Abstract
Thermogenic beige adipocytes are recognized as potential therapeutic targets for combating metabolic diseases. However, the metabolic advantages that they offer are compromised with aging. Here we show that treating mice with estrogen (E2), a hormone that decreases with age, can counteract the age-related decline in beige adipogenesis when exposed to cold temperature while concurrently enhancing energy expenditure and improving glucose tolerance in mice. Mechanistically, we found that nicotinamide phosphoribosyl transferase (NAMPT) plays a pivotal role in facilitating the formation of E2-induced beige adipocytes, which subsequently suppresses the onset of age-related endoplasmic reticulum (ER) stress. Furthermore, we found that targeting NAMPT signaling, either genetically or pharmacologically, can restore the formation of beige adipocytes by increasing the number of perivascular adipocyte progenitor cells. Conversely, the absence of NAMPT signaling prevents this process. Together, our findings shed light on the mechanisms regulating the age-dependent impairment of beige adipocyte formation and underscore the E2-NAMPT-controlled ER stress pathway as a key regulator of this process.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The RNA-seq dataset generated and analyzed during the current study is available under accession number GSE261481. The rest of the data generated or analyzed during this study are all included in the published article and its supplementary information files. Source data are provided with this paper. All other data are available from the corresponding authors upon reasonable request.
References
Spiegelman, B. M. & Flier, J. S. Obesity and the regulation of energy balance. Cell 104, 531–543 (2001).
Friedman, J. M. Obesity: causes and control of excess body fat. Nature 459, 340–342 (2009).
Jura, M. & Kozak, L. P. Obesity and related consequences to ageing. Age (Dordr) 38, 23 (2016).
Finucane, M. M. et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377, 557–567 (2011).
Stout, M. B., Justice, J. N., Nicklas, B. J. & Kirkland, J. L. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda) 32, 9–19 (2017).
Porter Starr, K. N. & Bales, C. W. Excessive body weight in older adults. Clin. Geriatr. Med. 31, 311–326 (2015).
Bluher, M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298 (2019).
Scherer, P. E. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55, 1537–1545 (2006).
Kajimura, S., Spiegelman, B. M. & Seale, P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559 (2015).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Xue, S., Lee, D. & Berry, D. C. Thermogenic adipose tissue in energy regulation and metabolic health. Front. Endocrinol. (Lausanne) 14, 1150059 (2023).
Frontini, A. et al. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim. Biophys. Acta 1831, 950–959 (2013).
Sidossis, L. S. et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 22, 219–227 (2015).
Weir, G. et al. Substantial metabolic activity of human brown adipose tissue during warm conditions and cold-induced lipolysis of local triglycerides. Cell Metab. 27, 1348–1355 (2018).
van der Lans, A. A. et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123, 3395–3403 (2013).
Chondronikola, M. et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099 (2014).
Finlin, B. S. et al. Human adipose beiging in response to cold and mirabegron. JCI Insight 3, e121510 (2018).
Berry, D. C. et al. Cellular aging contributes to failure of cold-induced beige adipocyte formation in old mice and humans. Cell Metab. 25, 166–181 (2017).
Benvie, A. M. et al. Age-dependent Pdgfrβ signaling drives adipocyte progenitor dysfunction to alter the beige adipogenic niche in male mice. Nat. Commun. 14, 1806 (2023).
Geach, T. Adipose tissue: reversing age-related decline in beiging. Nat. Rev. Endocrinol. 13, 64 (2017).
Rogers, N. H., Landa, A., Park, S. & Smith, R. G. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell 11, 1074–1083 (2012).
Tajima, K. et al. Mitochondrial lipoylation integrates age-associated decline in brown fat thermogenesis. Nat Metab. 1, 886–898 (2019).
Nguyen, H. P. et al. Aging-dependent regulatory cells emerge in subcutaneous fat to inhibit adipogenesis. Dev. Cell 56, 1437–1451 (2021).
Hammes, S. R. & Levin, E. R. Impact of estrogens in males and androgens in females. J. Clin. Invest. 129, 1818–1826 (2019).
Steiner, B. M. & Berry, D. C. The regulation of adipose tissue health by estrogens. Front. Endocrinol. (Lausanne) 13, 889923 (2022).
Torres Irizarry, V. C., Jiang, Y., He, Y. & Xu, P. Hypothalamic estrogen signaling and adipose tissue metabolism in energy homeostasis. Front. Endocrinol. (Lausanne) 13, 898139 (2022).
Morselli, E. et al. Impact of estrogens and estrogen receptor-α in brain lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 315, E7–E14 (2018).
Brzozowski, A. M. et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389, 753–758 (1997).
Wu, A. et al. Age-related changes in estradiol and longitudinal associations with fat mass in men. PLoS ONE 13, e0201912 (2018).
Burger, H. G., Hale, G. E., Robertson, D. M. & Dennerstein, L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum. Reprod. Update 13, 559–565 (2007).
Nelson, J. F., Felicio, L. S., Osterburg, H. H. & Finch, C. E. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol. Reprod. 24, 784–794 (1981).
Koebele, S. V. & Bimonte-Nelson, H. A. Modeling menopause: the utility of rodents in translational behavioral endocrinology research. Maturitas 87, 5–17 (2016).
Heine, P. A., Taylor, J. A., Iwamoto, G. A., Lubahn, D. B. & Cooke, P. S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl Acad. Sci. USA 97, 12729–12734 (2000).
Brooks, H. L., Pollow, D. P. & Hoyer, P. B. The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology (Bethesda) 31, 250–257 (2016).
Rogers, N. H., Perfield, J. W. 2nd, Strissel, K. J., Obin, M. S. & Greenberg, A. S. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150, 2161–2168 (2009).
Lizcano, F. & Guzman, G. Estrogen deficiency and the origin of obesity during menopause. Biomed. Res. Int. 2014, 757461 (2014).
Lambrinoudaki, I., Paschou, S. A., Armeni, E. & Goulis, D. G. The interplay between diabetes mellitus and menopause: clinical implications. Nat. Rev. Endocrinol. 18, 608–622 (2022).
Heianza, Y. et al. Effect of postmenopausal status and age at menopause on type 2 diabetes and prediabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 17 (TOPICS 17). Diabetes Care 36, 4007–4014 (2013).
Mauvais-Jarvis, F., Clegg, D. J. & Hevener, A. L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 34, 309–338 (2013).
Almey, A., Hafez, N. M., Hantson, A. & Brake, W. G. Deficits in latent inhibition induced by estradiol replacement are ameliorated by haloperidol treatment. Front. Behav. Neurosci. 7, 136 (2013).
Das, S. K. et al. Estrogenic responses in estrogen receptor-α deficient mice reveal a distinct estrogen signaling pathway. Proc. Natl Acad. Sci. USA 94, 12786–12791 (1997).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Ganz, J. et al. A novel specific PERK activator reduces toxicity and extends survival in Huntington’s disease models. Sci. Rep. 10, 6875 (2020).
Madhavan, A. et al. Pharmacologic IRE1/XBP1s activation promotes systemic adaptive remodeling in obesity. Nat. Commun. 13, 608 (2022).
Blackwood, E. A. et al. Pharmacologic ATF6 activation confers global protection in widespread disease models by reprograming cellular proteostasis. Nat. Commun. 10, 187 (2019).
Berry, D. C., Jiang, Y. & Graff, J. M. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat. Commun. 7, 10184 (2016).
Lee, Y. H., Petkova, A. P., Mottillo, E. P. & Granneman, J. G. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491 (2012).
Covarrubias, A. J., Perrone, R., Grozio, A. & Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 22, 119–141 (2021).
Yoshino, J., Mills, K. F., Yoon, M. J. & Imai, S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536 (2011).
Hasmann, M. & Schemainda, I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 63, 7436–7442 (2003).
Vishvanath, L. et al. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 23, 350–359 (2016).
Jiang, Y., Berry, D. C. & Graff, J. Distinct cellular and molecular mechanisms for β3 adrenergic receptor induced beige adipocyte formation. eLife 6, e30329 (2017).
Chen, Y. et al. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 565, 180–185 (2019).
Rosenwald, M., Perdikari, A., Rulicke, T. & Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 (2013).
Cohen, P. & Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 22, 393–409 (2021).
Shao, M. et al. Cellular origins of beige fat cells revisited. Diabetes 68, 1874–1885 (2019).
Oguri, Y. et al. CD81 controls beige fat progenitor cell growth and energy balance via FAK signaling. Cell 182, 563–577 (2020).
Long, J. Z. et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 19, 810–820 (2014).
Park, J. et al. Progenitor-like characteristics in a subgroup of UCP1+ cells within white adipose tissue. Dev. Cell 56, 985–999(2021).
Schwarz, D. S. & Blower, M. D. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell. Mol. Life Sci. 73, 79–94 (2016).
Yuliana, A. et al. Endoplasmic reticulum stress impaired uncoupling protein 1 expression via the suppression of peroxisome proliferator-activated receptor γ binding activity in mice beige adipocytes. Int. J. Mol. Sci. 20, 274 (2019).
Lee, J.-M. et al. Reduction in endoplasmic reticulum stress activates beige adipocytes differentiation and alleviates high fat diet-induced metabolic phenotypes. Biochim. Biophys. Acta 1867, 166099 (2021).
Xie, N. et al. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 5, 227 (2020).
Stromsdorfer, K. L. et al. NAMPT-mediated NAD+ biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep. 16, 1851–1860 (2016).
Nielsen, K. N. et al. NAMPT-mediated NAD+ biosynthesis is indispensable for adipose tissue plasticity and development of obesity. Mol Metab. 11, 178–188 (2018).
Yamaguchi, S. et al. Adipose tissue NAD+ biosynthesis is required for regulating adaptive thermogenesis and whole-body energy homeostasis in mice. Proc. Natl Acad. Sci. USA 116, 23822–23828 (2019).
Mina, A. I. et al. CalR: a web-based analysis tool for indirect calorimetry experiments. Cell Metab. 28, 656–666 (2018).
Wang, W. et al. Constitutive GLI1 expression in chondrosarcoma is regulated by major vault protein via mTOR/S6K1 signaling cascade. Cell Death Differ. 28, 2221–2237 (2021).
Wu, R. et al. Genetically prolonged beige fat in male mice confers long-lasting metabolic health. Nat. Commun. 14, 2731 (2023).
Acknowledgements
We thank S. Imai for kindly providing the NAMPT floxed mouse strain. We thank members of the Y.J. laboratory for mouse genotyping and technical help, with special thanks to P. Bhalla and A. Elsabbagh. We thank C. R. Adams and J. Purcell for assistance with mouse husbandry; S. J. Green and the Research Resources Center for RT–PCR analysis; the Metabolic Phenotyping Core for analytical and phenotypical mouse measurements; and members of the Y.J. laboratory for helpful comments on the manuscript. The graphical abstract (Extended Data Fig. 10c) was created using BioRender, and scheme illustrations in Fig. 2a and Extended Data Fig. 5b were adapted from previous work19 and redeveloped using BioRender. This work was supported by National Institutes of Health (NIH) grants K01 DK11177, R03 DK127149 and R01 DK132398 and Pilot Support grant P30DK020595 to Y.J., NIH grant R01HL148756 to S.-G.O. and NIH grant R01DK123098 to P.X.
Author information
Authors and Affiliations
Contributions
Y.J. and J.P. conceived and designed the experiments. J.P. and R.H. conducted most of the experiments. Y.Q., S.X., A.S.E.-S., M.I., J.W., Q.S., Z.S., G.Y., A.M., Y.H., B.L., J.C., S.-G.-O. and P.X. contributed to the study design, data interpretation and manuscript writing. Z.X. and Z.C. performed interaction analysis between age and treatment types.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Michael Stout, Jonas Treebak and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
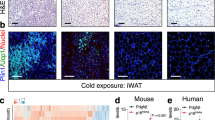
Extended Data Fig. 1 Age-related decline and restoration of serum E2 levels in male mice.
Serum E2 levels were compared between 2-month-old (Young) and 12-month-old (Old) males and assessed 4 and 24 hours after intraperitoneal injections of E2 (10 μg/Kg) to 12-month-old males by one way-ANOVA followed by Tukey’s post-hoc test. Animal number: Young (n = 5), Old (n = 4), Old E2 4 h (n = 5), Cold E2 (n = 4). Data information: All results are presented as means ± SEM.
Extended Data Fig. 2 Estrogen treatment ameliorates some metabolic dysfunctions associated with aging.
a, Body lean mass composition. Animal number: RT Veh (n = 4), RT E2 (n = 4), Cold Veh (n = 9), Cold E2 (n = 10). b-c, Weight of adipose tissues (b), ANOVA summary (IGW; Interaction P = 0.039, Temperature P = 0.103, Treatment P < 0.0001, PGW; Interaction P = 0.862, Temperature P = 0.056, Treatment P = 0.0002, by two way-ANOVA followed by Tukey’s post-hoc test), and other tissues (c). Animal number: RT Veh (n = 4), RT E2 (n = 4), Cold Veh (n = 9), Cold E2 (n = 10). d-e, Serum and hepatic triglyceride by two-tailed unpaired Student’s t-test (D), and serum and hepatic cholesterol (e). Animal number: Cold Veh (n = 9), Cold E2 (n = 10). f-j, Oxygen consumption and quantification (f), ANOVA summary (Time, Treatment and Temperature; Interaction P = 0.07, Time P < 0.0001, Treatment and Temperature P = 0.006, Light; Interaction P = 0.56, Temperature P = 0.79, Treatment P < 0.0001, Dark; Interaction P = 0.35, Temperature P = 0.011, Treatment P < 0.0001, by two way-ANOVA followed by Tukey’s post-hoc test). Carbone dioxygen consumption and quantification (g), ANOVA summary (Time, Treatment and Temperature; Interaction P = 0.28, Time P = 0.006, Treatment and Temperature P < 0.0001, Light; Interaction P < 0.0001, Treatment P = 0.039, Temperature P < 0.0001, Dark; Interaction P < 0.0001, Treatment P = 0.0019, Temperature P < 0.0001, by two way-ANOVA followed by Tukey’s post-hoc test). RER (H), ANOVA summary (Interaction P < 0.0001, Temperature P < 0.0001, Treatment P < 0.0001), food intake (I), ANOVA summary (Interaction P = 0.51, Temperature P < 0.0001, Treatment P = 0.41, by two way-ANOVA followed by Tukey’s post-hoc test), and physical activity (j), Animal number: RT Veh (n = 5), RT E2 (n = 4), Cold Veh (n = 6), Cold E2 (n = 7). Data information: All results are presented as means ± SEM.
Extended Data Fig. 3 Estrogen treatment has minimal impact on BAT thermogenesis upon cold exposure.
a, Representative H&E staining of BAT from vehicle (Veh) or E2-treated (10 µg/Kg) groups upon cold exposure. Scale bar, 100 μM. b, qPCR analysis of mRNA expression of thermogenic genes in BAT from vehicle or E2-treated groups by two-tailed unpaired Student’s t-test (n = 7 per group). Data information: All results are presented as means ± SEM.
Extended Data Fig. 4 Estrogen does not significantly impact cold-induced beige adipocyte formation in aged female mice.
a-b, Body weight and the difference in body fat composition by two-tailed unpaired Student’s t-test. Animal number: Veh (n = 8), E2 (n = 10). c, Representative images of H&E staining and UCP1 immunofluorescence staining in the middle part of iWAT. Scale bar, 100 μm. Data information: All results are presented as means ± SEM.
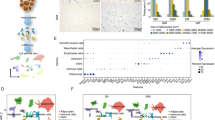
Extended Data Fig. 5 ERα but not ERβ antagonist abolishes estrogen-promoted beige adipogenesis in old SVF cells.
a, qPCR analysis of estrogen receptor-related gene expression between young and old SVF cells (n = 3 per group) by two-tailed unpaired Student’s t-test. b, Schematic illustration. SVF cells isolated from iWAT of 12-month-old male mice were pre-treated with either vehicle or E2 (10 nM) or a combination with ERα antagonist MPP (10μM) and E2, or a combination with ERβ antagonist PHTPP (10μM) and E2 before differentiating into beige adipocytes. c, Representative images of UCP1 immunofluorescence staining. Scale bar, 100 μM. d, Quantification of UCP1 by one way-ANOVA followed (n = 5 per group) by Tukey’s post-hoc test. e, qPCR analysis of the thermogenic gene expression (n = 3 per group) by one way-ANOVA followed by Tukey’s post-hoc test. Data information: All results are presented as means ± SEM.
Extended Data Fig. 6 ER stress inducer tunicamycin reduces energy expenditure in young mice.
a, Downregulated gene ontology (GO) cellular component to E2 by two-tailed unpaired Student’s t-test. b, Downregulated GO reactome pathway to E2 by two-tailed unpaired Student’s t-test. c, The lean mass composition in vehicle and tunicamycin treated group (n = 5 per group). d-h, Oxygen consumption and quantification (d), ANOVA summary (Interaction P = 0.798, Treatment P < 0.0001, Time P = 0.657, by two way-ANOVA followed by Tukey’s post-hoc test), Carbone dioxygen consumption and quantification (e), ANOVA summary (Interaction P = 0.788, Treatment P < 0.0001, Time P = 0.174, by two way-ANOVA followed by Tukey’s post-hoc test), RER (f), food intake (g), and physical activity (h) (n = 7 per group). i, Quantification of basal respiration, ATP-turnover, and maximum respiratory capacity of the samples in panel (n = 7 per group). ANOVA summary (Basal; Interaction P = 0.178, PBS verse Tunicamycin P < 0.0001, Vehicle verse Estrogen P = 0.432, ATP-Linked; Interaction P = 0.505, PBS verse Tunicamycin P < 0.0001, Vehicle verse Estrogen P = 0.763, Maximum; Interaction P = 0.119, PBS verse Tunicamycin P < 0.0001, Vehicle verse Estrogen P = 0.047, by two way-ANOVA followed by Tukey’s post-hoc test). Data information: All results are presented as means ± SEM.
Extended Data Fig. 7 NAD+ pathway analysis in E2-treated aged iWAT.
a-c, Upregulated gene ontology (GO) cellular component to E2 (a), upregulated gene ontology (GO) molecular function to E2 (b), and heat map of a list of NAD pathway gene expression (c) (n = 3 per group), by two-tailed unpaired Student’s t-test. d, Quantification of the percentage of RFP+ cells. Animal number: Control Veh (n = 8), Control E2 (n = 6), NAMPT Het Veh (n = 8), NAMPT Het E2 (n = 8). ANOVA summary (Interaction P = 0.997, Condition P < 0.0001, Treatment P < 0.0001, by two way-ANOVA followed by Tukey’s post-hoc test). e, Quantification of the percentage of RFP+ cells that express endogenous UCP1 (n = 6 per group). ANOVA summary (Interaction P = 0.896, Condition P < 0.0001, Treatment P < 0.0001, by two way-ANOVA followed by Tukey’s post-hoc test). Data information: All results are presented as means ± SEM.
Extended Data Fig. 8 FK866 treatment has minimal effect on BAT thermogenesis upon cold exposure.
a, Representative H&E staining of BAT from E2-treated (10 µg/Kg) or combination with FK866 (10 mg/Kg) groups upon cold exposure. Scale bar, 100 μM. b, qPCR analysis of mRNA expression of thermogenic genes in BAT from E2-treated or combination with FK866 groups (n = 4 per group) by two-tailed unpaired Student’s t-test. Data information: All results are presented as means ± SEM.
Extended Data Fig. 9 NAD+ levels in SVF cells are modulated by NMN or FK866 treatment.
Comparison of NAD+ levels in young versus aged SVF cells, treated with NMN (50 µM) or FK866 (300 nM), shows a decline in the aged cells, an effect that is altered by the treatments. Animal number: Young Veh (n = 5), Young NMN (n = 4), Young FK866 (n = 4), Old Veh (n = 5), Old NMN (n = 4), Old FK866 (n = 4). ANOVA summary (Interaction P < 0.0001, Age P < 0.0001, Treatments P < 0.0001, by two way-ANOVA followed by Tukey’s post-hoc test). Data information: All results are presented as means ± SEM.
Extended Data Fig. 10 NMN treatment has minimal effect on BAT thermogenesis upon cold exposure.
a, Representative H&E staining of BAT from vehicle or NMN-treated (50 mg/Kg) groups upon cold exposure. Scale bar, 100 μM. b, qPCR analysis of mRNA expression of thermogenic genes in BAT from vehicle or NMN-treated groups by two-tailed unpaired Student’s t-test. Animal number: Veh (n = 5), NMN (n = 4). c, The illustration of the mechanisms of E2-restored beige adipogenesis in aging mice. Data information: All results are presented as means ± SEM.
Supplementary information
Supplementary Table 1
The primer sequences for qPCR in this study.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data, unprocessed western blots.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data.
Source Data Fig. 8
Statistical Source Data, unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 9
Statistical Source Data.
Source Data Extended Data Fig. 10
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, J., Hu, R., Qian, Y. et al. Estrogen counteracts age-related decline in beige adipogenesis through the NAMPT-regulated ER stress response. Nat Aging 4, 839–853 (2024). https://doi.org/10.1038/s43587-024-00633-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-024-00633-z
This article is cited by
-
Adipose tissue ageing: implications for metabolic health and lifespan
Nature Reviews Endocrinology (2025)
-
Endoplasmic Reticulum Stress Inhibition Promotes Mitophagy via Miro1 Reduction to Rescue Mitochondrial Dysfunction and Protect Dopamine Neurons in Parkinson’s Disease
Cellular and Molecular Neurobiology (2025)