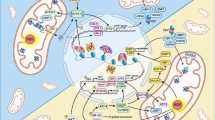
Abstract
The mitochondrial unfolded protein response (UPRmt), a mitochondria-to-nucleus retrograde pathway that promotes the maintenance of mitochondrial function in response to stress, plays an important role in promoting lifespan extension in Caenorhabditis elegans1,2. However, its role in mammals, including its contributions to development or cell fate decisions, remains largely unexplored. Here, we show that transient UPRmt activation occurs during somatic reprogramming in mouse embryonic fibroblasts. We observe a c-Myc-dependent, transient decrease in mitochondrial proteolysis, accompanied by UPRmt activation at the early phase of pluripotency acquisition. UPRmt impedes the mesenchymal-to-epithelial transition (MET) through c-Jun, thereby inhibiting pluripotency acquisition. Mechanistically, c-Jun enhances the expression of acetyl-CoA metabolic enzymes and reduces acetyl-CoA levels, thereby affecting levels of H3K9Ac, linking mitochondrial signalling to the epigenetic state of the cell and cell fate decisions. c-Jun also decreases the occupancy of H3K9Ac at MET genes, further inhibiting MET. Our findings reveal the crucial role of mitochondrial UPR-modulated MET in pluripotent stem cell plasticity. Additionally, we demonstrate that the UPRmt promotes cancer cell migration and invasion by enhancing epithelial-to-mesenchymal transition (EMT). Given the crucial role of EMT in tumour metastasis3,4, our findings on the connection between the UPRmt and EMT have important pathological implications and reveal potential targets for tumour treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data and materials that support the findings of this study are available from the corresponding author (X.L.) upon reasonable request. The RNA-seq and CUT&Tag raw data have been deposited in the Genome Sequence Archive at the Beijing Institute of Genomics Data Center at the Chinese Academy of Sciences (RNA-seq accession number: CRA014321; CUT&Tag accession number: CRA018794). The RNA-seq data for the hepatic differentiation of human ES cells are available in the GEO (accession number: GSE70741). All other relevant data supporting the key findings of this study are available within the article and its Supplementary Information files. Source data are provided with this paper.
Code availability
There was no custom code used.
References
Houtkooper, R. H. et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497, 451–457 (2013).
Jensen, M. B. & Jasper, H. Mitochondrial proteostasis in the control of aging and longevity. Cell Metab. 20, 214–225 (2014).
Gerstberger, S., Jiang, Q. & Ganesh, K. Metastasis. Cell 186, 1564–1579 (2023).
Yang, S., Zhang, J. J. & Huang, X. Y. Mouse models for tumor metastasis. Methods Mol. Biol. 928, 221–228 (2012).
Mahmoudi, S. & Brunet, A. Bursts of reprogramming: a path to extend lifespan? Cell 167, 1672–1674 (2016).
Núñez-Delicado, E. et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167, 1719 (2016).
Orkin, S. H. & Hochedlinger, K. Chromatin connections to pluripotency and cellular reprogramming. Cell 145, 835–850 (2011).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Chen, T., You, Y., Jiang, H. & Wang, Z. Z. Epithelial-mesenchymal transition (EMT): a biological process in the development, stem cell differentiation, and tumorigenesis. J. Cell. Physiol. 232, 3261–3272 (2017).
Diepenbruck, M. & Christofori, G. Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr. Opin. Cell Biol. 43, 7–13 (2016).
Milmoe, N. J. & Tucker, A. S. Craniofacial transitions: the role of EMT and MET during head development. Development 148, dev196030 (2021).
Li, R. et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 7, 51–63 (2010).
Nichols, J. & Smith, A. Naive and primed pluripotent states. Cell Stem Cell 4, 487–492 (2009).
Baker, C. L. & Pera, M. F. Capturing totipotent stem cells. Cell Stem Cell 22, 25–34 (2018).
Wanet, A., Arnould, T., Najimi, M. & Renard, P. Connecting mitochondria, metabolism, and stem cell fate. Stem Cells Dev. 24, 1957–1971 (2015).
Ware, C. B. et al. Derivation of naïve human embryonic stem cells. Proc. Natl Acad. Sci. USA 111, 4484–4489 (2014).
Xu, X. et al. Mitochondrial regulation in pluripotent stem cells. Cell Metab. 18, 325–332 (2013).
Ying, Z. et al. Transient activation of mitoflashes modulates nanog at the early phase of somatic cell reprogramming. Cell Metab. 23, 220–226 (2016).
Ying, Z. et al. Short-term mitochondrial permeability transition pore opening modulates histone lysine methylation at the early phase of somatic cell reprogramming. Cell Metab. 28, 935–945 (2018).
Owusu-Ansah, E., Song, W. & Perrimon, N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 155, 699–712 (2013).
Merkwirth, C. et al. Two conserved histone demethylases regulate mitochondrial stress-induced longevity. Cell 165, 1209–1223 (2016).
Shao, L. W. et al. Histone deacetylase HDA-1 modulates mitochondrial stress response and longevity. Nat. Commun. 11, 4639 (2020).
Tian, Y. et al. Mitochondrial stress induces chromatin reorganization to promote longevity and UPRmt. Cell 165, 1197–1208 (2016).
Zhu, D. et al. NuRD mediates mitochondrial stress-induced longevity via chromatin remodeling in response to acetyl-CoA level. Sci. Adv. 6, eabb2529 (2020).
Fiorese, C. J. et al. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr. Biol. 26, 2037–2043 (2016).
Horibe, T. & Hoogenraad, N. J. The CHOP gene contains an element for the positive regulation of the mitochondrial unfolded protein response. PLoS ONE 2, e835 (2007).
Quiros, P. M. et al. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J. Cell. Biol. 216, 2027–2045 (2017).
Zhao, Q. et al. A mitochondrial specific stress response in mammalian cells. EMBO J. 21, 4411–4419 (2002).
Luo, H. et al. Mitochondrial stress-initiated aberrant activation of the NLRP3 inflammasome regulates the functional deterioration of hematopoietic stem cell aging. Cell Rep. 26, 945–954 (2019).
Qiu, X. et al. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 12, 662–667 (2010).
Mohrin, M. et al. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science 347, 1374–1377 (2015).
Mohrin, M., Widjaja, A., Liu, Y., Luo, H. & Chen, D. The mitochondrial unfolded protein response is activated upon hematopoietic stem cell exit from quiescence. Aging Cell 17, e12756 (2018).
Wang, C. L. et al. The mitochondrial unfolded protein response regulates hippocampal neural stem cell aging. Cell Metab. 35, 996–1008 (2023).
Munch, C. & Harper, J. W. Mitochondrial unfolded protein response controls matrix pre-RNA processing and translation. Nature 534, 710–713 (2016).
Guo, Q. et al. Mitochondrial proteostasis stress in muscle drives a long-range protective response to alleviate dietary obesity independently of ATF4. Sci. Adv. 8, eabo0340 (2022).
Hernandez, G. et al. MitoTimer: a novel tool for monitoring mitochondrial turnover. Autophagy 9, 1852–1861 (2013).
Perez, M. J. et al. Loss of function of the mitochondrial peptidase PITRM1 induces proteotoxic stress and Alzheimer’s disease-like pathology in human cerebral organoids. Mol. Psychiatry 26, 5733–5750 (2021).
Taouktsi, E. et al. Organismal and cellular stress responses upon disruption of mitochondrial Lonp1 protease. Cells 11, 1363 (2022).
Sutandy, F. X. R., Gossner, I., Tascher, G. & Munch, C. A cytosolic surveillance mechanism activates the mitochondrial UPR. Nature 618, 849–854 (2023).
Chen, K. et al. Gadd45a is a heterochromatin relaxer that enhances iPS cell generation. EMBO Rep. 17, 1641–1656 (2016).
Banyard, J. & Bielenberg, D. R. The role of EMT and MET in cancer dissemination. Connect. Tissue Res. 56, 403–413 (2015).
Li, Q. et al. A sequential EMT–MET mechanism drives the differentiation of human embryonic stem cells towards hepatocytes. Nat. Commun. 8, 15166 (2017).
Zorzan, I. et al. The transcriptional regulator ZNF398 mediates pluripotency and epithelial character downstream of TGF-β in human PSCs. Nat. Commun. 11, 2364 (2020).
Wang, Y. et al. TFAP2C facilitates somatic cell reprogramming by inhibiting c-Myc-dependent apoptosis and promoting mesenchymal-to-epithelial transition. Cell Death Dis. 11, 482 (2020).
Feng, J. et al. Sin3a drives mesenchymal-to-epithelial transition through cooperating with Tet1 in somatic cell reprogramming. Stem Cell Res. Ther. 13, 29 (2022).
Liu, J. et al. The oncogene c-Jun impedes somatic cell reprogramming. Nat. Cell Biol. 17, 856–867 (2015).
Cicchini, C. et al. Epigenetic control of EMT/MET dynamics: HNF4α impacts DNMT3s through miRs-29. Biochim. Biophys. Acta 1849, 919–929 (2015).
Lin, W.-H. et al. STAT3 phosphorylation at Ser727 and Tyr705 differentially regulates the EMT–MET switch and cancer metastasis. Oncogene 40, 791–805 (2021).
Sheta, R. et al. Hic-5 regulates epithelial to mesenchymal transition in ovarian cancer cells in a TGFβ1-independent manner. Oncotarget 8, 82506–82530 (2017).
Samavarchi-Tehrani, P. et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 7, 64–77 (2010).
Wang, C., Li, Q. & He, Y. MicroRNA‑21‑5p promotes epithelial to mesenchymal transition by targeting SRY‑box 17 in endometrial cancer. Oncol. Rep. 43, 1897–1905 (2020).
Liao, B. et al. MicroRNA cluster 302–367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J. Biol. Chem. 286, 17359–17364 (2011).
Shpilka, T. & Haynes, C. M. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 19, 109–120 (2018).
Moussaieff, A. et al. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 21, 392–402 (2015).
Luo, R. X. & Dean, D. C. Chromatin remodeling and transcriptional regulation. J. Natl Cancer Inst. 91, 1288–1294 (1999).
Hou, C. et al. Lysine demethylase 1B (Kdm1b) enhances somatic reprogramming through inducing pluripotent gene expression and promoting cell proliferation. Exp. Cell. Res. 420, 113339 (2022).
Wang, F., Kou, Z., Zhang, Y. & Gao, S. Dynamic reprogramming of histone acetylation and methylation in the first cell cycle of cloned mouse embryos. Biol. Reprod. 77, 1007–1016 (2007).
Wille, C. K. et al. DOT1L is a barrier to histone acetylation during reprogramming to pluripotency. Sci. Adv. 9, eadf3980 (2023).
Rajagopalan, K. N. et al. Metabolic plasticity maintains proliferation in pyruvate dehydrogenase deficient cells. Cancer Metab. 3, 7 (2015).
Sebastian, C. & Mostoslavsky, R. The various metabolic sources of histone acetylation. Trends Endocrinol. Metab. 28, 85–87 (2017).
Wellen, K. E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009).
Goudarzi, A. The recent insights into the function of ACAT1: a possible anti-cancer therapeutic target. Life Sci. 232, 116592 (2019).
Tillander, V., Alexson, S. E. H. & Cohen, D. E. Deactivating fatty acids: Acyl-CoA thioesterase-mediated control of lipid metabolism. Trends Endocrinol. Metab. 28, 473–484 (2017).
Wang, Y. et al. Acetyl-CoA carboxylases and diseases. Front. Oncol. 12, 836058 (2022).
Hirai, S., Bourachot, B. & Yaniv, M. Both Jun and Fos contribute to transcription activation by the heterodimer. Oncogene 5, 39–46 (1990).
Shen, K. et al. The germline coordinates mitokine signaling. Cell 187, 4605–4620 (2024).
Le, R. et al. Enhanced telomere rejuvenation in pluripotent cells reprogrammed via nuclear transfer relative to induced pluripotent stem cells. Cell Stem Cell 14, 27–39 (2014).
Zhu, X. et al. Fine-tuning of PGC1alpha expression regulates cardiac function and longevity. Circ. Res. 125, 707–719 (2019).
Passos, J. F. & von Zglinicki, T. Mitochondria, telomeres and cell senescence. Exp. Gerontol. 40, 466–472 (2005).
Macfarlan, T. S. et al. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487, 57–63 (2012).
Hescheler, J. et al. Embryonic stem cells: a model to study structural and functional properties in cardiomyogenesis. Cardiovasc. Res. 36, 149–162 (1997).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Zhang, Y. et al. Model-based analysis of ChIP–seq (MACS). Genome Biol. 9, R137 (2008).
Acknowledgements
We are grateful to all members of X. Liu’s laboratory for useful discussions. We thank the J. Liu lab at the Guangzhou Institutes of Biomedicine and Health for the c-Jun overexpression plasmids. We also thank the entire staff of the Public Instrument Center at Guangzhou Institutes of Biomedicine and Health, CAS. Our work was financially supported by the National Key Research and Development Program of China (2023YFE0210100), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0480000), the National Natural Science Foundation projects of China (32025010, 32488301, 92254301, 92357302, 92157202, 32241002, 32261160376, 32100619, 32170747, 32322022, 32370782, 32371007, 32300608, 32300620, 32471358, 32461160288), the National Key Research and Development Program of China (2024YFA0916400, 2022YFE0210100, 2024YFA1802302, 2022YFA1103800), the NSFC/RGC Joint Grant Scheme 2022/2023 (N_CUHK 428/22), Major Project of Guangzhou National Laboratory (GZNL2024A03006, GZNL2024B01003) the Key Research Program, CAS (ZDBS-ZRKJZ-TLC003), the CAS Project for Young Scientists in Basic Research (YSBR-075), the Guangdong Province Science and Technology Program (2023B0303000023, 2023B1111050005, 2023A1515030231, 2022A1515110493, 2023B1212060050, 2021B1515020096, 2022A1515110951, 2023B1212120009, 2024A1515010782, 2024B1515040020, 2024A1515030120, 2023TQ07A024, 2024A1515012839), the Guangzhou Science and Technology Program (202206060002, 2023A04J0414, 2025A04J2106, 2025A04J7110, 2025A04J5485, 2023A04J0863, 2023A04J0727), Health@InnoHK funding support from the Innovation Technology Commission of the Hong Kong SAR, CAS Youth Innovation Promotion Association (to Y. Wu and K.C.) and the Basic Research Project of Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
X.L. conceived, designed and supervised the project. Z.Y. and Y.X. designed and carried out most experiments. Z.Y. and Y.X. carried out most data analyses. Z.L. carried out experiments involving cancer cells and Hsp60 and performed data analysis. Y.H. and Y.D. carried out RNA-seq, CUT&Tag and metabolome analyses. T.T., X.H., Q.L., G. Liu, Z.R. and W.L. performed western blot experiments. J.G., S.Z., Y.Y., Q.M., L.L., Y. Wu, Y.L. (Chinese Academy of Sciences), M.M. and W.L. participated in cell culture. J.W. helped with flow cytometery. Z.R., G.X., B.L., C.L. and Y.L. (The University of Hong Kong) participated in data analysis. Y. Wang, D.Q., W.W., G. Lu, D.P. and W.-Y.C. gave suggestions. X.L. and Z.Y. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Danica Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Transient increased UPRmt inhibits pluripotency acquisition.
a, The relative band densities of Fig. 1a; n = 4; **P = 0.0078 (D3 vs D0), *P = 0.019 (D5 vs D3). b, The expression of Hsp60 at days 0, 2, 4, 6, and 8 during mESCs to EBs differentiation. c, The expression of Hsp60 at days 0, 3, 5, and 8 during somatic cell reprogramming with TOMM20 as an internal reference. d, The expression of Hsp60 in EpiSCs, mESCs and 2CLCs. e, The relative band densities of Fig. 1d; n = 4; **P = 0.0042. f, The expression of Hsp60 after Flag, Sox2, Klf4 or Oct4 transduced at days 0 and 3. g, The relative band densities of Fig. 1e; n = 4; ***P = 0.00043 (Flag vs SKOM), ***P = 0.0006 (SKO vs SKOM). h, The expression of Hsp60 at days 0, 3, 5, and 8 during SKO mediated somatic cell reprogramming. i, The relative band densities of Fig. 1f; n = 4 (Control vs CDDOme) or n = 3 (Control vs knockdown of Lonp1 or Pitrm1); **P = 0.0039, *P = 0.019 (Control siRNA vs Lonp1 siRNA), *P = 0.013 (Control siRNA vs Pitrm1 siRNA). j, The expression of Hsp60 and Hsp70 after knockdown of Hsp60 at day 3 of reprogramming. k, The relative numbers of GFP+ colonies after knockdown of Hsp60; n = 5; ***P = 7.74 × 10−5. Data are presented as mean ± SEM; * P < 0.05; ** P < 0.01; *** P < 0.001. Unpaired two-tailed student’s t test (a, e, g, i, and k). n represents the number of biological replicates.
Extended Data Fig. 2 GO terms of differentially expressed genes in control and UPRmt activated cells.
a, GO terms of downregulated genes in CDDOme treated cells. b, GO terms of upregulated and downregulated genes in Lonp1 or Pitrm1 knockdown cells. Statistical significance was determined by two-way analysis of variance, and wilcoxon testing was used to correct for multiple testing.
Extended Data Fig. 3 UPRmt inhibits MET during reprogramming.
a, The relative band densities of Fig. 2b; n = 3 (Control vs CDDOme) or n = 4 (Control vs knockdown of Lonp1 or Pitrm1); *P = 0.045 (E-cadherin, Control vs CDDOme), *P = 0.027 (E-cadherin, Control siRNA vs Lonp1 siRNA), **P = 0.0022 (E-cadherin, Control siRNA vs Pitrm1 siRNA); *P = 0.045 (Slug, Control vs CDDOme), *P = 0.038 (Slug, Control siRNA vs Lonp1 siRNA), **P = 0.0045 (Slug, Control siRNA vs Pitrm1 siRNA). b, Real time PCR analysis of the MET relative genes after CDDOme treatment or Lonp1 or Pitrm1 knockdown at day 3 of reprogramming; n = 4 (Control vs CDDOme) or n = 3 (Control vs knockdown of Lonp1 or Pitrm1); For Control vs CDDOme, ***P (E-cadherin)= 2.44 × 10−5, **P (Epcam)= 0.005, **P (Slug)= 0.0022, *P (Snail)= 0.03, **P (Zeb1)= 0.0025; For Control siRNA vs Lonp1 siRNA, **P (E-cadherin)= 0.0033, ***P (Epcam)= 0.0006, *P (Slug)= 0.028, *P (Snail)= 0.025, *P (Zeb1)= 0.015; For Control siRNA vs Pitrm1 siRNA, *P (E-cadherin)= 0.048, *P (Epcam)= 0.021, **P (Slug)= 0.0053, *P (Snail)= 0.034, *P (Zeb1)= 0.016. c, Western blot analysis of Hsp60, Hsp70, E-cadherin and Slug after Hsp60 knockdown at days 3 of somatic cell reprogramming. d, e, Representative images (d) and quantification (e) of scratch assays on day 3 after CDDOme treatment or Lonp1 or Pitrm1 knockdown during reprogramming; Scale bar: 100 μm; n = 3; **P = 0.0091 (Control vs CDDOme), *P = 0.03 (Control siRNA vs Lonp1 siRNA), *P = 0.017 (Control siRNA vs Pitrm1 siRNA). f, g, Representative images (f) and quantification (g) of scratch assays at day 3 after Hsp60 knockdown during reprogramming; Scale bar: 200 μm; n = 3; *P = 0.043. Data are presented as mean ± SEM; * P < 0.05; ** P < 0.01; *** P < 0.001. Unpaired two-tailed student’s t test (a, b, e, and g). n represents the number of biological replicates.
Extended Data Fig. 4 UPRmt regulates MET and EMT during epithelial cell reprogramming or cells differentiation.
a, The expression of Hsp60 at days 0, 3, 5, and 8 during mammary epithelial cell (MEC) reprogramming. b, Western blot analysis of E-cadherin and Slug after CDDOme treatment or knockdown of Lonp1 or Pitrm1 at day 3 of MEC reprogramming. c, AP staining analysis of the efficiency of the MEC reprogramming after CDDOme treatment or knockdown of Lonp1 or Pitrm1. d, Plots of UPRmt responsive gene expression and epithelial genes at days 0, 1, 2, 3, 5, 7, 9 and 11 during human embryonic stem cells differentiation to hepatocytes. The black lines represent the average expression levels of these genes (6 genes) by day. e, Western blot analysis of E-CADHERIN and SLUG after CDDOme treatment or Lonp1 or Pitrm1 knockdown at day 3 and day 7 during human embryonic stem cells differentiation to hepatocytes. f, Western blot analysis of E-cadherin and Slug at days 0, 5, 7 and 10 during the differentiation of mouse embryonic stem cells into cardiac cells. g, Western blot analysis of E-cadherin and Slug after CDDOme treatment or Lonp1 or Pitrm1 knockdown at day 7 and 10 during the differentiation of mouse embryonic stem cells into cardiac cells.
Extended Data Fig. 5 UPRmt enhances EMT in cancer cells.
a, Western blot analysis of HSP60, E-CADHERIN and SLUG after CDDOme treatment in indicated concentration for 24 hours in the lung cancer cell line H1299. b, Representative images and quantification of scratch assays after CDDOme treatment in H1299; Scale bar: 100 μm; n = 3; **P = 0.0097. c, Representative images and quantification of invasion assays after CDDOme treatment in H1299; Scale bar: 100 μm; n = 3; *P = 0.017. d, Western blot analysis of HSP60, E-CADHERIN and SLUG after CDDOme treatment in indicated concentration for 24 hours in the liver cancer cell line HepG2. e, Representative images and quantification of invasion assays after CDDOme treatment in HepG2; Scale bar: 100 μm; n = 3, *P = 0.027. Data are mean ± SEM; * P < 0.05; ** P < 0.01. Unpaired two-tailed student’s t test (b, c, and e). n represents the number of biological replicates.
Extended Data Fig. 6 c-Jun is involved in UPRmt activation in reprogramming.
a, The expression of Hsp60 at day 3 with Atf4, Atf5 or Chop knockdown. b, The expression of Hsp60 at day 3 with Atf4, Atf5 or Chop overexpression. c, The expression of Hsp60 at day 3 with miR-200c, miR-21a or miR-367 inhibitor. d, The relative band densities of Fig. 2c; n = 3; *P = 0.025. e, The relative band densities of Fig. 2d; n = 3; *P = 0.017 (Control siRNA vs c-Jun siRNA 1), ***P = 0.00018, *P = 0.048 (Control siRNA vs c-Jun siRNA 3). f, The relative band densities of Fig. 2e; n = 3; *P = 0.048 (Control vs CDDOme), *P = 0.026 (Control siRNA vs Lonp1 siRNA), *P = 0.016 (Control siRNA vs Pitrm1 siRNA). g, The expression of c-Jun, Sirt7 and Atf5 at days 0, 3, 5, and 8 during SKOM mediated somatic cell reprogramming. h, The relative band densities of Fig. 2f; n = 4; For E-cadherin, left, *P = 0.02 (Control siRNA vs c-Jun siRNA), *P = 0.034 (Control siRNA vs Control siRNA plus CDDOme), **P = 0.0077 (Control siRNA plus CDDOme vs c-Jun siRNA plus CDDOme); middle, *P = 0.034 (Control siRNA vs c-Jun siRNA), **P = 0.0096 (Control siRNA vs Lonp1 siRNA), *P = 0.011 (Lonp1 siRNA vs c-Jun siRNA plus Lonp1 siRNA); right, *P = 0.031 (Control siRNA vs c-Jun siRNA), *P = 0.031 (Control siRNA vs Pitrm1 siRNA), *P = 0.047 (Pitrm1 siRNA vs c-Jun siRNA plus Pitrm1 siRNA); For Slug, left, *P = 0.016 (Control siRNA vs c-Jun siRNA), *P = 0.022 (Control siRNA vs Control siRNA plus CDDOme), **P = 0.0082 (Control siRNA plus CDDOme vs c-Jun siRNA plus CDDOme); middle, *P = 0.037 (Control siRNA vs c-Jun siRNA), *P = 0.045 (Control siRNA vs Lonp1 siRNA), *P = 0.031 (Lonp1 siRNA vs c-Jun siRNA plus Lonp1 siRNA); right, **P = 0.0053 (Control siRNA vs c-Jun siRNA), *P = 0.036 (Control siRNA vs Pitrm1 siRNA), *P = 0.026 (Pitrm1 siRNA vs c-Jun siRNA plus Pitrm1 siRNA). Data are mean ± SEM; * P < 0.05; ** P < 0.01; *** P < 0.001. Unpaired two-tailed student’s t test (d-f, and h). n represents the number of biological replicates.
Extended Data Fig. 7 Metabolomics analysis after UPRmt activation during reprogramming.
a, Metabolomics analysis with CDDOme treatment at day 3 of reprogramming. Orange dots indicate upregulated metabolites, whereas the green dot indicates the lone downregulated metabolite, acetyl-CoA. Unpaired two-tailed student’s t test. b, Heatmap showing the levels of metabolites in control and CDDOme treated cells. Red indicates upregulated metabolites, whereas blue indicates downregulated metabolite.
Extended Data Fig. 8 Activated UPRmt decreases histone acetylation and occupancy at MET genes.
a, Western blot analysis of H3Ac, H3K4Ac, H3K14Ac, H3K18Ac and H3K27Ac with or without UPRmt activation at day 3 of reprogramming. b, The relative band densities of Fig. 3b; n = 4 (Control vs CDDOme); n = 3 (Control vs knockdown of Lonp1 or Pitrm1); *P = 0.014 (Control vs CDDOme), *P = 0.017 (Control siRNA vs Lonp1 siRNA), *P = 0.029 (Control siRNA vs Pitrm1 siRNA). c, The relative band densities of Fig. 3c; CDDOme treatment: *P = 0.028 (Acetate), **P = 0.0091 (Citrate), *P = 0.043 (Pyruvate); Lonp1 siRNA: *P = 0.04 (Acetate), *P = 0.021 (Citrate), *P = 0.022 (Pyruvate); Pitrm1 siRNA: *P = 0.048 (Acetate), *P = 0.047 (Citrate), *P = 0.0497 (Pyruvate); n = 3. d, CUT&Tag analysis of H3K9Ac binding to epithelial genes with or without UPRmt activation at day 3 of reprogramming. e, The relative band densities of Fig. 3e; CDDOme treatment: *P = 0.016 (Acetate), *P = 0.047 (Citrate), *P = 0.022 (Pyruvate); Lonp1 siRNA: *P = 0.02 (Acetate), **P = 0.0018 (Citrate), *P = 0.016 (Pyruvate); Pitrm1 siRNA: *P = 0.01 (Acetate), *P = 0.03 (Citrate), ***P = 4.76 × 10−5 (Pyruvate); n = 3. Data are mean ± SEM; * P < 0.05; ** P < 0.01; *** P < 0.001. Unpaired two-tailed student’s t test (b, c, and e). n represents the number of biological replicates.
Extended Data Fig. 9 Expression analysis of histone acetylation, acetyl-CoA and acetyl-CoA associated enzymes with or without c-Jun knockdown after UPRmt activation.
a, The relative band densities of Fig. 4a; left, *P = 0.037 (Control siRNA vs Control siRNA plus CDDOme), *P = 0.029 (Control siRNA plus CDDOme vs c-Jun siRNA plus CDDOme); middle, *P = 0.013 (Control siRNA vs Lonp1 siRNA), *P = 0.029 (Lonp1 siRNA vs c-Jun siRNA plus Lonp1 siRNA); right, **P = 0.0015 (Control siRNA vs Pitrm1 siRNA), *P = 0.047 (Pitrm1 siRNA vs c-Jun siRNA plus Pitrm1 siRNA); n = 3. b, Western blot analysis of H3K27Ac after UPRmt activation with or without c-Jun knockdown at day 3 of reprogramming. c, d, Relative acetyl-CoA levels analysis after c-Jun overexpression (c) or knockdown (d) at day 3 of reprogramming; *P = 0.017 (c); *P = 0.019 (d); n = 3. e, qPCR analysis of acetyl-CoA metabolic enzymes (Acat1, Acat2 and Acot12) in CDDOme-treated or Lonp1 or Pitrm1 knockdown cells with or without c-Jun knockdown at day 3 of reprogramming; For (CDDOme plus Control siRNA) vs (CDDOme plus c-Jun siRNA), *P = 0.028 (Acat1), *P = 0.015 (Acat2), *P = 0.016 (Acot12); For (Lonp1 siRNA plus Control siRNA) vs (Lonp1 siRNA plus c-Jun siRNA), *P = 0.016 (Acat1), *P = 0.015 (Acat2), **P = 0.0048 (Acot12); For (Pitrm1 siRNA plus Control siRNA) vs (Pitrm1 siRNA plus c-Jun siRNA), **P = 0.0072 (Acat1), *P = 0.045 (Acat2), *P = 0.011 (Acot12); n = 3. f, qPCR analysis of acetyl-CoA generating enzymes (Acss2, Acly and Pdha1) and metabolic enzyme (Acaca) after UPRmt activation with or without c-Jun knockdown at day 3 of reprogramming; n = 4. Data are mean ± SEM; * P < 0.05; ** P < 0.01. Unpaired two-tailed student’s t test (a and c-f). n represents the number of biological replicates.
Extended Data Fig. 10 c-Jun regulates acetyl-CoA levels by binding to the gene loci of acetyl-CoA metabolic enzymes during UPRmt activation.
a, The relative band densities of Fig. 4c; *P = 0.018 (Control siRNA vs c-Jun siRNA); *P = 0.013 (Control siRNA vs Control siRNA plus CDDOme), *P = 0.024 (Control siRNA plus CDDOme vs c-Jun siRNA plus CDDOme); *P = 0.049 (Control siRNA vs Lonp1 siRNA), *P = 0.035 (Lonp1 siRNA vs c-Jun siRNA plus Lonp1 siRNA); **P = 0.0022 (Control siRNA vs Pitrm1 siRNA), **P = 0.0015 (Pitrm1 siRNA vs c-Jun siRNA plus Pitrm1 siRNA); n = 3. b, Relative acetyl-CoA levels in Acat1, Acat2 or Acot12 knockdown cells with c-Jun overexpression at day 3 of reprogramming; *P = 0.04 (Acat1 siRNA), *P = 0.012 (Acat2 siRNA), *P = 0.015 (Acot12 siRNA); n = 3. c, ChIP-qPCR analysis of c-Jun binding to Acat1, Acat2 and Acot12 with or without UPRmt activation at day 3 of reprogramming; For CDDOme vs Control, *P = 0.033 (Acat1), **P = 0.0063 (Acat2), **P = 0.0018 (Acot12); For Lonp1 siRNA vs control siRNA, *P = 0.047 (Acat1), *P = 0.014 (Acat2), *P = 0.01 (Acot12); For Pitrm1 siRNA vs control siRNA, *P = 0.022 (Acat1), *P = 0.037 (Acat2), *P = 0.011 (Acot12); n = 4. Data are mean ± SEM; * P < 0.05; ** P < 0.01. Unpaired two-tailed student’s t test (a-c). n represents the number of biological replicates.
Supplementary information
Supplementary Tables 1–3
Primers and antibodies.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ying, Z., Xin, Y., Liu, Z. et al. The mitochondrial unfolded protein response inhibits pluripotency acquisition and mesenchymal-to-epithelial transition in somatic cell reprogramming. Nat Metab 7, 940–951 (2025). https://doi.org/10.1038/s42255-025-01261-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-025-01261-6
This article is cited by
-
UPRmt controls the mesenchymal-to-epithelial transition
Nature Metabolism (2025)