Abstract
Some natural compounds derived from medicinal plants show anti-tumor activity with high efficacy and safety, low toxicity and residual levels etc. The aim of this study was to select natural compounds and biomarkers having high inhibitory effects against A549 adenocarcinoma cells. A total of eight natural compounds having pure plant origin were initially screened, purchased, and their potential anti-cancer activities were comprehensively and systematically evaluated against A549 lung adenocarcinoma cells. The maximum non-cytotoxic concentration (MNTC) and 50% cytotoxic concentration (CC50) of the eight compounds against A549 cells were obtained by cytopathological and MTT assays, respectively. Using Cisplatin as a positive control, the effect of selected compounds were elucidated on the proliferation, migration and invasion of A549 cells by MTT, wound healing and invasion assays, respectively. AnnexinV-FITC/PI, JC-1, ROS and Cell Cycle Kits were used to detect the pro-apoptotic mechanism of A549 cells induced by the tested compounds. qRT-PCR and RNA-seq were used to investigate the effective biomarkers involved in the inhibition process. The results showed that Curcumin, Osthole, Paeonol, Cepharanthine and Cisplatin significantly reduced the proliferation, migration and invasion abilities of A549 cells in a dose-dependent manner. Post 48 h of treatment, Osthole inhibited the metastatic ability of A549 cells by regulating mitochondrial apoptosis, arresting A549 cell in G1-phase and inhibiting release of ROS, while Curcumin, Paeonol and Cepharanthine did not showed the same response. It was therefore elucidated that Osthole was the optimal natural compound showing powerful anti-inhibitory properties against A549 cells. Moreover, the expressions of EGF, IL-2 and IL-10 genes were significantly decreased in Osthole treated group, while IL-6 gene was significantly increased. This study suggested that EGF gene has the potential to be used as a biomarker for Osthole treatment against A549 cells, involved in mitochondrial apoptosis and ROS down-regulation, inhibiting proliferation and epithelial mesenchymal transition (EMT), inflammation and immune processes in A549 cells providing a foundation to develop Osthole as a potential target drug to prevent the occurrence and development of lung adenocarcinoma.
Similar content being viewed by others
Introduction
World widely, the incidence and mortality rates due to lung cancer have been very high and is increasing every year1. Pathologically, lung cancer is divided into small cell lung cancer and non-small cell lung cancer (NSCLC). NSCLC is the most common type of lung cancer, including diffused squamous cell carcinoma, squamous cell carcinoma and adenocarcinoma2,3. Lung adenocarcinoma accounts 45-50% of the total NSCLC which is increasing every year, and thus possess a serious threat to human health4.
Surgery, transplantation, chemotherapy and radiotherapy are the main therapeutic options to prolong the survival rate and improve the quality of life of cancer patients5. However, as the early clinical symptoms are hidden and atypical, the best time to intervene the cancer is therefore missed which results in a short survival time for patients6,7. Although molecular targeted therapy has made great progress in recent years, still the average 5-year survival rate of patients with NSCLC is as low as 15%8. The main reason for reduced annual survival and cure rates are canner cell metastasis, and is thus considered as one of the global public health problems9.
Epithelial mesenchymal transition (EMT) is a cellular process in which epithelial cells are transformed into mesenchymal cells, increasing cell invasion and migration, which results in loss of epithelial cell markers while gain in mesenchymal cell markers10. Studies11,12 have shown that transforming growth factor (TGF-β1) and epidermal growth factor (EGF) are the primary cytokine involved in inducing EMT. TGF-β1/Smad signaling pathway promotes the occurrence of EMT by increasing and decreasing the expression of mesenchymal and epithelial markers, respectively11,13,14. EGF mediates JAK-STAT signaling pathway to inhibit the growth and aggressiveness of tongue squamous cell carcinoma15. At the same time, it also regulates the expression of other transcription factors including Slug, ZEB, Twist and Snail.
Okon et al.16 studied that TGF-β-induces EMT is dependent on ROS, and an increase of ROS promotes oxidation, inflammation, as well as the occurrence and progression of EMT. EGF inhibits apoptosis, and promotes ROS production, cell proliferation and EMT17. ROS are mainly produced in mitochondria18. Regulatory factors such as Sirtuin 1 (SIRT1), peroxisome proliferator-activated receptor gamma coactivator-1-alpha (PGC-1α), mitochondrial transcription factor A (TFAM) and nuclear respiration factors 1 (NRF-1) are involved in maintaining the normal physiological functions of mitochondria18,19.
Natural compounds are safe, highly efficient with low toxicity and residual levels, and possessed anti-oxidative20,21,22, anti-inflammatory23, anti-cancer7, anti-virus24,25,26, pro-apoptosis27 activities and improve body’s immunity28 through targeting multiple pathways and targets. It has been reported that many natural compounds isolated from different medicinal plants have been extensively used for the treatment of numerous chronic diseases and cancer by scavenging free radicals, quenching ROS, regulate apoptosis and other activities29. There are various types of natural compounds, and Karthika et al.30 found that Curcumin could treat and relieve colorectal cancer. It has also been found that Curcumin exhibits anticancer effects against breast cancer cells and breast cancer stem cells31. Wang et al.32 found that Glycyrrhizic acid induced apoptosis of gastric cancer cells by regulating DNA damage and apoptosis in HCC cells33. Jia et al.34 found that Tea saponins could be used as a potential drug for ovarian cancer. It has also been found that Osthole35 and Baicalin36 induces apoptosis of lung cancer cells, Curcumol inhibits cell proliferation37, Paeonol inhibits cell metastasis38, and Cepharanthine regulates cell autophagy39. However, the comparative effects of these different natural compounds on the same type of cancer cells, especially lung cancer cells, were still unclear. Meanwhile, research and development of anti-lung cancer drugs are of great concern for the medical field and medical security departments of various governments40,41. So far, no milestone has been achieved in the development of drugs for the treatment of lung cancer.
Compared with the above research results, this study uniquely selected eight natural compounds having potential anticancer activity from initial screening of several natural compounds with clear chemical structures, traditional medicinal plant sources and specific biological activities. The compounds were studied to check their anti-proliferative, anti-migratory and anti-invasive properties against lung adenocarcinoma cell model A549 through in vitro cell experiments and bioinformatics, The optimal level of natural compound against A549 cells and the potential biomarkers were screened out in the selected eight natural compounds. Moreover, this study anonymously applied multiple experimental methods were applied to explore the anti-lung cancer effects of natural compounds in a more systematic and comprehensive way. This is a baseline study which will provide a foundation for the development of safe, efficient and novel compounds with anti-lung cancer effects.
Results
Cytopathic effect
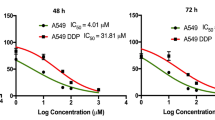
MTT assay was used to investigate the cytopathic rate (CR) of 8 selected compounds and Cisplatin (positive control drug) to find out its MNTC and CC50 values. The chemical structures of selected compounds were shown in Fig. 1A. The results showed that different compounds with different concentrations possessed different cytotoxic effect against A549 cells. MNTC and CC50 values of different compounds and Cisplatin showed different values as shown in Table 1. No obvious cytopathic effect was observed when A549 cells were treated with the maximum solubility concentration (MSC) of Curcumol (0.04 mg/mL) and Glycyrrhizic acid (8 mg/mL) within the tested concentrations. Therefore, these two compounds were screened negative and were not considered in the follow-up study.
Dose-response curves of 8 natural compounds. (A) The chemical structure of 8 natural compounds. (B) Dose-response curves of all tested compounds in cytotoxicity assay. 10 of gradient dilutions of each compound was prepared by 2-fold serial dilution and incubated with cells for 72 h. Morphological changes and dose-response curve of the tested compounds used in A549 shown the cytopathic effect were increased with the increased concentration of compounds.
Curcumin, Osthole, Baicalin, Paeonol, Cepharanthine, Tea saponin, and Cisplatin (0.016 mg/mL, positive control) showed significant cytopathic effects with progressive changes in cells shape which became wrinkled, shrunken and ruptured at the end. Dose-response curves and CC50 value of the tested compounds were shown in Fig. 1B; Table 1, respectively after calculating the CR value. The “S” typed curves showed that the relationship was dose-dependent with an increase in the concentration of the compound, the morphology of the cells became worst.
Anti-proliferative effect
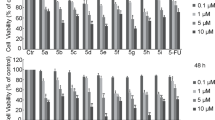
The proliferation rate (Fig. 2A-F) of A549 cells treated with compounds for 24, 48 and 72 h showed that high concentrations of Curcumin (Fig. 2A) and Baicalin (Fig. 2C) significantly inhibited cell proliferation (p ˂ 0.001) time dependently. However, the medium and low concentrations of Curcumin showed non-significant effect (p > 0.05), while that of Baicalin significantly promoted cell proliferation (p ˂ 0.001). Figure 2B, D and E concluding that Osthole, Paeonol and Cepharanthine had significantly inhibited A549 cells proliferation at 48 h in a dose-dependent manner (p ˂ 0.001). As Tea saponin showed non significant effect on the proliferation of A549 cells (p > 0.05) as shown in Fig. 2F, and was therefore eliminated from further experiments.
Curcumin, Osthole, Baicalin, Paeonol and Cepharanthine inhibited proliferation of A549 cells. Cell proliferation rate of A549 cells treated with compounds for 24, 48 and 72 h were evaluated by MTT. Compared with A549 control group, the results of 24, 48 and 72 h treatment with tested compounds were as follows: (A) Curcumin 0.02 mg/mL treatment for 24, 48 and 72 h significantly inhibited the proliferation of A549 cells (p ˂ 0.001), while Curcumin 0.01 mg/mL and 0.005 mg/mL treatment group showed non significant difference (p > 0.05). (B, D, E) The proliferation of A549 cells were significantly inhibited dose-dependent in Osthole, Paeonol and Cepharanthine treated group (p ˂ 0.05). (C) With the increase of incubation time, Curcumin 1.0 mg/mL treatment group significantly inhibited the proliferation of A549 cells (p ˂ 0.001), 0.5 mg/mL treatment group had non significant effect (p > 0.05), while 0.25 mg/mL treatment group significantly promoted the proliferation of A549 cells (p ˂ 0.001). (F) Tea saponin had non significant effect on the proliferation of A549 cells (p > 0.05). Data were presented as mean ± SEM (n = 3). * p < 0.05, ** p < 0.01, and *** p < 0.001.
Inhibition of A549 cells migrations
Migration ability of cells has a direct nexus with disease progress42. Compared with the cell control group, the mobility of A549 cells treated with Cisplatin and five compounds for 24 h was significantly reduced in a dose-dependent manner. However, non significant change was observed in the medium-dose, and significant increase in the low-dose Baicalin groups (Fig. 3B, p ˂ 0.05). Cisplatin and five compounds including high and medium concentrations of Curcumin significantly reduced the mobility of A549 cells when treated for 48 h. However, a non significant change was observed in the low-dose Curcumin group, and a significant increase was seen in the medium and low-dose Baicalin group (Fig. 3C, p ˂ 0.05). Post 72 h of treatment, Cisplatin and five compounds significantly reduced the mobility of A549 cells, non significant change in the low-dose Curcumin and middle-dose Baicalin group, and a significant increase in the low-dose Baicalin group (Fig. 3D, p ˂ 0.05) were observed. Compared with Cisplatin group, high-dose Curcumin, Osthole and Paeonol (24, 48, 72 h) inhibited the mobility of A549 cells better than Cisplatin group (p ˂ 0.05). In conclusion, these results showed that the migration ability of A549 cells were significantly reduced in a dose-dependent manner after treatment with Cisplatin, Osthole (high, medium, low), Curcumin (high, medium), Paeonol and Cepharanthine as shown in Fig. 3A-D. and the inhibitory effects of Curcumin, Osthole and Paeonol on the migration of A549 cells were better than that of positive control Cisplatin group.
The five tested compounds inhibited the migration of A549 cells. (A, B) Compared with the A549 cell group, the migration of A549 cells was inhibited to varying degrees by the five tested compounds. Different letters indicate significant differences between groups, p < 0.05. Data were presented as mean ± SEM (n = 3). Different letters (a, b, c, d, etc.) indicated significant differences between the groups, p < 0.05.
Inhibition of A549 cells invasion
Invasion is a key feature of cancer cells towards metastasis42. Compared with the control group, the number of cells invading transwell membrane in the high concentration treatment group of Curcumin and Cepharanthine were significantly reduced after 48 h of treatment (p ˂ 0.01), while non significant change was seen in the medium and low concentration treatment groups (p > 0.05). However, the number of cells invading the transwell membrane were significantly decreased in a dose-dependent manner in the high, medium and low concentration treatment groups of Osthole and Paeonol (p ˂ 0.001, 0.01 and 0.05). Non significant change was observed in the number of cells invading the transwell membrane in all the three concentration groups of Baicalin (p > 0.05). Based on the abovementioned results (Fig. 4A, B), Curcumin, Osthole, Paeonol and Cepharanthine were further used to explore the mechanism of inhibition.
The four tested compounds inhibited the invasion of A549 cells. (A, B) Compared with the A549 cell group, invasion of A549 cells were significantly inhibited by Curcumin, Osthole, Paeonol and Cepharanthine with dose-dependent effect, p < 0.05. Data were presented as mean ± SEM (n = 3). * p < 0.05, ** p < 0.01, and *** p < 0.001.
Tested compounds regulated cells apoptosis, promoted mitochondrial apoptosis, augmented ROS generation in A549 cells
Annexin-V positive cell percentage is a key marker for cell apoptosis43. In Fig. 5A and B, compared with the A549 control group (1.69%±1.13%), the apoptotic rates were significantly increased (p < 0.05) in a dose dependent manner by Curcumin (9.96%±0.14%), Osthole (16.23%±1.34%), Paeonol (9.48%±0.68%), Cepharanthine (11.37%±1.20%) and Cisplatin (8.79%±1.18%). Compared with the Cisplatin positive drug control group, the high concentrations of Osthole (16.23%±1.34%) and Cepharanthine (11.37%±1.20%) significantly increased A549 cells apoptosis (p < 0.05), while non significant difference was observed between the medium and low concentrations of Osthole (8.85%±1.25% and 7.90%±0.01%) and Cepharanthine (7.90%±1.13% and 6.93%±0.32%). However, compared with the Cisplatin, non significant difference was observed between the high, medium and low concentrations groups of Curcumin and Paeonol. These results indicated that the pro-apoptotic effects of Osthole and Cepharanthine high concentrations were significantly better than Cisplatin treatment group (p < 0.05).
Tested compounds promoted the cell apoptosis via mitochondria pathway and augmented ROS generation in A549 cells. (A, B) After cells were treated with four tested compounds and Cisplatin for 48 h. The apoptosis rate was evaluated by Annexin V/PI staining followed by Flow cytometry. The results showed that Curcumin, Osthole, Paeonol, Cepharanthine and Cisplatin treatment groups significantly promoted the apoptosis rate of A549 cells, p < 0.05. (C, D) The value of MMP in each treated group after being staining with JC-1 kit were detected by Flow cytometry. MMP value is expressed in R2. The results showed that compared with the A549 control group, MMP values were significantly higher in groups treated with Curcumin, Osthole, Paeonol, Cepharanthine and Cisplatin, p < 0.05. (E, F) Compared with the A549 group, the cells treated with Curcumin, Osthole, Paeonol, Cepharanthine and Cisplatin for 48 h. Flow cytometry showed that the ROS value of Osthole and Paeonol treatment group was significantly decreased with an increase concentration, while the ROS value of Curcumin and Cepharanthine treatment group was significantly increased. Data were presented as mean ± SEM (n = 3). Different letters (a, b, c, d, etc.) indicated significant differences between the groups, p < 0.05.
Compared with the A549 control group, the MMP values were significantly increased in the group treated with Curcumin, Osthole, Paeonol, Cepharanthine and Cisplatin in a dose-dependent response (Fig. 5C, D, p < 0.05). Surprisingly, the MMP values of Osthole and Cepharanthine treated groups were down-regulated while that of Curcumin and Paeonol treated groups were up-regulated with decreasing concentration. The pro-apoptotic mechanism of compounds explored that Osthole and Cepharanthine significantly regulate cell apoptosis through mitochondrial pathway (p < 0.05), while mitochondrial pathway was not the main pathway of Curcumin and Paeonol to promote A549 cells apoptosis.
The mechanism of Curcumin, Osthole, Paeonol and Cepharanthine was further explored in mitochondrial apoptosis to find the relationship between MMP and ROS. Compared with A549 cell control group, treatment of different tested compounds and Cisplatin resulted in stronger DCF-fluorescence intensity in A549 cells (p < 0.05), as shown in Fig. 5E and F which indicated an enhanced intracellular ROS generation. However, it was curious that with the decreased concentration, the intracellular ROS values in Osthole and Paeonol treated groups were up-regulated while that of Curcumin and Cepharanthine treated groups were down-regulated. Compared with Cisplatin group, ROS values in high-dose and medium-dose Osthole and Paeonol treated groups were down-regulated (p < 0.05).
Osthole triggers cell cycle arrest in A549 cells
To further explore whether the inhibitory effect of Osthole on A549 cells was due to its induction of cell-cycle arrest, Flow cytometry analysis was performed (Fig. 6A). Compared with A549 cells control group (Fig. 6B, C), Osthole (0.02 and 0.01 mg/mL) significantly increased the number of G1-phase cells (p < 0.05). At the same time, the number of S-phase cells in 0.02 mg/mL Osthole group was significantly decreased, and the number of G2-phase cells in 0.02, 0.01 mg/mL Osthole group were significantly decreased (p < 0.05). These results suggest that Osthole mainly blocked A549 cells in G1-phase.
Effects of Osthole on cell cycle of A549 cells. (A) The cells were treated with different concentration of Osthole to estimate the cell cycle arrest by Flow cytometry. (B, C) Effect of Osthole (0.02, 0.01, 0.005 mg/mL) on cell cycle treated for 48 h. Data were presented as mean ± SEM (n = 3). Different letters (a, b, c, d, etc.) indicated significant differences between the groups, p < 0.05.
mRNA expression was detected by quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR results showed that compared with bronchial epithelial cell (BEAS-2B cell) group, TGF-β1, Smad, SIRT1, PGC-1α, TFAM, NRF-1, α-SMA, Vimentin, E-cadherin, CK18, ZO-1, EGF, Slug, ZEB, Snail, STAT3, STAT1, IL-1β, TNF-α, IL-2, and IL-10 genes expression were significantly increased (p < 0.05), and N-cadherin, AQP5, IL-6 genes expression were significantly decreased (p < 0.05) in A549 cell control group, while Twist gene showed non-significant differences (p > 0.05). Compared with A549 cell control group, TGF-β1, Smad, α-SMA, Vimentin, N-cadherin, E-cadherin, ZO-1, EGF, Slug, ZEB, STAT3, SIRT1, PGC-1α, TFAM, NRF-1, IL-1β, TNF-α, IL-2, and IL-10 genes expression were significantly decreased (p < 0.05), and IL-6 gene was significantly increased (p < 0.05) in Osthole treated group (0.02 mg/mL), while AQP5, CK18, Twist, Snail, STAT1 genes showed non-significant differences (Fig. 7, p > 0.05).
mRNA expression was detected by RNA-seq
In addition, we also performed RNA-seq. Compared with BEAS-2B cell group, RNA-seq results showed that TGF-β1, Smad, E-cadherin, ZO-1, EGF, IL-2 and IL-10 genes expression were significantly increased (p < 0.05), and Vimentin, N-cadherin, AQP5, Slug, ZEB, STAT1, SIRT1, TFAM, NRF-1, IL-1β, IL-6 genes expression were significantly decreased (p < 0.05) in A549 cell control group, while α-SMA, Twist, STAT3, PGC-1α and TNF-α genes showed non-significant differences (p > 0.05). Compared with A549 cell control group, Smad, N-cadherin, E-cadherin, ZO-1, EGF, STAT3, TFAM, IL-2, IL-10 genes expression were decreased significantly (p < 0.05), and TGF-β1, α-SMA, STAT1, IL-6 genes were increased significantly (p < 0.05) in Osthole treated group (0.02 mg/mL), while Vimentin, AQP5, Slug, ZEB, Twist, SIRT1, PGC-1α, NRF-1, IL-1β, TNF-α genes showed non-significant differences (Fig. 8, p > 0.05). However, the results of RNA-seq were not completely consistent with those of qRT-PCR. Trends were consistent only in Smad, N-cadherin, E-cadherin, AQP5, ZO-1, EGF, IL-2, IL-6, IL-10 genes.
Identification of differentially expresses genes (DEGs)
PCA (Fig. 9A) demonstrated significant differences among groups B (BEAS-2B group), A (A549 cell group) and O (Osthole treated group). DEGs were defined using the criteria of |log2FC| ≥ 1 and padjust < 0.05 for demarcation. Venn diagram showed that there were 902 DEGs shared by groups B, A and O (Fig. 9B). Compared with group B, a total of 7595 DEGs were identified in group A, of which 3237 were up-regulated and 4358 down-regulated genes. Compared with group A, a total of 2192 DEGs were identified in group O, of which 1009 were up-regulated and 1183 down-regulated genes. Compared with group B, a total of 8613 DEGs were identified in group O, of which 4802 were up-regulated and 3811 down-regulated genes, and the volcano plot in Fig. 9C-E were shown.
PCA analysis plot, Venn analysis plot, Volcano plot of DEGs. B stands for BEAS-2B group, A stands for A549 cell group, O stands for Osthole treated group (0.02 mg/mL). (A) PCA analysis plot of groups B, A and O. (B) Identification of overlapping DEGs of group B, A and O. 902 overlapping DEGs were obtained from the Venn analysis plot. (C) Volcano plot of group B and A DEGs. (D) Volcano plot of group O and A DEGs. (E) Volcano plot of group O and B DEGs. Gray indicates up-regulated genes, blue indicates down-regulated genes. |log2FC| ≥ 1 and padjust < 0.05.
Cluster analysis heat plot and protein–protein interaction (PPI) network
The cluster analysis heat plot of mRNAs was shown in Fig. 10A. 25 genes were searched in the online String platform for protein interaction analysis, and a PPI network (Fig. 10B) was constructed. The results showed 25 nodes and 159 interaction relationships, with an average of 12.7 average degree value.
Cluster analysis heat plot, PPI network, GO, KEGG pathway annotation and enrichment analysis. B stands for BEAS-2B group, A stands for A549 cell group, O stands for Osthole treated group (0.02 mg/mL). (A) Each column represents a sample. Red indicates up-regulated genes, blue indicates down-regulated genes. |log2FC| ≥ 1 and padjust < 0.05. (B) Protein interaction analysis was performed on 25 genes, and 25 nodes were obtained. The more connections, the more important it is. GO annotation (C), KEGG annotation (D), GO enrichment (E) and KEGG enrichment (F) analysis of 25 genes.
GO, KEGG pathway annotation and enrichment analysis
25 genes were annotated for using GO and KEGG databases for further analysis. In the three main categories of GO classification consisting of MF, CC and BP, binding (GO:0005488), organelle (GO:0043226), cell part (GO:0044464), and cellular process (GO:0009987) were dominant (Fig. 10C). According to KEGG analysis, these genes had been annotated in Cancer: overview, Immune system, Cell growth and death, and Signal transduction (Fig. 10D). The top 3 GO enrichment pathways included positive regulation of transcription, DNA-templated, positive regulation of nucleic acid-templated transcription, and positive regulation of RNA biosynthetic process, which were associated with the occurrence and development of lung cancer (Fig. 10E). KEGG enrichment results showed that the 25 genes were mainly enriched in Th17 cell differentiation, C-type lectin receptor signaling pathway, FoxO signaling pathway, and JAK-STAT signaling pathway, which were associated with cell proliferation, apoptosis and immunity (Fig. 10F).
Discussion
Although great progress has been made in basic and clinical research, cancer is still the main cause of deaths throughout the world. Tumor metastasis is one of the main factors that make it difficult to cure44, reduce the annual survival rate and increase the recurrence rate9. Natural compounds are nature presents with a valuable wealth and material sources to prevent and treat human diseases. In drug development and clinical therapy, natural compounds extracted from medicinal plants have the effect that cannot be ignored in the treatment of diseases including cancer45,46. The aim of this study was to screen out the optimal anti-cancer natural compounds and to highlight the potential biomarkers involved. In comparison with other research results, this study applied multiple experimental methods to explore the anti-lung cancer effects of natural compounds in a more systematic and comprehensive way, which will provide a foundation for the development of safe, efficient and novel compounds with anti-lung cancer effects.
In view of the potential anti-tumor activity of natural compounds in clinical treatment, eight natural compounds including Curcumin, Osthole, Curcumol, Baicalin, Paeonol, Glycyrrhizic acid, Tea saponin and Cepharanthine were selected and purchased commercially. According to the experimental design, selected compounds were gradually explored against A549 cells activity. Finally, the optimal compound against A549 cells was selected in eight natural compounds. Meanwhile, Cisplatin47,48, the most commonly used broad-spectrum anti-cancer drug, was selected as a positive control drug, and the feasibility of the trial results was evaluated by the degree of drug action in each group.
Based on the results of proliferation, wound healing and matrigel invasion assays, Osthole at 0.02, 0.01 and 0.005 mg/mL, Paeonol at 0.25, 0.125, and 0.0625 mg/mL, Curcumin at 0.02 mg/mL and Cepharanthine at 0.005 mg/mL showed significant inhibitory effect against A549 cells in a dose-dependent manner. Numerous studies have confirmed that Osthole possesses anti-cancer activities, including colorectal cancer49, ovarian cancer50, prostate cancer51 and so on52. Paeonol inhibits cell proliferation, migration and invasion53. Curcumin inhibits the proliferation of A549 cells by down-regulating lncRNA UCA154, and the migration and invasion of A549 cells through suppression of PI3K/AKT/mTOR signaling pathway55. Cepharanthine also possesses antitumor activity in multiple types of cancer, including liver cancer56 via activating a number of oncogenic pathways or signaling nodes such as NF‑κB, multidrug resistance protein 1 (MRP1), PI3K/Akt, AMPK, JNK1/2, and DNA damage repair57. Cepharanthine treatment results in the inhibition of cell viability, migration, and proliferation in HCC cells in a dose-dependent manner58. Our experimental results are consistent with those reported by the above mentioned studies.
Previous studies have shown that natural compounds induce apoptosis of A549 cells59 and human breast cancer cells60. Annexin-V positive cell percentage is a key marker of cell apoptosis43. Mitochondria have a central role in cell apoptosis with loss of mitochondrial MMP is its marker of apoptosis61,62. In present study, the values of cell apoptosis and MMP were detected by Flow cytometry using Annexin V/PI double staining and JC-1 detection kit. The results showed that compared with A549 cell control group, Osthole, Curcumin, Cepharanthine and Paeonol promoted MMP value, which was consistent with the findings of Yao et al.63. In this study, MMP value in the Osthole and Cepharanthine treatment group were gradually decreased, while MMP value in the Curcumin and Paeonol treatment group were gradually increased with the decrease of drugs concentration. Therefore, Curcumin and Paeonol may promote apoptosis through multiple apoptotic pathways including the mitochondrial pathway. At the same time, we speculate that Osthole promotes A549 cells apoptosis through the mitochondrial pathway.
Mitochondrial dysfunction occurs in many lung diseases, and its structure and function are mainly reflected by MMP, ROS and mitochondrial morphology20,61. To further evaluate whether ROS was involved in the process of mitochondrial apoptosis promoted by the tested compounds, the values of ROS were analyzed by Flow cytometry using ROS staining kit. Compared with the A549 cell control group, Osthole, Curcumin, Cepharanthine and Paeonol treatment groups promoted ROS production, which were inconsistent with the results of Yao et al.63. It may be due to the inconsistent drug concentration and effect of the drug itself on A549 cells. However, with the increase of drug concentration, ROS values in Osthole and Paeonol treatment groups were down-regulated, while Curcumin and Cepharanthine were not. These results suggested that Osthole not only promoted mitochondrial apoptosis, but also inhibited the release of ROS. These results were consistent with the previous reports64,65. In order to further investigate the inhibitory effect of Osthole on A549 cells Flow cytometry was utilized to determine cell cycle. Our results showed that Osthole induced cell cycle arrest. Therefore, we speculated that Osthole was the optimal natural compound in eight natural compounds against A549 cells which downregulate ROS through mitochondrial pathway, induce cell cycle arrest, promote cell apoptosis, and thus exert its ability to inhibit A549 cell proliferation, invasion, and migration.
Literature review showed that Osthole promoted apoptosis of A549 cells66. It was found35,67 that Osthole inhibited the proliferation of A549 cells, induced G2/M block and cell apoptosis. Xu et al.68 found that the growth, proliferation, migration and invasion of A549 cells were inhibited by Osthole treatment. Although studies have shown an inhibitory effect of Osthole on A549 cells, still the above studies were partial. However, in this study, Cisplatin was used as a positive control to elucidate the effect of selected compounds on the proliferation, migration and invasion of A549 cells using MTT, wound healing and invasion assays, respectively. Meanwhile, AnnexinV-FITC/PI, JC-1, ROS and Cell Cycle Kits were used to detect the pro-apoptotic mechanism of A549 cells induced by tested compounds. The results of our study explained the effect of Osthole on A549 cells in a more comprehensive and systematic way.
In order to further explore the hidden molecular mechanism by which Osthole inhibited proliferation, invasion and migration of A549 cells, qRT-PCR and RNA-seq were used to detect the expression levels of related genes. The results of the two methods were consistent only in Smad, N-cadherin, E-cadherin, AQP5, ZO-1, EGF, IL-2, IL-6, IL-10 genes. In the present study, GO enrichment revealed that these genes were primarily associated with positive regulation of transcription, DNA-templated, positive regulation of nucleic acid-templated transcription, and positive regulation of RNA biosynthetic process. KEGG enrichment results showed that 25 genes were mainly enriched in Th17 cell differentiation, C-type lectin receptor signaling pathway, FoxO signaling pathway, and JAK-STAT signaling pathway. Studies have shown that these pathways are related to cell immunity69, apoptosis, proliferation70, metastasis and invasion15,71. Therefore, we speculated that 25 genes were involved in immunity, apoptosis, proliferation, metastasis and invasion. In this study, EGF gene was enriched in FoxO and JAK-STAT signaling pathways, and the expression level of EGF was significantly increased in A549 cell control group compared with BEAS-2B cell group, while significantly decreased in Osthole treated group compared with A549 cell control group by qRT-PCR and RNA-seq. Studies have shown that inducing EGF gene expression may upregulate ROS17, inhibit cell apoptosis, induce cell proliferation72, promote EMT and enhance cell migration and invasion ability73. These results demonstrated that Osthole may inhibit EGF gene expression through FoxO and JAX-STAT signaling pathways, downregulate ROS, promote apoptosis and inhibit proliferation of A549 cells, inhibit the development of EMT and metastasis and invasion processes. Therefore, we speculate that the EGF gene has the potential to be used as a biomarker for the treatment of A549 cells with Osthole. In addition, our RNA-seq results showed that IL-2, IL-6 and IL-10 genes were enriched in Th17 cell differentiation and C-type lectin receptor signaling pathway. The results of qRT-PCR and RNA-seq showed that compared with BEAS-2B cell group, IL-2 and IL-10 genes expression were significantly increased, and IL-6 genes expression were significantly decreased in A549 cell control group. Compared with A549 cell control group, the expression levels of IL-2 and IL-10 genes were significantly decreased, and the expression levels of IL-6 gene were significantly increased in Osthole treated group, suggesting that Osthole may inhibit the expression of IL-2 and IL-10 genes, promote the expression of IL-6 gene, affect the inflammatory and immune processes of A549 cells.
Conclusions
In summary, this study demonstrated that Osthole was the optimal natural compound against A549 cells in eight natural compounds which played an important role to inhibit the proliferation, migration and invasion of A549 cells by promoting mitochondrial apoptosis and inhibiting release of ROS, and affect the inflammatory and immune processes of A549 cells. EGF gene had the potential to be develop as a biomarker for Osthole against the therapy of A549 lung cancer cells, which was involved in mitochondrial apoptosis and ROS down-regulation, inhibition of proliferation and EMT, inflammation and immune processes in A549 cells (Fig. 11).
A biomarker of Osthole inhibiting proliferation, EMT, inflammatory and immune of A549 cells. EGF gene is involved in mitochondrial apoptosis and ROS down-regulation, inhibition of proliferation and EMT, inflammation and immune processes in A549 cells, has the potential to be a biomarker for Osthole the therapy of A549 lung cancer cells.
Materials and methods
Natural compounds
The selected compounds including Curcumin, Osthole, Curcumol, Baicalin, Paeonol, Cepharanthine, Glycyrrhizic acid and Tea saponin were purchased from Nanjing Zelang Biotechnology Co., Ltd, China. Cisplatin with ≥ 98.5% purity was used as a positive control drug, purchased from Beijing Solarbio Technology Co., Ltd, China. The chemical structures of selected eight compounds were shown in Fig. 1A. Compounds information including chemical name, purity, classification, main plant source, cosolvent and Lot number were shown in Table 2.
Cell culture
A549 and BEAS-2B cells purchased from China Center for Type Culture Collection (CCTCC, Wuhan, China) were cultured at 37℃ with 5% CO2, passaged in Dulbecco’s modified eagle’s medium (DMEM, Hyclone, USA), containing 10% fetal bovine serum (FBS, BI, Israel, 10% DMEM). The cells were maintained in DMEM containing 2% FBS (2% DMEM) and preserved in liquid nitrogen in the laboratory. When the cells were 80% confluent, the cells were trypsinized, centrifuged and mixed into suspension for subsequent tests.
Reagents
The reagents used were: DMEM (Hyclone, USA, Cat. No. SH30022.01), FBS (BI, Israel, Cat. No. 04-001-1 A), DMSO (Solarbio, China, Cat. No. D8371), MTT (Solarbio, China, Cat. No. 298-93-1), 0.1% Crystal violet solution (Solarbio, China, Cat. No. G1064), Annexin V-FITC/PI kit (Keygen Biotech, China, Cat. No. KGA107), Mitochondrial membrane potential assay kit with JC-1 (Beyotime, China, Cat. No. C2006), Reactive Oxygen Species (ROS) assay kit (Beyotime, China, Cat. No. S0033Ss).
Cytotoxicity assay
The viability of A549 cells were assessed with MTT (4 mg/mL in PBS)74. 1.0 × 106 cells/mL were seeded into 96-well plates (100 µL/well). After 24 h of incubation, when the cells were 80% confluent, each of the eight tested compounds were sequentially diluted up to 8–10 concentrations by twofold dilution and 100 µL of each diluent were respectively added to each well according to the experimental design. The cell control and Cisplatin (positive control drug) groups were established at the same time. Each experiment was repeated thrice with four replicas for each concentration. The dissolution method of 8 tested compounds and Cisplatin were shown in Table 2.
The cytopathological effect (CPE) was monitored under microscope and photographed (DMIL LED, LEICA, Germany) with proper intervals. After incubation at 37℃ with 5% CO2 for 72 h, the cells were treated with 20 µL MTT and incubated again for 4 h. The medium was discarded and the resulting formazan crystals were dissolved in 150 µL of DMSO and incubated again for 30 min. The generated color was quantified by measuring the optical density (OD) value at 490 nm using a microplate spectrophotometric microtiter plate reader (EnSpire™2300, Thermo, USA). MNTC was calculated according to the formula of Cytopathic ratio (CR) = [(OD control - OD test)/ OD] ×100%. The data of CR was then analyzed by GraphPad PrismTM5.0 soft (Inc. California, USA) to determine the CC50 of each of the 8 tested compounds and Cisplatin against A549 cells. The MNTC and CC50 on A549 cells were calculated using GraphPad Prism™ 5.0.
Cell proliferation assay
The proliferative rate of A549 cells was detected by MTT assay74. The MNTC value for each of the 8 tested compounds and Cisplatin were determined and two-fold serial dilution of each compound (high, medium, and low concentration) were made with 2% DMEM. The A549 cells were seeded in 96-well plates (100 µL/well) and incubated for 24 h at 37°C with 5% CO2. When A549 cells of the 96-well plates were confluent up to 80%, the cells were treated with three MNTC concentrations of Curcumin (0.02, 0.01, 0.05), Osthole (0.02, 0.01, 0.05), Baicalin (1.0, 0.5, 0.25), Paeonol (0.25, 0.125, 0.0625), Cepharanthine (0.005, 0.0025, 0.00125) and Tea saponin (0.02, 0.01, 0.005). The positive control (Cisplatin) and the Control groups were treated with 0.016 Cisplatin and 2% DMEM, respectively. All the concentrations were in mg/mL with 100 µL/well. After 24 h, 48 h and 72 h of incubation with 37°C and 5% CO2, cells were treated with MTT and DMSO as described in the MTT assay. Cell proliferation rate at different time points were calculated according to the OD value and using the equation: cell proliferation (CR)= [ 1- (OD control - OD test) / OD] ×100%[23, 24,75,76. The cell proliferation histograms were generated through GraphPad Prism™ 5.0 software. Each experiment was performed in triplicate with three replicas in each group.
Wound healing assay
Wound-healing assay was performed to evaluate the effect of selected compounds and Cisplatin on A549 cells migration capability in 2D space77. Briefly, the A549 cells at mid-log phase were seeded at a density of 1 × 106 cells/mL in 6-well plate (2 mL/well) and cultured till the cells were 100% confluent. With a 200 µL pipette tip, a straight scratch was produced by denuding strips of the cell monolayer. The supernatant was discarded, cells were gently washed three times with PBS and were treated with high, medium and low concentrations of each selected compound for 24, 48 and 72 h with A549 cells and Cisplatin used as control groups. Post incubated at 37°C and 5% CO2, wound healing images were photographed at the above mentioned time intervals. The equipment used for wound healing images and Invasion images acquisition was LEICA DMiL LED (objective lenses: N PLAN 10X/0.25). These micrographs were photographed at a resolution of 1024*768 with the cameras of DFC295, and the image acquisition was obtained using LAS X software, which enhanced the images to 300 dpi. The lamp used for transmitted light BF- contrast imaging was 10 W LED illumination. The migrated distance and repaired area of the scratched area by A549 cells were quantitated and compared with that of the control groups using Image J software. Three independent experiments were performed in triplicate.
Invasion assay
Transwell chamber (Corning, China) was used to investigate the anti-invasion properties of the selected compounds using A549 cells in the 3D gel78. Briefly, Matrigel (Corning, China) was completely dissolve at 4 °C overnight, 1 mL and 200 µL of pipette tips, 1.5 mL EP tube and transwell-24 cell culture plate were placed in the refrigerator for pre-cooling. Performing all the steps on ice, Matrigel was diluted with cold DMEM (1:10), 30 µL of the diluted Matrigel was poured vertically in the upper portion of each transwell chamber and incubated at 37 °C with 5% CO2 for 2 h so that Matrigel could be gathered and coat the transwell upper portion. 500 µL of 20% FBS medium was added to the bottom chamber and 100 µL of the A549 cell suspension was seeded in the upper portion of transwell chamber at a density of 2.5 × 105 cells/mL (100 µL/well). At the same time, high, medium and low concentrations of each tested compound (100 µL/well) were successively added into the upper chamber of transwells. Cisplatin and DMEM were added into the positive control and cell control groups, respectively. Post 48 h of incubation, the medium was discarded, washed three times with PBS, and the residual cells on upper surface of well were gently removed using cotton swab. The cells on the lower portion of the upper chamber were fixed with cold methanol before staining with 0.1% crystal violet for 30 min at room temperature. In each experiment, the number of migrated cells on the underside of the filter in five random microscopic fields were counted and photographed under microscope (100X). The specific information on Invasion images acquisition and processing was the same as the wound healing test. Besides, fluorescein isothiocyanate (FITC)-labeled cells were sequentially excited with a WU excitation block and a WB excitation. Mercury lamp was used for reflection of fluorescence imaging. Each in dependent experiment was performed in triplicate with three replicas in each group.
Apoptosis with a flow cytometer
Annexin V-FITC/PI assay was used to evaluate the apoptotic effects of the 4 tested compounds and Cisplatin on A549 cells79. When the cells were confluent up to 80%, high, medium and low concentrations of each compound, Cisplatin and 2% DMEM (cell control) were successively added to the 6-well plate. The cells of each group were incubated for 48 h at 37°Cand 5% CO2. Cell samples were collected and washed twice with PBS, then cells were suspended by adding 500 µL buffer, and mixed with 5 µL Annexin V-FITC, then mixed with 5 µL propidium lodide and incubated for 15 min in the dark. Data were analyzed and plotted by Flow cytometry. Each experiment was performed in triplicate.
Mitochondrial membrane potential (MMP) by JC-1
JC-1 assay kit was used to evaluate changes of MMP of A549 cells treated with four tested compounds and Cisplatin79. When the cells were 80% confluent in the 6-well plate, the supernatant was discarded, and compounds of high, medium and low concentration were added with cell control and Cisplatin positive control groups. Post incubation of 48 h, cells were collected and centrifuged. According to the manufacturer instructions, 0.5 mL of working solution was added at one time, mixed upside down for several times and incubated at 37°C for 20 min. The cells were then washed with JC-1 staining buffer, centrifuged resuspended and analyzed by Flow cytometry.
ROS by flow cytometer assay
ROS of A549 cells treated with compounds and Cisplatin were detected by Flow cytometry80. When cells in the 6-well plates were 80% confluent, the four tested compounds were added successively with cell control and Cisplatin control groups set at the same time. Post 48 h of incubation, cell samples were collected and treated with ROS assay kit. 1 mL of diluted fluorescent probe DCFH-DA was added successively, incubated at 37°C for 20 min and washed with serum-free medium 3 times. Intracellular ROS levels were measured by measuring the fluorescence of DCF using Flow cytometer.
Cell cycle by flow cytometer assay
In order to further explore the mechanism of A549 cell apoptosis promoted by Osthole, the cell cycle was detected by Flow cytometry81. A549 lung cancer cells at a density of 1.25 × 105 cells /mL were seeded in 6-well plates (2.5 × 105 cells/well) and cultured until a monolayer was formed at 37℃ with 5% CO2 for 24 h. Post incubation, 2% DMEM and Osthole (0.02, 0.01, 0.05) were added (2 mL/well) for 48 h. Each experiment was repeated three times with three repetitions per concentration. The adherent cells were trypsinized and released from the monolayer using EDTA-free pancreatic enzyme digestion solution, harvested and centrifuged. The Cell Cycle Analysis Kit and Flow cytometry were utilized to detect cell cycle in each group.
Quantitative real-time polymerase chain reaction (qRT-PCR)
A549 cells were cultured according to the experimental groups. After the cells were confluent, they were trypsinized, collected and total RNA was isolated with the TRIzol reagent (Takara, Kusatsu, Japan). Reverse transcription was performed with a PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Kusatsu, Japan). The obtained cDNA was subjected to qRT-PCR for holding at 95°C for 10 min followed by up to 40 cycles at 95°C for 15 s, 50–60°C for 30 s, and 72°C for 30 s using 2 × SYBR Green qPCR Master Mix (Bimake.cn, Munich, Germany). To confirm the specificity of the amplification reaction, after the last amplification cycle a melting curve was drawn. The mRNA level was quantified with the LightCycler® 96 System (Roche, Basel, Switzerland). The relative expression level of RNAs was analyzed with the 2–ΔΔCT method. The sequences of primers used are listed in Table 3.
RNA sequencing (RNA-seq) and PPI network analysis
The samples were provided to Majorbio for transcriptome sequencing. Sequencing data uploaded to NCBI repository (https://www.ncbi.nlm.nih.gov/) with the accession number PRJNA1027498. The data of RNA-seq were analyzed using the online platform of Majorbio Cloud Platform (www.majorbio.com). The PCA analysis plot, Venn analysis plot, Volcano plot, Cluster analysis heat plot, GO and KEGG functional annotation plot, GO and KEGG pathway enrichment plot of mRNAs were drawn using this platform. The GO describes the biological function of genes that were subdivided into molecular function (MF), cellular component (CC) and biological process (BP). KEGG serves as a comprehensive resource database that integrates information on genes, chemistry, and system function through bioinformatics82,83. The interactions between proteins were analyzed with String online platform (https://string-db.org/).
Statistical analysis
Chemical formulas were drawn by KingDraw software (http://www.kingdraw.com/). CC50 was calculated using nonlinear regression and the results of “log (inhibitor) vs. response-variable slope” were analyzed using GraphPad PrismTM5.0 (GraphPad Software, Inc. California, USA). The images captured with the help of a microscope for wound healing and invasion assays were analyzed with Image J (National Institutes of Health, USA). Data of MTT, cell proliferation, wound healing, invasion, Annexin V-FITC/PI, JC-1 assay and qRT-PCR were analyzed by GraphPad PrismTM5.0 and the data were represented as the mean ± standard error of the mean (SEM) of at least 3 repeated experiments (n = 3). Different letters (a, b, c, d, etc.) indicate statistically significant difference to other groups (p ˂ 0.05), and significant probability values were indicated as * p < 0.05, ** p < 0.01, and *** p < 0.001. The data of RNA-seq were performed using the online platform of Majorbio Cloud Platform (www.majorbio.com). The PPI network was constructed using the String database.
Data availability
The data generated or analysed during this study are included in this published article and its supplementary information files. In addition, the datasets generated and/or analysed during the current study are available in the NCBI (https://www.ncbi.nlm.nih.gov) repository. The accession number is PRJNA1027498.
Abbreviations
- A549:
-
Human lung adenocarcinoma cell line
- CPE:
-
Cytopathologic effect
- CR:
-
Cytopathic ratio
- CC50 :
-
50% cytotoxic concentration
- EMT:
-
Epithelial mesenchymal transition
- MTT:
-
3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MNTC:
-
Maximum no-cytotoxic concentration
- MSC:
-
The maximum solubility concentration
- MMP:
-
Mitochondrial membrane potential
- OD:
-
Optical density
- PPI:
-
Network protein–protein interaction network
- ROS:
-
Reactive oxygen species
- RNA-seq:
-
RNA sequencing
References
Sun, Y. Q. et al. METTL3 promotes chemoresistance in small cell lung cancer by inducing mitophagy. J. Exp. Clin. Cancer Res. 42, 65–84. https://doi.org/10.1186/s13046-023-02638-9 (2023).
Yang, C. et al. EHMT2-mediated transcriptional reprogramming drives neuroendocrine transformation in non-small cell lung cancer. Proc. Natl. Acad. Sci. U S A. 121, e2317790121. https://doi.org/10.1073/pnas.2317790121 (2024).
Casagrande, G. M. S., Silva, M. O., Reis, R. M. & Leal, L. F. Liquid biopsy for lung cancer: Up-to-date and perspectives for screening programs. Int. J. Mol. Sci. 24, 2505–2527. https://doi.org/10.3390/ijms24032505 (2023).
Gettinger, S. et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J. Clin. Oncol. 36, 1675–1684. https://doi.org/10.1200/JCO.2017.77.0412 (2018).
Hirsch, F. R. et al. Lung cancer: current therapies and new targeted treatments. Lancet 389, 299–311. https://doi.org/10.1016/S0140-6736(16)30958-8 (2017).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Preuss, K. et al. Using quantitative imaging for personalized medicine in pancreatic cancer: A review of radiomics and deep learning applications. Cancers (Basel). 14, 1654–1687. https://doi.org/10.3390/cancers14071654 (2022).
Kimbrough, E. O. et al. Sex-and age-associated differences in genomic alterations among patients with advanced non-small cell lung cancer (NSCLC). Cancers (Basel). 16, 2366–2379. https://doi.org/10.3390/cancers16132366 (2024).
Yue, J. et al. The multifaceted mechanisms of dihydrotanshinone I in the treatment of tumors. Biomed. Pharmacother. 175, 116635–116645. https://doi.org/10.1016/j.biopha.2024.116635 (2024).
Ang, H. L. et al. Mechanism of epithelial-mesenchymal transition in cancer and its regulation by natural compounds. Med. Res. Rev. 43, 1141–1200. https://doi.org/10.1002/med.21948 (2023).
Sun, Y. et al. Resveratrol inhibits the migration and metastasis of MDA-MB-231 human breast cancer by reversing TGF-β1-induced epithelial-mesenchymal transition. Molecules 24, 1131–1147. https://doi.org/10.3390/molecules24061131 (2019).
He, S. Y. et al. MiR-199a-3p/5p participated in TGF-β and EGF induced EMT by targeting DUSP5/MAP3K11 in pterygium. J. Transl Med. 18, 332–351. https://doi.org/10.1186/s12967-020-02499-2 (2020).
Xu, F. Y., Liu, C. W., Zhou, D. D. & Zhang, L. TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 64, 157–167 (2016).
Shi, P. et al. The flavonoids from the fruits of Psoralea corylifolia and their potential in inhibiting metastasis of human non-small cell lung cancers. Bioorg. Chem. 150, 107604–107618. https://doi.org/10.1016/j.bioorg.2024.107604 (2024).
Duan, M. L. et al. Obovatol inhibits the growth and aggressiveness of tongue squamous cell carcinoma through regulation of the EGFmediated JAKSTAT signaling pathway. Mol. Med. Rep. 18, 1651–1659. https://doi.org/10.3892/mmr.2018.9078 (2018).
Okon, I. S., Coughlan, K. A., Zhang, M., Wang, Q. X. & Zou, M. H. Gefitinib-mediated reactive oxygen specie (ROS) instigates mitochondrial dysfunction and drug resistance in lung cancer cells. J. Biol. Chem. 290, 9101–9110. https://doi.org/10.1074/jbc.M114.631580 (2015).
Huo, Y. N. et al. The protective effect of EGF-activated ROS in human corneal epithelial cells by inducing mitochondrial autophagy via activation TRPM2. J. Cell. Physiol. 235, 7018–7029. https://doi.org/10.1002/jcp.29597 (2020).
Zhao, Y. et al. NAD + improves cognitive function and reduces neuroinflammation by ameliorating mitochondrial damage and decreasing ROS production in chronic cerebral hypoperfusion models through Sirt1/PGC-1α pathway. J. Neuroinflammation. 18, 207–223 (2021).
Scarpulla, R. C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 88, 611–638. https://doi.org/10.1186/s12974-021-02250-8 (2008).
Zhao, P. et al. Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int. J. Mol. Med. 36, 633–644. https://doi.org/10.3892/ijmm.2015.2260 (2015).
Wang, Q. et al. Evaluation of Cross-Talk and alleviate potential of cytotoxic factors induced by deoxynivalenol in IPEC-J2 cells interference with curcumin. Int. J. Mol. Sci. 25, 6984–7004. https://doi.org/10.3390/ijms25136984 (2024).
Dai, J. P. et al. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int. Immunopharmacol. 54, 177–187. https://doi.org/10.1016/j.intimp.2017.11.009 (2018).
Niu, Y. J., Dong, Q. M. & Li, R. H. Matrine regulates Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating the NF-κB signaling. Cell. Biol. Int. 41, 611–621. https://doi.org/10.1002/cbin.10763 (2017).
Xu, Y. L. et al. Matrine combined with osthole inhibited the PERK apoptosis of splenic lymphocytes in PCV2-infected mice model. BMC Vet. Res. 19, 26–40. https://doi.org/10.1186/s12917-023-03581-9 (2023).
Xu, Y. L. et al. The combined usage of matrine and osthole inhibited endoplasmic reticulum apoptosis induced by PCV2. BMC Microbiol. 20, 303–316. https://doi.org/10.1186/s12866-020-01986-2 (2020).
Xu, Y. L. et al. Cepharanthine and crcumin inhibited mitochondrial apoptosis induced by PCV2. BMC Vet. Res. 16, 345–356. https://doi.org/10.1186/s12917-020-02568-0 (2020).
Ma, Q. et al. Versatile function of NF-ĸB in inflammation and cancer. Exp. Hematol. Oncol. 13, 68–96. https://doi.org/10.1186/s40164-024-00529-z (2024).
Wang, Y. F., Dan, K., Xue, X. L., Chen, B. T. & Chen, C. Curcumin assists anti-EV71 activity of IFN-α by inhibiting IFNAR1 reduction in SH-SY5Y cells. Gut Pathog. 14, 8–17. https://doi.org/10.1186/s13099-022-00481-5 (2022).
Boy, H. I. A. et al. Recommended medicinal plants as source of natural products: a review. Digit. Chin. Medi. 1, 131–142. https://doi.org/10.1016/S2589-3777(19)30018-7 (2018).
Karthika, C. et al. Curcumin as a great contributor for the treatment and mitigation of colorectal cancer. Exp. Gerontol. 152, 111438–111449. https://doi.org/10.1016/j.exger.2021.111438 (2021).
Ros, M. et al. Inhibition of cancer stem-like cells by curcumin and other polyphenol derivatives in MDA-MB-231 TNBC cells. Int. J. Mol. Sci. 25, 7446–7467. https://doi.org/10.3390/ijms25137446 (2024).
Wang, H. et al. Glycyrrhizic acid inhibits proliferation of gastric cancer cells by inducing cell cycle arrest and apoptosis. Cancer Manag Res. 12, 2853–2861. https://doi.org/10.2147/CMAR.S244481 (2020).
Wang, W. S. et al. DNA damage and NF-κB inactivation implicate glycyrrhizic acid-induced G1 phase arrest in hepatocellular carcinoma cells. J. Food Biochem. 46, e14128. https://doi.org/10.1111/jfbc.14128 (2022).
Jia, L. Y. et al. Inhibitory effects of total triterpenoid saponins isolated from the seeds of the tea plant (Camellia sinensis) on human ovarian cancer cells. Molecules 22, 1649–1665. https://doi.org/10.3390/molecules22101649 (2017).
Xu, X. M., Zhang, M. L., Zhang, Y. & Zhao, L. Osthole induces lung cancer cell apoptosis through Inhibition of inhibitor of apoptosis family proteins. Oncol. Lett. 12, 3779–3784. https://doi.org/10.3892/ol.2016.5170 (2016).
Sui, X. B. et al. Baicalin induces apoptosis and suppresses the cell cycle progression of lung cancer cells through downregulating Akt/mTOR signaling pathway. Front. Mol. Biosci. 7, 602282–602291. https://doi.org/10.3389/fmolb.2020.602282 (2021).
Ma, C. J. et al. Curcumol repressed cell proliferation and angiogenesis via SP1/mir-125b-5p/VEGFA axis in non-small cell lung cancer. Front. Pharmacol. 13, 1044115–1044130. https://doi.org/10.3389/fphar.2022.1044115 (2022).
Lv, J. et al. Paeonol inhibits human lung cancer cell viability and metastasis in vitro via miR-126-5p/ZEB2 axis. Drug Dev. Res. 83, 432–446. https://doi.org/10.1002/ddr.21873 (2022).
Li, G., Qiao, K., Xu, X. & Wang, C. Cepharanthine regulates autophagy via activating the p38 signaling pathway in lung adenocarcinoma cells. Anti-cancer Agents Med. Chem. 22, 1523–1529. https://doi.org/10.2174/1871520621666210903163407 (2022).
Kitai, H. et al. Combined inhibition of KRASG12C and mTORC1 kinase is synergistic in non-small cell lung cancer. Nat. Commun. 15, 6076–6091. https://doi.org/10.1038/s41467-024-50063-z (2024).
Yu, X. Q. et al. Chamaejasmenin E from Stellera Chamaejasme induces apoptosis of hepatocellular carcinoma cells by targeting c-Met in vitro and in vivo. Bioorg. Chem. 119, e105509. https://doi.org/10.1016/j.bioorg.2021.105509 (2022).
Khan, A. et al. Recombinant porcine NK-lysin inhibits the invasion of hepatocellular carcinoma cells in vitro. Int. J. Biol. Macromol. 140, 1249–1259. https://doi.org/10.1016/j.ijbiomac.2019.08.212 (2019).
Saddiq, A. A. et al. Curcumin, thymoquinone, and 3, 3’-diindolylmethane combinations attenuate lung and liver cancers progression. Front. Pharmacol. 13, e936996. https://doi.org/10.3389/fphar.2022.936996 (2022).
Alshehri, A. et al. In vitro evaluation of antioxidant, anticancer, and anti-inflammatory activities of ethanolic leaf extract of adenium obesum. Front. Pharmacol. 13, e847534. https://doi.org/10.3389/fphar.2022.847534 (2022).
Yi, J. J. et al. Potential of natural products as radioprotectors and radiosensitizers: opportunities and challenges. Food Funct. 12, 5204–5218. https://doi.org/10.1039/d1fo00525a (2021).
Clark, A. M. Natural products as a resource for new drugs. Pharm. Res. 13, 1133–1144. https://doi.org/10.1023/a:1016091631721 (1996).
Yildirim, A., Fidan, E. & Ozdemir, F. Hearing loss after the first low dose administration of cisplatin. J. Coll. Physicians Surg. Pak. 32, 1216–1218. https://doi.org/10.29271/jcpsp.2022.09.1216 (2022).
Eskicioglu, H. E. et al. Comparison of cytotoxic and ototoxic effects of lipoplatin and cisplatin in neuroblastoma in vivo tumor model. J. Int. Adv. Otol. 18, 392–398. https://doi.org/10.5152/iao.2022.21268 (2022).
Song, J., Ham, J., Park, W., Song, G. & Lim, W. Osthole impairs mitochondrial metabolism and the autophagic flux in colorectal cancer. Phytomedicine 125, 155383–155396. https://doi.org/10.1016/j.phymed.2024.155383 (2024).
Bae, H., Lee, J. Y., Song, J., Song, G. & Lim, W. Osthole interacts with an ER-mitochondria axis and facilitates tumor suppression in ovarian cancer. J. Cell. Physiol. 236, 1025–1042. https://doi.org/10.1002/jcp.29913 (2021).
Song, J., Ham, J., Song, G. & Lim, W. Osthole suppresses cell growth of prostate cancer by disrupting redox homeostasis, mitochondrial function, and regulation of TiRNAHisGTG. Antioxid. (Basel). 13, 669–685. https://doi.org/10.3390/antiox13060669 (2024).
Shokoohinia, Y. et al. Potential anticancer properties of osthol: A comprehensive mechanistic review. Nutrients 10, 36–43. https://doi.org/10.3390/nu10010036 (2018).
Wang, Y. et al. Paeonol repurposing for cancer therapy: from mechanism to clinical translation. Biomed. Pharmacother. 165, 115277–115297. https://doi.org/10.1016/j.biopha.2023.115277 (2023).
Wang, W. H. et al. Curcumin inhibits proliferation and enhances apoptosis in A549 cells by downregulating LncRNA UCA1. Pharmazie 73, 402–407. https://doi.org/10.1691/ph.2018.8402 (2018).
Wang, N. Z., Feng, T., Liu, X. N. & Liu, Q. Curcumin inhibits migration and invasion of non-small cell lung cancer cells through up-regulation of miR-206 and suppression of PI3K/AKT/mTOR signaling pathway. Acta Pharm. 70, 399–409. https://doi.org/10.2478/acph-2020-0029 (2022).
Seubwai, W. et al. Cepharanthine exerts antitumor activity on cholangiocarcinoma by inhibiting nf-kappab. Cancer Ence. 101, 1590–1595. https://doi.org/10.1111/j.1349-7006.2010.01572.x (2010).
Bailly, C. Cepharanthine: An update of its mode of action, pharmacological properties and medical applications. Phytomedicine 62, e152956. https://doi.org/10.1016/j.phymed.2019.152956 (2019).
Feng, F. et al. In vivo cepharanthine inhibits hepatocellular carcinoma cell growth and proliferation by regulating amino acid metabolism and suppresses tumorigenesis. Int. J. Biol. Sci. 17, 4340–4352. https://doi.org/10.7150/ijbs.64675 (2021).
Endo, H., Inoue, I., Masunaka, K., Tanaka, M. & Yano, M. Curcumin induces apoptosis in lung cancer cells by 14-3-3 protein-mediated activation of bad. Biosci. Biotechnol. Biochem. 84, 2440–2447. https://doi.org/10.1080/09168451.2020 (2020).
Gao, S. M., Li, X. Y., Ding, X., Qi, W. W. & Yang, Q. F. Cepharanthine induces autophagy, apoptosis and cell cycle arrest in breast cancer cells. Cell. Physiol. Biochem. 41, 1633–1648. https://doi.org/10.1159/000471234 (2017).
Armstrong, J. S. Mitochondrial membrane permeabilization: the sine qua Non for cell death. Bioessays 28, 253–260. https://doi.org/10.1002/bies.20370 (2006).
Kroemer, G., Galluzzi, L. & Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87, 99–113. https://doi.org/10.1152/physrev.00013.2006 (2007).
Yao, Q. H. et al. Curcumin induces the apoptosis of A549 cells via oxidative stress and MAPK signaling pathways. Int. J. Mol. Med. 36, 1118–1126. https://doi.org/10.3892/ijmm.2015.2327 (2015).
Tang, J. H., Liu, J. L. & Zhang, X. H. The role of osthole on TGF-β-Induced lung epithelium apoptosis injury and epithelial-mesenchymal transition-mediated airway remodeling in pediatric asthma. J. Healthc. Eng. 2022, e7099097. https://doi.org/10.1155/2022/7099097 (2022).
Wang, J. et al. Osthole induces apoptosis and caspase-3/GSDME-dependent pyroptosis via NQO1-mediated ROS generation in HeLa cells. Oxid. Med. Cell. Longev. 2022, e8585598. https://doi.org/10.1155/2022/8585598 (2022).
Yang, S. et al. Osthole: an up-to-date review of its anticancer potential and mechanisms of action. Front. Pharmacol. 13, 945627–945637. https://doi.org/10.3389/fphar.2022.945627 (2022).
Xu, X., Zhang, Y., Qu, D., Jiang, T. & Li, S. Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells by modulating PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 30, 33–40. https://doi.org/10.1186/1756-9966-30-33 (2011).
Xu, X. M. et al. Osthole suppresses migration and invasion of A549 human lung cancer cells through Inhibition of matrix metalloproteinase-2 and matrix metallopeptidase-9 in vitro. Mol. Med. Rep. 6, 1018–1022. https://doi.org/10.3892/mmr.2012 (2012).
Damasceno, L. E. A. et al. PKM2 promotes Th17 cell differentiation and autoimmune inflammation by fine-tuning STAT3 activation. J. Exp. Med. 217, e20190613. https://doi.org/10.1084/jem.20190613 (2020).
Farhan, M. et al. FOXO signaling pathways as therapeutic targets in cancer. Int. J. Biol. Sci. 13, 815–827. https://doi.org/10.7150/ijbs.20052 (2017).
Dilmac, S. et al. SIRT1/FOXO signaling pathway in breast cancer progression and metastasis. Int. J. Mol. Sci. 23, e10227. https://doi.org/10.3390/ijms231810227 (2022).
Lin, C., Qin, Y., Zhang, H., Gao, M. Y. & Wang, Y. F. EGF upregulates RFPL3 and hTERT via the MEK signaling pathway in nonsmall cell lung cancer cells. Oncol. Rep. 40, 29–38. https://doi.org/10.3892/or.2018.6417 (2018).
Huang, K. et al. Correlation between FAK and EGF-induced EMT in colorectal cancer cells. J. Oncol. e5428920. (2020). https://doi.org/10.1155/2020/5428920 (2020).
Stepanenko, A. A. & Dmitrenko, V. V. Pitfalls of the MTT assay: direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 574, 193–203. https://doi.org/10.1016/j.gene.2015.08.009 (2015).
Zheng, J. G. et al. In vitro screening of traditional Chinese medicines compounds derived with anti-encephalomyocarditis virus activities. Biotechnol. Bioproc E. 25, 181–189. https://doi.org/10.1007/s12257-019-0354-5 (2022).
Zheng, J. G. et al. Curcumol inhibits encephalomyocarditis virus by promoting IFN-β secretion. BMC Vet. Res. 17, 318–327. https://doi.org/10.1186/s12917-021-03015-4 (2021).
Martinotti, S. & Ranzato, E. Scratch wound healing assay. Methods Mol. Biol. 2109, 225–229. https://doi.org/10.1007/7651_2019_259 (2020).
Huang, X. et al. CHSY3 promotes proliferation and migration in gastric cancer and is associated with immune infiltration. J. Transl Med. 21, 474–494. https://doi.org/10.1186/s12967-023-04333-x (2023).
Xiao, Y. et al. Geniposide ameliorated dexamethasone-induced endoplasmic reticulum stress and mitochondrial apoptosis in osteoblasts. J. Ethnopharmacol. 291, 115154–115165. https://doi.org/10.1016/j.jep.2022.115154 (2022).
Zhou, F. et al. Oridonin promotes endoplasmic reticulum stress via TP53-repressed TCF4 transactivation in colorectal cancer. J. Exp. Clin. Cancer Res. 42, 150–169. https://doi.org/10.1186/s13046-023-02702-4 (2023).
Mouna, R. et al. Antiproliferative activity, cell-cycle arrest, apoptotic induction and LC-HRMS/MS analyses of extracts from two Linum species. Pharm. Biol. 60, 1491–1501. https://doi.org/10.1080/13880209.2022.2102196 (2022).
Wang, W., Zhang, J., Wang, Y., Xu, Y. & Zhang, S. Identifies microtubule-binding protein CSPP1 as a novel cancer biomarker associated with ferroptosis and tumor microenvironment. Comput. Struct. Biotechnol. J. 20, 3322–3335. https://doi.org/10.1016/j.csbj.2022.06.046 (2022).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for tax-onomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Acknowledgements
We thank Professor Jing Liu, Henan Provincial Engineering Center for Tumor Molecular Medi-cine, School of Basic Medical Sciences, Henan University for their assistance in the experi-mental design.
Funding
This work was supported by the Doctoral Research Funding of Xinxiang Medical College (Grant No. XYBSKYZZ202168), Key Scientific Research Project of Colleges and Universities of Henan Province (Grant No. 23A320040), Key Scientific and Technological Research Projects of Henan Province (Grant No. 222102310701), National Natural Science Foundation of China (Grant No. U1904127). Financial support personnel all have a role in the design of the study, collection, analysis, interpretation of data or in the writing of this manuscript, and approved the final manuscript.
Author information
Authors and Affiliations
Contributions
Author ContributionsY.X. and R.S. conducted investigation, supervision and project administration. Y.X., L.C. and R.S. designed this work. Y.X., L.C., D.L. and X.L. carried out the experiments. Y.X., L.C., B.X., C.L, A.K. and X.L. analyzed the data and prepared the figures. Y.X. wrote the original draft. R.S. critically reviewed the manuscript. Y.X., X.L. participated in funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, Y., Chen, L., Liu, D. et al. The inhibitory effect of Osthole on A549 lung adenocarcinoma cells and its biomarker. Sci Rep 15, 12948 (2025). https://doi.org/10.1038/s41598-025-97305-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97305-8