescence at 488/530 nm (green) and 549/595 nm (red) was monitored by high-content screening. The loss of MMP was reflected by the ratio of aggregates (red fluorescence) to monomer (green fluorescence).
Transmission electron microscopy (TEM)
The neurons were harvested in 1 mm3 and fixed in ice-cold 2.5% glutaraldehyde. Next, samples were dehydrated in ethanol (with 3% uranyl acetate), and embedded in a mixture of epoxy resin and propylene oxide for 24 h. After a 70-nm-thick sample section was stained with lead citrate, the neuronal morphology was detected under a transmission electron microscope (HITACHI H-7650).
Western blot analysis
The Western blot assay was performed as described in our previous study44.The collected tissues or cells were lysed with lysis buffer (Protein Biotechnology, China) containing, RIPA, PMSF and protease inhibitors (Sigma-Aldrich, USA), followed by centrifugation for 10 min. The supernatant was collected and the protein concentration was determined using the BCA protein kit (Beyotime Institute Biotechnology, China). The proteins obtained were separated on 12% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes (Immobilon-P, Millipore, Switzerland) by the wet transfer method, followed by blocking the membranes with 5% skim milk powder for 1 h. Subsequently, the membranes were incubated at 4 °C overnight with primary antibodies against Nrf2 (1:1000 dilution; Cell Signaling Technology, Inc., USA), HO-1(1:1000 dilution; Cell Signaling Technology, Inc., USA), Bcl-2(1:1000 dilution; Cell Signaling Technology, Inc., USA), and β-actin (1:1000 dilution; Cell Signaling Technology, Inc., USA). The membranes were followed by incubation with fluorescent secondary antibody (1:10000 dilution) (LI-COR Biosciences, USA) for 1 h at room temperature. Images were obtained using the Odyssey system (LI-COR Biosciences). The relative expression of target proteins was expressed as the ratio of the gray value of each target protein band to the gray value of the internal reference band. The final statistical results were presented as the ratio of the relative expression of the proteins in the treatment group to the relative expression of the proteins in the Control group.
Statistical analysis
IBM SPSS software version 18.0 was used for data analysis. All data are expressed as the mean ± standard deviation. The normality of the data distribution was tested by the Shapiro-Wilk normality test. Homogeneity of variance was evaluated by the Brown-Forsythe test. Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test (only if F was significant and there was no significant variance inhomogeneity), or Kruskal-Wallis test with Dunn’s post hoc test (if the data did not conform to a normal distribution) for multi-group comparisons. P < 0.05 was considered statistically significant.
Results
LPS led to cognitive dysfunction and anxiety‑like behaviors in rats
The Morris water maze (MWM) test, a widely used measure of learning and memory, was used to evaluate cognitive impairment. Exploratory activity and anxiety-like behavior were evaluated by the Open Field Test (OFT). During the training phase of MWM, the escape latency time of rats in the LPS groups was gradually decreased (Fig. 3A), but the reduction was not as great as that of the Control group. In the probe trial, rats exposed to LPS showed less time spent in the target quadrant and platform crossover number (P < 0.05) (Fig. 3B and C). Moreover, among the three LPS groups, the LPS5 group had the largest decrease in time spent in the target quadrant and platform crossover number (P < 0.05) (Fig. 3B and C). It indicated that LPS led to learning and memory impairments. The results of OFT showed that both of the total distance, the time spent in the center, frequency of crossing and rearing were significantly decreased in the LPS5 group as compared to the Control group (P < 0.05) (Fig. 3D and E), which reflects the fact that 5 mg/kg LPS caused anxiety‑like behaviors in rats. Based on the results of MWM and OFT, 5 mg/kg LPS was used for the subsequent experiments to evaluate the neuroprotection of remimazolam in rats.
LPS caused cognitive dysfunction and anxiety-like behaviors in rats. (A,B) The Morris Water Maze (MWM) test was used to evaluate cognitive impairment. The results showed that (A) the reduction in the escape latency time of rats in the LPS groups was not as great as in the Control group, and (B) the time spent in the target quadrant and platform crossover number in the LPS groups were decreased, suggesting LPS-induced cognitive impairment. (C) Representative swimming traces of rats during probe trials of MWM test. (D) The Open Field Test (OFT) was used to investigate effect of LPS on anxiety-like behaviors. The total distance, the frequency of rearing, the time spent in central zone, and the frequency of crossing were reduced in the rats treated with LPS, suggesting that LPS induced anxiety-like behavior. (E) The representative tracking images of rats in the OFT. Results are shown as means ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001.
Remimazolam attenuated LPS-induced behavioral deficits in rats
To investigate the effects of remimazolam on LPS-induced behavioral changes in rats, MWM and OFT were conducted. As shown in Fig. 4A, the escape latency time of rats in the LR10 groups was shortened during the training phase of MWM, which was similar to the Control group. Treatment with 10 mg/kg remimazolam markedly increased the time spent in the target quadrant and the platform crossover compared with the administration of LPS in the probe trial (P < 0.05) (Fig. 4B and C). In addition, the time spent in the target quadrant in the LR20 group was found to be shorter than that in the LR10 group (P < 0.05) (Fig. 4B and C). In OFT, the total distance, the time spent in the center, frequency of crossing and rearing in the LR10 group were significantly increased (P < 0.05) when compared to the LPS group (Fig. 4D and E). The time spent in the centre of the LR5 and LR20 groups was found to be less than that of the LR10 group. (P < 0.05) (Fig. 4D and E). Although there were no differences in the above parameters in the MWM and OFT in the LR5 or LR20 groups compared to the LPS group, it showed a trend of elevation. The results revealed that remimazolam could ameliorate the anxiety-like behavior, exploratory activity impairment, and spatial and learning behavior impaired by LPS administration.
Remimazolam attenuated LPS-induced cognitive dysfunction and anxiety-like behaviors. (A,B) The Morris Water Maze (MWM) test results showed that (A) treatment with remimazolam shortened the escape latency time of rats, and (B) markedly increased the time spent in the target quadrant and the platform crossover, suggesting an ameliorative effect of remimazolam on LPS-induced cognitive impairment. (C) Representative swimming traces of rats during probe trials of MWM test. (D) An increase in total distance, frequency of rearing, time spent in the central zone and frequency of crossing in the Open Field Test (OFT) indicated a positive effect of remimazolam on LPS-induced anxiety-like behaviors. (E) The representative tracking images of rats in the OFT. Results are shown as means ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001.
Remimazolam reversed neuroapoptosis induced by LPS in rat hippocampus
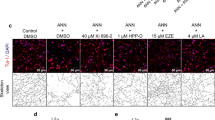
The impact of remimazolam on neuronal apoptosis in the hippocampus was evaluated by using TUNEL assay. Compared to the Control group, the proportion of apoptotic neurons was enhanced significantly in the hippocampal CA1 and DG regions after LPS administration, whereas both 5, 10 and 20 mg/kg remimazolam treatment markedly decreased LPS-induced neuronal apoptosis (P < 0.05) (Fig. 5A, B and C). Furthermore, the results revealed that a dose of 10 mg/kg of remimazolam exhibited the most potent inhibitory effect on apoptosis, in comparison with doses of 5 and 20 mg/kg (P < 0.05) (Fig. 5A, B and C).
Remimazolam inhibited neuroapoptosis and oxidative stress induced by LPS in rat hippocampus. (A) Neuronal apoptosis in the hippocampus was determined by TUNEL assay. Brain Sections are stained with TUNEL (green) and counterstained with DAPI (blue). The scale bar represents 50 μm. (B,C) Remimazolam decreased the rate of apoptotic neurons (TUNEL-positive cell) in the DG and CA1. (D,E) The levels of Superoxide Dismutase (SOD) and Malondialdehyde (MDA) in the hippocampus were measured to assess the role of remimazolam on LPS-induced oxidative stress. Results are shown as means ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001.
Then we analyzed the expression of the apoptosis-related protein Bcl-2 in rat hippocampus. The results of western blot revealed that the expression of Bcl-2 was lower in the LPS group in comparison with the Control group (P < 0.05), whereas 10 and 20 mg/kg remimazolam treatment greatly ameliorated the decrease of Bcl-2 level in LPS-induced rats (P < 0.05) (Fig. 6A and B). In addition, the results showed that a dose of 10 mg/kg of remimazolam led to a greater increase in Bcl-2 levels than a dose of 20 mg/kg of remimazolam (P < 0.05) (Fig. 6A and B). These results indicated that the pro-apoptosis effects of LPS were significantly reversed by the treatment with remimazolam in rat hippocampus.
Remimazolam mitigated the decrease of Bcl2, Nrf2 and HO-1 expression in LPS-treated rat hippocampus. (A) The expression of Bcl2, Nrf2 and HO-1 were detected by Western blotting assay and quantified by densitometry. (B–D) The quantification analysis of Bcl2, Nrf2 and HO-1 in the hippocampus. The final statistical results were presented as the ratio of the relative expression of the proteins in the treatment group to the relative expression of the proteins in the Control group. Results are shown as means ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001.
Remimazolam elevated SOD level and reduced MDA level in LPS-treated rat hippocampus
Excessive oxidative response is one of the most important driving forces in LPS-induced neurotoxicity. The levels of SOD and MDA reflect the levels of oxidative stress. To determine the effect of remimazolam on LPS-evoked oxidative stress in the hippocampus, we measured the levels of SOD and MDA with assay kits. The results were shown in Fig. 5D and E. The MDA level in LPS group was higher than that in Control group, while the SOD level was lower (P < 0.05). The 5, 10, and 20 mg/kg doses of remimazolam were found to remarkably reduce the MDA levels (P < 0.05). However, a statistically significant reduction in MDA was not observed when these three doses were compared (P > 0.05). The data also revealed that the administration of remimazolam in doses of 5, 10 and 20 mg/kg resulted in a significant increase in the SOD level in comparison with LPS administration (P < 0.05). Of note, the 10 mg/kg dose of remimazolam elicited a more pronounced response in SOD elevation than the 5 mg/kg and 20 mg/kg doses (P < 0.05).
Remimazolam mitigated the decrease of Nrf2/HO-1 in LPS-treated rat hippocampus
Western blot was used to investigate the effects of remimazolam on the expression of Nrf2 and HO-1, known to be important in antioxidant response, cell survival, and apoptosis, after LPS exposure in rat hippocampus. A statistically significant difference between groups for Nrf2 and HO-1 was shown in Fig. 6. The expression of Nrf2 and HO-1 in the LPS group were decreased than that in the Control group (P < 0.05). Compared to the LPS exposure, both the Nrf2 and HO-1 protein levels increased in rats treated with 10 mg/kg of remimazolam (P < 0.05).
Remimazolam ameliorated the morphology damage of primary hippocampal neurons induced by LPS
In the present study, immunofluorescent staining with microtubule-associated protein 2 (MAP2, a neuron marker), neuron-specific nuclear protein (NeuN), and DAPI were used for identification and purity analyses of primary hippocampal neurons (Fig. 2B). Under a fluorescence microscope, MAP2 staining showed that neuronal cytoskeletal extensions overlapped each other. The purity of primary hippocampal neurons was 95.4%±2.1% by measuring the rate of NeuN and DAPI staining overlap. As shown in Fig. 7, TEM revealed that normal cellular morphology was observed in the primary hippocampal neurons in the Control group. The mitochondria were round, dumbbell or rod shaped, and the mitochondrial ridge was visible. After being exposed to LPS, primary hippocampal neurons displayed a large amount of vacuolar degeneration, chromatin margination, and cell shrinkage. There were fewer and more scattered mitochondria that could be found. The ridge of mitochondria disappeared obviously. Treatment with remimazolam significantly alleviated the LPS-induced neuronal morphology damage, with fewer degenerating vacuoles and more visible mitochondrial ridges observed under TEM.
Remimazolam ameliorated LPS-induced morphology damage of primary hippocampal neurons. The transmission electron microscopy (TEM) images showed neuronal damage induced by LPS in the hippocampus CA1 region, which manifested as extensive vacuolar degeneration, chromatin margination, and cell shrinkage. Remimazolam improves these morphological changes. The scale bar represents 200 nm.
LPS led to neuronal damage and accumulation of ROS
The cell viability and the apoptosis rates of primary hippocampal neurons were detected to evaluate neuronal damage induced by LPS. The primary hippocampal neurons were treated with LPS. The results revealed that LPS at concentrations of 0.1, 1, 10, 100 µM reduced the viability of neurons (P < 0.05) (Fig. 8A). Similarly, Flow Cytometry assay revealed that LPS exposure led to an increased apoptotic rate of primary hippocampal neurons (P < 0.05) (Fig. 8B and C). The apoptotic rate was higher in cells admisnistrated by 10, 100 µM LPS than that by 0.1 µM LPS (P < 0.05) (Fig. 8B and C). In addition, the level of intracellular ROS was evaluated by the changes in DCF fluorescence intensity using flow cytometry, which showed that 1, 10, 100 µM LPS caused the ROS burst in primary hippocampal neurons (P < 0.05) (Fig. 8D). In particular, 10 and 100 µM LPS resulted in a more than 1-fold increase in intracellular ROS levels. There was no difference between 10 and 100 µM LPS in the apoptotic rate and intracellular ROS level (P > 0.05). These results suggested that LPS led to neuronal damage and accumulation of ROS. 10 µM LPS was select for the subsequent experiments to evaluate the neuroprotection of remimazolam in vitro.
LPS exposure led to a decrease in cell viability, an increased apoptotic rate and an accumulation of intracellular ROS in primary hippocampal neurons. (A) The cells were incubated with 0.1, 1, 10, or 100µM LPS for 24 h, respectively. The cell viability of primary hippocampal neurons was measured using Cell Counting Kit-8 (n = 6). (B,C) The neuronal apoptosis was quantified by flow cytometry. The apoptotic rate of primary hippocampal neurons was determined by the ratio of V-APC+/7-AAD + cells and V‐APC +/ 7-AAD − cells to the total number of cells (n = 3). (D) The levels of intracellular ROS were detected by flow cytometry using 2,7-Dichlorofluorescein-diacetate (DCFH-DA) staining (n = 3). Results are shown as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Remimazolam ameliorated apoptosis and the decrease in the viability of primary hippocampal neurons induced by LPS
The primary hippocampal neurons were treated with LPS with or without treatment with different concentrations of remimazolam, as the drug treatment described above. Then the viability and apoptosis of primary hippocampal neurons was detected by the CCK-8 assay and flow cytometry assay with Annexin V-APC+/7‐AAD − staining, respectively. The results revealed that treatment with remimazolam at the concentration from 5µM to 50µM had a lower apoptotic rate compared to that treated with LPS (P < 0.05) (Fig. 9A and B). And 5, 10, 20, and 50µM remimazolam exerted similar anti-apoptotic effects (P > 0.05). However, there was no effect of 1µM remimazolam on neuroapoptosis induced by LPS (P > 0.05). CCK-8 assay revealed that 10 µM, 20 µM, and 50 µM remimazolam treatment increased neuron viability (P < 0.05). No significant difference in neuronal viability was found between LPS, 1µM and 5µM remimazolam treatment (P > 0.05) (Fig. 9C).
Remimazolam ameliorated LPS-induced neuroapoptosis, accumulation of intracellular ROS and increased cell viability. (A–C) The cells were exposed to 10 µM LPS for 24 h (LPS group), or alternatively for 0.5 h, followed by incubation with or without 1, 5, 10, 20, 50 µM remimazolam. Cell apoptosis was quantified by flow cytometry using the Annexin V-APC/7‐AAD double‐staining and cell viability was measured using Cell Counting Kit-8. The results indicated (B) remimazolam reduced the apoptotic rate of primary hippocampal neurons (n = 3) and (C) increased neuronal viability (n = 6). (D) ROS levels were assessed by detecting the amount of fluorescence in the FITC channel using the Dichlorofluorescein-diacetate (DCFH-DA) assay (n = 3). Remimazolam decreased the intracellular ROS level. Results are shown as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Remimazolam relieved the accumulation of ROS and the decrease in the MMP induced by LPS
In this study, the DCFH-DA assay was used to measure the effects of remimazolam on LPS-induced ROS production. Treatment of remimazolam at concentrations of 5µM, 10µM, 20µM and 50µM decreased the level of ROS production compared with that exposed to LPS (P < 0.05) in the primary hippocampal neurons. However, no statistically significant difference was observed in the reduction of intracellular ROS levels caused by remimazolam in the concentration range of 5 µM to 50 µM (P > 0.05) (Fig. 9D). In addition, 1µM remimazolam could not attenuate the accumulation of ROS induced by LPS (P > 0.05) (Fig. 9D). It suggested that this effect seemed to be not dose-dependent, as 50µM remimazolam did not exert the greatest ability to inhibit ROS production.
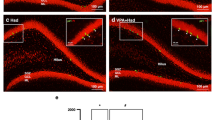
Mitochondrial dysfunction was considered to be a reason for the continuous production of ROS. And excessive production of ROS also causes damage to mitochondria with the loss of MMP. We next evaluated the MMP in primary hippocampal neurons. The results indicated that LPS caused a reduction of MMP in neurons (P < 0.05) (Fig. 10). Treatment with remimazolam at the concentration of 10µM, 20µM, and 50µM, but not at 1µM and 5µM significantly reversed the reduction of MMP after LPS exposure (P < 0.05) (Fig. 10). In comparison with the 10 µM remimazolam concentration, the 20 µM and 50 µM concentrations exhibited enhanced efficacy in increasing MMP (P < 0.05) (Fig. 10).
Remimazolam attenuated LPS-induced reduction of the mitochondrial membrane potential (MMP). (A) The MMP of cells was measured using a JC-1 assay. The representative images of JC-1 aggregates and JC-1 monomers reflexed the MMP of primary hippocampal neurons. The scale bar represents 200 nm. (B) The ratio of JC-1 aggregates to JC-1 monomers was increased in neurons administrated with remimazolam, suggesting that remimazolam could improve MMP. Results are shown as means ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Remimazolam mitigated the down-regulation of Nrf2, and HO-1 induced by LPS in primary hippocampal neurons
Western blot was used to test the expression of Nrf2/HO-1 signaling pathway in response to remimazolam against LPS-induced neurotoxicity in primary hippocampal neurons. A significantly lower Nrf2 and HO-1 levels were observed in the neurons exposed to LPS, compared with the control neurons (P < 0.05) (Fig. 11). LPS-induced inhibition of Nrf2 and HO-1 expression were rescued to varying degrees by 10µM to 50µM remimazolam (P < 0.05) (Fig. 11). The higher concentrations of remimazolam, such as 20µM and 50µM, exert a greater ability to influence the expression of the factors mentioned above than 10µM (P < 0.05) (Fig. 11).
Remimazolam mitigated the decrease of Nrf2 and HO-1 expression in primary hippocampal neurons. (A) The expression of Nrf2 and HO-1 were detected by Western blotting assay and quantified by densitometry. (B) The quantification analysis of Nrf2 and HO-1 in primary hippocampal neurons. The final statistical results were presented as the ratio of the relative expression of the proteins in the treatment group to the relative expression of the proteins in the Control group. Results are shown as means ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Nrf2 inhibitor reversed the protective effects of remimazolam in primary hippocampal neurons
ML385 (an Nrf2 inhibitor) was administered in primary hippocampal neurons exposure to LPS 1 h before the administration of remimazolam to investigate whether remimazolam reversed the neurotoxicity induced by LPS via Nrf2 pathway. As shown in Fig. 12A, the remimazolam treatment markedly reduced the higher apoptosis rate of primary hippocampal neurons observed in LPS group, while this effect was significantly reversed by the administration with ML385 (P < 0.05). DCFH-DA assay revealed that remimazolam attenuated LPS-induced ROS burst in primary hippocampal neurons (P < 0.05). However, inhibition of Nrf2 with ML385 reversed the effect of remimazolam, which led to an increase in intracellular ROS level as compared to LR group (P < 0.05) (Fig. 12B). Moreover, MMP in LR group was higher than that in the LPS group in primary hippocampal neurons (P < 0.05) (Fig. 13). In contrast, remimazolam-induced higher MMP was markedly ameliorated by ML385 (P < 0.05) (Fig. 13). Western blot showed that remimazolam up-regulated the expression of Nrf2, HO-1 and Bcl-2, as compared to LPS group (P < 0.05) (Fig. 14). Conversely, the effects of remimazolam on Nrf2, HO-1 and Bcl-2 were reversed by ML385 administration (P < 0.05) (Fig. 14). There was no difference among the Control group, DMSO group and remimazolam alone group (R group) for cell apoptosis, intracellular ROS level, MMP and Nrf2/HO-1 expression. All these results suggested that the remimazolam protected against LPS-induced neurotoxicity probably via Nrf2 pathway.
Nrf2 inhibitor ML385 reversed the protective effects of remimazolam on apoptosis and ROS production of primary hippocampal neurons. (A,B) The cells were exposed to 5 µM ML385 for 0.5 h, after which 10 µM LPS was administered, followed by 20 µM remimazolam for 24 h. (A) Cell apoptosis was quantified by flow cytometry using the Annexin V-APC/7‐AAD double‐staining (n = 3). (B) The levels of intracellular ROS were detected by flow cytometry using 2,7-Dichlorofluorescein-diacetate (DCFH-DA) staining (n = 3). Results are shown as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Nrf2 inhibitor ML385 reversed the enhancing effect of remimazolam on the mitochondrial membrane potential (MMP). (A) The MMP of cells was measured using a JC-1 assay. The representative images of JC-1 aggregates and JC-1 monomers reflexed the MMP of primary hippocampal neurons. The scale bar represents 200 nm. (B) The ratio of JC-1 aggregates to JC-1 monomers was decreased in neurons administrated with ML385 prior to remimazolam, suggesting that ML385 inhibited the elevation of MMP by remimazolam. Results are shown as means ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
The effects of remimazolam on the Bcl-2, Nrf2 and HO-1 expression of primary hippocampal neurons were reversed by Nrf2 inhibitor ML385. (A) The expression of Bcl-2, Nrf2 and HO-1 were detected by Western blotting assay and quantified by densitometry. (B–D) The quantification analysis of Bcl-2, Nrf2 and HO-1 in primary hippocampal neurons. The final statistical results were presented as the ratio of the relative expression of the proteins in the treatment group to the relative expression of the proteins in the Control group. Results are shown as means ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The present study aimed at investigating the neuroprotective effects of remimazolam on LPS-induced neuronal injury. In vivo, we found that LPS induced cognitive dysfunction and anxiety‑like behaviors, which were attenuated by remimazolam. Treatment with remimazolam also present less neuroapoptosis, lower MDA level, higher SOD level, and elevated Nrf2/HO-1 expression in rats hippocampus. In vitro, our results revealed that LPS induced neuroapoptosis and cell morphology damage, decreases cell viability and MMP, and increased the production of ROS in primary hippocampal neurons, which could be attenuated by remimazolam. It is also observed that remimazolam exerted neuronal protection accompanied by increases in the levels of Nrf2, and HO-1. What’s more, Nrf2 inhibitor ML385 reversed the protection of remimazolam on hippocampal neurons. Taken together, our findings indicated that remimazolam alleviated LPS-induced neurotoxic injury, which was associated with activation of Nrf2/HO-1 signaling.
Sepsis, leading to life-threatening organ dysfunction, has become one of the major threats to the prognosis and survival of patients in the perioperative period and ICU. Sepsis-induced brain dysfunction often leads to deterioration of mental state and cognition abnormalities. The pathological process of neurological deficit induced by sepsis involves redox imbalance, immunity dysregulation, and mitochondrial dysfunction, leading to neuroinflammation and neuronal apoptosis45,46.
Remimazolam has shown anti-inflammatory effects in several studies. It was reported that remimazolam could attenuate inflammatory response in sepsis-associated acute liver injury in vivo and in vitro. The underlying mechanism may be related to activation of peripheral benzodiazepine receptors and suppression of macrophage p38 phosphorylation28. In the model of myocardial ischemia/reperfusion (I/R) injury, remimazolam inhibits macrophage M1 polarization and reduces inflammation by inhibiting NF-ĸB pathway and p50 and p65 phosphorylation, ultimately attenuating myocardial I/R injury and improving cardiac function40. In another study, remimazolam was found to reduce LPS-induced TNF-α, IL-6, and IL-1β release, inhibit MAPK pathway activation, and improve survival in endotoxemic mice, indicating that remimazolam may be beneficial for septic patients with uncontrolled inflammatory responses29. Our previous studies have shown that remimazolam has a beneficial effect on the neuronal function of cerebral ischemia-reperfusion in rats, manifested by inhibition of NLRP3 inflammasome-dependent pyroptosis and reducing the release of inflammatory factors, resulting in a reduction in morphological damage of cerebral cortical neurons and an improvement in neurological function44. In the present study, we used LPS to build sepsis model in vivo and in vitro. Considering that sepsis-induced neuronal injury and cerebral ischemia-reperfusion injury share similar pathophysiological processes, such as neuroinflammatory response, oxidative stress, mitochondrial dysfunction, etc., we speculated that remimazolam may also affect neuronal injury induced by LPS. We found that LPS administration leads to learning and memory impairment and anxiety‑like behaviors in rats. Remimazolam treatment prevented neurocognitive dysfunction and anxiety induced by LPS. The results indicated remimazolam had a protective role in sepsis.
As we known, the hippocampus plays an important role in many neurological functions, such as memory, learning and cognition. It is considered that cognitive dysfunction results from early sepsis-related insults of the hippocampus47. Besides, the hippocampus is shown to be associated with mood disorders48,49. Therefore, we further explored the neuron morphological and functional changes in the hippocampus after administration of LPS and remimazolam. Our results showed that LPS caused neuroapoptosis obviously in hippocampus. The expression of Bcl-2, an anti-apoptotic factor, was markedly down-regulated in hippocampus after LPS administration. Remimazolam decreased the rate of apoptosis and increased the level of Bcl-2 in hippocampus of rats administrated with LPS. Besides, the decrease in cell viability, and the increase in neuroapoptosis, confirmed the neurotoxic effects of LPS in cultured hippocampal neurons, and remimazolam effectively reduced neuroapoptosis rate, attenuated morphology damage, and increased cell viability, ultimately promoting cell survival, supporting the effects of remimazolam against LPS-induced cell toxicity, which consistent with our in vivo finding. These results suggested the beneficial effects of remimazolam on LPS-induced neuronal damage. Furthermore, the highest dose of 20 mg/kg remimazolam in the present study was not found to be the most effective in terms of behavioral changes, apoptosis and SOD levels. Similar results were obtained in in vitro experiments. The findings of this study indicated that the neuroprotective effect of remimazolam does not appear to be dose-dependent, which was consistent with the results of previous studies44. Therefore, the exact dose-effect relationship of the neuroprotection of remimazolam needs to be explored in more in-depth studies in the future.
The mechanism of remimazolam to protect against LPS-induced neuronal damage is not understood. It is well known that the damage to neurons by oxidative stress was considered one of the initial and important processes for the development of sepsis-induced brain injury50. Inflammatory response to LPS caused oxidative stress and accumulation of ROS which subsequently led to peroxidation of protein and lipid, organelle damage, and mitochondrial dysfunction that culminated in neuronal death51. Brain tissues are particularly vulnerable to oxidative stress7. Therefore, attenuation of oxidative stress-induced toxicity through antioxidation, maintaining mitochondrial health, and anti-apoptosis has become the main targets of neuroprotective therapy52,53,54. Given the importance of oxidative stress in sepsis-related brain injury, we investigated the effects of remimazolam on LPS-induced SOD and MDA changes, which reflected the level of oxidative stress damage. Low level of SOD and high level of MDA were observed in rat hippocampus administration by LPS. While remimazolam elevated the SOD level and reduced the MDA level in hippocampus of rats administrated by LPS. The effects of remimazolam against oxidative stress were confirmed in cultured hippocampal neurons. We found that neurons exposed to LPS resulted in oxidative stress by inducing a rise in ROS production and a reduction of MMP. While remimazolam exerted its protective effects against oxidative stress by decreasing the production of ROS and improving MMP, thus attenuating mitochondrial dysfunction, inhibiting apoptosis, and increasing cellular viability, contributing to the recovery of neuronal injury.
Nrf2 is a major transcription factor to regulate cell antioxidant response. LPS-induced ROS generation was regulated by the Nrf2 signaling pathway55. When suffering stress stimulation, Nrf2 detaches from Keap1 and translocates to the nucleus, and causes the transcriptional increase of antioxidant proteins, subsequently modulating oxidative stress, mitochondrial function, apoptosis, and inflammation56. HO-1 is an Nrf2 target gene, which exerts anti-oxidative functions via various mechanisms including inhibition of ROS production57. It is reported that Nrf2/HO-1 exerted neuroprotective effects against cerebral ischemia damage may be achieved by the instigation of antioxidant mechanisms17. Another study demonstrated that ROS production regulated by the Nrf2 signaling pathway was involved in preventing apoptosis, inflammation, and oxidative stress to protect against spinal cord and brain injury58,59,60. Therefore, we speculated that remimazolam regulates oxidative stress by activating the Nrf2 pathway. Our results showed that LPS led to a reduction in the protein levels of Nrf2 and HO-1, consistent with previous findings which indicated the Nrf2/HO-1 pathway involved in LPS-induced brain damage61,62,63. While remimazolam attenuated the decreased expression of Nrf2 and HO-1 in hippocampus of rats administrated by LPS. Similar results were observed in primary hippocampal neurons, that remimazolam treatment could increase the levels of Nrf2 and HO-1 in neurons exposed to LPS. To further confirm our hypothesis, we inhibited Nrf2 expression by using ML385 in cultured hippocampal neurons. The effects of remimazolam on LPS-induced neuronal injury was reversed by ML385, which significantly increased the apoptosis rate and intracellular ROS level of primary hippocampal neurons, as well as enhancing damage to MMP, companied by a down-regulation of Nrf2, HO-1 and Bcl-2. Taken together, our results indicated that the protection of remimazolam against LPS-induced neuronal injury was probably via regulation of Nrf2 signaling pathway.
The present study has limitations. We just investigated the effects of remimazolam on rat hippocampus and primary hippocampal neurons, while the influence of remimazolam on other brain regions and other types of cells did not clear. Remimazolam at the highest dose in the present study did not show the strongest neuroprotective effect. Therefore, further exploration is required into the remimazolam at different doses, especially lower doses and the effects on other brain regions in order to provide a more comprehensive understanding of its neurological effects. Besides, we did not use more methods to activate or block upstream and downstream factors of Nrf2 pathway for further exploration. It is possible that the effects of remimazolam are not entirely due to the Nrf2 pathway. Other mechanisms may be involved. Recent study has demonstrated that remimazolam could inhibit MAPK/ERK signalling, thereby reducing liver ischemia-reperfusion (I/R) -induced TNF-α and IL-6 release, as well as hepatocyte apoptosis. Consequently, it can be suggested that remimazolam exerts an anti-inflammatory role in protecting the liver from ischemia-reperfusion injury43. Remimazolam also exhibited anti-apoptotic effects on lung endothelial cells and epithelial cells, with the potential to mitigate acute lung injury. This effect may be associated with the activation of the PI3K/AKT pathway64. Another study has shown that remimazolam has a protective effect on cardiac ischemia-reperfusion (I/R) injury. Remimazolam can improve I/R-induced cardiac dysfunction by reducing oxidative stress and mitochondrial damage, inhibiting the expression and release of inflammatory factors, increasing the value of ejection fraction and reducing the size of myocardial infarction, and the NLRP3/IL-1β pathway is involved65. Therefore, future experiments are crucial to fully discuss the antioxidation and anti-inflammatory effect of remimazolam, as well as the exact molecular mechanisms.
In conclusion, this study suggested that remimazolam attenuated LPS-induced oxidative stress and neuronal injury, potentially through the Nrf2 signaling pathway. These results provide a theoretical basis for remimazolam as a promising drug for the sedation of septic patients.
Data availability
Relevant data generated or analyzed during this study are shown within the figures of this paper.
References
Cecconi, M., Evans, L., Levy, M. & Rhodes, A. Sepsis and septic shock. Lancet 392, 75–87. https://doi.org/10.1016/S0140-6736(18)30696-2 (2018).
Ren, C., Yao, R. Q., Zhang, H., Feng, Y. W. & Yao, Y. M. Sepsis-associated encephalopathy: a vicious cycle of immunosuppression. J. Neuroinflammation. 17, 14. https://doi.org/10.1186/s12974-020-1701-3 (2020).
Mazeraud, A. et al. Neuroanatomy and physiology of brain dysfunction in sepsis. Clin. Chest. Med. 37, 333–345. https://doi.org/10.1016/j.ccm.2016.01.013 (2016).
Iwashyna, T. J., Ely, E. W., Smith, D. M. & Langa, K. M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304, 1787–1794. https://doi.org/10.1001/jama.2010.1553 (2010).
Winters, B. D. et al. Long-term mortality and quality of life in sepsis: a systematic review. Crit. Care Med. 38, 1276–1283. https://doi.org/10.1097/CCM.0b013e3181d8cc1d (2010).
Annane, D. & Sharshar, T. Cognitive decline after sepsis. Lancet Respiratory Med. 3, 61–69. https://doi.org/10.1016/s2213-2600(14)70246-2 (2015).
Wang, X. & Michaelis, E. K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2 https://doi.org/10.3389/fnagi.2010.00012 (2010).
Dyson, A. et al. An integrated approach to assessing nitroso-redox balance in systemic inflammation. Free Radic Biol. Med. 51, 1137–1145. https://doi.org/10.1016/j.freeradbiomed.2011.06.012 (2011).
Hill, R. L., Singh, I. N., Wang, J. A. & Hall, E. D. Time courses of post-injury mitochondrial oxidative damage and respiratory dysfunction and neuronal cytoskeletal degradation in a rat model of focal traumatic brain injury. Neurochem Int. 111, 45–56. https://doi.org/10.1016/j.neuint.2017.03.015 (2017).
Rizwan, H., Pal, S., Sabnam, S. & Pal, A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 241, 117148. https://doi.org/10.1016/j.lfs.2019.117148 (2020).
Robledinos-Anton, N., Fernandez-Gines, R., Manda, G. & Cuadrado, A. Activators and inhibitors of NRF2: A review of their potential for clinical development. Oxid. Med. Cell. Longev. 2019, 9372182. https://doi.org/10.1155/2019/9372182 (2019).
Ahmed, S. M., Luo, L., Namani, A., Wang, X. J. & Tang, X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 585–597. https://doi.org/10.1016/j.bbadis.2016.11.005 (2017).
Zhang, B. et al. Icariin attenuates neuroinflammation and exerts dopamine neuroprotection via an Nrf2-dependent manner. J. Neuroinflammation. 16 https://doi.org/10.1186/s12974-019-1472-x (2019).
Calkins, M. J. et al. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 11, 497–508. https://doi.org/10.1089/ARS.2008.2242 (2009).
Kyung, S., Lim, J. W. & Kim, H. alpha-Lipoic acid inhibits IL-8 expression by activating Nrf2 signaling in Helicobacter pylori-infected gastric epithelial cells. Nutrients 11 https://doi.org/10.3390/nu11102524 (2019).
Deng, S. et al. Albumin reduces oxidative stress and neuronal apoptosis via the ERK/Nrf2/HO-1 pathway after intracerebral hemorrhage in rats. Oxid. Med. Cell. Longev. 2021, 8891373. https://doi.org/10.1155/2021/8891373 (2021).
Khan, M. S. et al. Inhibition of JNK alleviates chronic hypoperfusion-related ischemia induces oxidative stress and brain degeneration via Nrf2/HO-1 and NF-kappaB signaling. Oxid. Med. Cell Longev. 2020, 5291852 https://doi.org/10.1155/2020/5291852 (2020).
Lou, Y. et al. Houttuynia cordata thunb. And its bioactive compound 2-undecanone significantly suppress benzo(a)pyrene-induced lung tumorigenesis by activating the Nrf2-HO-1/NQO-1 signaling pathway. J. Exp. Clin. Cancer Res. 38, 242. https://doi.org/10.1186/s13046-019-1255-3 (2019).
Ren, C. et al. Betulin alleviates the inflammatory response in mouse chondrocytes and ameliorates osteoarthritis via AKT/Nrf2/HO-1/NF-kappaB axis. Front. Pharmacol. 12, 754038. https://doi.org/10.3389/fphar.2021.754038 (2021).
He, D. et al. Camptothecin regulates microglia polarization and exerts neuroprotective effects via activating AKT/Nrf2/HO-1 and inhibiting NF-κB pathways in vivo and in vitro. Front. Immunol. 12, 619761. https://doi.org/10.3389/fimmu.2021.619761 (2021).
Zhao, X. J. et al. Oridonin ameliorates traumatic brain Injury-Induced neurological damage by improving mitochondrial function and antioxidant capacity and suppressing neuroinflammation through the Nrf2 pathway. J. Neurotrauma. 39, 530–543. https://doi.org/10.1089/neu.2021.0466 (2022).
Ou, Z. et al. Albiflorin alleviates neuroinflammation of rats after MCAO via PGK1/Nrf2/HO-1 signaling pathway. Int. Immunopharmacol. 137, 112439. https://doi.org/10.1016/j.intimp.2024.112439 (2024).
Liu, Y. et al. Ramelteon ameliorates LPS-induced hyperpermeability of the blood-brain (BBB) by activating Nrf2. Inflammation 44, 1750–1761. https://doi.org/10.1007/s10753-021-01451-w (2021).
Tse, A. H. W., Ling, L., Lee, A. & Joynt, G. M. Altered pharmacokinetics in prolonged infusions of sedatives and analgesics among adult critically ill patients: A systematic review. Clin. Ther. 40, 1598–1615e1592. https://doi.org/10.1016/j.clinthera.2018.07.021 (2018).
Sneyd, J. R., Gambus, P. L. & Rigby-Jones, A. E. Current status of perioperative hypnotics, role of benzodiazepines, and the case for Remimazolam: a narrative review. Br. J. Anaesth. 127, 41–55. https://doi.org/10.1016/j.bja.2021.03.028 (2021).
Kilpatrick, G. J. Remimazolam: Non-clinical and clinical profile of a new sedative/anesthetic agent. Front. Pharmacol. 12, 690875. https://doi.org/10.3389/fphar.2021.690875 (2021).
Upton, R. N., Martinez, A. M. & Grant, C. Comparison of the sedative properties of CNS 7056, Midazolam, and Propofol in sheep. Br. J. Anaesth. 103, 848–857. https://doi.org/10.1093/bja/aep269 (2009).
Fang, H. et al. Remimazolam reduces sepsis-associated acute liver injury by activation of peripheral benzodiazepine receptors and p38 Inhibition of macrophages. Int. Immunopharmacol. 101, 108331. https://doi.org/10.1016/j.intimp.2021.108331 (2021).
Liu, X. et al. Remimazolam protects against LPS-Induced endotoxicity improving survival of endotoxemia mice. Front. Pharmacol. 12, 739603. https://doi.org/10.3389/fphar.2021.739603 (2021).
Herrera, A. J., Castano, A., Venero, J. L., Cano, J. & Machado, A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol. Dis. 7, 429–447. https://doi.org/10.1006/nbdi.2000.0289 (2000).
Sun, Y. B. et al. Dexmedetomidine inhibits astrocyte pyroptosis and subsequently protects the brain in in vitro and in vivo models of sepsis. Cell. Death Dis. 10, 167. https://doi.org/10.1038/s41419-019-1416-5 (2019).
Haileselassie, B. et al. Mitochondrial dysfunction mediated through dynamin-related protein 1 (Drp1) propagates impairment in blood brain barrier in septic encephalopathy. J. Neuroinflammation. 17 https://doi.org/10.1186/s12974-019-1689-8 (2020).
Huang, W. Y. et al. NADPH oxidase 2 as a potential therapeutic target for protection against cognitive deficits following systemic inflammation in mice. Brain Behav. Immun. 84, 242–252. https://doi.org/10.1016/j.bbi.2019.12.006 (2020).
Duan, M. et al. Remimazolam suppresses oxidative stress and apoptosis in cerebral ischemia/reperfusion injury by regulating AKT/GSK-3β/NRF2 pathway. Drug Des. Devel Ther. 19, 111–128. https://doi.org/10.2147/dddt.S478692 (2025).
Zhang, Y. et al. Midazolam ameliorates acute liver injury induced by carbon tetrachloride via enhancing Nrf2 signaling pathway. Front. Pharmacol. 13, 940137. https://doi.org/10.3389/fphar.2022.940137 (2022).
Bi, W. et al. PSMC5 regulates microglial polarization and activation in LPS-induced cognitive deficits and motor impairments by interacting with TLR4. J. Neuroinflammation. 20, 277. https://doi.org/10.1186/s12974-023-02904-9 (2023).
Beheshtimanesh, Z. & Rajaei, Z. Neuroprotective effects of Sesamol against LPS-induced spatial learning and memory deficits are mediated via anti-inflammatory and antioxidant activities in the rat brain. Avicenna J. Phytomed. 13, 213–222. https://doi.org/10.22038/ajp.2022.21403 (2023).
Savran, M. et al. Agomelatine could prevent brain and cerebellum injury against LPS-induced neuroinflammation in rats. Cytokine 127, 154957. https://doi.org/10.1016/j.cyto.2019.154957 (2020).
Liu, Y. et al. Design, synthesis, and biological evaluation of novel CNS 7056 derivatives as sedatives in rats and rabbits. Chem. Biol. Drug Des. 88, 38–42. https://doi.org/10.1111/cbdd.12731 (2016).
Xu, H. et al. Remimazolam attenuates myocardial ischemia-reperfusion injury by inhibiting the NF-ĸB pathway of macrophage inflammation. Eur. J. Pharmacol. 965, 176276. https://doi.org/10.1016/j.ejphar.2023.176276 (2024).
Tu, Y. et al. Dexmedetomidine attenuates the neurotoxicity of propofol toward primary hippocampal neurons in vitro via Erk1/2/CREB/BDNF signaling pathways. Drug Des. Devel Ther. 13, 695–706. https://doi.org/10.2147/dddt.S188436 (2019).
Kilpatrick, G. J. et al. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology 107, 60–66. https://doi.org/10.1097/01.anes.0000267503.85085.c0 (2007).
Shi, Y., Deng, H., Zhang, Z., Zhu, X. & Zeng, Z. Remimazolam protects the liver from ischemia-reperfusion injury by inhibiting the MAPK/ERK pathway. BMC Anesthesiol. 24, 251. https://doi.org/10.1186/s12871-024-02641-3 (2024).
Shi, M. et al. Protective effects of remimazolam on cerebral ischemia/reperfusion injury in rats by inhibiting of NLRP3 inflammasome-dependent pyroptosis. Drug Des. Devel Ther. 16, 413–423. https://doi.org/10.2147/DDDT.S344240 (2022).
Mazeraud, A. et al. Septic-associated encephalopathy: a comprehensive review. Neurotherapeutics 17, 392–403. https://doi.org/10.1007/s13311-020-00862-1 (2020).
Manabe, T. & Heneka, M. T. Cerebral dysfunctions caused by sepsis during ageing. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-021-00643-7 (2021).
Semmler, A. et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J. Neurol. Neurosurg. Psychiatry. 84, 62–69. https://doi.org/10.1136/jnnp-2012-302883 (2013).
Rawat, R., Tunc-Ozcan, E., McGuire, T. L., Peng, C. Y. & Kessler, J. A. Ketamine activates adult-born immature granule neurons to rapidly alleviate depression-like behaviors in mice. Nat. Commun. 13, 2650. https://doi.org/10.1038/s41467-022-30386-5 (2022).
Spencer-Segal, J. L. et al. Sepsis survivor mice exhibit a behavioral endocrine syndrome with ventral hippocampal dysfunction. Psychoneuroendocrinology 117, 104679. https://doi.org/10.1016/j.psyneuen.2020.104679 (2020).
Catarina, A. V., Branchini, G., Bettoni, L., De Oliveira, J. R. & Nunes, F. B. Sepsis-associated encephalopathy: from pathophysiology to progress in experimental studies. Mol. Neurobiol. 58, 2770–2779. https://doi.org/10.1007/s12035-021-02303-2 (2021).
Wu, C. C. & Bratton, S. B. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox Signal. 19, 546–558. https://doi.org/10.1089/ars.2012.4905 (2013).
Wang, Y., Li, B. & Zhang, X. Scutellaria Barbata D. Don (SBD) protects oxygen glucose deprivation/reperfusion-induced injuries of PC12 cells by up-regulating Nrf2. Artif. Cells Nanomed. Biotechnol. 47, 1797–1807. https://doi.org/10.1080/21691401.2019.1610413 (2019).
Naoi, M., Wu, Y., Shamoto-Nagai, M. & Maruyama, W. Mitochondria in neuroprotection by phytochemicals: bioactive polyphenols modulate mitochondrial apoptosis system, function and structure. Int. J. Mol. Sci. 20 https://doi.org/10.3390/ijms20102451 (2019).
Ren, J. et al. Tricetin protects against 6-OHDA-induced neurotoxicity in Parkinson’s disease model by activating Nrf2/HO-1 signaling pathway and preventing mitochondria-dependent apoptosis pathway. Toxicol. Appl. Pharmacol. 378, 114617. https://doi.org/10.1016/j.taap.2019.114617 (2019).
Bian, H. et al. Dihydrolipoic acid protects against lipopolysaccharide-induced behavioral deficits and neuroinflammation via regulation of Nrf2/HO-1/NLRP3 signaling in rat. J. Neuroinflammation. 17, 166. https://doi.org/10.1186/s12974-020-01836-y (2020).
Li, M., Zhou, F., Xu, T., Song, H. & Lu, B. Acteoside protects against 6-OHDA-induced dopaminergic neuron damage via Nrf2-ARE signaling pathway. Food Chem. Toxicol. 119, 6–13. https://doi.org/10.1016/j.fct.2018.06.018 (2018).
Bijjem, K. R., Padi, S. S. & lal Sharma, P. Pharmacological activation of Heme Oxygenase (HO)-1/carbon monoxide pathway prevents the development of peripheral neuropathic pain in Wistar rats. Naunyn Schmiedebergs Arch. Pharmacol. 386, 79–90. https://doi.org/10.1007/s00210-012-0816-1 (2013).
Wang, J. L., Luo, X. & Liu, L. Targeting CARD6 attenuates spinal cord injury (SCI) in mice through inhibiting apoptosis, inflammation and oxidative stress associated ROS production. Aging (Albany NY). 11, 12213–12235. https://doi.org/10.18632/aging.102561 (2019).
Muhammad, T., Ikram, M., Ullah, R., Rehman, S. U. & Kim, M. O. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-kappaB signaling. Nutrients. 11 https://doi.org/10.3390/nu11030648 (2019).
Cong, P. et al. Resveratrol ameliorates thoracic blast exposure-induced inflammation, Endoplasmic reticulum stress and apoptosis in the brain through the Nrf2/Keap1 and NF-kappaB signaling pathway. Injury 52, 2795–2802. https://doi.org/10.1016/j.injury.2021.08.019 (2021).
He, D. et al. Camptothecin regulates microglia polarization and exerts neuroprotective effects via activating AKT/Nrf2/HO-1 and inhibiting NF-kappaB pathways in vivo and in vitro. Front. Immunol. 12, 619761. https://doi.org/10.3389/fimmu.2021.619761 (2021).
Wu, P. S. et al. Anti-inflammatory activity of 8-hydroxydaidzein in LPS-stimulated BV2 microglial cells via activation of Nrf2-antioxidant and attenuation of Akt/NF-kappaB-inflammatory signaling pathways, as well as inhibition. J. Agric. Food Chem. 66, 5790–5801. https://doi.org/10.1021/acs.jafc.8b00437 (2018).
Yang, S. et al. Anti-inflammatory effects of higenamine (Hig) on LPS-activated mouse microglia (BV2) through NF-kappaB and Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 85, 106629. https://doi.org/10.1016/j.intimp.2020.106629 (2020).
Li, R. et al. Remimazolam inhibits apoptosis of endothelial and epithelial cells by activating the PI3K/AKT pathway in acute lung injury. Int. Immunopharmacol. 147, 113949. https://doi.org/10.1016/j.intimp.2024.113949 (2025).
Liu, X. et al. Remimazolam alleviates myocardial ischemia/reperfusion injury and inflammation via Inhibition of the NLRP3/IL–1β pathway in mice. Int. J. Mol. Med. 55 https://doi.org/10.3892/ijmm.2025.5498 (2025).
Funding
This work was supported by Guangxi Science and Technology Base and Talent Special Project (No. AD25069060), Guangxi Key Research and Development Program (No. AB24010066), National Natural Science Foundation of China (No. 82460239), Special Fund of Neurotoxicity of General Anesthetics and Its Prevention and Treatment Innovation Team of the First Affiliated Hospital of Guangxi Medical University (No. YYZS2022001).
Author information
Authors and Affiliations
Contributions
Y.W. and Z.Z. designed the study, performed the experiments. Y.W. wrote the first draft and the revised the manuscript. Z.Z., S.P. and Y. Y. performed the experiments and analyzed data. T.L. and J. C. Provide technical support for the experiments. Y.X. conceived and supervised the study and modified the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wei, Y., Pan, S., Zhou, Z. et al. Remimazolam attenuated lipopolysaccharide-induced behavioral deficits and neuronal injury via activation of the Nrf2 pathway. Sci Rep 15, 13784 (2025). https://doi.org/10.1038/s41598-025-95379-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95379-y