Abstract
This study examined the relationship between homologous recombination deficiency (HRD) and variations in non-BRCA1/2 homologous recombination repair (HRR) genes. 27.3% (132/483) of the patients with ovarian, breast, endometrial, prostate, and pancreatic cancers carrying non-BRCA1/2 HRR variations were HRD + . Germline mutations were associated with significantly higher HRD+ rates than somatic mutations, while biallelic alterations did not show stronger associations with HRD compared to monoallelic alterations. High HRD+ rates (66.7–100.0%) were associated with variations in PALB2, RAD51C/D, and RAD54L, while low HRD+ rates (0–37.5%) corresponded with variations in PTEN, ATM, BRIP1, CDK12, and NBN, which may be influenced by variation grade and tissue origin. HRD positivity was mutually exclusive with HER2+ status in breast cancer and with TMB-H/MSI-H in endometrial cancer. Overall, these findings highlight the different strengths of the correlation between non-BRCA1/2 HRR gene variations and HRD and guide HRD testing in cases of “BRCA1/2-wildtype” results.
Similar content being viewed by others
Introduction
Homologous recombination deficiency (HRD) arises as a phenotypic consequence of defects in the homologous recombination repair (HRR) pathway, characterized by the inability of cells to repair double-stranded DNA (dsDNA) breaks via high-fidelity HRR mechanisms1,2. HRD is commonly observed in several cancers, such as ovarian, breast, pancreatic, and prostate cancers, rendering these tumors highly susceptible to platinum-based drugs and poly (ADP-ribose) polymerase inhibitors (PARPi)3,4. While the deleterious variants of BRCA1/2, the breast cancer susceptibility genes, remain the primary biomarkers for predicting response to PARPi2, HRD has recently been established as an officially recognized biomarker for PARPi use in combination with bevacizumab as a first-line maintenance therapy in ovarian cancer, as recommended by NCCN Guidelines. This advancement highlights the increasing clinical need for HRD status testing5,6.
Cells with HRD rely more heavily on the error-prone non-homologous end-joining pathway to repair dsDNA breaks, leading to the accumulation of genomic scars7. These scars are measured by the genomic instability score (GIS), which combines three metrics: telomeric allelic imbalance (TAI)8, loss of heterozygosity (LOH)9, and large-scale state transitions (LST)10. Current diagnostic HRD tests define HRD-positive (HRD + ) status as the presence of a deleterious mutation in BRCA1/2 or a GIS of ≥4211. Notably, a substantial portion of HRD+ tumors with high GIS lacks deleterious BRCA1/2 mutations, referred to as non-BRCA1/2 HRD-associated tumors12. Variations in certain non-BRCA1/2 HRR genes are also important causes of HRD13, such as PALB2 and RAD51C/D12,14. However, the specific strength of the association between different non-BRCA1/2 HRR genes and HRD remains poorly characterized.
In routine clinical practice, we observed that patients with variations in different non-BRCA1/2 HRR genes exhibited varying HRD+ rates. If this trend is observed consistently, it could help clinicians determine when to recommend an additional, costly HRD test for their patients. Therefore, in this study, we aimed to comprehensively investigate the associations between HRD positivity and different non-BRCA1/2 HRR genes, considering factors such as variation origin, variation class, hit type, and cancer type. The findings of this study may help clinicians in developing a framework to categorize patients for appropriate molecular testing.
Results
Patient characteristics overall and by gene panel
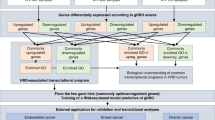
Among the 483 patients included in this study, 371 and 112 patients with non-BRCA1/2 HRR mutations were from gene panels 1 and 2, respectively (Fig. 1). Most patients were diagnosed with ovarian (218, 45.1%) or endometrial cancer (172, 35.6%), whereas smaller proportions of patients were diagnosed with breast (65, 13.5%), prostate (20, 4.1%), and pancreatic (8, 1.7%) cancers. No somatic class I mutations were identified, and no significant differences were observed between the two gene panels regarding age at diagnosis (>50 vs. ≤50, p = 0.1929) or the ratios of somatic variation classes (class II vs. class III, p = 0.4320) (Table 1). The proportion of ovarian cancer cases (90.1% vs. 31.5%, p < 0.0001), HRD+ rate (58.0% vs. 18.3%, p < 0.0001) and the ratio of germline variations (80.4% vs. 7.0%, p < 0.0001) were significantly higher in gene panel 2 than in gene panel 1, whereas the proportions of endometrial cancer cases (5.4% vs. 44.7%, p < 0.0001), breast cancer cases (0.9% vs. 17.3%, p < 0.0001), and pathogenic/likely pathogenic (P/LP) germline variations (23.3% vs. 92.3%, p < 0.0001) were markedly higher in gene panel 1 compared to the gene panel 2 (Table 1).
We previously detected 80 cases (75 with ovarian cancer and five with endometrial cancer) using gene panels 1 and 2. The consistency rate of HRD status was 97.5% (78/80, Supplementary Table 1), and the consistency rate of 15 cases with BRCA1/2 deleterious variations was 100.0%, supporting the integration of data from two gene panel groups to enrich the sample size and composition.
HRD prevalence based on non-BRCA1/2 HRR gene variation origin and hit type
In clinical practice, both P and LP germline mutations in BRCA1/2 are approved indications for PARPi15. According to our data, no significant differences were observed in HRD+ rates (50.0% vs. 48.0%, p = 0.8939) (Supplementary Fig. 1a), or GISs (38.5 vs. 35.4, p = 0.6619) between the P and LP groups, regardless of the HRD+ status (58.4 vs. 54.4, p = 0.3901) or HRD- status (18.5 vs. 18.2, p = 0.9572) (Supplementary Fig. 1b). The data above support the integration of cases with P and LP germline mutations in the subsequent analysis.
The HRD+ rate for the overall cohort was 27.3% (132/483). Patients with germline variations in non-BRCA1/2 HRR genes exhibited a significantly higher HRD+ rate than those with somatic mutations (49.6% vs. 20.4%, p < 0.0001; Fig. 2a). Within the germline group, P/LP non-BRCA1/2 HRR variations were associated with a similar HRD+ rate to those of variants of uncertain significance (VUS) (48.9% vs. 50.0%, p = 0.8548), regardless of whether they were biallelic alterations (BAs, 58.3% vs. 50.0%, p = 0.4807) or monoallelic alterations (MAs, 38.1% vs. 50.0%, p = 0.3379). The HRD+ rate of patients with germline BAs was numerically but not statistically higher than that of patients with germline MAs (58.3% vs. 38.1%, p = 0.1754) (Fig. 2b). Within the somatic group, patients with class III variations had a significantly higher HRD+ rate than those with class II variations (33.6% vs. 13.3%, p < 0.0001), regardless of whether they were BAs (33.6% vs. 10.6%, p < 0.0001) or MAs (33.6% vs. 18.8%, p = 0.0202). The HRD+ rate of patients with somatic BAs was similar to that of patients with somatic MAs (10.6% vs. 18.8%, p = 0.0809) (Fig. 2c). Here, we observed close associations between HRD+ rate and germline non-BRCA1/2 HRR mutations; however, the hit type (BAs or MAs) did not affect the HRD+ rate.
a Patients with germline non-BRCA1/2 HRR variations demonstrated a significantly higher HRD+ rate than those with somatic variations. b Patients with germline P/LP non-BRCA1/2 HRR variations had a similar HRD+ rate to those with VUS, regardless of whether they were BAs or MAs. c Patients with class III variations had a significantly higher HRD+ rate than those with class II variations, regardless of whether they were BAs or MAs. GIS, TAI, LOH, and LST scores in HRD+ groups with germline (d) and somatic e variations, and in HRD- groups with germline (f) and somatic (g) variations. BAs biallelic alterations, GIS genomic instability score, HRD homologous recombination deficiency, LOH loss of heterozygosity, LP likely pathogenic, LST large-scale state transitions, MAs monoallelic alterations, P pathogenic, TAI telomeric allelic imbalance, VUS variants of uncertain significance. * p < 0.05; ** p < 0.01; ****p < 0.0001; ns not significant.
GIS, TAI, LOH, and LST score distributions based on non-BRCA1/2 HRR gene variation origin, hit type, and HRD status
In the HRD+ group with germline variations, GIS (62.2 vs. 56.0, p = 0.0410) and TAI scores (20.7 vs. 17.7, p = 0.0231) for tumors with VUS were significantly higher than those for tumors with P/LP variations (Fig. 2d). In the HRD-negative (HRD-) group with germline variations, GIS, TAI, LOH, and LST scores of the cases with BAs were all significantly higher than those of the cases with MAs. Similar with HRD+ tumors with germline mutations, the TAI score for HRD- tumors with germline VUS were significantly higher than that for tumors with P/LP variations (10.6 vs. 6.6, p = 0.0177, Fig. 2e), indicating that the TAI score is more sensitive than the LOH or LST scores in cases with germline non-BRCA1/2 HRR mutations. In contrast, no significant differences in these scores were observed between tumors with class III and II variations in the HRD+ group (Fig. 2f). In the HRD- group with somatic variations, GIS (17.3 vs. 8.4, p < 0.0001), TAI (5.1 vs. 2.5, p < 0.0001), LOH (4.9 vs. 2.1, p < 0.0001), and LST (7.3 vs. 3.8, p < 0.0001) scores for tumors with class III variations were significantly higher than those for tumors with class II variations, no matter they were BAs or MAs (all p < 0.0001, Fig. 2g). Here, we found that somatic BAs were linked with higher HRD-related scores than MAs, though the hit type actually did not affect the HRD+ rate.
Strengths of association between different non-BRCA1/2 HRR variants and HRD
Variations in different HRR genes may exhibit different strengths of association with HRD+ rates. To test this hypothesis, HRD+ rates were independently analyzed for each non-BRCA1/2 HRR gene after excluding cases with mutations in more than one HRR gene (i.e., co-occurring HRR mutations). For germline variants (Fig. 3a), high HRD+ rates were associated with P/LP variations in PALB2 (4/4, 100.0%), RAD51C (5/7, 71.4%), and RAD51D (4/6, 66.7%), as well as those with VUS in RAD54L (6/6, 100.0%), FANCD2 (3/3, 100.0%), and ATR (3/4, 75.0%). Conversely, low HRD+ rates were associated with P/LP variations in ATM (0/2, 0.0%) and BRIP1 (1/4, 25.0%), as well as those with VUS in EMSY (0/4, 0.0%), NBN (0/3, 0.0%), and CDK12 (0/2, 0.0%).
a HRD+ rate associated with germline P/LP variations and VUS of individual non-BRCA1/2 HRR gene. b HRD+ rate associated with somatic II and III variations of individual non-BRCA1/2 HRR gene. c HRD+ rate related with germline P/LP BA and MA variations of individual non-BRCA1/2 HRR gene. d HRD+ rate related with somatic II BA and MA variations of individual non-BRCA1/2 HRR gene. BAs biallelic alterations, HRD homologous recombination deficiency, LP likely pathogenic, MAs monoallelic alterations, P pathogenic, VUS variants of uncertain significance.
For somatic variants (Fig. 3b), high HRD+ rates were associated with tumors with class II variations in RAD54L (2/2, 100.0%) and class III variations in FANCD2 (3/3, 100.0%). Conversely, low HRD+ rates were associated with tumors with class II variations in PTEN (7/110, 6.4%), as well as those with class III variations in ATM (0/7, 0.0%), CDK12 (1/10, 10.0%), EMSY (1/8, 12.5%), NBN (2/11, 18.2%), and BRIP1 (3/8, 37.5%). When considering the hit type, germline BAs of RAD51C (5/6, 83.3%) and RAD51D (4/5, 80.0%), and MAs of PALB2 (3/3, 100.0%) were strongly associated with high HRD+ rates (Fig. 3c). Both somatic BAs (4/78, 5.1%) and MAs (3/32, 9.4%) of PTEN were related to low HRD+ rates (Fig. 3d).
The low number of cases with other HRR mutations precluded meaningful comparisons. We downloaded TCGA somatic mutations and paired HRD scores to compensate for this limitation. Excluding cases with co-occurring HRR mutations, 245 and 234 cases with somatic non-BRCA1/2 HRR variations from the present study and TCGA data were included. Compared to the present study, the ratio of cases with pancreatic cancer was higher, while the ratio of cases with breast cancer was lower in the TCGA cohort (Fig. 4a). Using a cut-off of 42, the rate of HRD+ was lower in the TCGA group than in our cohort (15.4% vs. 23.3%, p = 0.0372, Fig. 4b). Upon integration, class II variations in RAD54L (3/4, 75.0%) and class III variations in RAD51D (2/3, 66.7%) were associated with high rates of HRD + . Conversely, class II variations in PTEN (11/181, 6.1%) and ATM (2/19, 10.5%), and class III variations in ATM (3/28, 10.7%), PTEN (4/32, 12.5%), CDK12 (2/16, 12.5%), and NBN (2/12, 16.7%) were associated with low rates of HRD+ (Fig. 4c).
For germline variations, we integrated data from the present study and seven previous studies12,14,16,17,18,19,20 focusing on germline P/LP variations of HRR genes to increase sample size and analysis reliability (Table 2). After integration, the number of cases with germline P/LP variations of ATM, BRIP1, CHEK2, PALB2, and RAD51B/C/D increased significantly. Upon integration, germline P/LP variations of PALB2 (24/26, 92.3%), RAD51C (16/25, 64.0%), and RAD51D (12/18, 66.7%) were robustly associated with high HRD+ rates, while germline P/LP variations of ATM (1/40, 2.5%), CHEK2 (1/17, 5.9%), BRIP1 (1/8, 12.5%), RAD51B (1/8, 12.5%) were steadily associated with low HRD+ rates, particularly in prostate and pancreatic cancers (Table 2).
HRD prevalence based on individual non-BRCA1/2 HRR gene and cancer type
The HRD+ rates and corresponding average GIS, TAI, LOH, and LST scores were explored based on cancer type. As shown in the OncoPlots, the HRD+ rates were 47.7%, 40.0%, 21.5%, 3.5%, and 0% in the ovarian, prostate, breast, endometrial, and pancreatic cancers, respectively (Fig. 5a–e). PTEN was the most frequently mutated gene in endometrial (84.3%), breast (20.0%), and ovarian (12.4%) cancers, followed by ATM in endometrial (22.1%) and ovarian (11.0%) cancers, most of which were somatic variations. We observed a high frequency of germline BAs in RAD51D (60.0%) and RAD51C (50.0%) in ovarian cancer, and a high frequency of somatic BAs in PTEN in breast (76.9%), endometrial (75.2%), and ovarian (66.7%) cancers. Among the overall cohort, 25.8% of the cases had mutations of more than one non-BRCA1/2 HRR gene. Interestingly, the HRD+ rate of the cases with co-occurring HRR mutations was not higher than that of the cases with isolated HRR gene mutation (20.8% vs. 29.6%, p = 0.0625), indicating that multiple HRR mutations may not produce significant synergistic effects on HRD.
The mutation incidences of non-BRCA1/2 HRR genes, the number, origin, and hit type of variations in each case, and GIS composition (TAI, LOH and LST) in each case in ovarian (a), endometrial (b), breast (c), prostate (d), and pancreatic (e) cancers. BA biallelic alteration, Ger germline, GIS genomic instability score, HRD homologous recombination deficiency, LOH loss of heterozygosity, LST large-scale state transitions, MA monoallelic alteration, P/LP pathogenic and likely pathogenic, Som somatic, TAI telomeric allelic imbalance, VUS variants of uncertain significance, WT wild type.
As we can see, germline mutations mainly existed in cases with ovarian cancer (87.0%, 80/92). Within ovarian cancer, germline mutations of RAD51C/D, RAD54B/L, and FANCD2 were associated with high HRD+ rates (66.7%–100.0%), while germline mutations of CDK12, EMSY, FANCI, NBN, RAD51B, MRE11, BRIP1, and RAD50 were associated with low HRD+ rates (0%–33.3%) (Fig. 6a). Although the small numbers of cases with the other four caner types limited the evaluation of the influence of tissue origin on HRD+ rate corresponding to individual genes, published data suggest that germline P/LP variations of PALB2 was associated with high HRD+ rate, while germline P/LP variations of ATM, and CHEK2 were associated with low HRD+ rates in prostate and pancreatic cancers (Table 2).
a The GIS, HRD+ rate, and case frequency based on germline variations of individual non-BRCA1/2 HRR gene in BC, OC, PAC, PRC and EC. b The GIS, HRD+ rate, and case frequency based on somatic II and III variations of individual non-BRCA1/2 HRR gene in BC, OC, PAC, PRC, and EC. The numbers in the left, center, and right panels represent the mean GIS, HRD+ rate, and percentage of cases per cancer type, respectively. The numbers in parentheses next to gene symbols indicate the number of cases. BC breast cancer, EC endometrial cancer, GIS genomic instability score, OC ovarian cancer, PAC pancreatic cancer, PRC prostate cancer.
Considering the HRD+ rates in cases with somatic II and III were significantly different (Fig. 2c), we separated the two grades of somatic mutations when drawing the heatmap (integrated with somatic mutation data from TCGA). Somatic mutations in PTEN and ATM were robustly related to low rates of HRD+ across all five cancer types; nonetheless, the other gene mutations had different association strengths depending on the cancer type. For instance, somatic class III mutations in ATR and BAP1 were more strongly associated with higher HRD+ rates and GISs in ovarian cancer than in breast cancer. Somatic class III mutations in FANCD2 were more strongly related to high HRD+ rates and GISs in breast and ovarian cancers than in pancreatic and prostate cancers. Somatic class II mutations in CDK12 were correlated with low HRD+ rates in breast and prostate cancers but not in ovarian cancer. Somatic class II mutations in RAD51D were linked to high HRD+ rates and GISs in ovarian cancer. Somatic class III mutations in RAD51D were related to low HRD+ rates and GISs in ovarian cancer; conversely, these mutations were related to high HRD+ rates and GISs in breast and prostate cancer (Fig. 6b). These observations confirm that certain mutations may exert different effects in different cancer types, thus the tissue origin, along with variation origin and grade, should be taken into account when judging its association with HRD.
Triple-negative breast cancer (TNBC) has been previously reported to exhibit much higher signature scores than the ER/PR+ and HER2+ subtypes21. However, our results showed only numerical, but not statistically significant, differences in the HRD+ rate between TNBC and the other subtypes (34.8% vs. 14.6%, p = 0.0614, Table 3). Although no significant differences were observed in GIS (34.3 vs. 24.2, p = 0.0631) or LST scores (15.8 vs. 11.7, p = 0.1528), patients with TNBC had slightly higher TAI (8.9 vs. 6.1, p = 0.0404) and LOH (9.6 vs. 6.4, p = 0.0386) scores than those with non-TNBC (Supplementary Fig. 2). Notably, 14 cases of HRD+ breast cancer were HER2-, and eight HER2+ cases were HRD- (Table 3), which demonstrated the mutual exclusivity of HER2+ status and HRD in breast cancer.
Associations between HRD and TMB-H/MSI-H in non-BRCA1/2 HRR mutant cases
As demonstrated previously, endometrial cancer is characterized by high tumor mutational burden and high microsatellite instability (TMB-H/MSI-H). Here, we found that the average GIS (7.6 vs. 35.1) and TAI (2.0 vs. 8.2), LOH (2.2 vs. 10.8), and LST (3.4 vs. 16.2) scores for patients with endometrial cancer were significantly lower than those of patients with other cancer types (all p < 0.0001), particularly in the HRD- group (GIS: 5.8 vs. 18.2, TAI: 1.5 vs. 4.4, LOH: 1.7 vs. 6.4, LST: 2.6 vs. 7.4; all p < 0.0001; Supplementary Table 2), suggesting that effect of TMB-H/MSI-H status on HRD+ rate should be further analyzed.
TMB status, TMB value, and MSI status were available from the gene panel 1 data (n = 371, Table 4), with 26.1% of cases classified as TMB-H and 13.5% as MSI-H. The results indicated that the HRD+ rate of non-BRCA1/2 HRR mutant cases was significantly lower in the TMB-H (11.3% vs. 20.8%, p = 0.0384) and MSI-H (4.0% vs. 20.6%, p = 0.0049) subgroups than in their TMB-low (TMB-L) and microsatellite stable (MSS) counterparts. Consistently, the average TMB value in the HRD+ group was markedly lower than that in the HRD- group (6.1 vs. 22.2 mutations/Mb, p = 0.0384).
Given that endometrial cancer accounted for a high proportion of TMB-H and MSI-H cases (69.1% and 86.0%, respectively), we separately investigated the associations between TMB-H/MSI-H and HRD scores in patients with endometrial cancer and patients with the other cancers. The GIS, TAI, LOH, and LST scores were significantly lower in the TMB-H/MSI-H subsets than in the TMB-L and MSS subsets both in patients with all included cancers (Fig. 7a, all p < 0.0001) and in patients with endometrial cancer (Fig. 7b, all p < 0.05) but were similar in patients with non-endometrial cancers (Fig. 7c). These observations support the previous hypothesis that defects affecting single strand repair (MSI-H) are mutually exclusive with defects affecting double strand repair (HRD)22, which is specifically evident in endometrial cancer.
The GIS and TAI, LOH, and LST scores were significantly lower in the TMB-H/MSI-H subsets than in the TMB-L and MSS subsets, both in patients with all included cancers (a) and in patients with endometrial cancer (b), but were similar in patients with non-endometrial cancers (c). ** p < 0.01; ****p < 0.0001. ns not significant.
Variations in the six most common non-BRCA1/2 HRR genes in HRD+ cases
To explore if any “hot spot” exists, we analyzed the locations of both germline and somatic variations on the non-BRCA1/2 HRR genes. The six most frequently observed non-BRCA1/2 HRR genes in HRD+ cases were RAD54L (n = 12), ATR (n = 11), FANCD2 (n = 11), FANCA (n = 10), RAD51C (n = 8), and RAD51D (n = 6). The specific amino acid changes in these genes were displayed according to their ___domain locations. Although the number of HRD+ cases was relatively small, several protein alterations appeared repeatedly, including RAD51D K91Ifs*13 (n = 4), RAD54L L621P (n = 2), ATR A1488V (n = 2), FANCA A1442T (n = 2), and RAD51C T132Nfs*23 (n = 2) (Fig. 8a–f). The recurrent appearance of the above variations may increase their value on HRD prediction.
The specific mutations in RAD54L (a), ATR (b), FANCD2 (c), FANCA (d), RAD51C (e) and RAD51D (f) observed in the HRD+ cases. Amino acid changes and frequencies were annotated using MutationMapper (http://www.cbioportal.org/mutation_mapper. Blue, germline variations; black, somatic variations; red, germline and somatic variations.
Discussion
The identification of HRD plays a crucial role in guiding the selection of patients who would benefit from targeted therapies; however, previous research has primarily focused on BRCA1/2 mutations, rendering clinicians understandably inquisitory about the likelihood of HRD in patients with non-BRCA1/2 HRR mutations. In the present study, we found that patients with mutations in PALB2, RAD51C/D, and RAD54L exhibited a tendency toward high HRD+ rates (66.7–100.0%), whereas those with variations in PTEN, ATM, BRIP1, CDK12, and NBN had low HRD+ rates (0–37.5%). Germline mutations were associated with significantly higher HRD+ rates than somatic mutations, while the hit type, namely BAs or MAs, did not affect HRD+ rates significantly. The HRD+ rates associated with individual HRR genes might be influenced by variation grade and tissue origin, offering potentially valuable insights that may guide clinicians in cases of “BRCA1/2-wildtype” results23,24.
Notably, patients with germline variations in non-BRCA1/2 HRR genes exhibited significantly higher HRD rates compared to those with somatic variations (49.6% vs 20.4%, p < 0.0001, Fig. 2a). We found that the somatic II PTEN mutations were related to very low HRD+ rates (6.4%), regardless of whether they were BAs (5.1%) or MAs (9.4%) (Fig. 3b, d). Therefore, the high proportion of cases with PTEN mutations (114/245, 46.5%, excluding cases with co-occurring HRR mutations) may partly explain the lower HRD+ rate observed in the somatic group. When PTEN mutations were excluded from both the germline and somatic groups, the discrepancy narrowed but was still significant (37.4% vs. 51.6%, p = 0.0351). This result is logical, because germline alterations are static and long-lasting. In contrast, somatic alterations provide only a snapshot of the tumor at a specific stage and can be influenced by various factors, including sampling site, tumor purity, and chemotherapy treatment25. As previously reported, PTEN is a tumor suppressor gene critical for the maintenance of genome stability26. A PTEN inhibitor was reported to increase double-strand breaks through the modulation of the MRE11-RAD50-NBN complex and enhance the inhibitory effect of olaparib on breast cancer cells27. A pan-cancer analysis by E. Rempel et al. reported that PTEN was the most frequently affected gene in breast cancer (34%) and prostate cancer (59%), and HRD scores were significantly higher in PTEN-mutated ovarian cancer (fold change = 1.4) and prostate cancer (fold change = 1.3)22. Additionally, Pérez-Villatoro et al. found that tumors with somatic PTEN mutations exhibited higher levels of ovaHRDscar, a type of HRD score built on TCGA ovarian cancer multi-omics dataset, compared to the reference group28. According to our data, PTEN was the most frequently affected gene in ovarian, breast, and endometrial cancer (Fig. 5); however, somatic mutations of PTEN were associated with very low HRD+ rates. Both the HRD+ rate (0% vs. 25%, p < 0.0001) and average GIS (5.5 vs. 25.6, p < 0.0001) were significantly lower in PTEN-mutated endometrial cancer cases compared to PTEN-mutated non-endometrial cancer cases. Therefore, the discrepancy in PTEN-related HRD+ rates observed in the current study and previous reports could be partly explained by the high ratio of endometrial cancer cases (77.1%) within the PTEN-mutated group. Regrettably, there were too few cases of germline PTEN mutations in the current study to draw any meaningful conclusions. It has been found that some HRD-related genes were specific to certain races. For instance, mutations of ATM, BRCA2, POLE, and TOP2B were more prevalent in ‘White’ and ‘Asian’ populations, while PTEN and EGFG mutations were more frequent in the ‘White’ and ‘African American/Black’ populations29. The data analyzed by E. Rempel et al.22 were obtained from TCGA, while our data were obtained from a Chinese population; therefore, the race-specific factor may partly explain the observed difference in the PTEN mutation rate and HRD+ rate in PTEN somatic variants, in addition to the limited sample size of the current study.
Our results also indicated that GIS and TAI, LOH, and LST scores varied based on different non-BRCA1/2 HRR variation classes (germline P/LP vs. VUS, somatic II vs. III). It may seem counterintuitive that class III variations were more strongly associated with HRD than class II variations. The exact reason for this discrepancy is unclear; however, it is possible that somatic class II/III variations could be “passenger” mutations rather than “driver” mutations, given that the true mechanisms underlying the HRD phenotype are complex, involving a combination of genetic and epigenetic changes (underlying causes of HRD), genomic scars and mutational signatures (consequences of HRD), and HRR activity7,30. Therefore, the value of somatic variations should be interpreted cautiously, particularly for class III variations with little pathogenic evidence. A former study conducted by Guillaume Beinse et al.31 analyzed the association between a transcriptomic model and genomic scars in gynecological cancers and did not focus on individual HRR genes but rather captured gene expression levels in HRD-related pathways (nuclear structure, chromatin remodeling, and so on), which also helps explain why some tumors exhibit HRD features even without BRCA1/2 mutations. We agreed with Guillaume Beinse et al.31 in that a gradient of intermediate levels of deficiency exist beyond BRCA1/2-altered tumors, just as we found the variations of several non-BRCA1/2 genes, such as ATR and FANCA/C, had intermediate strengths of association with HRD+ rates (33.3–66.7%). Since somatic or germline mutations in HRR genes are not found in all HRD tumors and VUS can be difficult to interpret, the transcriptional features, which might be the consequential reflect of specific HRR gene variations, may serve as a supplementary predictor of HRD status in cases without HRR variations.
Several HRR genes, including RAD51C/D, PALB2, BRIP1, BARD1, CHEK2, ATM, H2AX, MRE11, and those associated with Fanconi anemia, have been previously recognized as contributors to a “BRCAness” phenotype in 30–50% of high-grade serous ovarian cancer cases32,33,34. However, the strength of the correlation between mutations in these genes and the benefits of PARPi has been variable32. Our findings revealed that tumors with P/LP variations in PALB2 and RAD51C/D accounted for a high proportion of HRD+ cases (72.2%, 13/18), whereas tumors with P/LP variations in BRIP1 constituted a lower proportion of HRD+ cases (5.6%, 1/18), consistent with previous research results12,14. Notably, RAD51D c.270_271dup p.K91Ifs*13 was detected in four HRD+ cases (both germline and somatic) in the present study. This specific frameshift mutation was detected in 1.7% (13/781) of Chinese patients with ovarian cancer, was the most common mutation in RAD51D35, and has been linked to steady platinum and PARPi benefits in ovarian cancer36,37. Based on these findings, we hypothesize that this mutation may be a founder mutation in the Chinese population.
Furthermore, 9/10 patients with germline variations and 4/17 patients with somatic variations in RAD54L were classified as HRD + . The RAD51/RAD54 complex is essential for activating dsDNA break repair38. Rad54, an ATP-dependent motor protein, dissociates Rad51 from dsDNA, the product complex of DNA strand exchange39. The combined depletion of RAD54L and RAD54B or overexpression of RAD51 could impede replication and promote chromosome segregation defects40. RAD54L P/LP variations have been associated with outcomes in patients with metastatic castration-resistant prostate cancer in the “HRR non-BRCA” cohort, which showed better results than the BRCA cohort but poorer outcomes than the non-HRR cohort23. Additionally, a missense mutation in RAD54L (c.604 C > T) was identified in one carrier with a family history of ovarian cancer, as well as in a 29-year-old patient with breast cancer with a family history of ten breast cancer cases41. Although the function of RAD54L in hereditary breast and ovarian cancer syndromes remains unclear, certain functional RAD54L mutations may play a role in the formation of HRD.
GI is a well-established hallmark of cancer, with HRD and MSI representing two distinct forms of GI based on different repair mechanisms42. Previous studies have demonstrated that tumors with MSI-H exhibit low levels of genomic scars across colon, gastric, and endometrial cancers, with particularly low genomic scar levels in endometrial cancer, with median TAI, LOH, and LST scores of 3.0, 2.0, and 2.0, respectively21. Consistently, our analysis revealed a mutual exclusivity between HRD and TMB-H/MSI-H status, particularly in endometrial cancer, with median TAI, LOH, and LST scores of 2.0, 2.2, and 3.4, respectively (Supplementary Table 2). This evident tendency toward mutual exclusivity indicates that in MSI-H tumors, MSI may precede the GI that generate genomic scars. This observation suggests that HRD testing may be unnecessary for MSI-H cancers.
TNBC has been reported to exhibit significantly higher signature scores than the ER/PR+ and HER2+ subtypes, with median TAI, LOH, and LST values of 27.0, 21.5, and 21.0, respectively21. However, our data showed only numerically, but not statistically, significant higher HRD+ rates in TNBCs than in non-TNBCs (34.8% vs. 14.6%). The median TAI, LOH, and LST values for TNBCs were 8.9, 9.6, and 15.8, respectively, which were lower than previously reported. This discrepancy may be caused by the relatively small number of included TNBC cases (14 cases) and the different gene panels used in the two studies. Notably, our analysis revealed a mutual exclusivity between HRD and HER2+ status in breast cancer, which seems reasonable because tumors are generally promoted by only one strong functional driver gene variation during a specific period43,44. This observation also suggests that HER2+ breast cancer may not require additional HRD testing in clinical practice.
In the present study, only 20 and eight patients with prostate and pancreatic cancer, respectively, harboring non-BRCA1/2 HRR variations were included, which precluded meaningful statistical comparisons of associations with HRD of different non-BRCA1/2 HRR variants. After integration with TCGA data, the number of prostate cancer cases increased from 20 to 39, and the number of pancreatic cancer cases increased from 8 to 35. We observed that germline P/LP variations of ATM and CHEK2 were associated with low HRD+ rates in prostate cancer and pancreatic cancer. Although the low frequency of HRR pathway gene alterations was also limited other HRD-related studies in these two malignancies, some useful clues can be obtained from large-scale studies45. In prostate cancer, BRCA2 (6.76%), ATM (4.50%), and CHEK2 (1.92%) were most frequently mutated HRR genes46, and tumors with germline BRCA2 mutations had higher HRD scores (median = 27). In contrast, tumors with germline ATM (median = 16.5, p = 0.029) or CHEK2 (median = 9.0, p < 0.001) mutations had significantly lower HRD scores16. A meta-analysis suggested that HRD occurs in 14.5–16.5% of pancreatic cancer cases47, and the HRD+ rate was lower in tumors with deleterious non-BRCA1/2 HRR mutations than those with deleterious BRCA1/2 mutations22. This confirms that the cutoff for HRD in prostate and pancreatic cancers may need to be defined differently based on treatment effectiveness.
This study has some limitations. First, the data on progression-free survival remains immature owing to the relatively short follow-up period. Therefore, further long-term studies are needed to determine whether the prognosis differs between HRD+ patients with BRCA1/2 mutations and those with non-BRCA1/2 HRR mutations. Such studies should also assess the prognostic implications of these genetic alterations and their potential effect on treatment response. Additionally, the two gene panels used in this study do not cover all genes involved in the HRR pathway; therefore, the associations between HRD and other non-BRCA1/2 HRR genes not investigated in this study remain uncertain. BLM, FANCE, FANCF, and WRN are only covered by gene panel 1 but not gene panel 2, which might generate bias in the HRD+ rate of cases carrying mutations of these four genes. Finally, the retrospective, single-institutional nature and limited sample size of this analysis may restrict the generalizability of the findings. These factors highlight the need for caution when interpreting the observed associations and emphasize the necessity for larger, multi-center studies to validate these findings and assess their clinical relevance.
In conclusion, this study demonstrated the varying strength of association between different non-BRCA1/2 HRR mutations and HRD across several cancer types. These findings suggest that specific genetic alterations could guide molecular testing and treatment strategies in a cancer type-specific setting. Nevertheless, further research is needed to explore the underlying mechanisms and prognostic effects of non-BRCA1/2 HRR gene variations in larger patient populations.
Methods
Patient selection and data collection
HRD and non-BRCA1/2 HRR gene sequencing results for patients diagnosed with ovarian, breast, endometrial, prostate, and pancreatic cancers through pathological examination at the Sun Yat-sen University Cancer Center (SYSUCC, Guangzhou, China) between June 1, 2023, and September 30, 2024, were retrospectively investigated. The HRD status was detected by gene panels 1 and 2. Cases with germline or somatic alterations in at least one of 30 non-BRCA1/2 HRR genes were included in the final analysis (Fig. 1). Among the 30 non-BRCA1/2 HRR genes, 26 genes are shared by both gene panel 1 (covering 520 genes) and gene panel 2 (covering 36 genes), including ABRAXAS1 (FAM175A), ATM, ATR, BAP1, BARD1, BRIP1, EMSY (C11ORF30), CDK12, CHEK1, CHEK2, FANCA, FANCC, FANCD2, FANCI, FANCL, MRE11, NBN, PALB2, PPP2R2A, PTEN, RAD50, RAD51B, RAD51C, RAD51D, RAD54B, and RAD54L. The other four genes, BLM, FANCE, FANCF, and WRN, only covered by gene panel 1, were also included due to their involvement in a previous large-scale HRD-related study22.
HRD status, GIS, TAI, LOH, and LST scores, variated genes, variation class, protein changes, patient age, and cancer type were systematically collected. Additionally, TMB value, TMB status, and MSI status, which can be obtained from gene panel 1 but not gene panel 2, were also evaluated. The use of clinical and NGS data was approved by the Ethics Committee of the Sun Yat-Sen University Cancer Center (approval number B2020-344-01). All patients provided written informed consent, and the study was performed in accordance with the guidelines outlined in the Declaration of Helsinki.
DNA isolation and capture-based targeted DNA sequencing
Formalin-fixed paraffin-embedded tumor specimens were processed for DNA extraction using 8–10 slides of 5 μm thickness, and the final slide was stained with hematoxylin and eosin (H&E). Two independent pathologists reviewed the H&E-stained slide to confirm the pathological diagnosis and ensure tumor cellularity (at least 30%). Genomic DNA was isolated from the tumor specimens and matched peripheral white blood cells. DNA concentration was measured using the dsDNA HS assay kit (Thermo Fisher Scientific, Waltham, MA) with a Qubit Fluorometer. Sequencing was performed using gene panel 1 (OncoScreenPlusTM, Burning Rock, Guangzhou, China)48 and gene panel 2 (Precision Scientific, Beijing, China)49. Detailed experimental procedures and bioinformatic analysis have been described previously50.
Variation classification and hit type identification of non-BRCA1/2 HRR genes
Germline variants were classified as P/LP or VUS according to the American College of Medical Genetics and Genomics recommendations for interpreting sequence variations51. Somatic variants were classified as class I/II (variants of strong/potential clinical significance) or class III (variants of unknown clinical significance) according to the categories of clinical and/or experimental evidence51. It has been established that BAs, rather than ‘single-hits’ or MAs, of HRR genes beyond BRCA1/2 are strongly associated with phenotypic functional HR deficiency and LST score in breast cancer52, and with elevated genome-wide LOH in breast, ovarian, pancreatic, and prostate cancers53. In light of that, we further identified BAs based on the following criteria49: (i) deleterious mutation in one allele and LOH in the other, (ii) two deleterious mutations in the same gene. The deleterious variations in only one allele without LOH in the other allele were considered as MAs, where “deleterious” refers to germline P/LP or somatic II variations. Cases with co-occurring HRR mutations were excluded from hit type identification and individual gene analyses as other researchers did previously54,55, otherwise, the gene-specific HRD+ rate and zygosity on the patient level could not be assessed.
GIS calculation
GIS is calculated as the sum of TAI, LOH, and LST. The TAI score measures the frequency of allelic imbalance that do not cross the centromere and extends to the ends of the chromosome telomeres. LOH occurs when a normal gene copy is lost due to the deletion of a large chromosome segment. The LOH score is calculated as the count of LOH regions that are greater than 15 Magabytes (Mb) but less than the entire length of the chromosome. The LST score quantifies the number of chromosomal breakpoints between two adjacent regions, each at least 10 Mb in length, with a distance of no more than 3 Mb, and not passing through the centromere.
The GI algorithm of gene panel 1 utilized over 9000 single-nucleotide polymorphisms (SNPs) from a 520-gene panel, estimating allele-specific copy numbers with a custom script based on logR and median coverage. Minor allele frequency and logR data were segmented using circular binary segmentation, and a probabilistic model was used to estimate tumor copy number, purity, and ploidy. LOH, TAI, and LST were calculated as described previously, with the GIS being the sum of these metrics48.
The development of the HRD assay for gene panel 2 has been previously detailed49. In brief, the GI algorithm calculated the GIS in four steps. First, the genome was split into segments, and heterozygous SNPs were selected based on sequencing depth and allele frequency. Second, four parameters were estimated using maximum likelihood estimation: major allele count per segment, minor allele count per segment, tumor purity, and tumor ploidy. Third, LOH, TAI, and LST scores were calculated based on these parameters. Finally, the GIS was derived by summing the LOH, TAI, and LST scores.
A positive GIS was defined by a cutoff value of ≥42 for both gene panels 1 and 2. The positive and negative predictive values of the HRD tests, as claimed by the manufacturers, are 98.4% and 96.2% for gene panel 1, and 100% and 100% for gene panel 2, respectively.
TMB and MSI evaluation
TMB and MSI were evaluated using gene panel 1 data. TMB was calculated as the ratio of the total number of nonsynonymous mutations detected to the total coding region size (1.003 Mb). Only mutations with an allelic fraction ≥2% were included, and the mutation count did not include hot spot mutation events, copy number variations, structural variations, or germline SNPs48. MSI status was determined using a read-count-distribution-based method, as described previously56.
Online data acquisition
We downloaded somatic mutation information from the somatic mutation profile published by the Multi-Center Mutation Calling in Multiple Cancers (MC3) project57 (mc3.v0.2.8.PUBLIC.maf), and obtained HRD scores from the supplemental data of a previous paper, in which more than 10,000 tumors comprising 33 cancer types from TCGA were analyzed for immunogenomic characteristic58, and matched the somatic mutations to the HRD scores based on the TCGA_sample_ID. Thereafter, we eliminated low-quality variations (t_depth <25, t_alt_count <3, or t_alt_count/t_depth <0.05) after filtering by five cancer types and 30 non-BRCA1/2 HRR genes (Fig. 1) focused in the present study. Excluding cases with co-occurring HRR mutations, we extracted 234 cases whose somatic non-BRCA1/2 HRR mutations and HRD scores were both available. A cutoff of ≥42 was used to define the HRD+ status of TCGA.
Statistical analysis
The clinical characteristics of cases were evaluated using the two gene panels, and the associations between TMB/MSI status and HRD status were compared using the chi-square test. Additionally, differences in GIS, TAI, LOH, and LST scores, and TMB values across different groups were assessed using a Mann–Whitney test. The oncoplots were drawn using R software version 4.4.0 and the oncoplot function in the maftools package version 2.20.0 (https://github.com/PoisonAlien/maftools). Statistical significance was determined based on two-tailed tests with a p < 0.05. All statistical analyses were performed using GraphPad Prism version 9.5.0 (GraphPad Software, San Diego, CA, USA).
Data availability
Raw sequencing data files of patients cannot be publicly shared under the obtained institutional review board approval, as patients did not consent to share raw sequencing data beyond the research and clinical terms. The datasets that support the conclusions of this article are available in the Research Data Deposit repository (No. RDDA2024644861, http://www.researchdata.org.cn/).
Code availability
The R code used for generating oncoplots is publicly available (oncoplot function in the maftools package version 2.20.0 (https://github.com/PoisonAlien/maftools). However, the custom code for calculating the GISs is proprietary.
References
Magadeeva, S. et al. Assessing the phenotype of a homologous recombination deficiency using high resolution array-based comparative genome hybridization in ovarian cancer. Int. J. Mol. Sci. 24, 17467 (2023).
Yamamoto, H. & Hirasawa, A. Homologous recombination deficiencies and hereditary tumors. Int. J. Mol. Sci. 23, 348 (2022).
Luo, Y. et al. Neoadjuvant PARPi or chemotherapy in ovarian cancer informs targeting effector Treg cells for homologous-recombination-deficient tumors. Cell 187, 4905–4925.e24 (2024).
Guffanti, F., Mengoli, I. & Damia, G. Current HRD assays in ovarian cancer: differences, pitfalls, limitations, and novel approaches. Front. Oncol. 14, 1405361 (2024).
Liu, J. et al. NCCN Guidelines® insights: ovarian cancer/fallopian tube cancer/primary peritoneal cancer, Version 3.2024. J. Natl Compr. Cancer Netw. 22, 512–519 (2024).
Incorvaia, L. et al. Theranostic biomarkers and PARP-inhibitors effectiveness in patients with non-BRCA associated homologous recombination deficient tumors: Still looking through a dirty glass window?. Cancer Treat. Rev. 121, 102650 (2023).
Ratnaparkhi, R., Javellana, M., Jewell, A. & Spoozak, L. Evaluation of homologous recombination deficiency in ovarian cancer. Curr. Treat. Options Oncol. 25, 237–260 (2024).
Birkbak, N. J. et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2, 366–375 (2012).
Abkevich, V. et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br. J. Cancer 107, 1776–1782 (2012).
Popova, T. et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 72, 5454–5462 (2012).
Herzog, T. J. et al. Testing for homologous recombination repair or homologous recombination deficiency for poly (ADP-ribose) polymerase inhibitors: a current perspective. Eur. J. Cancer 179, 136–146 (2023).
Kahn, R. M. et al. Pathogenic germline variants in non-BRCA1/2 homologous recombination genes in ovarian cancer: analysis of tumor phenotype and survival. Gynecol. Oncol. 180, 35–43 (2024).
D’Angelo, E., Espinosa, I., Felicioni, L., Buttitta, F. & Prat, J. Ovarian high-grade serous carcinoma with transitional-like (SET) morphology: a homologous recombination-deficient tumor. Hum. Pathol. 141, 15–21 (2023).
Torres-Esquius, S. et al. Prevalence of homologous recombination deficiency among patients with germline RAD51C/D breast or ovarian cancer. JAMA Netw. Open 7, e247811 (2024).
Tew, W. P. et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J. Clin. Oncol. 38, 3468–3493 (2020).
Lotan, T. L. et al. Homologous recombination deficiency (HRD) score in germline BRCA2- versus ATM-altered prostate cancer. Mod. Pathol. 34, 1185–1193 (2021).
Mandelker, D. et al. Genomic profiling reveals germline predisposition and homologous recombination deficiency in pancreatic acinar cell carcinoma. J. Clin. Oncol. 41, 5151–5162 (2023).
Xie, F. et al. RAD51B harbors germline mutations associated with pancreatic ductal adenocarcinoma. JCO Precis. Oncol. 6, e2100404 (2022).
Lee, J. E. A. et al. Molecular analysis of PALB2-associated breast cancers. J. Pathol. 245, 53–60 (2018).
Golan, T. et al. Genomic features and classification of homologous recombination deficient pancreatic ductal adenocarcinoma. Gastroenterology 160, 2119–2132.e9 (2021).
Marquard, A. M. et al. Pan-cancer analysis of genomic scar signatures associated with homologous recombination deficiency suggests novel indications for existing cancer drugs. Biomark. Res. 3, 9 (2015).
Rempel, E. et al. Pan-cancer analysis of genomic scar patterns caused by homologous repair deficiency (HRD). NPJ Precis. Oncol. 6, 36 (2022).
Olmos, D. et al. Treatment patterns and outcomes in metastatic castration-resistant prostate cancer patients with and without somatic or germline alterations in homologous recombination repair genes. Ann. Oncol. 35, 458–472 (2024).
Bazan Russo, T. D. et al. Polθ: emerging synthetic lethal partner in homologous recombination-deficient tumors. Cancer Gene. Ther. 31, 1619–1631 (2024).
Ellison, G. et al. An evaluation of the challenges to developing tumor BRCA1 and BRCA2 testing methodologies for clinical practice. Hum. Mutat. 39, 394–405 (2018).
Mendes-Pereira, A. M. et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol. Med. 1, 315–322 (2009).
Qiu, L. et al. PTEN inhibition enhances sensitivity of ovarian cancer cells to the poly (ADP-ribose) polymerase inhibitor by suppressing the MRE11-RAD50-NBN complex. Br. J. Cancer 131, 577–588 (2024).
Perez-Villatoro, F. et al. Optimized detection of homologous recombination deficiency improves the prediction of clinical outcomes in cancer. NPJ Precis. Oncol. 6, 96 (2022).
Hsiao, Y. W. & Lu, T. P. Race-specific genetic profiles of homologous recombination deficiency in multiple cancers. J. Pers. Med. 11, 1287 (2021).
Paulet, L. et al. Cracking the homologous recombination deficiency code: how to identify responders to PARP inhibitors. Eur. J. Cancer 166, 87–99 (2022).
Beinse, G. et al. Discovery and validation of a transcriptional signature identifying homologous recombination-deficient breast, endometrial and ovarian cancers. Br. J. Cancer 127, 1123–1132 (2022).
Mangogna, A. et al. Homologous recombination deficiency in ovarian cancer: from the biological rationale to current diagnostic approaches. J. Pers. Med. 13, 284 (2023).
Toh, M. & Ngeow, J. Homologous recombination deficiency: cancer predispositions and treatment implications. Oncologist 26, e1526–e1537 (2021).
Pennington, K. P. et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 20, 764–775 (2014).
Yao, H., Li, N. & Yuan, H. Clinical characteristics and survival analysis of Chinese ovarian cancer patients with RAD51D germline mutations. BMC Cancer 22, 1337 (2022).
Xu, J. et al. RAD51D secondary mutation-mediated resistance to PARP-inhibitor-based therapy in HGSOC. Int. J. Mol. Sci. 24, 14476 (2023).
Ishihara, E. et al. Four cancer cases with pathological germline variant RAD51D c.270_271dup. J. Obstet. Gynaecol. Res. 50, 1742–1747 (2024).
Tong, Y. et al. Histone methyltransferase KMT5C drives liver cancer progression and directs therapeutic response to PARP inhibitors. Hepatology 80, 38–54 (2024).
Li, X. et al. Rad51 and Rad54 ATPase activities are both required to modulate Rad51-dsDNA filament dynamics. Nucleic Acids Res. 35, 4124–4140 (2007).
Mason, J. M. et al. RAD54 family translocases counter genotoxic effects of RAD51 in human tumor cells. Nucleic Acids Res. 43, 3180–3196 (2015).
Felicio, P. S. et al. Whole-exome sequencing of non-BRCA1/BRCA2 mutation carrier cases at high-risk for hereditary breast/ovarian cancer. Hum. Mutat. 42, 290–299 (2021).
Sokol, E. S. et al. PARP inhibitor insensitivity to BRCA1/2 monoallelic mutations in microsatellite instability-high cancers. JCO Precis. Oncol. 6, e2100531 (2022).
Cisowski, J., Sayin, V. I., Liu, M., Karlsson, C. & Bergo, M. O. Oncogene-induced senescence underlies the mutual exclusive nature of oncogenic KRAS and BRAF. Oncogene 35, 1328–1333 (2016).
Seth, R. et al. Concomitant mutations and splice variants in KRAS and BRAF demonstrate complex perturbation of the Ras/Raf signalling pathway in advanced colorectal cancer. Gut 58, 1234–1241 (2009).
Takamatsu, S. et al. Utility of homologous recombination deficiency biomarkers across cancer types. JCO Precis. Oncol. 6, e2200085 (2022).
Heeke, A. L. et al. Prevalence of homologous recombination–related gene mutations across multiple cancer types. JCO Precis. Oncol. 2018, 1–13 (2018).
Casolino, R. et al. Homologous recombination deficiency in pancreatic cancer: a systematic review and prevalence meta-analysis. J. Clin. Oncol. 39, 2617–2631 (2021).
Feng, J. et al. Combination of genomic instability score and TP53 status for prognosis prediction in lung adenocarcinoma. NPJ Precis. Oncol. 7, 110 (2023).
Li, L. et al. HRD effects on first-line adjuvant chemotherapy and PARPi maintenance therapy in Chinese ovarian cancer patients. NPJ Precis. Oncol. 7, 51 (2023).
Li, Y. et al. SWI/SNF complex gene variations are associated with a higher tumor mutational burden and a better response to immune checkpoint inhibitor treatment: a pan-cancer analysis of next-generation sequencing data corresponding to 4591 cases. Cancer Cell Int. 22, 347 (2022).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Mutter, R. W. et al. Bi-allelic alterations in DNA repair genes underpin homologous recombination DNA repair defects in breast cancer. J. Patho. 242, 165–177 (2017).
Westphalen, C. B. et al. Pan-cancer analysis of homologous recombination repair-associated gene alterations and genome-wide loss-of-heterozygosity score. Clin. Cancer Res. 28, 1412–1421 (2022).
Barnicle, A. et al. Patterns of genomic instability in > 2000 patients with ovarian cancer across six clinical trials evaluating olaparib. Genome Med. 16, 145 (2024).
Pujade-Lauraine, E. et al. Homologous recombination repair gene mutations to predict olaparib plus bevacizumab efficacy in the first-line ovarian cancer PAOLA-1/ENGOT-ov25 Trial. JCO Precis. Oncol. 7, e2200258 (2023).
Zhu, L. et al. A novel and reliable method to detect microsatellite instability in colorectal cancer by next-generation sequencing. J. Mol. Diagn. 20, 225–231 (2018).
Ellrott, K. et al. Scalable open science approach for mutation calling of tumor exomes using multiple genomic pipelines. Cell Syst. 6, 271–281.e7 (2018).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830.e14 (2018).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant number 82002561), and the Guangdong Basic and Applied Basic Research Foundation (Grant numbers 2024A1515012191). The funder played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript. The authors thank Ting Hou and Jing Zhao (Burning Rock Biotech, Guangzhou, China), Fancheng Kong and Kun Yang (Precision Scientific Co., Ltd., Beijing, China), for their support in the revision process.
Author information
Authors and Affiliations
Contributions
Y.L. analyzed and interpreted the data, and was a major contributor in writing the manuscript. X.Y. and H.C. collected the data. F.W. and L.Y. designed the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Yang, X., Cai, H. et al. Homologous recombination deficiency among patients with germline or somatic non-BRCA1/2 homologous recombination repair gene variations. npj Precis. Onc. 9, 192 (2025). https://doi.org/10.1038/s41698-025-00999-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-025-00999-2