Abstract
Prymnesins produced by an algal bloom of Prymnesium parvum led to the death of several hundred tons of freshwater fish in the Oder River in summer 2022. We investigated effects on aquatic life and human cell lines from exposure to extracts of contaminated water collected during the fish kill. We detected B-type prymnesins and >120 organic micropollutants. The micropollutants occurred at concentrations that would cause the predicted mixture risk quotient for aquatic life to exceed the acceptable threshold. Extracts of water and filters (biomass and particulates) induced moderate effects in vivo in algae, daphnids and zebrafish embryos but caused high effects in a human neuronal cell line indicating the presence of neurotoxicants. Mixture toxicity modelling demonstrated that the in vitro neurotoxic effects were mainly caused by the detected B-type prymnesins with minor contributions by organic micropollutants. Complex interactions between natural and anthropogenic toxicants may underestimate threats to aquatic ecosystems.
Similar content being viewed by others
Main
In summer 2022 a harmful algal bloom (HAB) led to a catastrophic fish kill in the Oder River (Poland, Germany), where 249 metric tons of dead fish were retrieved in Poland alone1. This fish kill led to the eradication of up to 60% of fish2,3 plus up to 85% of mussel biomass and up to 85% of snail biomass in the Oder River3. It was a compound event caused by high salinity, high temperature, low stream flow and nutrients discharged via wastewater and agricultural run-off that triggered a bloom of the golden algae Prymnesium parvum4. At the peak of the bloom, P. parvum made up more than half of the phytoplankton biomass in the Oder River, producing mainly three B-type prymnesins4, which are known to be highly toxic to fish5,6 and directly damage fish gills7. In addition to their ichthyotoxicity, they are also cytotoxic, neurotoxic and haemolytic in nanomolar concentrations5,8,9. The main mode of action is saponin-like disturbance of the membrane function leading to ion leakage8. P. parvum isolated and grown in laboratory cultures have been tested for their non-target toxicity to fish5,6 and Daphnia magna5. The pH dependence of the observed effect indicated that basic properties of prymnesins lead to an increased toxicity at higher pH values5, which is relevant because the pH typically increases under eutrophic conditions due to photosynthesis.
The ichthyotoxic and haemolytic activity of P. parvum depends on the growth conditions, most likely due to differences in the production of prymnesins and potentially other toxins in response to nutrient availability8. Detailed investigations of the toxic mechanisms of prymnesins are rare due to late identification of the causative toxins in P. parvum and lack of purified standards8. Hill et al.10 demonstrated how salinity and nutrients impact on the formation of prymnesins in P. parvum and how the exposure to laboratory-cultured P. parvum increased oxidative stress biomarkers and swimming responses in fish embryos. P. parvum culture medium that presumably contained prymnesins activated antioxidant response in fish gill and liver cells11. Rasmussen et al.9 and Varga et al.12 detected severe cytotoxicity of isolated prymnesins in vitro on rainbow trout RTgill-W1 cells at nanomolar concentrations.
Little is known about the adverse impacts of prymnesins on human health, but swimmers have reported allergy-like symptoms in an affected brackish-water lake13. Crude and purified algal extracts triggered lysis of human erythrocytes and inhibition of the uptake of the neurotransmitters into synaptic vesicles in rat brains14,15. Human intestinal cells (HCEC-1CT) were only slightly less sensitive to P. parvum extracts compared with RTgill-W1 when exposed in vitro12.
P. parvum typically triggers HABs in brackish estuarine systems16,17 but its occurrence in inland waters with enhanced salinity has also been regularly reported18. The 2022 fish kill in the Oder River occurred in a freshwater system, which was impacted by high industrial discharges of sodium chloride2. The multifactorial causes leading to the fish kill were extensively investigated in several studies1,2,3,4,19,20,21. It was concluded that the combination of unfavourable factors such as eutrophication and nutrient inputs, hydrological conditions, temperature, salinity from mining and industry pollution and river regulation created the optimal conditions for the development of the golden algal bloom3. Freshwater systems are additionally threatened by organic micropollutants from wastewater, surface and agricultural run-off.
The objective of the present study was to quantify complex mixtures of micropollutants extracted from water, biomass and particulate matter and impacted fish sampled during the time of the fish kill. The organic extracts, which—as will be demonstrated—contained complex mixtures of B-type prymnesins and micropollutants, were also dosed to non-target aquatic organisms, that is, algae, daphnids and fish embryos that had not yet developed functional gills22. Human neuronal cell lines served as a model for effects on humans after exposure of river water through skin contact and ingestion. The focus of this study was on the mixture interactions of prymnesins and micropollutants and how micropollutants could have exacerbated the effects of the algal toxins. A detailed analysis of the detection of prymnesins and all inorganic parameters is given in an accompanying paper4.
Sampling was opportunistic and could not be planned because of the nature of the catastrophic event. The fish kill in the Oder River was reported in Germany on 9 August 2022. After mercury was reported not to be exceeded on 14 August 2022 and other causes were suspected, among them pesticides and micropollutants, a sampling team from Leibniz Institute of Freshwater Ecology and Inland Fisheries (IGB) collected two sets of water-grab samples in Küstrin/Kostrzyn and Frankfurt (Oder) on 16 August 2022, and a team from Helmholtz Centre for Environmental Research (UFZ) collected water at Schwedt/Szwed, Mescherin and Gryfino on 17 August 2022 (Fig. 1) and purchased fish from local fisheries. In addition, juvenile sturgeons from a restoration project were investigated.
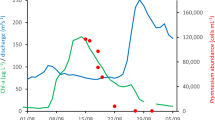
Contribution of individual chemicals for the total water sample extracts (water + filter (biomass and particles)) of samples 1–5 (Supplementary Table 8). The top left insert shows the relative mean contribution of the top ten chemicals to the mixture risk quotient (RQ). The insert on the bottom shows the ___location of the sampling sites.
Micropollutants detected in water, biomass and particulate matter
Sample locations (Fig. 1) and inorganic parameters are described in Supplementary Table 1 and Supplementary Text 1. Only chlorothalonil was detected in the SORBSTAR passive sampler extracts with the analytical method for 27 hydrophobic pesticides (Supplementary Text 2 and Supplementary Table 2). In the solid-phase extracts (SPE) of the water filtrates (codes W1, W2, W3, W4, W5; Fig. 1 and Supplementary Table 1), 125 out of 410 targeted chemicals were detected (Supplementary Table 3) and 43 out of 426 targeted chemicals (Supplementary Table 4 and Supplementary Text 3) in the solvent extract of the filters (codes F1, F2, F3, F4, F5; Supplementary Table 1), which constitute algal biomass and particulate matter, with 32 chemicals common in the water SPE and the filter solvent extract. Among the chemicals with largest concentrations were the flame retardant tris(1-chloro-2-propyl)phosphate, the polymer additive hexa(methoxymethyl)melamine and the corrosion inhibitor 1H-benzotriazole with maximum concentrations of 1,735 ng l−1, 1,046 ng l−1 and 663 ng l−1, respectively. Samples from the two main locations (Fig. 1), one site close to Frankfurt (Oder) collected on 16 August 2022 (samples W1, W2), the other site further downstream in Schwedt/Szwed and Mescherin/Gryfino a day later (samples W3, W4, W5), each clustered together (Supplementary Fig. 1). Fewer chemicals were detected at the Frankfurt (Oder) sites (W1, W2), but for some chemicals, concentrations were higher than at the downstream sites (W3, W4, W5). Regarding the filter extracts (details in Supplementary Text 3 and Supplementary Fig. 2), more chemicals were detected in the filter samples F3, F4, F5 than in F1 and F2. More hydrophobic chemicals (for example, orlistat (log Kow 5.3), salinomycin (log Kow 8.53)) were found in the filter extracts as compared to the water extracts, including most of the permanently positively charged alkylammonium compounds and the surfactant lauramidopropylbetaine, which bind strongly to organic matter23.
Most micropollutants detected in the Oder River (Supplementary Fig. 3) are likely to stem from wastewater treatment plant (WWTP) effluents because most occurred also in WWTP effluents from 56 European WWTPs24 but typically in lower concentrations as it would be expected from the comparably high urban discharge fraction of approximately 23% of treated wastewater at base flow (gauging station Frankfurt (Oder)).
Notable exceptions were hexa(methoxymethyl)melamine, 2,4-dichlorophenol and tetraglyme, which had presumably direct industrial inputs, and 2,4-dichlorophenoxyacetic acid, desethylterbutylazine, metazachlor-oxalamic acid, metazachlor ethane sulfonic acid and chlorotoluron, which are pesticides or pesticide metabolites that had presumably direct input from agricultural run-off. As already indicated in the official German report21, the concentrations of chemicals were not unusually high. Although some concentration spikes were noted in the first half of August, these cannot have led to the observed fish kill but might have contributed to the increased vulnerability of the affected aquatic life. Our samples were taken when a peak of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid was trailing out that had been observed in Hohenwutzen and Frankfurt (Oder) before 15 August 2022 (ref. 21).
Micropollutants detected in fish
Because the exposure of fish to organic micropollutants is not well described by chemical analysis of cocktails extracted from water, the comprehensive exposure to fish can only be estimated by experimentally analysing the exposed fish themselves. As dead and affected fish could not be sampled, we opted for buying fish that had slight symptoms but had been in clean water before sacrificing them and extracting the organic micropollutants from their tissues. Therefore, as expected, we did not find prymnesins in the fish extracts, but we can assume that the mostly relatively hydrophobic micropollutants do not fluctuate as much within days and are still representative for the exposure during the period of the fish kill. Of the 625 analysed chemicals, 137 were detected in muscle or gills of chubs or ide or sturgeons from the site, 72 thereof were detected in the muscle samples, 107 were detected in the gills of chub and/or ide and 98 in the sturgeons. The concentrations in the samples are provided in Supplementary Tables 5 and 6 and more details are in Supplementary Text 4.
Mixture risk for aquatic organisms caused by detected micropollutants
The risk for aquatic organisms of the detected chemicals can be estimated as risk quotient (RQ) by dividing the aqueous concentration by the predicted no effect concentrations (PNEC), which are safe levels of individual chemicals that should not trigger any effect in any aquatic species. PNECs for freshwater organisms were collected from literature and estimated by Finckh et al.25 and are reprinted in Supplementary Table 8. No PNECs are available for prymnesins; therefore they could not be included in this evaluation. The concentrations in the SPE extracts of the water and the solvent extracts of the filters were added up for each detected chemical i to obtain total aqueous concentrations. The resulting RQi of all detected chemicals i (Supplementary Table 7) can be summed up to obtain the mixture risk quotient RQmix. The calculated RQmix were 16.4 and 16.5 for the samples from Frankfurt (Oder) (W1 + F1 and W2 + F2), 17.1 for W3 + F3 from Schwedt/Szwed, 22.0 for sample 4 from Mescherin (W4 + F4) and 22.4 for sample 5 from Gryfino (W5 + F5). All RQmix values exceeded the safe RQ of 1 substantially, indicating potential risk for aquatic organisms already due to micropollutants. As the Oder River is highly impacted by wastewater treatment plant effluents2, such a high potential risk by micropollutants does not come to a surprise.
The contribution of individual chemical’s RQi to the RQmix is depicted in Supplementary Fig. 5 for all individual samples. As they did not differ very much, Fig. 1 depicts the mean contribution from both, water and particles. The polymer additive hexa(methoxymethyl)melamine, of which the mode of action is unknown25, made up the highest contribution of 59% to 65% to the RQmix. As the PNEC for this chemical is only a predicted value, this contribution bears some uncertainty. The second-most relevant chemical is the herbicide nicosulfuron with 7% to 12% contribution to RQmix. The PNEC for nicosulfuron is an annual average environmental quality standard of the Water Framework Directive and hence reliable. Nicosulfuron alone already exceeded the RQ of 1. Nicosulfuron had rarely been detected in WWTP effluents25 and could be directly released from agriculture. The contribution of additional chemicals is smaller but not negligible as demonstrated in the heat map in Fig. 1. A reduction of micropollutant loads would lead to higher resilience of the aquatic community against the consequences of warming and salinization.
Prymnesins detected in water, biomass, particulate matter and fish
At the time of sampling on 16 and 17 August 2022, it was not yet known that prymnesins from P. parvum had caused the fish kill, and accordingly the extraction methods had not been optimized for high yields of prymnesins, but targeted organic micropollutants. It was still possible to obtain an estimate of the concentrations of prymnesins that was sufficient to estimate their contribution to mixture toxicity measured in the same extracts, but the real concentrations are probably even higher.
Parallel to our sampling, P. parvum was microscopically detected and confirmed later by Sanger sequencing of the internal transcripted spacer region using eDNA samples4. The first recorded cell density was around 110,000 cells ml−1 in a sample taken on 15 August 2022 at Hohenwutzen just after the peak of the bloom4.
High-resolution mass spectrometry (HRMS) analysis revealed the presence of up to three different prymnesins in the water and particulate matter samples: the B-type prymnesin backbone with one incorporated chlorine atom and the analogues with one or two attached hexose moieties (Supplementary Fig. 6). It was striking that the commonly co-occurring analogues with attached pentose units were not observed. In the fish and gill extracts, no prymnesins or prymnesin-like compounds could be detected by liquid chromatography coupled to HRMS (LC-HRMS). More details in Supplementary Text 5.
Only the filter extracts were above the limit of detection in the semi-quantitative determination of prymnesins according to Svenssen et al.26. We filled the data gaps by adjusting for the ratio of peak areas of the filter and water extracts of the LC-HRMS analysis. The resulting concentrations (Supplementary Table 8 and Supplementary Fig. 7) ranged from F5 + W5 of 2.7 nmol l−1, F4 + W4 of 4.9 nmol l−1 and F5 + W5 of 8.9 nmol l−1 to F1 + W1 of 30.0 nmol l−1 (individual concentrations in Supplementary Table 8), which agreed well with the unfiltered water concentration of 39.2 nmol l−1 (ref. 4) and F2 + W2 of 52.9 nmol l−1 (unfiltered 63.7 nmol l−1)4.
Prymnesins could not be detected in fish. This can have various reasons, most prominently that the sample preparation was not targeting prymnesins as it was not known at the time of sampling that prymnesins were the culprit. It might also be possible that prymnesins were never present in these fish (they showed only slight symptoms) or fish had depurated them before sampling. As government restriction orders and high uncertainty about the degree of degradation of the dead and floating fish prevented their analysis, we purchased or obtained fish that had shown signs of intoxication but had potentially depurated any toxicants as they were kept alive in tanks before we bought and sampled the fish. Even (relatively polar) micropollutants could have been excreted during that time. Nevertheless, we detected a substantial quantity and diversity of chemicals in these fish (Supplementary Fig. 4 and Supplementary Table 5), which clearly indicates the multiple stress fish face in rivers. We have no proof that this additional body burden of micropollutants on its own impacted the fish, but the cocktail of pharmaceuticals, fragrance materials, pesticides, disinfectants and so on in a fish could have impacted the fish health.
In vivo toxicity towards algae, water flea and zebrafish embryos
Only the water extract could be tested for algal toxicity with the chlorophyte Scenedesmus vacuolatus (details in Supplementary Text 6, Supplementary Table 9 and Supplementary Fig. 8) because the filter extracts contained particles in the size of microalgae, which disturbed the algal toxicity test. The water samples (Supplementary Fig. 9) had a different response pattern than the positive control, the herbicide diuron (Supplementary Fig. 8), indicating that the phytotoxicity was non-specific and not caused by specific inhibition of photosynthesis.
The concentration–immobilization curves for the water flea Daphnia magna are depicted in Supplementary Fig. 11 and the thereof-derived effect concentration for 10% immobilization (EC10) for samples W1, F3, F4, F5 ranged from a relative enrichment factor (REF) of 4 to >10 (highest tested concentration was REF 10) (Supplementary Table 10) with details in Supplementary Text 7.
The zebrafish embryo (Danio rerio) is frequently used as a model for detecting developmental and acute neurotoxicity. The only parameter, for which concentration-dependent effects of the water extracts were observed in the zebrafish, was the photomotor response (PMR) (Supplementary Text 8, Supplementary Fig. 12, EC50 in Supplementary Table 11). The PMR has been previously shown to be sensitive to compounds interfering with the function and development of the nervous system27,28. The screening of the particulate matter samples induced no effects in all end-points if compared with the particulate matter blank control except for the locomotor response for F5 (Supplementary Table 11).
The algal toxicity was the most sensitive in vivo end-point (Fig. 2a), followed by immobilization of D. magna (Supplementary Table 10). The water extracts had to be enriched more than ten times to startle the photomotor response in D. rerio (Supplementary Table 11). In the government report21 of the Oder River fish kill, direct toxicity assessments with water samples was reported but no effects were observed for the bioluminescence inhibition test with Aliivibrio fischeri, algae and daphnia assays, which is consistent with the need to dose the extracts in the present study at REF > 1 to observe effects.
a, Comparison of effect concentrations for in vivo bioassays (EC10 for 10% growth inhibition in green algae (green, squares; Supplementary Table 9), EC10 for 10% immobilization in Daphnia magna (gold upward triangles; Supplementary Table 10), 50% effect concentration EC50 in the zebrafish (Danio rerio) behavioural assay for PMR (blue downward triangles; Supplementary Table 11)). There was not enough sample available to test W1 and W2 in D. magna or the zebrafish embryo, and testing was not possible for the filter samples in the algal toxicity assay, and the PMR in D. rerio was not significantly different from control up to REF 4 for the filtrate samples (unpaired t-test P = 0.6771). b, Comparison of effect concentrations in the in vitro neurotoxicity assay with EC10 for neurite outgrowth inhibition (10% reduction in neurite length, magenta diamonds) and inhibitory concentration IC10 for 10% cytotoxicity (teal circles) in the differentiated neuronal cell line SH-SY5Y (Supplementary Table 12).
For algal toxicity, the relationship between photosynthesis and growth inhibition compared well with previous results of a similar assay for WWTP effluents and river water29: the higher WWTP effluent contribution, the more non-specific were the effects (Supplementary Fig. 10), which agrees with the highly WWTP effluent-impacted Oder River. SPE extracts from the Lambro and Po rivers in Italy30 and water from the Sava River in Croatia31 tested on Daphnia magna showed similar effects as the Oder water samples tested here. In previous studies, river water also did not cause any acute Danio rerio embryo toxicity32, whereas WWTP effluent caused effects on PMR33 and triggered other stress responses33,34. The similarity of the in vivo effects to normal river water could also stem from the fact that the photolabile prymnesins35,36 were rapidly degraded in the in vivo bioassays, which were all conducted under artificial light.
In vitro neurotoxicity in human cell lines
Cytotoxicity and inhibition of neurite outgrowth in differentiated SH-S5Y5 cells were measured as in vitro indicator of mixtures of neurotoxic chemicals37. Concentration–response curves are depicted in Supplementary Fig. 13 for water extracts, Supplementary Fig. 14 for filter extracts and Supplementary Fig. 15 for fish extracts. All water and filter extracts samples were cytotoxic at an REF of 1.7 to 15 and decreased the neurite outgrowth at an REF of 0.4 to 4.2 in the neurotoxicity assays, and blanks were clean (Supplementary Table 12).
The in vitro effects were observed at lower concentrations than in vivo effects (Fig. 2b). The cytotoxicity was higher in the filter extracts than in corresponding water extracts, but the effect on neurite outgrowth inhibition was more pronounced in the water extracts than in the filter extracts (Fig. 2b). The in vitro neurotoxicity data of the Oder River water extracts were at the higher end of effect potency (Fig. 3) when comparing with literature data on surface water sampled during rain events and WWTP effluent38.
Comparison of the IC10 for cytotoxicity and EC10 for neurite outgrowth inhibition in the neurotoxicity assay with differentiated neuronal cell line SH-SY5Y (Supplementary Table 12) with literature data of surface water during rain events (grey upward triangles) and WWTP effluent (grey downward triangles)38 and water (blue diamonds) and filter (green circles) extracts in the Oder River. The broken line indicates a specificity ratio SR of 1, the dotted line an SR of 10.
The specificity ratio (SR), which is a measure of how more sensitive neurite outgrowth inhibition is as compared to cytotoxicity (SR = IC10(cytotoxicity)/EC10(neurite outgrowth inhibition)37), ranged from 3.5 to 9.5 (mean 5.3 ± 2.1) for the water extracts (Supplementary Table 12). The SR was around 1 (mean 0.91 ± 0.34) for the filter extracts (Supplementary Table 12), which means that the effect on the neurites was not specific but caused indirectly by cytotoxicity. The water extracts had similar SR (3.6 to 8.5) as the data from literature, but the SRs of the filter extract were close to 1 (range 0.5 to 1.3), indicating non-specific neurotoxicity.
Most of the fish extracts were not cytotoxic but inhibited the neurite outgrowth (Supplementary Table 12). Extracts without clean-up were 19 ± 15 times more potent than samples with clean-up (Supplementary Fig. 16), indicating that the clean-up led to loss of chemicals or of matrix.
Data for the neurotoxicity assay were available for 11 of the detected chemicals, and six were active and five inactive. We measured additional 13 chemicals that had high occurrence and/or concentrations, and nine of them were active and four were inactive (Supplementary Table 13). The relative effect potency (REP) of the 16 bioactive and detected micropollutants were low, ranging from 2.4 × 10−6 to 1.1 × 10−3 (Supplementary Table 13) and their specificity ratios SR ranged from 0.74 to 3.9, indicating low specificity.
There were no B-type prymnesin standards available, but an A-type prymnesin solution previously sold as haemolysis standard (Sigma Aldrich), which had an IC10 for cytotoxicity of 1.28 nM and an EC10 for neurite outgrowth inhibition of 1.45 nM (Supplementary Fig. 17 and Supplementary Table 13), which means prymnesins were very potent and had a high cytotoxicity but did not act specifically on neurites (SR = 0.88). This low SR and very high potency is an indication that the neurotoxicity of the filter extracts could be dominated by the prymnesins, whereas the water extracts are rather dominated by specifically acting neurotoxicants.
For another cytotoxicity assay using rainbow trout gill cells (RT-gillW1), the A-type prymnesin was 28 times more potent than the B-type prymnesin after 3 h and 17 times more potent after 24 h of exposure12. We used the potency ratio of 17 in RT-gillW1 after 24 h EC10(B-type)/EC10(A-type) to predict the IC10 for cytotoxicity of 22.1 nM and an EC10 for neurite outgrowth inhibition of 25.1 nM.
Contribution of prymnesins and micropollutants to the mixture effects
Despite concentrations of micropollutants that were substantially higher than prymnesin concentrations, they had only a small contribution to the predicted mixture effects expressed as bioanalytical equivalent concentrations (BEQ) with the reference chemical narciclasine, that is, narciclasine-EQchem (equation (2)). In the water extracts, an average of 9% of the measured biological effect expressed as narciclasine-EQbio (equation (1)) could be explained by prymnesins, whereas less than 0.01% could be explained by the 16 bioactive and detected micropollutants (Supplementary Table 14 and Fig. 4a). The contribution for all individual samples is shown in Supplementary Fig. 18: W1 and W2 had a higher fraction of narciclasine-EQbio explained by prymnesins than W3, W4, W5.
a,b, Mean BEQ of water extracts (a), filter extracts (b). Data in Supplementary Table 14, detailed figures for individual samples in Supplementary Fig. 18. On the left: the distribution between bioanalytical equivalent concentrations BEQchem from the detected chemicals (blue), from prymnesins (green) and unknown BEQchem (grey, BEQchem(unknown) = BEQbio − BEQchem(chemicals) − BEQchem(prymnesins)). On the right: apportionment of BEQchem(chemicals) by individual chemicals. The reference chemical for the neurotoxicity assay was narciclasine; hence BEQ are narciclasine-EQ.
In the filter extracts F3, F4 and F5, an average of 66% of the effect was caused by prymnesins (Supplementary Table 14 and Fig. 4b). F1 and F2 could not be evaluated with the iceberg model as bioactive micropollutants were not detected in these samples, but prymnesin concentrations were among the highest and would have explained 200% of the narciclasine-EQbio in F1 (F2 could not be quantified due to false positive microscope images from cell debris). An uncertainty analysis of the iceberg model indicates that the potency of B-type prymnesins is probably overestimated (Supplementary Text 9).
It has to be taken into consideration though that prymnesins are photolabile35,36, and degradation might have occurred during processing, which could have underestimated the observed effects. In contrast to the in vivo assays, which were conducted under artificial light, the 24 h exposure of human neuronal cells were conducted in the dark and the isolated prymnesin solution and the water extracts were treated exactly the same way.
Hexa(methoxymethyl)melamine had a substantial but lower contribution to the micropollutant’s mixture effects than in the RQ analysis, presumably because the predicted PNEC was only estimated, whereas the in vitro neurotoxicity was an experimental value. Carbamazepine, 1H-benzotriazole and tris(2-chloroisopropyl)phosphate also had a substantial contribution to the mixture effect (Fig. 4a and Supplementary Fig. 18a–e). Different chemicals were mixture effect drivers in the filter extracts, mainly surfactants and phosphate flame retardants (Fig. 4b and Supplementary Fig. 18f–h). Iceberg modelling was not possible for the fish extracts as only two bioactive chemicals could be included (tris(1-chloro-2-propyl)phosphate and tri(butoxyethyl) phosphate) and their contribution to narciclasine-EQbio was negligible.
This analysis clearly shows that the detected micropollutants constitute just the tip of the iceberg, many bioactive micropollutants are most likely unknown. It is striking that the prymnesins have such a high contribution to the mixture effects, but it cannot be excluded that other natural toxins had also contributed to the mixture effect. For instance the cyanobacteria toxins microcystin LR and YR were also identified in Oder water samples collected during the fish kill21. Cyanotoxins also cause neurotoxic effects as has been demonstrated with the neuroblastoma cell line SH-SY5Y (ref. 39).
Conclusions
HABs involving P. parvum have been reported to occur frequently in the estuaries of rivers, brackish-water lakes, but also in commercial aquaculture. So far the actual prymnesin toxins have only once been directly detected in a brackish lake40, but the presence of P. parvum has been confirmed on multiple occasions and has been associated to fish kills in both lakes and rivers18. Samples taken from the Oder River during the fish kill event in 2022 contained high concentrations of B-type prymnesins although the peak of the fish kill had presumably occurred a few days earlier with the first dead fish sighted in Germany on 9 August 2022. A count of dead fish near our sampling site, carried out by a Polish group just 1 day after our sampling, showed that a substantial number of dead fish were still washed up on the riverbanks between Zatoń Dolna ( >200) and Widuchowa ( <50) (ref. 3). Accordingly, it can also be assumed that the P. parvum peak was already at its tail end, and the toxic impact of prymnesins would have been even higher during the peak of the event. This is also evidenced by the chlorophyll A peaking to over 300 µg l−1 between 1 and 4 August 2022 (ref. 4), whereas being at or lower than 100 µg l−1 on 17 August 2022 (Supplementary Table 1 and ref. 4). The prymnesins’ toxic effects were exacerbated by the high micropollutant loads that clearly led to mixture effects in cytotoxicity and neurotoxicity assays based on differentiated human neuronal cells, although they were not deemed to be contributing to the actual fish kill21 due to the high ichthyotoxicity of prymnesins that exceeded the contribution by micropollutants by far.
Nevertheless, the estimated mixture risk quotients for aquatic organisms revealed exceedance of the risk quotient threshold of RQ = 1 by several individual chemicals. The RQmix was clearly exceeding the threshold of 1 even if only 30 organic micropollutants were included in the model, despite presumably hundreds and thousands of organic chemicals probably present.
The neurite outgrowth inhibition of prymnesins equalled that of the potent reference compound narciclasine. However, it was non-specific with SR close to 1, calling for more research on the impact of prymnesins on drinking water and human recreational use of water bodies, including research on the exposure routes such as skin contact and oral uptake, toxicokinetics and more mechanistic investigations on the molecular mechanisms of toxicity and more diverse human health-related end-points. High in vitro effects indicate primarily a hazard potential but may not be used alone to conclude risks. However, according to the concept of adverse outcome pathways, molecular initiating events and cellular effects are a prerequisite for the manifestation of effects in vivo41.
The study has demonstrated how ad hoc sample campaigns during HAB outbreaks can provide important insights on the drivers of toxicity in water samples, which is highly relevant for risk management and mitigation. Under climate change scenarios, society needs to brace against future compound extreme events, such as heat waves combined with long periods of sunshine and low flows42, because the harmful algal bloom in the Oder River in 2022 clearly had diverse and wide-ranging adverse impacts beyond the highly visible fish kills.
Methods
Water sampling and direct measurements
Water was sampled at the locations and coordinates given in Supplementary Table 1. Grab samples were taken in pre-washed glass bottles and immediately transported to the laboratory in cool boxes. Sample codes, basic physico-chemical parameters (pH), nutrients, chlorophyll A content and inorganic ion concentrations are provided in Supplementary Table 1 and Supplementary Text 10. For pigment analysis, samples were filtered onto glass fibre filters (GFF, 0.7 µm, Whatman) and measured by high performance liquid chromatography after ethanolic extraction43.
Extraction
All water samples were stored in glass bottles in the refrigerator in the dark until further processing and exposure to direct sunlight was kept to a minimum. Water was filtered with (several) glass microfibre filters (GFF, 0.7 µm, Whatman). The filtered water samples were split in three aliquots. Aliquots (100 ml) were extracted for pyrethroid analysis without pH adjustment. The rest was split in two equal aliquots of which one was adjusted to pH 7. Water was enriched with solid-phase extraction with an enrichment factor of 1,000 (details in Supplementary Text 11). The filters were combined, freeze dried and extracted with methanol by shaking and ultrasonication (Supplementary Text 12).
Fish
Two chubs (Squalius cephalus, fresh weight approximately 2 kg) were purchased from a local fisherman in Schwedt/Szwed who had caught the fish alive by electrofishing on 8 August 2022, when conductivity had already increased but the fish had no symptoms yet. These fish were kept alive in a tributary of the Oder River until 17 August 2022, when they were obtained by the research team, killed, frozen and transported to the laboratory. A 2-kg ide (Leusciscus idus) caught alive on 17 August 2022 in the morning with observed symptoms of intoxication by a (private) fisherman in Gryfino was kept in a pool of tap water for <12 h before killing, freezing and transported to the laboratories for extraction. The chubs and the ide were dissected to sample muscle and gills separately. In total 35 sturgeons (Acipenser oxyrinchus) were obtained from a restoration project reintroducing sturgeons into the Oder River (https://www.igb-berlin.de/en/sturgeon; refs. 44,45). Juvenile sturgeons (23 individuals, in total 25 gwet weight, 0.92 g mean individual wet weight) that had symptoms of intoxication were frozen on 10 August 2022 (sturgeon_exposed) and 12 healthy juvenile sturgeon (in total 7 gwet weight, 0.58 g mean individual wet weight) that had been kept in a rearing facility in a tributary of the Oder River were sampled 22 August 2022 (sturgeon_unexposed).
Homogenization of sturgeons, muscle and gills
The muscle of chubs and ide were homogenized using a blender, the gills of the chubs and ide and the whole juvenile sturgeons were homogenized using a cryo mill (Retsch) (Supplementary Text 13).
Extraction of sturgeons, muscle and gills
For extraction and clean-up a QuEChERS method was applied to homogenize sturgeons, gills and muscle. A blank was processed in the same way as the samples. Before analysis, the samples and the extraction blanks were spiked using an internal standard mix containing isotope-labelled compounds. A method-matched calibration was utilized for quantification (Supplementary Text 14).
Chemical analysis of micropollutants in water, particulate matter and fish
Targeted analysis of 27 pesticides in filtered water samples was performed by gas chromatography/tandem mass spectrometry measurements after Sorbstar extraction (details in Supplementary Text 15). The water SPE extracts, filter extracts, extracts of fish and fish organs were analysed for >600 chemicals with a target screening approach using liquid chromatography high-resolution mass spectrometry (LC-HRMS)25 (Supplementary Text 16).
Prymnesin analysis of all extracts
The presence and identity and the relative intensity of prymnesins were determined by LC-HRMS using a previously characterized extract of strain K-0081 (ref. 46) as retention time marker and an estimation of the concentrations of B-type prymnesins was performed as described by Svenssen et al.26 (Supplementary Text 17).
Toxicity assays
The concentrations of the extracts are given as relative enrichment factors (REF), which are defined as volume of original water sample dosed in form of its extract divided by the volume of the bioassay47. The concentration–response curves of the bioassays were fitted with a linear or a log-logistic concentration–response model according to ref. 47 and effect concentrations causing 10% (EC10) or 50% of effect (EC50) derived by interpolation.
Algal toxicity
Growth rate and photosynthetic capacity was quantified after a 24 h exposure to the water extracts in a synchronized culture of the unicellular green algae S. vacuolatus according to ref. 48 (Supplementary Text 18).
Immobilization assays with Daphnia magna
Water and filter extracts were tested with Daphnia magna LRV0_1 (ref. 49) according to OECD 202 guideline50 (Supplementary Text 19).
Zebrafish embryo toxicity test
An automated zebrafish embryo test with dosing at 2 h post fertilization (hpf) and exposure through to 96 h hpf was conducted using a dilution series of the extract with the highest tested concentration referring to a REF of 15. The automated image-based assessment is described in ref. 51. For the filter extracts only one concentration per sample was tested and the concentration was selected according to the IC10 of the cytotoxicity assay with SH-SY5Y cells. Using video tracking and image analysis, morphological effects and changes in embryonic movement (spontaneous tail contractions, photomotor response and locomotor response) as potential indicator of neurotoxicity after 96 hpf were quantified. Effect concentrations were calculated based on log-logistic modelling of concentration–response data (Supplementary Text 20).
Neurotoxicity assay
The water and fish extracts were tested in human neuroblastoma SH-SY5Y cells to investigate their effects on developmental neurotoxicity37. After 24 h of exposure, neurite length and cell viability were quantified based on phase-contrast and fluorescence images, respectively, using an IncuCyte S3 live cell imaging system (Essen BioScience). The 10% effect concentrations on cell viability (IC10) and neurite outgrowth (EC10) were determined and their ratio, named specificity ratio (SR), indicates specificity of effects on neurite outgrowth (Supplementary Text 21).
Hierarchical clustering
The hierarchical clustering was performed in R (version 4.1.3) using the vegan package (version 2.6-4). For concentrations, the means of replicates were used if observations >2. Otherwise, values were set to 0. Bray–Curtis dissimilarity was used to generate distance matrices. Clusters and respective dendrograms for both samples and chemicals were generated by Wards hierarchical agglomerative clustering using the Ward2 clustering criterion.
Iceberg mixture modelling
Bioanalytical equivalent concentrations (BEQ) serve to express the toxicity as the concentration of a reference chemical that has the same effect as the mixture47. Narciclasine is used as reference chemical in the neurite outgrowth inhibition assay38 and the narciclasine equivalent concentrations from chemical analysis narciclasine-EQbio can be derived with equation (1).
The narciclasine equivalent concentrations from chemical analysis narciclasine-EQchem is the sum of the narciclasine-EQchem(i) of each individual bioactive and detected chemical (equation (2)) with its relative effect potency REPi defined by equation (3).
Data availability
The data that support the findings of this study are available within this article and the Supplementary Information.
Change history
17 September 2024
A Correction to this paper has been published: https://doi.org/10.1038/s44221-024-00325-3
References
Absalon, D., Matysik, M., Woznica, A. & Janczewska, N. Detection of changes in the hydrobiological parameters of the Oder River during the ecological disaster in July 2022 based on multi-parameter probe tests and remote sensing methods. Ecol. Indic. 148, 110103 (2023).
Sobieraj, J. & Metelski, D. Insights into toxic Prymnesium parvum blooms as a cause of the ecological disaster on the Odra River. Toxins 15, 403 (2023).
Szlauer-Łukaszewska, A. et al. Quantifying a mass mortality event in freshwater wildlife within the lower Odra River: insights from a large European river. Sci. Total Environ. 907, 167898 (2024).
Köhler, J. et al. Unpredicted ecosystem response to compound human impacts in a European river. Sci. Rep. 14, 16445 (2024).
Valenti, T. W. et al. A mechanistic explanation for pH-dependent ambient aquatic toxicity of Prymnesium parvum carter. Toxicon 55, 990–998 (2010).
Blossom, H. E. et al. Prymnesium parvum revisited: relationship between allelopathy, ichthyotoxicity, and chemical profiles in 5 strains. Aquat. Toxicol. 157, 159–166 (2014).
Qin, J. L., Hu, Z. X., Zhang, Q., Xu, N. & Yang, Y. F. Toxic effects and mechanisms of Prymnesium parvum (Haptophyta) isolated from the Pearl River Estuary, China. Harmful Algae 96, 101844 (2020).
Manning, S. R. & La Claire, J. W. Prymnesins: toxic metabolites of the golden alga, Prymnesium parvum carter (Haptophyta). Mar. Drugs 8, 678–704 (2010).
Rasmussen, S. A. et al. Chemodiversity of ladder-frame prymnesin polyethers in Prymnesium parvum. J. Nat. Prod. 79, 2250–2256 (2016).
Hill, B. N., Saari, G. N., Steele, W. B., Corrales, J. & Brooks, B. W. Nutrients and salinity influence Prymnesium parvum (UTEX LB 2797) elicited sublethal toxicity in Pimephales promelas and Danio rerio. Harmful Algae 93, 101795 (2020).
Franco, M. E., Hill, B. N., Brooks, B. W. & Lavado, R. Prymnesium parvum differentially triggers sublethal fish antioxidant responses in vitro among salinity and nutrient conditions. Aquat. Toxicol. 213, 105214 (2019).
Varga, E. et al. Cytotoxicity of Prymnesium parvum extracts and prymnesin analogs on epithelial fish gill cells RTgill-W1 and the human colon cell line HCEC-1CT. Arch. Toxicol. 98, 999–1014 (2024).
Lindholm, T., Ohman, P., Kurki-Helasmo, K., Kincaid, B. & Meriluoto, J. Toxic algae and fish mortality in a brackish-water lake in angstrom land, SW Finland. Hydrobiologia 397, 109–120 (1999).
Meldahl, A. S., Edvardsen, B. & Fonnum, F. Toxicity of 4 potentially ichthyotoxic marine phytoflagellates determined by 4 different test methods. J. Toxicol. Environ. Health 42, 289–301 (1994).
Mariussen, E., Nelson, G. N. & Fonnum, F. A toxic extract of the marine phytoflagellate Prymnesium parvum induces calcium-dependent release of glutamate from rat brain synaptosomes. J. Toxicol. Environ. Health A 68, 67–79 (2005).
Graneli, E., Edvardsen, B., Roelke, D. L. & Hagstrom, J. A. The ecophysiology and bloom dynamics of Prymnesium spp. Harmful Algae 14, 260–270 (2012).
Karlson, B. et al. Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae 102, 101989 (2021).
Roelke, D. L. et al. A chronicle of a killer alga in the west: ecology, assessment, and management of Prymnesium parvum blooms. Hydrobiologia 764, 29–50 (2016).
Free, G. et al. An EU Analysis of the Ecological Disaster in the Oder River of 2022—Lessons Learned and Research-Based Recommendations to Avoid Future Ecological Damage in EU Rivers, a Joint Analysis from DG ENV, JRC and the EEA (Publications Office of the European Union, accessed 8 August 2024); https://publications.jrc.ec.europa.eu/repository/handle/JRC132271
Slugocki, L. & Czerniawski, R. Water quality of the Odra (Oder) River before and during the ecological disaster in 2022: a warning to water management. Sustainability 15, 8594 (2023).
Wiederhold, J. et al. Untersuchungen zum Fischsterben in der Oder im August 2022 (Bundesanstalt für Gewässerkunde, 2023).
Jonz, M. G. & Nurse, C. A. Development of oxygen sensing in the gills of zebrafish. J. Exp. Biol. 208, 1537–1549 (2005).
Ishiguro, M. & Koopal, L. K. Surfactant adsorption to soil components and soils. Adv. Colloid Interface Sci. 231, 59–102 (2016).
Büttner, O., Jawitz, J. W., Birk, S. & Borchardt, D. Why wastewater treatment fails to protect stream ecosystems in Europe. Water Res. 217, 118382 (2022).
Finckh, S. et al. A risk based assessment approach for chemical mixtures from wastewater treatment plant effluents. Environ. Internat. 164, 107234 (2022).
Svenssen, D. K. et al. Development of an indirect quantitation method to assess ichthyotoxic B-type prymnesins from Prymnesium parvum. Toxins 11, 251 (2019).
Kokel, D. et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol. 6, 231–237 (2010).
Ortmann, J., Altenburger, R., Scholz, S. & Luckenbach, T. Photomotor response data analysis approach to assess chemical neurotoxicity with the zebrafish embryo. Altex-Alternat. Anim. Exp. 39, 82–94 (2022).
Glauch, L. & Escher, B. I. The combined algae test for the evaluation of mixture toxicity in environmental samples. Environ. Toxicol. Chem. 39, 2496–2508 (2020).
Galassi, S., Guzzella, L. & Croce, V. Screening organic micropollutants in surface waters by SPE extraction and ecotoxicological testing. Chemosphere 54, 1619–1624 (2004).
Malev, O. et al. Combining short-term bioassays using fish and crustacean model organisms with ToxCast in vitro data and broad-spectrum chemical analysis for environmental risk assessment of the river water (Sava, Croatia). Environ. Pollut. 292, 118440 (2022).
Schweizer, M. et al. The importance of sediments in ecological quality assessment of stream headwaters: embryotoxicity along the Nidda River and its tributaries in Central Hesse, Germany. Environ. Sci. Eur. 30, 22 (2018).
Gauthier, P. T. & Vijayan, M. M. Municipal wastewater effluent exposure disrupts early development, larval behavior, and stress response in zebrafish. Environ. Pollut. 259, 113757 (2020).
Babić, S. et al. Embryotoxic and genotoxic effects of sewage effluents in zebrafish embryo using multiple endpoint testing. Water Res. 115, 9–21 (2017).
James, S. V. et al. Sunlight amelioration of Prymnesium parvum acute toxicity to fish. J. Plankton Res. 33, 265–272 (2011).
Taylor, R. B., Hill, B. N., Langan, L. M., Chambliss, C. K. & Brooks, B. W. Sunlight concurrently reduces Prymnesium parvum elicited acute toxicity to fish and prymnesins. Chemosphere 263, 127927 (2021).
Lee, J., Escher, B. I., Scholz, S. & Schlichting, R. Inhibition of neurite outgrowth and enhanced effects compared to baseline toxicity in SH-SY5Y cells. Arch. Toxicol. 96, 1039–1053 (2022).
Lee, J. et al. Monitoring mixture effects of neurotoxicants in surface water and wastewater treatment plant effluents with neurite outgrowth inhibition in SH-SY5Y cells. ACS Environ. Au 2, 523–535 (2022).
Hinojosa, M. G. et al. Cytotoxic effects and oxidative stress produced by a cyanobacterial cylindrospermopsin producer extract versus a cylindrospermopsin non-producing extract on the neuroblastoma SH-SY5Y cell line. Toxins 15, 320 (2023).
Wagstaff, B. et al. Assessing the toxicity and mitigating the impact of harmful Prymnesium blooms in eutrophic waters of the norfolk broads. Environ. Sci. Technol. 55, 16538–16551 (2021).
Ankley, G. T. et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 29, 730–741 (2010).
Zscheischler, J. et al. Future climate risk from compound events. Nat. Clim. Change 8, 469–477 (2018).
Wilhelm, C. & Wiedemann, I. Evidence of protein bound chlorophyll c3 in a light-harvesting protein isolated from the flagellate alga Prymnesium parvum (Prymnesiophyceae). Photosynthetica 25, 249–255 (1991).
Friedrich, T., Reinartz, R. & Gessner, J. Sturgeon re-introduction in the upper and middle Danube River basin. J. Appl. Ichthyol. 35, 1059–1068 (2019).
Gessner, J., Tautenhahn, M., Spratte, S., Arndt, G. M. & von Nordheim, H. Development of a German Action Plan for the restoration of the European sturgeon Acipenser sturio L.—implementing international commitments on a national scale. J. Appl. Ichthyol. 27, 192–198 (2011).
Binzer, S. B. et al. A-, B- and C-type prymnesins are clade specific compounds and chemotaxonomic markers in Prymnesium parvum. Harmful Algae 81, 10–17 (2019).
Escher, B., Neale, P. & Leusch, F. Bioanalytical Tools in Water Quality Assessment, 2nd edn (IWA Publishing, 2021); www.iwapublishing.com/books/9781789061970/bioanalytical-tools-water-quality-assessment-2nd-edition
Rummel, C. D., Schafer, H., Jahnke, A., Arp, H. P. H. & Schmitt-Jansen, M. Effects of leachates from UV-weathered microplastic on the microalgae Scenedesmus vacuolatus. Anal. Bioanal.Chem. 414, 1469–1479 (2022).
Chaturvedi, A. et al. The hologenome of Daphnia magna reveals possible DNA methylation and microbiome-mediated evolution of the host genome. Nucleic Acids Res. 51, 9785–9803 (2023).
Test Guideline No. 202. Daphnia sp. Acute Immobilisation Test (Environmental Directorate, OECD, 2004).
Teixido, E., Kiessling, T. R., Kluver, N. & Scholz, S. Grouping of chemicals into mode of action classes by automated effect pattern analysis using the zebrafish embryo toxicity test. Arch. Toxicol. 96, 1353–1369 (2022).
Acknowledgements
This study was performed by an ad hoc formed group during the fish kill in the Oder River in August 2022 without formal funding. We acknowledge support for consumables from the research unit ‘Chemicals in the Environment’ at UFZ and the University of Vienna, Faculty of Chemistry. This work is also partly funded by the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement number 965406 (PrecisionTox). This output reflects only the author’s view, and the European Union cannot be held responsible for any use that may be made of the information contained therein. E.V. was supported by the joint international project I-5707 N of the Austrian Science Fund (FWF) and the French National Research Agency (ANR) (grant DOI 10.55776/I5707). We gratefully acknowledge access to the platform CITEPro (Chemicals in the Environment Profiler) funded by the Helmholtz Association for analytical and bioassay measurements and financial support from the Helmholtz POF IV Topic 9 ‘Healthy Planet—towards a non-toxic environment’. P. Frühauf and the Department of Analytical Chemistry at the University of Vienna are acknowledged for the provision of the fluorescence detector and we would like to express our gratitude to the Mass Spectrometry Center of the Faculty of Chemistry, University of Vienna, for technical support. We thank P. Hawelitschek and C. Schmalsch from IGB for help with field sampling.
Funding
Open access funding provided by Helmholtz-Zentrum für Umweltforschung GmbH - UFZ.
Author information
Authors and Affiliations
Contributions
B.I.E., S. Spahr and E.V. designed research. J.A., A.B., J.P.D., M. Krauss, N.K, M. König, J.L., S.L., X.L., T.S, S. Spahr, S. Scholz and E.V. performed research. B.I.E., S. Spahr and E.V. wrote the manuscript, and B.I.E., J.A., D.B., W.B., J.K.C., J.G., A.J., M. Krauss, S.L., K.R. and M.W. were in the ad hoc response team during the fish kill and contributed to the interpretation of the results and to the text. T.S. organized laboratory work and performed chemical analysis, data evaluation and research. A.B. and N.U. performed the chemical analysis using Sorbstar and the corresponding data evaluation. A.J. prepared the map. M.S-J. performed the algal toxicity experiments; X.L. and L.O., the daphnid toxicity; and S. Scholz and N.K., the fish embryo toxicity experiments. M. König and J.L. performed the in vitro toxicity experiments. G.B. performed the hierarchical clustering. All authors read and revised the manuscript and approved the submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Gustaaf Hallegraeff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Text 1–21, Fig. 1–19 and Table 15.
Supplementary Tables
Supplementary Tables 1–14.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Escher, B.I., Ahlheim, J., Böhme, A. et al. Mixtures of organic micropollutants exacerbated in vitro neurotoxicity of prymnesins and contributed to aquatic toxicity during a toxic algal bloom. Nat Water 2, 889–898 (2024). https://doi.org/10.1038/s44221-024-00297-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44221-024-00297-4