Abstract
Background
Musculoskeletal disorders pose major public health challenges, and accelerated biological aging may increase their risk. This study investigates the association between biological aging and musculoskeletal disorders, with a focus on sex-related differences.
Methods
We analyzed data from 172,332 UK Biobank participants (mean age of 56.03 ± 8.10 years). Biological age was calculated using the KDM-BA and PhenoAge algorithms based on blood biomarkers. Musculoskeletal disorders were diagnosed using the ICD-10 criteria, with sample sizes ranging from 1,182 to 23,668. Logistic regression assessed cross-sectional associations between age acceleration (AA) metrics and musculoskeletal disorders. Accelerated Failure Time (AFT) model was used for survival analysis to evaluate the relationships between AAs and musculoskeletal disorders onset. Models were adjusted for demographic, lifestyle, and socio-economic covariates. The threshold of P-values were set by the Holm-Bonferroni correction.
Results
Cross-sectional analyses reveal significant associations between AAs and fourteen musculoskeletal disorders. Survival analyses indicate that AAs significantly accelerate the onset of nine musculoskeletal disorders, including inflammatory polyarthropathies (RTKDM-BA = 0.993; RTPhenoAge = 0.983), systemic connective tissue disorders (RTKDM-BA = 0.987; RTPhenoAge = 0.980), spondylopathies (RTPhenoAge= 0.994), disorders of bone density and structure (RTPhenoAge= 0.991), gout (RTPhenoAge= 0.968), arthritis (RTPhenoAge= 0.991), pain in joint (RTPhenoAge= 0.989), low back pain (RTPhenoAge= 0.986), and osteoporosis (RTPhenoAge= 0.994). Sensitivity analyses are consistent with the primary findings. Sex-specific variations are observed, with AAs accelerating spondylopathies, arthritis, and low back pain in females, while osteoporosis is accelerated in males.
Conclusion
Accelerated biological aging is significantly associated with the incidence of several musculoskeletal disorders. These insights highlight the importance of biological age assessments in gauging musculoskeletal disorder risk, aiding early detection, prevention, and management.
Plain language summary
As we age, our bodies experience changes that can lead to health problems, including musculoskeletal disorders such as arthritis and back pain. This study explores how biological aging, a measure of how old our bodies seem based on biomarkers, affects the risk of developing these disorders. Using data from over 170,000 people, we found that faster biological aging is linked to an increased risk of several musculoskeletal disorders, and that these risks can vary between men and women. These findings could help identify people at risk earlier, leading to better prevention and treatment strategies.
Similar content being viewed by others
Introduction
As global populations age and life expectancy increases, the desire for extended, healthy lives becomes paramount1. This shift elevates the importance of studying age-related diseases in both scientific and public health contexts. Musculoskeletal disorders stand as the leading source of pain and disability worldwide, especially in the elderly demographic2. The bulk of musculoskeletal disorders comprises back and neck disorders, arthritic conditions, and soft tissue syndromes involving the tendons, ligaments, muscles, and cartilage. Although these disorders have notable prevalence and burden globally3, their significance is often downplayed. This is because they are infrequently fatal, deemed irreversible, and associated with aging4.
Aging is a progressive and irreversible pathophysiological process. However, the trajectories of age-related decline show marked variability across individuals5. Biological age offers a more precise assessment of human aging compared to chronological age6,7. Several metrics exist to define biological age, drawing primarily from histological data (like DNA methylation and proteomics) and clinical biomarkers from sources such as blood tests5. Significantly, algorithms that incorporate standard clinical parameters can adeptly predict morbidity and mortality8. For our study’s objective, we turned to two validated clinical-parameter algorithms: Klemera-Doubal method Biological Age (KDM-BA)9 and PhenoAge10. The former, KDM-BA, employs a multivariate analysis to balance the difference between chronological and estimated biological age across various biomarkers9. In contrast, PhenoAge is rooted in a weighted combination of select biomarkers that are aligned with known age-related mortality risks10. These two biological ages are essential for assessing aging and for standardizing the impact of aging on the musculoskeletal system.
Recent research has highlighted that biological aging is linked to musculoskeletal dysfunction11. A study has shown that senescent cells constitute integral components of the skeletal muscle regenerative niche, inhibiting regeneration across all life stages12, which may contribute to the onset of musculoskeletal disorders. While specific aging mechanisms have been connected to certain musculoskeletal diseases13,14, comprehensive research examining the broader impacts of aging on these disorders remains limited. Thus, it is imperative to quantify the effects of aging on the onset of musculoskeletal disorders.
Sex differences in the aging process15 and the development of musculoskeletal disorders16 have been observed. A recent study indicates significant sex disparities in the trajectories of skeletal muscle aging17. Factors contributing to age-related loss of muscle mass and strength, such as lower levels of mitochondrial-related proteins18, have been investigated and show varying impacts between sexes19. Therefore, exploring the impact of aging on musculoskeletal disorders while taking into account sex differences is crucial for developing targeted interventions for both men and women.
In this study, we examined the relationships between advanced biological aging and musculoskeletal disorder risks employing the accelerated failure time (AFT) model. Esteemed for analyzing time-to-event data20, the AFT model differs from prevalent proportional hazards models by modulating the time to an event based on covariates21. The AFT model, frequently referenced in diverse medical literature contexts22,23, promises sharper clarity than hazard ratios and retains its relevance even under non-proportional hazards. The findings clearly illustrate the extent to which biological age accelerates or decelerates the onset of pertinent events, as expressed by the time ratio (RT).
By analyzing a large prospective cohort over a decade, we assess how biological age influences the onset of musculoskeletal disorders, with a particular focus on potential sex differences. Cross-sectional analyses reveal significant associations between advanced biological aging and fourteen musculoskeletal disorders. Survival analyses indicate that accelerated aging significantly shortens the time to onset for nine disorders, with sex-specific variations observed. This research not only adds to the existing literature on aging and musculoskeletal disorders but also aims to inform public health strategies, ultimately contributing to improved quality of life for the aging population.
Methods
Study design and population
The UK Biobank is an ongoing prospective study that initially recruited 502,536 participants during the period of 2006 and 2010. Participants were aged 37–73 years at the baseline survey and multiple follow-up studies have been conducted on the participants. The database contains a wealth of biomedical data, including blood samples, physical measurements, and lifestyle questionnaires, as well as longitudinal medical records. Ethical approval for the UK Biobank was granted by the North West Multi-center Research Ethics Committee. No further ethical clearance was required for this study, as it involved the secondary use of anonymized data. All participants provided written informed consent. We applied for permission to access the UK biobank data, and access was granted under application number 4647824.
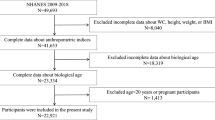
In this study, we initially considered 335,183 participants from the UK Biobank who had trait measurements required for the biological age algorithm at baseline. From these, we excluded 144,454 samples due to missing covariate values, and the detailed missing proportions for each covariate are provided in Supplementary Table S1. Further, we excluded 18,397 non-white participants, as the study specifically targets genetic and biological aspects pertinent to a specific population. As a result, data from 172,332 participants were utilized to evaluate the relationship between biological aging and the incidence of musculoskeletal disorders. Figure 1 presents the comprehensive design of our research, detailing the process of participant inclusion and exclusion.
Assessment of biological ages and age accelerations
To assess biological age, we utilized two rigorously validated algorithms, KDM-BA9 and PhenoAge10, which have been corroborated using the data from anthropometric measurements and biochemical markers collected in the UK Biobank25,26. The biomarkers used in the KDM-BA and PhenoAge calculations were measured during a single blood draw when participants were recruited. These algorithms generated biological age estimates based on a range of blood-based biochemical markers. Notably, algorithm parameters are determined distinctly for males and females.
The KDM-BA algorithm offers a predictive measure of an individual’s physiological state in relation to their chronological age. This metric is determined through a series of regression analyses using specific biomarkers compared to a standard age baseline. Specifically, KDM-BA calculations incorporated forced expiratory volume in one second (FEV1), systolic blood pressure (SBP), and an array of seven critical blood chemistry parameters (total cholesterol, glycated hemoglobin, blood urea nitrogen, albumin, creatinine, C-reactive protein, and alkaline phosphatase).
Conversely, the PhenoAge algorithm is rooted in multivariate analysis of mortality hazards. Originally developed through elastic-net Gompertz regression on a set of biomarkers in the NHANES III study, our adaptation of PhenoAge drew on a concise list of nine blood-based markers (lymphocyte proportion, mean cell volume, serum glucose, red cell distribution width, white blood cell count, albumin, creatinine, C-reactive protein, and alkaline phosphatase). Four of these were also used in the KDM-BA algorithm. The complete list of biomarkers used is provided in Supplementary Table S2. We removed any data entries with missing values prior to analysis.
To further explore the disparities in biological aging among participants, we used regression analysis to compare their computed biological ages against their actual chronological ages, as captured at the time of biomarker assessment. The derived residual values were subsequently designated as ‘age acceleration’ (AA). Within this study, AA served as an indicator of the biological aging rate in the population, signifying whether an individual’s physiological age appeared older (reflected by a positive value) or younger (reflected by a negative value) than their chronological age would suggest. At baseline, there was a strong correlation between participants’ biological ages and their chronological ages (Fig. S1). All computations were carried out using the “BioAge” package27 (https://github.com/dayoonkwon/BioAge) in R (version 4.3.0). More details can be found in the previous study27.
Assessment of musculoskeletal disorders
In this study, we focused on a sample population diagnosed with various musculoskeletal disorders, as classified under the International Classification of Diseases, 10th Edition (ICD-10) diagnostic criteria by the World Health Organization (WHO), corresponding to UK Biobank fields 41270 and 41280. The range of conditions included joint/bone replacement, inflammatory polyarthropathies, arthrosis, systemic connective tissue disorders, deforming dorsopathies, spondylopathies, disorders of synovium and tendon, disorders of bone density and structure, gout, arthritis, primary coxarthrosis, primary gonarthrosis, pain in the joint, osteophyte, spinal stenosis (encompassing both congenital and acquired forms), sciatica, low back pain, palmar fascial fibromatosis, rotator cuff syndrome, and osteoporosis. The detailed ICD-10 code definitions for these conditions can be found in Supplementary Table S3.
Survival time was calculated as the duration from the baseline assessment (UKBB field: 53) until the occurrence of the event of interest. We defined the baseline as the time when participants first attended the assessment centers. For participants who did not experience the event, survival time was censored at either the last known follow-up date, including death (UKBB field: 40,000), date lost to follow-up (UKBB field: 191), and the end of follow-up (October 29, 2022), whichever occurred first.
Measurements of covariates
In our study, we accounted for a nuanced set of covariates: age, sex, body mass index (BMI), smoking status, alcohol consumption, healthy physical activity status, vitamin D, Townsend deprivation index (TDI), and income. Smoking status was classified into current, former, and never-smokers (UKBB field: 20116). Alcohol consumption was assessed both qualitatively (ever consumed, UKBB field: 20117) and quantitatively (average weekly intake across beverage types, UKBB fields: 1568, 1578, 1588, 1598, 1608, and 5364). Non-drinkers were assigned a zero value for weekly consumption. For the rest of the participants, we utilized the average amount of various types of alcohol consumed weekly. BMI was calculated using the standard formula and segmented into underweight/normal (<25 kg/m2), overweight (25–30 kg/m2), and obese (≥30 kg/m2) categories. Physical activity levels (UKBB fields: 894 and 914) were assessed using the Metabolic Equivalent Task minutes, which were derived from the short International Physical Activity Questionnaire. We defined healthy physical activity as: ≥150 min/week moderate, or ≥75 min/week vigorous, or 150 min/week mixed (moderate and vigorous) activity. Vitamin D levels were ascertained from blood samples (UKBB field: 30,890). We employed the Townsend deprivation index (UKBB field: 22,189), a composite metric incorporating factors like unemployment, overcrowded households, non-car ownership, and non-home ownership. Higher scores indicate greater deprivation. Income was determined as average pre-tax household earnings (UKBB field: 738). Medication for cholesterol, blood pressure, diabetes, or taking exogenous hormones was included (UKBB field: 6153). Observations with any missing values of the covariates were excluded from this study.
Statistics and reproducibility
Cross-sectional associations: logistic regression
We first tested the cross-sectional associations of two AAs metrics (KDM-BA and PhenoAge) with 20 musculoskeletal disorders as the outcome variable using logistic regression (Analysis 1). We fit a series of models adjusting for increasing numbers of covariates. Model 1 included basic demographic variables, specifically age and sex. Model 2 expanded upon this by integrating lifestyle factors such as smoking status, alcohol consumption, and healthy physical activity status, along with body mass index (BMI). Model 3 further expanded on Model 2 by including vitamin D levels and socio-economic indicators like the TDI, and income. To account for multiple comparisons, we considered an association statistically significant based on the Holm–Bonferroni procedure, which set the family-wise error rate at 5% across 40 tests. All statistical analyses for the cross-sectional associations were performed using the “glm” function in R software (version 4.3.0).
Survival analysis: accelerated failure time (AFT) model
In the survival analysis (Analysis 2), an AFT model was employed, assuming a Weibull distribution for the survival times, to investigate the relationships between the two AAs and the onset of musculoskeletal disorders. The AFT model estimates how each biological age accelerates or decelerates the time to the event of interest. RT greater than 1 indicates that the event of interest is “accelerated,” meaning it occurs more quickly. Conversely, a time ratio less than 1 suggests that the event is “decelerated,” or takes longer to occur. Our analysis proceeded in a stepwise fashion, resulting in three progressively complex AFT models. The covariate settings are the same as in the logistic regression model. The covariate settings for these models mirrored those in the logistic regression analyses: Model 1 included basic demographics; Model 2 added lifestyle factors and BMI; and Model 3 further incorporated vitamin D levels and socio-economic indicators.
We conducted three sensitivity analyses to assess the robustness of our findings. First, we performed a sensitivity analysis based on Model 3, specifically excluding participants who had two years or less of follow-up to address potential issues of reverse causation. Secondly, we further adjusted Model 3 for medications related to cholesterol, blood pressure, diabetes, and exogenous hormone use. Finally, we addressed missing data through multiple imputations. We used the ‘mice’ package in R for Multiple Imputation by Chained Equations, employing the random forest method for imputation. After imputing the missing data, we re-ran Model 3 using the imputed dataset to evaluate the impact of missing data on our results.
Furthermore, we carried out sex-stratified analyses using Model 3 to explore potential sex-specific effects on the time-to-event outcomes. To control for multiple comparisons in the survival analysis, we considered an association statistically significant based on the Holm–Bonferroni procedure, which set the family-wise error rate at 5% across 40 tests. All survival analyses were performed using the ‘survival’ package in R (version 4.3.0).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Basic characteristics of the participants
In this study, data from 172,332 participants were used to assess the associations between biological aging and the incidence of musculoskeletal disorders. The mean (SD) age was 56.03 (8.10) years, with females comprising 48.02% (n = 82,754) of the study population. Baseline characteristics, including demographics and other relevant measures, are provided in Table 1. By applying a morbidity count threshold of 1000 cases, 20 musculoskeletal disorders were ultimately included in the study. During the 16-year follow-up, participants developed various musculoskeletal disorders as follows: 23,668 developed arthrosis, 9560 developed inflammatory polyarthropathies, and so forth. A comprehensive breakdown of the number of cases of each musculoskeletal disorder is also available in Supplementary Data 1 and 2.
Associations of the biological age with musculoskeletal disorders
In the logistic regression analysis, Model 3 demonstrated significant associations between AAs and fourteen musculoskeletal disorders, as detailed in Table 2. Specifically, advanced aging was associated with increased odds of all fourteen disorders, including inflammatory polyarthropathies (ORKDM-BA = 1.034; ORPhenoAge = 1.047), arthrosis (ORPhenoAge = 1.009), systemic connective tissue disorders (ORKDM-BA = 1.036; ORPhenoAge = 1.048), deforming dorsopathies (ORKDM-BA = 1.017), spondylopathies (ORKDM-BA = 1.009; ORPhenoAge = 1.016), disorders of bone density and structure (ORKDM-BA = 1.020; ORPhenoAge = 1.024), gout (ORKDM-BA = 1.085; ORPhenoAge = 1.087), arthritis (ORKDM-BA = 1.028; ORPhenoAge = 1.035), primary gonarthrosis (ORPhenoAge = 1.008), pain in joint (ORPhenoAge = 1.020), spinal stenosis (ORPhenoAge = 1.015), sciatica (ORPhenoAge = 1.020), low back pain (ORPhenoAge = 1.024), and osteoporosis (ORKDM-BA = 1.026; ORPhenoAge = 1.022). All p-values remained below the threshold set by the Holm–Bonferroni correction. Supplementary Data 1 offers an in-depth statistical breakdown across all models.
Prospective associations between biological aging and musculoskeletal disorders
Model 3 showed that AAs significantly accelerated the onset of nine musculoskeletal disorders. This is in line with the cross-sectional study results, demonstrating a positive correlation between the prevalence of these nine diseases and the magnitude of advanced aging. With each incremental unit of AA, the survival time for these diseases reduces markedly. For inflammatory polyarthropathies, the survival time diminishes by 0.7% (RTKDM-BA = 0.993) and by 1.7% (RTPhenoAge = 0.983). Systemic connective tissue disorders see a reduction of 1.3% (RTKDM-BA = 0.987) and 2.0% (RTPhenoAge = 0.980). The survival time for spondylopathies reduces by 0.6% (RTPhenoAge = 0.994). Disorders of bone density and structure experience a decline of 0.9% (RTPhenoAge = 0.991). Gout presents a more pronounced decrease of 3.2% (RTPhenoAge = 0.968). Arthritis exhibits a reduction of 0.9% (RTPhenoAge = 0.991), while pain in the joint and lower back pain shows reductions of 1.1% (RTPhenoAge = 0.989) and 1.4% (RTPhenoAge = 0.986), respectively. Lastly, osteoporosis manifests a reduction in survival time of 0.6% (RTPhenoAge = 0.994). All p-values met the significance criteria set by the Holm–Bonferroni correction. Supplementary Data 2 provides detailed statistics, including 95% confidence intervals for the estimates from three models.
The first sensitivity analysis confirmed the primary analysis, demonstrating that AAs increased the risk of earlier onset for seven disorders. In the second and third sensitivity analyses, eight of the nine significant associations were consistently replicated, further validating our findings. Detailed results of the sensitivity analysis can be found in Supplementary Data 3.
Sex-specific associations between biological aging and musculoskeletal disorders
We observed sex-specific variations in the associations between AAs and musculoskeletal disorders as depicted in Fig. 2. AAs were observed to accelerate the onset of four musculoskeletal disorders in both male and female subgroups, aligning with the primary analysis. These disorders comprised inflammatory polyarthropathies, systemic connective tissue disorders, disorders of bone density and structure, and gout. Notably, some associations were significant only in specific sex subgroups. In the female subgroup, AAs significantly accelerated the onset of spondylopathies (RTPhenoAge = 0.991, P = 2.67 × 10−4), arthritis (RTPhenoAge = 0.990, P = 4.01 × 10−5), and low back pain (RTPhenoAge = 0.983, P = 5.47 × 10−4), while decelerating the onset of arthrosis (RTKDM-BA = 1.007, P = 1.16 × 10−3). Conversely, in the male subgroup, AAs accelerated the onset of osteoporosis (RTPhenoAge = 0.982, P = 2.45 × 10−7). A detailed exposition of these statistics, inclusive of 95% confidence intervals, is provided in Supplementary Data 4.
Note: The analyses include data from n = 172,332 participants, of whom 82,754 (48.02%) are female. “*” indicates that the association is statistically significant after Holm–Bonferroni adjustment. Dots (centers of error bars): Point estimate; Error bar: 95% confidence intervals; Dash line: Reference line.
Discussion
This large study is the first to investigate the associations between blood-chemistry indicators of biological aging and the prevalence and incidence of musculoskeletal disorders among a cohort of 0.17 million midlife and older adults from the UK Biobank. The primary findings indicated that adults with a more advanced biological age exhibited an accelerated onset of nine musculoskeletal disorders, including inflammatory polyarthropathies, systemic connective tissue disorders, spondylopathies, disorders of bone density and structure, gout, arthritis, pain in joints, low back pain, and osteoporosis. Moreover, certain associations were discerned to be sex-specific.
Numerous studies and reports highlight the rising prevalence of musculoskeletal issues as individuals age28,29. In line with our findings, aging has been identified as a significant factor in the increased risk and severity of these disorders. For instance, a study contrasting muscle activation and postural control patterns in younger versus older computer users concluded that the elderly face a heightened risk of musculoskeletal problems30. Our research solidifies the causal linkage between aging and the onset of musculoskeletal conditions. The mechanism through which aging impacts the musculoskeletal system substantiates the plausibility of our findings. Age-related skeletal muscle dysfunction in structure and function is predominantly attributed to anabolic resistance31, mitochondrial dysfunction32, heightened oxidative stress33, inflammation34, and myokine dysfunction35. Building on these fundamental mechanisms of aging, it is crucial to explore their direct implications on specific musculoskeletal disorders.
We found that five of the nine musculoskeletal diseases were chronic immune-mediated diseases, including inflammatory polyarthropathies (encompassing gout and arthritis), systemic connective tissue disorders, and spondylopathies. In concordance with our study, the risk of inflammatory polyarthropathies36, systemic connective tissue disorders37, and spondylosis38 was found to escalate with age. One study points to inflammaging as the chronic low-grade inflammatory state that exists in older adults39. The increase in inflammation during the aging process may result from the following mechanisms intensified in elderly individuals, including immune disorders, enhanced intestinal permeability, augmented secretion of inflammatory mediators from visceral fat, altered immune resolution, and accumulation of senescent cells40. Accumulating evidence suggests that the elevated inflammatory state accompanying aging possesses the potential to trigger or facilitate the onset of significant age-related musculoskeletal diseases, which accelerate aging41,42.
Disorders of bone density and structure, as well as osteoporosis, have been found to be accelerated by advanced aging. Our findings have been corroborated by investigating the mechanisms through which aging precipitates osteoporosis. Genomic instability, a feature of aging43, results in DNA damage, promoting cell senescence, particularly in osteocytes and osteoprogenitors. Abnormal DNA repair systems, as seen in conditions like progeria, further exacerbate bone loss44. As individuals age, there’s a decline in bone formation by osteoblasts and an increase in bone resorption by osteoclasts. This imbalance leads to reduced bone mass and structural damage45. Furthermore, bone marrow mesenchymal stem cells, crucial for bone balance, show reduced differentiation into osteoblasts with age, further contributing to osteoporosis46,47.
Pain in the joint and lower back pain has been observed to be accelerated by advanced aging. The reported impact of age on pain prevalence in older individuals is inconsistent48. Aging affects pain experience. Older adults exhibited increased connectivity of pain-related sensory brain regions and diminished functional connectivity between key nodes of the descending pain inhibitory pathway49. Additionally, work-related strain accumulated over a lifetime may contribute to these differences in pain prevalence among older adults. Occupational factors such as repetitive movements, heavy lifting, and prolonged postures can lead to musculoskeletal wear and tear50, which may exacerbate pain symptoms in the elderly. This suggests that both biological aging and work-related factors play significant roles in the development and progression of joint pain and low back pain in older adults.
Sexual dimorphisms in the onset and progression of various musculoskeletal conditions in association with aging have been elucidated in numerous studies. We found that the accelerated onset of three musculoskeletal disorders, including spondylosis, arthritis, and low back pain, was uniquely observed in the female subgroup with advanced aging. Research has indicated that postmenopausal women may undergo a more rapid progression of spinal lesions51, an elevated risk of arthritis52, and a higher prevalence or intensity of low back pain53, attributed to declining estrogen levels. Conversely, the accelerated onset of osteoporosis was uniquely noted in the male subgroup with advanced aging. The decline in testosterone levels in men, alongside the reduction in insulin-like growth factor 1 (IGF-1) levels, may also contribute to bone loss associated with aging54. Moreover, we observed that advanced aging delayed the onset of arthrosis in females. This may be attributed to the likelihood that women experiencing advanced aging may be more proactive in seeking medical counsel during the pre-disease phase, potentially leading to early intervention. Replicating the findings with more diverse samples is imperative.
Our research highlights significant associations between accelerated biological aging and increased risks of several musculoskeletal disorders, underscoring the need for targeted screening protocols based on biological aging indicators to identify high-risk populations early. This approach not only supports preventive strategies but also aligns with personalized medicine by allowing tailored treatments that address specific aging-related risk factors. Furthermore, our findings may encourage further investigation into the mechanisms linking biological aging to musculoskeletal disorders, potentially leading to novel therapeutic targets.
This research had several strengths. This is the first study, to our knowledge, quantifying the effects of advanced aging on the onset of musculoskeletal disorders, underscoring the utility of biological age in assessing the risk of such diseases. This strategy enables us to discern the specific impacts of advanced aging and to forecast the risk of developing musculoskeletal disorders. Moreover, with its substantial sample size and extended follow-up period, this study pioneers in concurrently examining the manifestation of multiple musculoskeletal disorders in aging. However, this study also has limitations. Our participants comprised primarily middle-aged or older white adults, thereby constraining the generalizability of the findings to other age and ethnic cohorts. Although we addressed missing data through multiple imputations, the relatively high proportion of missingness in certain variables could introduce uncertainty. Nevertheless, sensitivity analyses demonstrated consistent results between the imputed and complete-case datasets, suggesting that missing data did not substantially bias our conclusions. Importantly, while our study identifies prospective associations between biological aging and musculoskeletal disorders, we acknowledge the limitations of observational data in establishing causality. Future research could refine methodologies to more robustly explore potential causal pathways. Additionally, merely about one-third of the enrolled participants engaged in the follow-up survey, and there might have been attrition of older individuals who were too ill to partake or who were demised during the follow-up period. Consequently, our findings are likely conservative.
In conclusion, we found that adults with an older biological age were at an elevated risk for musculoskeletal disorders. This investigation lends support to the hypothesis that biological aging may serve as a risk factor for musculoskeletal disorders in midlife and older adults. The findings propose avenues for future risk assessment of musculoskeletal disorders in older adults and highlight the potential for therapies targeting the biology of aging to contribute to the prevention of musculoskeletal disorders in later life.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. Eligible researchers may access UK Biobank data on www.ukbiobank.ac.uk upon registration. The source data for Fig. 2 is in Supplementary Data 4.
Code availability
The custom code used for the statistical analyses in this study is implemented in R software (version 4.3.0). No novel code was developed for the analysis of this study.
References
Beard, J. R. & Bloom, D. E. Towards a comprehensive public health response to population ageing. Lancet 385, 658–661 (2015).
Disease, G. B. D., Injury, I. & Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858 (2018).
Briggs, A. M. et al. Reducing the global burden of musculoskeletal conditions. Bull. World Health Organ. 96, 366–368 (2018).
Woolf, A. D. & Akesson, K. Understanding the burden of musculoskeletal conditions. The burden is huge and not reflected in national health priorities. BR. Med. J. 322, 1079–1080 (2001).
Li, Z. et al. Progress in biological age research. Fron.t Public Health 11, 1074274 (2023).
Murata, S. et al. Blood biomarker profiles and exceptional longevity: comparison of centenarians and non-centenarians in a 35-year follow-up of the Swedish AMORIS cohort. Geroscience https://doi.org/10.1007/s11357-023-00936-w (2023).
Levine, M. E. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J. Gerontol. A Biol. Sci. Med. Sci. 68, 667–674 (2013).
Li, X. et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. Elife https://doi.org/10.7554/eLife.51507 (2020).
Klemera, P. & Doubal, S. A new approach to the concept and computation of biological age. Mech. Ageing Dev. 127, 240–248 (2006).
Liu, Z. et al. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: A cohort study. PLoS Med. 15, e1002718 (2018).
Pabla, P., Jones, E. J., Piasecki, M. & Phillips, B. E. Skeletal muscle dysfunction with advancing age. Clin. Sci. (Lond.) 138, 863–882 (2024).
Moiseeva, V. et al. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature 613, 169–178 (2023).
Grunewald, M. et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science https://doi.org/10.1126/science.abc8479 (2021).
Chen, X. et al. METTL3-mediated m(6)A modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann. Rheum. Dis. 81, 87–99 (2022).
Hagg, S. & Jylhava, J. Sex differences in biological aging with a focus on human studies. Elife https://doi.org/10.7554/eLife.63425 (2021).
Tosi, L. L., Templeton, K., Pennington, A. M., Reid, K. A. & Boyan, B. D. Influence of sex and gender on musculoskeletal conditions and how they are reported. J. Bone Jt. Surg. Am. 106, 1512–1519 (2024).
de Jong, J. et al. Sex differences in skeletal muscle-aging trajectory: same processes, but with a different ranking. Geroscience 45, 2367–2386 (2023).
Lagerwaard, B., Nieuwenhuizen, A. G., Bunschoten, A., de Boer, V. C. J. & Keijer, J. Matrisome, innervation and oxidative metabolism affected in older compared with younger males with similar physical activity. J. Cachexia Sarcopenia Muscle 12, 1214–1231 (2021).
van der Hoek, M. D. et al. Intramuscular short-chain acylcarnitines in elderly people are decreased in (pre-)frail females, but not in males. FASEB J. 34, 11658–11671 (2020).
Wei, L. J. The accelerated failure time model: a useful alternative to the Cox regression model in survival analysis. Stat. Med. 11, 1871–1879 (1992).
Pang, M., Platt, R. W., Schuster, T. & Abrahamowicz, M. Flexible extension of the accelerated failure time model to account for nonlinear and time-dependent effects of covariates on the hazard. Stat. Methods Med. Res. 30, 2526–2542 (2021).
Dobson, J., Whitley, R. J., Pocock, S. & Monto, A. S. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 385, 1729–1737 (2015).
Collett, D. Modelling Survival Data in Medical Research. 2nd edn, (Chapman & Hall/CRC, 2003).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Kuo, C. L., Pilling, L. C., Liu, Z., Atkins, J. L. & Levine, M. E. Genetic associations for two biological age measures point to distinct aging phenotypes. Aging Cell 20, e13376 (2021).
Gao, X. et al. Accelerated biological aging and risk of depression and anxiety: evidence from 424,299 UK Biobank participants. Nat. Commun. 14, 2277 (2023).
Kwon, D. & Belsky, D. W. A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. Geroscience 43, 2795–2808 (2021).
Holmstrom, E. & Engholm, G. Musculoskeletal disorders in relation to age and occupation in Swedish construction workers. Am. J. Ind. Med. 44, 377–384 (2003).
Briggs, A. M. et al. Musculoskeletal health conditions represent a global threat to healthy aging: a report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist 56, S243–S255 (2016).
Hsiao, L. P. & Cho, C. Y. The effect of aging on muscle activation and postural control pattern for young and older computer users. Appl. Erg. 43, 926–932 (2012).
Angulo, J., El Assar, M., Alvarez-Bustos, A. & Rodriguez-Manas, L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 35, 101513 (2020).
Huang, Y. et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging (Albany NY) 11, 2217–2240 (2019).
Cannataro, R. et al. Sarcopenia: etiology, nutritional approaches, and miRNAs. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22189724 (2021).
Yamakawa, H., Kusumoto, D., Hashimoto, H. & Yuasa, S. Stem cell aging in skeletal muscle regeneration and disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21051830 (2020).
Severinsen, M. C. K. & Pedersen, B. K. Muscle-organ crosstalk: the emerging roles of myokines. Endocr. Rev. 41, 594–609 (2020).
Zhu, Y., Pandya, B. J. & Choi, H. K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 63, 3136–3141 (2011).
Spagnolo, P., Cordier, J. F. & Cottin, V. Connective tissue diseases, multimorbidity and the ageing lung. Eur. Respir. J. 47, 1535–1558 (2016).
Butler, J. S., Oner, F. C., Poynton, A. R. & O’Byrne, J. M. Degenerative cervical spondylosis: natural history, pathogenesis, and current management strategies. Adv. Orthop. 2012, 916987 (2012).
Nishikawa, H. et al. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. https://doi.org/10.3892/ijmm.2021.4989 (2021).
Liang, Z. et al. Inflammaging: the ground for sarcopenia? Exp. Gerontol. 168, 111931 (2022).
Duchesne, E., Dufresne, S. S. & Dumont, N. A. Impact of inflammation and anti-inflammatory modalities on skeletal muscle healing: from fundamental research to the clinic. Phys. Ther. 97, 807–817 (2017).
Pawelec, G., Goldeck, D. & Derhovanessian, E. Inflammation, ageing and chronic disease. Curr. Opin. Immunol. 29, 23–28 (2014).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Chen, Q. et al. DNA damage drives accelerated bone aging via an NF-kappaB-dependent mechanism. J. Bone Min. Res. 28, 1214–1228 (2013).
Parfitt, A. M. Age-related structural changes in trabecular and cortical bone: cellular mechanisms and biomechanical consequences. Calcif. Tissue Int. 36, S123–S128 (1984).
Farr, J. N. et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 23, 1072–1079 (2017).
Moerman, E. J., Teng, K., Lipschitz, D. A. & Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 3, 379–389 (2004).
Munch, T. et al. Pain and falls and fractures in community-dwelling older men. Age Ageing 44, 973–979 (2015).
Gonzalez-Roldan, A. M. et al. Age-related changes in pain perception are associated with altered functional connectivity during resting state. Front. Aging Neurosci. 12, 116 (2020).
Antwi-Afari, M. F. et al. A science mapping-based review of work-related musculoskeletal disorders among construction workers. J. Saf. Res. 85, 114–128 (2023).
Kalichman, L. et al. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine (Philos. Pa 1976) 34, 199–205 (2009).
Srikanth, V. K. et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 13, 769–781 (2005).
Hoy, D. et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 64, 2028–2037 (2012).
Andersen, A. E., Watson, T. & Schlechte, J. Osteoporosis and osteopenia in men with eating disorders. Lancet 355, 1967–1968 (2000).
Acknowledgements
This research has received financial support from the Natural Science Basic Research Plan in Shaanxi Province of China [2021JCW-08].
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, and analysis were performed by Wenming Wei and Xin Qi. The first draft of the paper was written by Wenming Wei. The figures and tables were made by Bolun Cheng, Na Zhang, Yijing Zhao, Xiaoyue Qin, and Dan He. The literature searches were performed by Xiaoge Chu, Sirong Shi, Qingqing Cai, Xuena Yang, Shiqiang Cheng, Peilin Meng, Jingni Hui, Chuyu Pan, Li Liu, Yan Wen, Huan Liu, and Yumeng Jia. The study design was performed by Feng Zhang.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. [Peer review reports are available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wei, W., Qi, X., Cheng, B. et al. A prospective study of associations between accelerated biological aging and twenty musculoskeletal disorders. Commun Med 4, 266 (2024). https://doi.org/10.1038/s43856-024-00706-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-024-00706-5