Abstract
Emerging infectious diseases, biodiversity loss, and anthropogenic environmental change are interconnected crises with massive social and ecological costs. In this Review, we discuss how pathogens and parasites are responding to global change, and the implications for pandemic prevention and biodiversity conservation. Ecological and evolutionary principles help to explain why both pandemics and wildlife die-offs are becoming more common; why land-use change and biodiversity loss are often followed by an increase in zoonotic and vector-borne diseases; and why some species, such as bats, host so many emerging pathogens. To prevent the next pandemic, scientists should focus on monitoring and limiting the spread of a handful of high-risk viruses, especially at key interfaces such as farms and live-animal markets. But to address the much broader set of infectious disease risks associated with the Anthropocene, decision-makers will need to develop comprehensive strategies that include pathogen surveillance across species and ecosystems; conservation-based interventions to reduce human–animal contact and protect wildlife health; health system strengthening; and global improvements in epidemic preparedness and response. Scientists can contribute to these efforts by filling global gaps in disease data, and by expanding the evidence base for disease–driver relationships and ecological interventions.
Key points
-
Human activities have created a planetary polycrisis that includes pandemics, climate change and the sixth mass extinction.
-
Climate change, land change, agriculture and wildlife use — the major threats to biodiversity — are also driving a global rise in infectious diseases.
-
Biodiversity loss is generally harmful to human health.
-
Interventions that target spillover interfaces for high-risk pathogens, such as avian influenza or coronaviruses, could prevent some future pandemics.
-
Even with these interventions, investments in health systems and pandemic preparedness will be an important part of living in the Anthropocene.
-
The world needs better real-time biosurveillance infrastructure to track pathogens across species and ecosystems.
Similar content being viewed by others
Introduction
One feature of the Anthropocene is a planetary dysbiosis, in which ecological relationships between hosts and microorganisms shift suddenly, with generally adverse consequences for human, animal and ecosystem health. Infectious disease outbreaks and cross-species transmission events are naturally occurring phenomena. But, as with changes in climate and biodiversity over the past several hundred years, the growing diversity and burden of emerging infectious diseases fall outside historical baselines1,2. For most of human history, pandemics were ‘once in a century’ events; since the start of the twentieth century, ten distinct pandemics have occurred, including two in the past fifteen years (Box 1). Every year, several new viruses reach human populations3, and the frequency of high-impact pathogen spillover events increases by an estimated 5% and resulting mortality increases by 9%4. Non-human animal populations are also increasingly vulnerable to emerging diseases, with epizootics and panzootics of diseases such as chytridiomycosis leading to unprecedented waves of extinctions.
The rising burden of emerging infectious diseases is one of many concurrent and interconnected human-induced changes in the biosphere1,5,6 (Fig. 1). Infectious disease emergence, biodiversity loss and anthropogenic global warming have all shown similar trends over the last few centuries. Global hotspots of emerging infectious diseases appear to follow classical biodiversity gradients: new zoonotic and vector-borne diseases have emerged at the fastest rate where mammal biodiversity is also high7. However, within ecological communities, loss of host and symbiont biodiversity can also increase pathogen transmission. Biodiversity loss and disease emergence also share many upstream drivers, including agricultural expansion, habitat loss, wildlife trade and climate change. Together, these changes form a ‘polycrisis’ — an interconnected web of rapidly accelerating transformations with no singular solution. The COVID-19 pandemic put these connections in the global spotlight to an unprecedented degree8,9,10. In its wake, some progress has been made towards multilateral action on biodiversity conservation and pandemic prevention, reflecting wider recognition of the links between biodiversity, sustainability and human health. However, planetary transformations have mostly continued as usual, and the risk of future pandemics continues to grow.
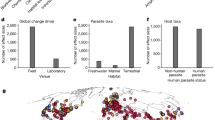
a, The annual number of spillover events of high-consequence zoonotic diseases increased steeply during the twentieth century4, diverging from historical baselines, similar to the temporal trends in species extinctions241 (b) and climate change (c). However, the apparent trend in spillover rates could be at least partly attributed to improvements in outbreak detection and reporting. d, Reports of emerging infectious diseases in humans (data points are coded by pathogen type) are more common in regions of the world with higher mammal biodiversity (number of zoonotic host species); however, outbreaks are also more likely to be detected by surveillance systems and described for the first time in Europe and North America compared to other regions. The effects of anthropogenic environmental change are felt worldwide, and — although high-biodiversity regions face unique risks — the threat posed by emerging infectious diseases is growing everywhere. For a full explanation of data sources and each specific component of the figure, see Supplementary Note 2.
In this Review, we synthesize current knowledge on the connections between biodiversity loss and disease emergence, their shared anthropogenic drivers, and their consequences for future epidemic and pandemic risks. We first introduce ecological perspectives on the multi-faceted relationship between biodiversity and infectious disease, including evidence that biodiversity loss can be a risk factor for zoonotic disease emergence. We next present a public health–oriented perspective on how biodiversity science can be used to monitor and manage infectious diseases, and discuss open challenges associated with pandemic prevention through biodiversity conservation. We conclude by describing how future work can clarify the connections between anthropogenic environmental change and infectious disease dynamics, to determine which nature-based solutions could reduce the risk of pandemics.
Biodiversity and infectious disease
Despite decades of calls for interdisciplinary frameworks and synthesis, ecological perspectives on infectious disease remain relatively fragmented. Macroecologists and systematists consider parasites and pathogens to be part of the sum total of biodiversity, documenting patterns in where and how parasite biodiversity has accumulated11,12. Community ecologists explore how species interactions, biodiversity gradients and even biodiversity loss shape disease dynamics over space and time13. Some conservation biologists study emerging infectious diseases as a growing threat to species survival, whereas a smaller community of practice is working to save the other >99% of parasites that are mostly harmless14,15. The One Health perspective bridges conservation biology, veterinary medicine and public health, focusing on strategies to reduce infectious disease risks at the interfaces among wildlife, livestock, companion animals and humans. Meanwhile, the planetary health approach emphasizes the connections among the climate crisis, the sixth mass extinction and emerging infectious diseases — and how these trends will continue to feed into each other over the coming century. Each of these perspectives paints a slightly different picture of emerging infectious diseases, the role of anthropogenic drivers and the possible ecological levers for intervention. In this section, we summarize the major perspectives on the multi-faceted relationship between biodiversity and disease.
Host biodiversity drives pathogen biodiversity
Pathogens and parasites account for a substantial fraction of global biodiversity. Parasitism has evolved over 200 times in at least 15 animal phyla16: there are hundreds of species of ticks, thousands of species of fleas, tens of thousands of species of parasitoid wasps17, several hundred thousand species of worms (a polyphyletic group) that parasitize vertebrate hosts18,19, and several million more worms and mites that parasitize invertebrates20. The diversity of fungal, bacterial and viral microparasites is even more vast, but harder to quantify; microorganisms are difficult to classify into discrete species, and many switch between mutualist, commensal or pathogenic states depending on their host’s microbiome composition, immune function or ecological context.
The relationship between host and parasite or pathogen diversity is scale- and system-dependent. At broad geographic and taxonomic scales, host species richness is tightly and positively correlated with parasite species richness21,22. This ‘diversity begets diversity’ effect is the result of both ecological and evolutionary processes. Any given parasite has a finite intrinsic host range, so on average, more diverse host communities can contain more possible parasites. Over time, parasites also diversify through a mix of cospeciation and host-switching, both of which are facilitated by host diversification22,23,24. However, within a given ecological community or region, the effect of host richness on parasite richness might be secondary to host evolutionary history, host traits (such as body size or immune phenotypes), and environmental conditions (such as climate or ecosystem type)25,26.
On the basis of these principles, tropical hotspots of vertebrate biodiversity are also presumed to be hotspots of parasite and pathogen biodiversity. However, the available data are mostly unfit for testing this hypothesis, given both the small fraction of parasite diversity that has been characterized, and the geographic and taxonomic biases of the underlying research (Box 2). For example, macroparasite discovery has been heavily biased towards high- and middle-income countries that invest in systematics research and collections infrastructure; the observed hotspots therefore primarily reflect research effort and capacity18,27,28,29. Viral discovery has been similarly biased towards high-income countries, but it has also been heavily shaped by public health priorities — especially large-scale investments in characterizing viruses with zoonotic potential30,31. As a result, sampling efforts have been idiosyncratic, with a sizeable gap in the Amazon basin and more broadly Latin America, compared to sub-Saharan Africa and southeast Asia.
Biodiversity drives disease emergence
The vast majority of animal pathogens will never pose a risk to human health, but a small fraction have the capacity to infect humans, given the opportunity. Over evolutionary timescales, this is the origin of almost all human infectious diseases, with very rare exceptions32,33. More than 70% of emerging infectious diseases have spread from animals to humans within the past several hundred years, with more than half coming from wildlife (as opposed to livestock or pets). Among emerging viruses specifically, almost 90% are zoonotic, and roughly two-thirds are the result of spillover from wildlife33. (On this point, many sources use incorrect citations, statistics, or both (Supplementary Note 1)). Viruses pose a unique and ongoing risk as potential zoonotic pathogens, because of their pace of diversification, propensity to cross species barriers, and potential to cause devastating epidemics starting from a single human case. Over 500 virus species have been recorded infecting both animal and human hosts34, but tens or hundreds of thousands of mammal viruses (and a small number of other vertebrate viruses) could be capable of human infection18,35.
Some animal groups seem to host a disproportionate number of known or potential zoonotic pathogens. One proposed explanation for these apparent ‘hyper-reservoirs’ is that some animal clades could harbour a higher overall pathogen diversity than others: for example, bats comprise 22% of mammals but host 35% of known mammal viruses, whereas rodents comprise 36% of mammals, but only account for 19% of their viral diversity. However, these patterns are nearly impossible to separate from sampling effort, especially in the case of bats, which have been uniquely targeted in virus surveillance efforts since the emergence of SARS-CoV in 2002 (refs. 31,36). Although per-species viral richness is similar across mammal orders22,37, some specific clades have higher-than-expected pathogen diversity; this could be a random outcome of evolutionary history, or the result of specific ecological traits, such as a fast pace of life or larger geographic range38.
Some animal clades also host pathogens with unique characteristics that increase their potential impact on human populations. Bats have particular immune adaptations that appear to facilitate an exceptional tolerance of virulent viruses, such as constitutive expression of IFNα and a dampened inflammatory response39,40. These traits create strong selective pressures on their pathogens41, potentially driving features that include a higher propensity for cross-species transmission and a higher intrinsic virulence — explaining why bats host many of the most virulent zoonotic viruses42. Similarly, viruses that are adapted to primate immune systems can be functionally ‘pre-adapted’ to humans, and so are more likely to be transmitted onwards after the first human case42,43,44.
At a global scale, the first records of new infectious diseases show a striking correspondence to mammal biodiversity gradients (Fig. 1d), with more newly described diseases in regions with medium to high mammal diversity. Novel infectious diseases are also more likely to be detected by surveillance systems and characterized by researchers in high-income countries, especially in North America and Europe; after adjusting for these biases, the correlation with biodiversity gradients becomes even stronger7,33. The association between higher host biodiversity and higher rates of disease emergence results from a higher underlying diversity of the pathogen community, as well as the large number of people and livestock living alongside biodiverse ecosystems45. However, there are exceptions to this pattern: most notably, the Neotropics should be a global hotspot of pathogens with zoonotic potential given their high mammal biodiversity, but novel epidemic viruses seem to emerge from wildlife very rarely in Latin America and the Caribbean46. This absence is particularly notable in the case of bats, which are most biodiverse in the Neotropics; based on the biogeography of clades that are tightly associated with high-consequence zoonotic viruses, spillover risk should be high in the Amazon basin37,47,48,49. However, to our knowledge, no epidemic of a bat-origin virus has been recorded in South America. These kinds of idiosyncrasy in the relationship between host biodiversity, pathogen biodiversity and disease emergence could be the result of specific coevolutionary history — for example, filoviruses (including Ebola and Marburg virus), henipaviruses (including Nipah and Hendra virus) and SARS-like coronaviruses have all been detected in bats in the Old World but not in the Neotropics (but see refs. 50,51) — or could be due to different socioecological pressures on, and pathways for, emergence (for example, bats might be less regularly consumed for protein in the Americas, and wildlife farming is less common than in east and southeast Asia).
Biodiversity loss can drive disease emergence
One of the most extensive debates in contemporary ecology revolved around whether biodiversity has a protective effect against infectious diseases52. This debate has been mostly resolved through an overwhelming body of empirical evidence and several meta-analyses. In general, differences between communities in baseline host biodiversity (in other words, natural biodiversity gradients) have an inconsistent — and, at the broadest spatial scales, often positive — effect on disease risk. However, the loss of host biodiversity within a given ecological community is typically followed by an increase in pathogen transmission53,54. This finding has been reproduced in observational and experimental studies, with terrestrial and marine systems, animal and plant hosts, directly transmitted and vector-borne pathogens, and different types of infectious agent54,55. However, in any given instance, this pattern could be the result of indirect association (biodiversity loss and disease emergence may share a driver such as habitat loss) or direct causation (biodiversity loss may directly increase pathogen prevalence in wildlife or spillover rates).
Biodiversity loss does not favour every pathogen or parasite equally: some decline or are lost alongside their hosts, whereas others become more prevalent as their hosts or vectors become more abundant. The fate of any given pathogen depends on the host competence of each available host species, their interactions with each other and their responses to anthropogenic disturbance. In general, anthropogenic change will increase disease risk if it favours host species that are important to pathogen transmission, or if it leads to the loss of other species. For example, loss of a keystone predator might lead to larger and more connected prey populations, increasing pathogen transmission (the ‘healthy herds effect’)56,57,58. Similarly, if environmental changes lead to the disproportionate loss of low-competence hosts (namely, those that serve as a sink or dead end for transmission), prevalence can increase in the remaining species (the ‘dilution effect’)59,60. For example, forest fragmentation favours the white-footed mouse (Peromyscus leucopus)61, which is a highly competent host for both the bacterium that causes Lyme disease and the ticks that transmit it; meanwhile, lower-competence hosts, such as opossums, become less abundant in disturbed landscapes62. The universality of the dilution effect and its relevance to human health have been heavily debated, but across ecosystems, the species that are most resilient to anthropogenic change are also more likely to be hosts of zoonotic diseases38,63. There are several explanations for this pattern, but many focus on life history: fast-lived animals that thrive in disturbed environments (‘weedy’ species such as the white-footed mouse) often undergo explosive population cycles that create episodes of high spillover risk64, and might also be subject to evolutionary trade-offs in immune investment65,66,67.
Not all parasites respond positively to anthropogenic change. Parasites are vulnerable to the loss of their hosts68,69,70, but can also be directly affected by environmental stressors that influence transmission or survival, especially in their free-living stages71,72,73. Paradoxically, decreases in total parasite richness can be accompanied by increased disease risk from specific pathogens. Sometimes, this decrease occurs because parasites are in direct competition, not just within host populations but within individual hosts (in other words, coinfection leads to worse disease outcomes for the host, limiting parasite transmission at high prevalences)74,75. In other cases, complex interactions between parasite infection and host immunity can reduce host susceptibility to infection with a more virulent pathogen73,76. Conservation strategies that proactively conserve hosts alongside a diverse parasite fauna could therefore help to protect them from the emergence of diseases that jeopardize their survival or even human health77. However, the field of parasite conservation biology, and broader scientific understanding of the ecological consequences of parasite biodiversity loss, is still in its infancy.
Disease can drive biodiversity loss
Emerging infectious diseases pose a growing problem for wildlife conservation78, with high-profile examples of mass mortality resulting from the introduction of novel pathogens or unusual outbreaks of endemic pathogens triggered by changing environmental conditions79. For example, during unusually warm and humid weather in 2015, an outbreak of an endemic and usually benign bacterium (Pasteurella multocida) in Kazakhstan was responsible for the loss of 60% of the global population of the saiga antelope (Saiga tatarica)80,81. Only two years later, the virulent peste des petits ruminants virus spread from livestock to saiga in Mongolia, leading to the loss of 80% of the local population82.
Epizootics of virulent pathogens are often self-limiting — as the number of susceptible hosts declines, infected hosts eventually die faster than they produce secondary infections — and, in isolation, are unlikely to cause the extinction of an entire species83. However, infectious disease can readily reduce wildlife populations to low levels, where they face an increased risk of extinction due to other factors; conversely, small and isolated populations such as island endemic species can be vulnerable to outright disease-induced extinction.
A subset of pathogens can also continue to spread through environmental reservoirs even as host populations reach critically low levels84. Indeed, several prominent disease-induced extinctions of wild animals are attributed to fungal pathogens that can persist in the environment. Since the late twentieth century, a panzootic of the chytrid fungus Batrachochytrium dendrobatidis has been responsible for the extinction of at least 90 amphibian species85. Another chytrid fungus, B. salamandrivorans, has been responsible for mass mortality events in some European salamander populations86 and could someday become a similarly global problem87. The fungus responsible for white-nose syndrome (Pseudogymnoascus destructans) has similarly led to the collapse of North American bat populations, with at least one species still at risk of extinction88. These pathogens have had unexpected repercussions for human health: the loss of Neotropical amphibians that feed on mosquito larvae might have increased malaria incidence in Costa Rica and Panama89, and the loss of insectivorous bats might have forced farmers to use more insecticide, leading to higher rates of infant mortality in the eastern USA90.
Beginning in 2020, highly pathogenic avian influenza A/H5NX sublineage 2.3.4.4b has been responsible for numerous mass mortality events in wild birds and mammals91, representing a potentially unprecedented panzootic threat. In 2023, 27% of the total population of Chilean Humboldt penguins (Spheniscus humboldti) were found dead, representing a nearly 2,000% year-to-date increase in mortality92. After several critically endangered California condors (Gymnogyps californianus) died from avian influenza in 2023, rapid efforts to develop an emergency vaccine were initiated.
Human pathogens also pose a growing risk to wildlife health. Human-to-animal pathogen spillback is limited by the same ecological and evolutionary bottlenecks as zoonotic spillover, as well as the asymmetry of many human–animal interactions (for example, humans eat other animals at a much higher frequency than the inverse). However, just as spillover rates are growing exponentially, spillback could also be a growing problem. This phenomenon is perhaps most visible in the global spread of SARS-CoV-2, which has been found in 35 animal species across 5 continents93. Whereas SARS-CoV-2 has generally had minimal effects on conservation, in other cases, the outcomes of pathogen spillback have been serious. For example, primates appear to be more vulnerable to multiple human infections than other mammals due to their evolutionary proximity to humans94, and respiratory pathogens that are relatively benign in humans (for example ‘common cold’ viruses) regularly cause serious mortality in great apes95,96. Primates living in sanctuaries or that become habituated to human landscapes seem to be particularly at risk94, but the effect on natural populations can still be substantial.
Conservation measures that limit human–wildlife contact are the primary defence against pathogen spillback. Surveillance for wildlife mortality and active pathogen surveillance are especially important where humans live alongside wildlife species or are expanding their reach into critical habitat for threatened species. Investment in human and livestock health around protected areas can also limit the level of exposure that wildlife face97. These measures, in turn, reduce the risks and effects of zoonotic spillover in high-biodiversity areas.
Common drivers and causal pathways
The same anthropogenic processes that are responsible for the biodiversity crisis are also implicated as the primary ecological drivers of disease emergence9,98,99,100,101. Land and climate change both mediate disease dynamics through organismal physiology and behaviour, and agriculture and wildlife trade and hunting act as distinct high-risk interfaces for animal-to-human pathogen transmission. However, the effect and importance of any given driver of biodiversity loss or disease emergence — and the relationship between the two — can be unique to a given pathogen, and even a given landscape. Putting these connections into context can help researchers to produce better risk assessments and identify points for intervention.
Upstream drivers
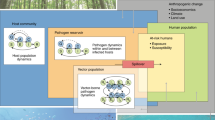
Since the 1960s, one-third of global land area has undergone anthropogenic land change102, in the form of conversion and fragmentation of intact forests and other ecosystems, of agricultural expansion and intensification, and of urbanization. Habitat loss from land change (and increasingly, climate change) is the single greatest threat to biodiversity103. Land change is also often cited as the primary driver of zoonotic spillover104. Habitat loss, fragmentation and degradation can cause nutritional stress and behavioural shifts that increase contact within and between species, all of which can lead to increased pathogen transmission104,105 (Fig. 2a). Habitat loss also pushes wildlife into human-used landscapes to seek resources, shelter or space63,106, leading to increased spillover risk.
Biodiversity loss and disease emergence often follow land conversion, but different schools of thought offer different insights into the underlying mechanisms. a, In a conceptual model based on ecoimmunology and community ecology104,105, land conversion sets off a cascade of organismal and community-level changes; over time, this increases the risk of zoonotic spillover. b, In a conceptual model based on landscape ecology and social–ecological systems theory63,107, spillover risk is shaped by the intensity and type of anthropogenic land use, the habitat requirements of important species in the pathogen life cycle, and the types of interface associated with spillover (for example, wildlife hunting or agriculture). Some variables are usually positively or negatively correlated over space with the degree of anthropogenic disturbance; other gradients might be unique to the ecology of a given landscape and pathogen. Neither model is comprehensive or universal, and both are compatible with other perspectives.
The response of a given pathogen to land change depends on its transmission ecology. Human cases of vector-borne zoonoses (such as yellow fever virus) appear to increase more consistently following land-use change than does spillover of directly transmitted pathogens (such as Ebola virus)5,107. However, risk can also decrease after land conversion if key wildlife hosts or vectors are poorly suited to human-altered landscapes or are excluded by synanthropic species54,108,109 (Fig. 2b). Land conversion is therefore sometimes associated with a regime shift between different assemblages of human pathogens. For example, in Brazil, the transition from rural to urban landscapes is accompanied by a shift from malaria and leishmaniasis to arboviruses such as dengue fever and Zika virus that are primarily transmitted by the Aedes aegypti mosquito109.
In addition to being the largest driver of deforestation, animal agriculture poses unique risks relative to other types of land use. Of the four facets of planetary change discussed here, agriculture had the earliest (and longest) effect on disease emergence; human and domesticated animals have had thousands of years to share viruses110. Livestock also account for more terrestrial biomass (around 630 million tonnes) than humans (around 390 million tonnes) or wild mammals (around 20 million tonnes)111; this abundance creates ample opportunities for pathogen circulation, evolution (including adaptation to mammalian immune systems) and cross-species contact. Livestock therefore often act as bridge or amplification hosts in the disease emergence process112,113,114. For example, in nearly half of all modern pandemics, emerging influenza virus subtypes infected poultry or other livestock before spreading to humans. Livestock can also be a source of pathogens that threaten wildlife populations, such as highly pathogenic avian influenza or tuberculosis115.
Compared to land change, climate change is often underestimated as a threat to both biodiversity and human health, both because climate-related risks are still accelerating, and because they can be hard to distinguish from other correlated trends, including improved surveillance. Rising temperatures, shifts in precipitation and severe storms could be responsible for increasing the risk of over half of human diseases116. So far, the best understood effect of climate change on disease risk has been an increase in the global burden of mosquito-borne diseases, which exhibit a well characterized unimodal transmission–temperature relationship117,118,119. Climate change is also implicated as a contributing factor in the rapid range expansion of the mosquito vectors of malaria and dengue fever120,121 and similar effects on ticks are also suspected, but less clearly established122,123. Climate-change-driven geographic range shifts are also creating new opportunities for interspecific contact among wildlife124,125, increasing risks related to epizootics and creating potential bridge hosts for zoonotic emergence126. In their native ranges, many species are also increasingly exposed to extreme temperatures they have never encountered127,128, posing a serious risk to species survival and ecosystem stability; the implications for disease transmission have barely been explored.
A final planetary driver of both biodiversity loss and emerging disease is wildlife use, including trade, farming and hunting. So far, human cases of fewer than 10% of emerging viruses have been traced back to wildlife use112, but some of these viruses pose a particularly serious risk — most notably, SARS-like coronaviruses in southeast Asia129,130,131,132. Wildlife trade affects a quarter of vertebrate species and has become a major threat to the survival of many species133. Wildlife farms, supply chains and live-animal markets all create unnatural conditions that can increase crowding and physiological stress, leading to higher rates of infection134,135 as well as unusual contact patterns between species136. Every stage of the commercial wildlife trade process also entails high-risk contact between humans and animals, and spillover events could be more likely to lead to epidemics if traded wildlife are brought into large population centres. Although generally lower-risk than commercial wildlife trade, subsistence hunting can also threaten species survival137,138 and create opportunities for pathogen spillover.
Although land change, agriculture, climate change and wildlife use are the most important ecological drivers of biodiversity loss and disease emergence54, several other facets of anthropogenic change are known — in more limited cases — to affect both processes. Invasive alien species are involved in 60% of modern extinctions139, and can bring pathogens into new regions. For example, two globally invasive mosquitoes (A. aegypti and Aedes albopictus) have become the primary vectors of several arboviruses, including dengue fever, yellow fever, chikungunya and Zika virus121,140. A third synanthropic mosquito from south Asia (Anopheles stephensi) now poses a similar risk of global invasion, and represents a growing threat to malaria eradication in Africa141,142. Pesticide pollution can cause ecosystem changes that increase disease risk; for example, fertilizer runoff favours invasive aquatic weeds, which create habitat for the snail vectors of schistosomiasis143. Finally, leakage of antibiotics into the environment is a major contributor to the global crisis of antimicrobial resistant bacteria and fungi144,145, selecting for the emergence of drug-resistant pandemic viruses before they ever spread from animals to humans146,147.
Case studies in causation
There is no canonical biodiversity–disease–driver relationship (Fig. 3). To illustrate the diversity of these interactions, we discuss four case studies that exemplify how variation in pathogen transmission mode, in wildlife host or vector identity, and in anthropogenic context can influence the dynamics of disease emergence.
a, Biodiversity loss can drive disease emergence, and vice versa; they also share many of the same upstream drivers. Three of these facets of anthropogenic global change are closely linked: agriculture is the single largest driver of land change, and agriculture and land change are both major drivers of climate change. The strength and direction of these relationships vary substantially on a disease-by-disease basis; case studies are shown for Lyme disease (b), Hendra virus (c), influenza (d), and coronaviruses (e). In b–e, solid lines indicate relationships supported by direct literature evidence, and dashed lines indicate relationships that are hypothesized but weakly supported, or that are more likely to be important in the future. To mitigate infectious disease risks, scientists need to establish these kinds of relationships, and identify case-by-case interventions that target the right drivers.
Lyme disease
Lyme disease is caused by the bacterium Borrelia burgdorferi, and primarily vectored by the blacklegged tick (Ixodes scapularis). Since it was first identified in 1975, the incidence of Lyme disease has grown substantially, primarily in the northeast and midwest regions of the USA. Land change and biodiversity loss have contributed to this trend: forest fragmentation has led directly to the loss of low-competence hosts such as opossums while favouring competent reservoirs such as mice, chipmunks and shrews61,62,148. Additionally, declines in key predator species such as red foxes have also increased the abundance of these competent hosts58. Although climate change has not been the primary driver, the growing burden of Lyme disease in the northeastern USA is at least partially attributable to rising temperatures, and is likely to continue to increase under future warming149.
Hendra virus
Hendra virus is a pathogen of Australian flying foxes (Pteropus spp.); spillover into humans occurs infrequently and does not lead to onward transmission. Human–bat interactions have increased as the bats’ winter habitats have been converted into agricultural land, and previously nomadic populations have settled in urban environments, creating more opportunities for Hendra virus spillover106. The loss of nomadic behaviour also reduces population connectivity and therefore allows immunity to wane, leading to larger epidemic sizes upon viral re-introduction106,150. Transmission dynamics are also probably affected by climate change. El Niño climate oscillations create years in which fruit resources are insufficient, driving bats into agricultural land to forage; this nutritional stress also increases seasonal pulses of viral shedding106,151. Habitat loss from extensive bushfires could lead to similar risks152. Finally, extreme heat associated with climate change killed over 72,000 bats, and caused widespread abandonment of pups in the summer of 2019–2020 (ref. 153); the implications of this kind of mortality event for disease dynamics are uncertain.
Influenza
Influenza A virus is the archetypal pathogen with pandemic potential and, increasingly, a global threat to biodiversity91,154. Viral strains undergo genetic drift and reassortment in poultry and other livestock, which also transmit the virus back to wild birds and humans. Although surveillance and prevention efforts often focus on farms, spillover has also been associated with poultry markets and the wild-bird trade, particularly in China and southeast Asia155,156. Anthropogenic drivers of influenza circulation in wild birds are comparatively understudied. The precipitous loss of the world’s wetlands might be forcing migratory waterfowl to congregate in smaller patches of intact habitat, leading to higher levels of transmission157,158; protected areas could reduce outbreak risk by reducing this pressure and separating waterfowl from domestic poultry. Despite speculation that climate change could be a contributing factor to the H5NX highly pathogenic avian influenza panzootic159, there is so far no evidence to support this hypothesis.
Coronaviruses
The Coronaviridae are an immensely diverse family of viruses found across mammals and birds, although some groups that have diversified in bats (particularly the subgenera Sarbecovirus and Merbecovirus) pose a distinct risk to human health. Fewer than a dozen coronaviruses have so far emerged in humans, of which only three have shown both high pathogenicity and pandemic potential: two severe acute respiratory syndrome coronaviruses (SARS-CoV and SARS-CoV-2), and Middle East respiratory syndrome coronavirus (MERS-CoV). The strongest available evidence indicates that SARS-CoV and SARS-CoV-2 both reached humans through the wildlife trade129,160,161, which also poses a serious threat to the conservation of suspected wildlife hosts. However, the majority of human coronaviruses — including MERS-CoV, several low-pathogenicity viruses, and the most recent additions, canine coronavirus and porcine deltacoronavirus — are known or suspected to have reached humans through livestock and companion animals162,163,164. Proposed connections between the specific origin of COVID-19 and land change165 or climate change166 are so far speculative. However, coronavirus prevalence is higher in wildlife-use-related contexts134,135 and in ecosystems with a greater human footprint167. One study found promising evidence of a dilution effect in the bat–coronavirus system in west Africa168, but more work is needed to establish whether interspecific variation in immunology contributes to differences in host competence and whether biodiversity might therefore have a protective effect.
Shared solutions for biodiversity and health
Within the next 25 years, the world is on track for at least 1.5 °C of warming and nearly 300 million hectares of tropical deforestation169. At the same time, based on current trends, a four-fold increase in the rate of zoonotic spillover, with 12 times as many deaths, is projected4. These problems call for a diverse set of solutions, ranging in scale from local initiatives170 to planetary governance171,172. Perspectives from ecology and biodiversity science can be leveraged to develop better surveillance infrastructure and ecosystem-based strategies for outbreak prevention, in tandem with renewed investments in public health and outbreak preparedness and response.
Biosurveillance and biodiversity monitoring
Surveillance is the backbone of public health. The One Health approach highlights the importance of monitoring pathogens not only in humans, but also in wildlife, domestic animals and environmental reservoirs such as soil, water and air. Given resource limitations, surveillance efforts should target the hosts and interfaces most associated with specific epidemic or pandemic risks, or the major data gaps that limit scientific inference. Machine learning models can also help to target sampling and monitoring efforts towards species that are most likely to host undiscovered pathogens48,49,173, are at the highest risk from viral spillback174,175, or are likely to display spillover-relevant behaviours such as living in human-built structures176.
Several technological advances are improving wildlife disease surveillance. For example, mobile apps such as the Spatial Monitoring and Reporting Tool (SMART)177 allow park rangers to report unusual mortality events. Platforms like Verena’s Pathogen Harmonized Observatory178 and the United States Geological Survey’s Wildlife Health Information Sharing Partnership179 allow researchers and managers to share wildlife disease data in real time. Non-invasive sampling methods also open up new horizons for biosurveillance: for example, air samples collected by drone can be used to monitor marine mammals180 or to sample live-animal markets or bat roosts without putting researchers at risk of pathogen exposure181. The advent of ‘next-generation biomonitoring’182,183,184, with its explicit adoption of artificial intelligence-enhanced image and sound analysis as well as environmental DNA and RNA data collection, is also increasing the volume and resolution of data that can be collected.
Biodiversity science has also become a critical source of data for public-health research and practice185. Geospatial data on disease hosts and vectors are regularly used to map disease transmission risk186,187, to identify surveillance gaps188, to reconstruct historical patterns, such as the spread of invasive vectors120,121, and to project future infectious disease risk under different scenarios of changing climate and land use126,189,190. Biodiversity repositories are usually the best available source of these data, although microorganisms remain under-represented in major biodiversity data platforms191 (Fig. 4a). Targeted efforts to recruit new data192,193, particularly from rich sources such as community science projects (for example, the Mosquito Alert app)194,195 and vector control agencies196, increase the value of these datasets for public health and for biodiversity research more broadly.
a, Geolocated occurrence records for Aedes aegypti, the primary urban vector of several viruses, including dengue, yellow fever and Zika. b, Geolocated occurrence records for horseshoe bats (Rhinolophus spp.), the primary known wildlife reservoir of SARS-like coronaviruses. Information contained in digital biodiversity infrastructure and museum collections is both a foundational resource for long-term ecological research and an open source of real-time epidemic intelligence and viral discovery. The photograph in a is reprinted from https://www.gbif.org/occurrence/3910014308, CC0 (https://creativecommons.org/publicdomain/zero/1.0/legalcode); the photograph in b is reprinted from https://www.gbif.org/occurrence/2432534405, CC0 (https://creativecommons.org/publicdomain/zero/1.0/). GBIF, Global Biodiversity Information Facility.
Museum collections can also support specimen-based research on infectious diseases197,198,199: by dissecting preserved animals, researchers can track long-term trends in their parasite communities and even test hypotheses about drivers such as climate change71,200,201. Preserved tissues can also be used to discover uncharacterized pathogens, including from species that are otherwise hard to sample (for example, rare and endangered species)202,203 (Fig. 4b).
Managing infectious disease risks
Public health and conservation can benefit from interventions that reduce pathogen transmission within wildlife populations or limit opportunities for cross-species transmission at the wildlife–livestock–human interface. Many public-health-oriented spillover prevention strategies include an educational component focused on living safely alongside wildlife204, especially when communities might otherwise rely on destructive interventions such as cullings that harm wildlife populations and can inadvertently increase spillover risk205,206,207. Other active strategies include wildlife and livestock vaccination or invasive species control208.
Ecologists have also called for ecosystem-based interventions that target the upstream drivers of disease emergence. Examples of these strategies include land-management decisions that preserve intact forests, supported by the preponderance of evidence that forest loss and fragmentation are often followed by an increase in zoonotic spillover risk. Other strategies that aim to reverse ecosystem changes, such as afforestation and reforestation106,209,210 or prescribed burns211, might achieve similar results, but there is only a small amount of primary research testing this assumption.
Among the range of interventions to restore ecosystems and reduce infectious disease risks, the most successful ‘win–win’ interventions for conservation and human health are those that are motivated by detailed knowledge of system dynamics, often from long-term case studies106; that involve locally led design and decision-making, aligned with pre-existing community priorities; and that are low-cost or, even better, aligned with existing economic incentives208,212,213. Without these factors, interventions are usually less successful and could have unintended negative consequences for human health, conservation, or both, as in the case of mosquito net fishing214, or unsuccessful restrictions on wildlife hunting and live-animal markets215,216.
Ecological strategies are only part of an effective strategy to combat emerging infectious diseases. Although popular narratives often frame spillover as the direct consequence of disordered relationships between humans and nature217,218, people are also regularly exposed to zoonotic and vector-borne diseases simply by living alongside other mammals, insects and biodiverse ecosystems107. The burden and consequences of those infections — namely, disease severity at the individual level and outbreak effects at the population level — are determined as much, if not more, by social, economic and political factors than by any facet of local ecology or global anthropogenic change219,220,221,222. Alleviating poverty and improving access to healthcare are recognized as prerequisites not only for improving population health, but also for sustainable development and use of natural resources170,223,224. Ecological solutions to manage disease risk will therefore be most effective in combination with ‘tried and true’ public health strategies — namely, health system strengthening225,226 and capacity building for outbreak preparedness and response227.
Unsolved problems for planetary governance
Despite the connections between biodiversity loss and emerging infectious diseases, global efforts on the two problems have historically run in isolation. Existing multilateral organizations (such as the World Health Organization (WHO)) and agreements (for instance, the International Health Regulations, in 2005) related to human health generally focus on outbreak preparedness and response, with less attention paid to prevention or the environmental determinants of health. Conversely, conservation-related organizations (for instance, the UN Environment Programme) and agreements (for instance, the Convention on Biological Diversity (CBD) in 1992) or the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) in 1973)) often address human health as a priority, but have usually been treated as ancillary to the global health security architecture.
The COVID-19 pandemic put zoonotic diseases and their drivers into the spotlight, with substantial associated changes in global governance. The United Nations’ new quadripartite partnership — a collaboration among WHO, the Food and Agriculture Organization, the World Organization for Animal Health and the UN Environment Programme — has established a One Health High-Level Expert Panel and produced a One Health Joint Plan of Action. This plan calls for improved scientific understanding of disease emergence; integration of human and animal disease surveillance systems, risk-assessment tools and triggers for action; national development of evidence-based legislation; and sustainable financing for One Health programmes. Meanwhile, the CBD secretariat has begun developing a global action plan on biodiversity and health, and the CITES secretariat has entered into a collaborative agreement with the World Organization for Animal Health, aimed at sharing technical expertise on wildlife trade and its risks to human health.
The upstream drivers of disease emergence pose a more complicated problem for global policy action. Biodiversity loss, deforestation, climate change, agricultural intensification and wildlife trade are all continuing to increase, and some experts have suggested that reversing these trends should be the highest priority for pandemic prevention efforts98,99,101. International environmental treaties — such as CBD, CITES, and the UN Framework Convention on Climate Change (in 1992), and related treaties (most notably, the Paris Agreement in 2015) — have all made substantial, but incomplete, progress in their respective areas. The growing cost of emerging infectious diseases has strengthened the case for action on environmental issues98,228, but these efforts still face an uphill battle against the overwhelming financial interests of extractive industries. Notably, consumer demand and corporate interests in the USA, Europe and China have often been a substantial barrier to the success of these treaties in the rest of the world229,230,231.
In any plausible scenario for global economic development and environmental change, it is unlikely that spillover rates will decrease within the next few decades: without significant improvements in both outbreak prevention and preparedness, epidemics and pandemics will continue to increase in frequency, effects and duration for at least a generation. Efforts to strengthen the global health security architecture are therefore a critical step to preparing for the health effects of anthropogenic environmental change. If adopted, the proposed WHO Pandemic Agreement is likely to acknowledge the One Health concept, and might establish obligations for the parties related to surveillance, workforce and policies aimed at zoonotic disease prevention. However, One Health programmes could cost an estimated US $22.0–31.2 billion per year98,99, presenting a barrier to more substantive action. Multilateral strategies to address issues such as wildlife trade are also unlikely to be well developed in the finalized text, but could still be achieved by an annex or protocol to the treaty232. Despite formidable challenges, the proposed Pandemic Agreement and the amendments to the revised International Health Regulations adopted in 2024 both represent major steps forward for public health emergency preparedness and response: in tandem, they could create ways to finance capacity building, increase compliance and cross-talk among national governments, and, most importantly, to ensure that vaccines and other countermeasures will be shared more equitably during future emergencies (Box 3).
Summary and future directions
Linkages between anthropogenic environmental change, biodiversity loss and disease emergence are widespread, and are often strong determinants of human and wildlife health outcomes. However, knowing that these general principles exist is not a substitute for system-specific knowledge. Scientists need to understand the ecological and evolutionary principles that apply on a case-by-case basis, and connect specific evidence to different public health objectives (such as pandemic prevention, reducing the burden of vector-borne disease or managing risks related to bat viruses). Data, evidence and interventions can exist in a dynamic feedback loop, supported by collaborations across biodiversity science, disease ecology, epidemiology and public health. In parallel, several important gaps in scientific research and synthesis need to be addressed.
First, disease ecologists need to develop a more taxonomically, geographically and ecologically diverse evidence base around biodiversity–disease-driver relationships. Wildlife disease research is heavily biased toward certain combinations of regions, hosts and pathogens: for example, rabies virus is disproportionately studied, particularly in vampire bats, relative to other bat viruses in the Americas233. These biases are both driven by data gaps, and perpetuate them (Box 2). Similarly, habitats with some degree of disturbance are better studied than pristine areas, which makes it harder to measure the effects of disturbance on disease dynamics, compared to balanced sampling designs. Most studies are also limited to single sampling events234; longitudinal study designs can document the effects of anthropogenic changes as they unfold, and demonstrate causality with a high degree of confidence, particularly if researchers also collect data that capture organismal responses beyond infection (for example, protein biomarkers of stress and immune function235). Most importantly, researchers should make a concerted effort to share raw, reusable, fine-scale spatial data on organism-level infection patterns236, particularly in the most data-deficient systems and parts of the world.
Second, more primary research is needed on the effects of biodiversity–disease-driver relationships on human health outcomes. The relationship between ecological change or biodiversity loss and disease outcomes is often found to be weaker in humans than in wildlife54,237, presumably because infectious-disease dynamics in humans are mediated by a number of other social and structural factors. An inclusive view of social–ecological systems, and additional comparative evidence across human disease systems, could challenge paradigms based primarily on wildlife studies and well known case studies107. Data availability remains the primary challenge: high-resolution data on infectious disease outbreaks are mostly unavailable to researchers, especially at a spatial scale that aligns with ecological processes such as forest clearing or livestock–wildlife contact. Efforts to compile data from the literature, collaboration with health ministries, and improved outbreak surveillance in remote communities would all help to close this gap.
Finally, research priorities in ecology and biodiversity science should be better aligned with public health priorities. Despite being motivated by pandemic prevention, many disease ecology studies focus on pathogen systems that pose a minimal pandemic threat (for example, Nipah virus or Lassa fever), whereas systems with higher risk (for example, influenza or primate viruses) are comparatively understudied. Similarly, more research is needed on the relationship between environmental change, biodiversity loss and neglected tropical diseases (including zoonoses such as leptospirosis, rabies and over a dozen helminthiases), which have a disproportionate burden on the world’s poorest populations238,239. Building relationships with national public health authorities and local communities to collaboratively identify local priorities for disease control and scientific research240 would help to decolonize the research process and spark scientific questions. Studies on the drivers of disease emergence also substantially outnumber studies that show that these trends can actually be reversed by proposed ecological solutions: more research is needed that measures the effects of interventions such as ecosystem restoration on human and wildlife disease outcomes209,210. These studies will help make the case to decision-makers that ecosystem-based strategies are scientifically sound and have a high return on investment.
Data availability
No original data were generated during the course of this study, but all data required to reproduce the figures here can be found at: https://github.com/viralemergence/pnpc.
Code availability
All code developed in this study can be found at: https://github.com/viralemergence/pnpc. The minimal statistical analysis and all other data and plotting related analysis were conducted in R version 4.3.2 (2023-10-31) (R Core Team 2023) using the rstanarm package (R Core Team 2023; Goodrich et al. 2024). All other packages we used are referenced in the repository’s README file.
References
Daszak, P., Cunningham, A. A. & Hyatt, A. D. Emerging infectious diseases of wildlife-threats to biodiversity and human health. Science 287, 443–449 (2000).
Wolfe, N. D., Dunavan, C. P. & Diamond, J. Origins of major human infectious diseases. Nature 447, 279–283 (2007).
Woolhouse, M., Scott, F., Hudson, Z., Howey, R. & Chase-Topping, M. Human viruses: discovery and emergence. Phil. Trans. R. Soc. Lond. B 367, 2864–2871 (2012).
Meadows, A. J., Stephenson, N., Madhav, N. K. & Oppenheim, B. Historical trends demonstrate a pattern of increasingly frequent and severe spillover events of high-consequence zoonotic viruses. BMJ Glob. Health 8, e012026 (2023).
Keesing, F. et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652 (2010).
Baker, R. E. et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 20, 193–205 (2022).
Allen, T. et al. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 8, 1124 (2017).
Lawler, O. K. et al. The COVID-19 pandemic is intricately linked to biodiversity loss and ecosystem health. Lancet Planet. Health 5, e840–e850 (2021).
Glidden, C. K. et al. Human-mediated impacts on biodiversity and the consequences for zoonotic disease spillover. Curr. Biol. 31, R1342–R1361 (2021).
Kock, R. A. & Caceres-Escobar, H. Situation Analysis on the Roles and Risks of Wildlife in the Emergence of Human Infectious Diseases (IUCN, 2022).
Stephens, P. R. et al. The macroecology of infectious diseases: a new perspective on global-scale drivers of pathogen distributions and impacts. Ecol. Lett. 19, 1159–1171 (2016).
Huang, S., Farrell, M. & Stephens, P. R. Infectious disease macroecology: parasite diversity and dynamics across the globe. Phil. Trans. R. Soc. Lond. B 376, 20200350 (2021).
Johnson, P. T. J., de Roode, J. C. & Fenton, A. Why infectious disease research needs community ecology. Science 349, 1259504 (2015).
Carlson, C. J. et al. A global parasite conservation plan. Biol. Conserv. 250, 108596 (2020).
Rubio‐Godoy, M. & de León, G. P. Equal rights for parasites: Windsor 1995, revisited after ecological parasitology has come of age. Biol. Conserv. https://doi.org/10.1016/j.biocon.2023.110174 (2023).
Weinstein, S. B. & Kuris, A. M. Independent origins of parasitism in Animalia. Biol. Lett. 12, 20160324 (2016).
Dolphin, K. & Quicke, D. L. J. Estimating the global species richness of an incompletely described taxon: an example using parasitoid wasps (Hymenoptera: Braconidae). Biol. J. Linn. Soc. Lond. 73, 279–286 (2008).
Carlson, C. J., Dallas, T. A., Alexander, L. W., Phelan, A. L. & Phillips, A. J. What would it take to describe the global diversity of parasites? Proc. R. Soc. B 287, 20201841 (2020).
Dobson, A. P., Lafferty, K. D., Kuris, A. M., Hechinger, R. F. & Jetz, W. Homage to Linnaeus: how many parasites? How many hosts? Proc. Natl Acad. Sci. USA 105, 11482–11489 (2008).
Larsen, B. B., Miller, E. C., Rhodes, M. K. & Wiens, J. J. Inordinate fondness multiplied and redistributed: the number of species on Earth and the new pie of life. Q. Rev. Biol. 92, 229–265 (2017).
Dallas, T. A. et al. Gauging support for macroecological patterns in helminth parasites. Glob. Ecol. Biogeogr. 27, 1437–1447 (2018).
Mollentze, N. & Streicker, D. G. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc. Natl Acad. Sci. USA. 117, 9423–9430 (2020).
Geoghegan, J. L., Duchêne, S. & Holmes, E. C. Comparative analysis estimates the relative frequencies of co-divergence and cross-species transmission within viral families. PLoS Pathog. 13, e1006215 (2017).
Hoberg, E. P. & Brooks, D. R. A macroevolutionary mosaic: episodic host‐switching, geographical colonization and diversification in complex host–parasite systems. J. Biogeogr. 35, 1533–1550 (2008).
Wallace, M. et al. Making sense of the virome in light of evolution and ecology. Preprint at EcoEvoRxiv https://doi.org/10.32942/X21K8T (2024).
Kamiya, T., O’Dwyer, K., Nakagawa, S. & Poulin, R. Host diversity drives parasite diversity: meta‐analytical insights into patterns and causal mechanisms. Ecography 37, 689–697 (2014).
Poulin, R. & Jorge, F. The geography of parasite discovery across taxa and over time. Parasitology 146, 168–175 (2019).
Jorge, F. & Poulin, R. Poor geographical match between the distributions of host diversity and parasite discovery effort. Proc. Biol. Sci. 285, 20180072 (2018).
Quicke, D. L. J. We know too little about parasitoid wasp distributions to draw any conclusions about latitudinal trends in species richness, body size and biology. PLoS ONE 7, e32101 (2012).
Rosenberg, R., Johansson, M. A., Powers, A. M. & Miller, B. R. Search strategy has influenced the discovery rate of human viruses. Proc. Natl. Acad. Sci. USA 110, 13961–13964 (2013).
Gibb, R. et al. Mammal virus diversity estimates are unstable due to accelerating discovery effort. Biol. Lett. 18, 20210427 (2022).
Lu, L. et al. Temporal dynamics, discovery, and emergence of human-transmissible RNA viruses. Mol. Biol. Evol. 41, msad272 (2024).
Jones, K. E. et al. Global trends in emerging infectious diseases. Nature 451, 990–993 (2008).
Carlson, C. J. et al. The Global Virome in One Network (VIRION): an atlas of vertebrate–virus associations. mBio 13, e0298521 (2022).
Carroll, D. et al. The global virome project. Science 359, 872–874 (2018).
Wille, M., Geoghegan, J. L. & Holmes, E. C. How accurately can we assess zoonotic risk? PLoS Biol. 19, e3001135 (2021).
Olival, K. J. et al. Host and viral traits predict zoonotic spillover from mammals. Nature 546, 646–650 (2017).
Albery, G. F. et al. Urban-adapted mammal species have more known pathogens. Nat. Ecol. Evol. 6, 794–801 (2022).
Zhou, P. et al. Contraction of the type I IFN locus and unusual constitutive expression of IFN-α in bats. Proc. Natl. Acad. Sci. USA 113, 2696–2701 (2016).
Irving, A. T., Ahn, M., Goh, G., Anderson, D. E. & Wang, L.-F. Lessons from the host defences of bats, a unique viral reservoir. Nature 589, 363–370 (2021).
Brook, C. E., Rozins, C., Guth, S. & Boots, M. Reservoir host immunology and life history shape virulence evolution in zoonotic viruses. PLoS Biol. 21, e3002268 (2023).
Guth, S. et al. Bats host the most virulent—but not the most dangerous—zoonotic viruses. Proc. Natl Acad. Sci. USA 119, e2113628119 (2022).
Guth, S., Visher, E., Boots, M. & Brook, C. E. Host phylogenetic distance drives trends in virus virulence and transmissibility across the animal–human interface. Phil. Trans. R. Soc. Lond. B 374, 20190296 (2019).
Walker, J. W., Han, B. A., Ott, I. M. & Drake, J. M. Transmissibility of emerging viral zoonoses. PLoS ONE 13, e0206926 (2018).
Cincotta, R. P., Wisnewski, J. & Engelman, R. Human population in the biodiversity hotspots. Nature 404, 990–992 (2000).
de Thoisy, B., Gräf, T., Mansur, D. S., Delfraro, A. & Dos Santos, C. N. D. The risk of virus emergence in South America: a subtle balance between increasingly favorable conditions and a protective environment. Annu. Rev. Virol. 11, 43–65 (2024).
Brierley, L., Vonhof, M. J., Olival, K. J., Daszak, P. & Jones, K. E. Quantifying global drivers of zoonotic bat viruses: a process-based perspective. Am. Nat. 187, E53–E64 (2016).
Han, B. A. et al. Undiscovered bat hosts of filoviruses. PLoS Negl. Trop. Dis. 10, e0004815 (2016).
Becker, D. J. et al. Optimising predictive models to prioritise viral discovery in zoonotic reservoirs. Lancet Microbe 3, e625–e637 (2022).
Schulz, J. E. et al. Serological evidence for Henipa-like and Filo-like viruses in Trinidad bats. J. Infect. Dis. 221, S375–S382 (2020).
Hernández, L. H. A. et al. First genomic evidence of a Henipa-like virus in Brazil. Viruses 14, 2167 (2022).
Halsey, S. Defuse the dilution effect debate. Nat. Ecol. Evol. 3, 145–146 (2019).
Halliday, F. W., Rohr, J. R. & Laine, A.-L. Biodiversity loss underlies the dilution effect of biodiversity. Ecol. Lett. 23, 1611–1622 (2020).
Mahon, M. B. et al. A meta-analysis on global change drivers and the risk of infectious disease. Nature 629, 830–836 (2024).
Huang, Z. Y. X., Yu, Y., VAN Langevelde, F. & DE Boer, W. F. Does the dilution effect generally occur in animal diseases? Parasitology 144, 823–826 (2017).
Gallagher, S. J. et al. Healthy but smaller herds: predators reduce pathogen transmission in an amphibian assemblage. J. Anim. Ecol. 88, 1613–1624 (2019).
Lopez, L. K. et al. A healthy but depleted herd: predators decrease prey disease and density. Ecology 104, e4063 (2023).
Levi, T., Kilpatrick, A. M., Mangel, M. & Wilmers, C. C. Deer, predators, and the emergence of Lyme disease. Proc. Natl. Acad. Sci. USA 109, 10942–10947 (2012).
Johnson, P. T. J., Preston, D. L., Hoverman, J. T. & Richgels, K. L. D. Biodiversity decreases disease through predictable changes in host community competence. Nature 494, 230–233 (2013).
Huang, Z. Y. X., VAN Langevelde, F., Estrada-Peña, A., Suzán, G. & DE Boer, W. F. The diversity–disease relationship: evidence for and criticisms of the dilution effect. Parasitology 143, 1075–1086 (2016).
Allan, B. F., Keesing, F. & Ostfeld, R. S. Effect of forest fragmentation on Lyme disease risk. Conserv. Biol. 17, 267–272 (2003).
Keesing, F. et al. Hosts as ecological traps for the vector of Lyme disease. Proc. Biol. Sci. 276, 3911–3919 (2009).
Gibb, R. et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature 584, 398–402 (2020).
Ecke, F. et al. Population fluctuations and synanthropy explain transmission risk in rodent-borne zoonoses. Nat. Commun. 13, 7532 (2022).
Previtali, M. A. et al. Relationship between pace of life and immune responses in wild rodents. Oikos 121, 1483–1492 (2012).
Albery, G. F. & Becker, D. J. Fast-lived hosts and zoonotic risk. Trends Parasitol. 37, 117–129 (2021).
Plourde, B. T. et al. Are disease reservoirs special? Taxonomic and life history characteristics. PLoS ONE 12, e0180716 (2017).
Farrell, M. J., Stephens, P. R., Berrang-Ford, L., Gittleman, J. L. & Davies, T. J. The path to host extinction can lead to loss of generalist parasites. J. Anim. Ecol. 84, 978–984 (2015).
Esser, H. J., Herre, E. A., Kays, R., Liefting, Y. & Jansen, P. A. Local host–tick coextinction in neotropical forest fragments. Int. J. Parasitol. 49, 225–233 (2019).
Sitko, J. & Heneberg, P. Systemic collapse of a host–parasite trematode network associated with wetland birds in Europe. Parasitol. Res. 119, 935–945 (2020).
Wood, C. L. et al. A reconstruction of parasite burden reveals one century of climate-associated parasite decline. Proc. Natl. Acad. Sci. USA 120, e2211903120 (2023).
Carlson, C. J. et al. Parasite biodiversity faces extinction and redistribution in a changing climate. Sci. Adv. 3, e1602422 (2017).
Neely, W. J. et al. Host-associated helminth diversity and microbiome composition contribute to anti-pathogen defences in tropical frogs impacted by forest fragmentation. R. Soc. Open Sci. 11, 240530 (2024).
Johnson, P. T. J., Preston, D. L., Hoverman, J. T. & LaFonte, B. E. Host and parasite diversity jointly control disease risk in complex communities. Proc. Natl. Acad. Sci. USA 110, 16916–16921 (2013).
Ezenwa, V. O. & Jolles, A. E. Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales. Science 347, 175–177 (2015).
Sweeny, A. R. et al. Experimental parasite community perturbation reveals associations between Sin Nombre virus and gastrointestinal nematodes in a rodent reservoir host. Biol. Lett. 16, 20200604 (2020).
Dougherty, E. R. et al. Paradigms for parasite conservation. Conserv. Biol. 30, 724–733 (2016).
Tompkins, D. M., Carver, S., Jones, M. E., Krkošek, M. & Skerratt, L. F. Emerging infectious diseases of wildlife: a critical perspective. Trends Parasitol. 31, 149–159 (2015).
Sanderson, C. E. & Alexander, K. A. Unchartered waters: climate change likely to intensify infectious disease outbreaks causing mass mortality events in marine mammals. Glob. Chang. Biol. 26, 4284–4301 (2020).
Kock, R. A. et al. Saigas on the brink: multidisciplinary analysis of the factors influencing mass mortality events. Sci. Adv. 4, eaao2314 (2018).
Fereidouni, S. et al. Mass die-off of saiga antelopes, Kazakhstan, 2015. Emerg. Infect. Dis. 25, 1169–1176 (2019).
Pruvot, M. et al. Outbreak of peste des petits ruminants among critically endangered Mongolian saiga and other wild ungulates, Mongolia, 2016–2017. Emerg. Infect. Dis. 26, 51–62 (2020).
De Castro, F. & Bolker, B. Mechanisms of disease‐induced extinction. Ecol. Lett. 8, 117–126 (2005).
Bonhoeffer, S., Lenski, R. E. & Ebert, D. The curse of the pharaoh: the evolution of virulence in pathogens with long living propagules. Proc. Biol. Sci. 263, 715–721 (1996).
Fisher, M. C. & Garner, T. W. J. Chytrid fungi and global amphibian declines. Nat. Rev. Microbiol. 18, 332–343 (2020).
Stegen, G. et al. Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature 544, 353–356 (2017).
Castro Monzon, F., Rödel, M.-O., Ruland, F., Parra-Olea, G. & Jeschke, J. M. Batrachochytrium salamandrivorans’ amphibian host species and invasion range. Ecohealth 19, 475–486 (2022).
Hoyt, J. R., Kilpatrick, A. M. & Langwig, K. E. Ecology and impacts of white-nose syndrome on bats. Nat. Rev. Microbiol. 19, 196–210 (2021).
Springborn, M. R., Weill, J. A., Lips, K. R., Ibáñez, R. & Ghosh, A. Amphibian collapses increased malaria incidence in Central America. Environ. Res. Lett. 17, 104012 (2022).
Frank, E. G. The economic impacts of ecosystem disruptions: costs from substituting biological pest control. Science 385, eadg0344 (2024).
Xie, R. et al. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature 622, 810–817 (2023).
Muñoz, G. et al. Stranding and mass mortality in Humboldt penguins (Spheniscus humboldti), associated to HPAIV H5N1 outbreak in Chile. Prev. Vet. Med. 227, 106206 (2024).
Nerpel, A. et al. SARS-ANI: a global open access dataset of reported SARS-CoV-2 events in animals. Sci. Data 9, 438 (2022).
Fagre, A. C. et al. Assessing the risk of human-to-wildlife pathogen transmission for conservation and public health. Ecol. Lett. 25, 1534–1549 (2022).
Weary, T. E. et al. Common cold viruses circulating in children threaten wild chimpanzees through asymptomatic adult carriers. Sci. Rep. 14, 10431 (2024).
Köndgen, S. et al. Pandemic human viruses cause decline of endangered great apes. Curr. Biol. 18, 260–264 (2008).
Hopkins, S. R. et al. Editorial essay: protected areas and One Health. Park. Recreat. 30, 6–13 (2024).
Bernstein, A. S. et al. The costs and benefits of primary prevention of zoonotic pandemics. Sci. Adv. 8, eabl4183 (2022).
Dobson, A. P. et al. Ecology and economics for pandemic prevention. Science 369, 379–381 (2020).
Vora, N. M. et al. Want to prevent pandemics? Stop spillovers. Nature 605, 419–422 (2022).
Vora, N. M. et al. Interventions to reduce risk for pathogen spillover and early disease spread to prevent outbreaks, epidemics, and pandemics. Emerg. Infect. Dis. 29, 1–9 (2023).
Winkler, K., Fuchs, R., Rounsevell, M. & Herold, M. Global land use changes are four times greater than previously estimated. Nat. Commun. 12, 2501 (2021).
IPBES. Summary for Policymakers of The Global Assessment Report on Biodiversity and Ecosystem Services (IPBES, 2019).
Plowright, R. K. et al. Land use-induced spillover: a call to action to safeguard environmental, animal, and human health. Lancet Planet. Health 5, e237–e245 (2021).
Becker, D. J. et al. Macroimmunology: the drivers and consequences of spatial patterns in wildlife immune defence. J. Anim. Ecol. 89, 972–995 (2020).
Eby, P. et al. Pathogen spillover driven by rapid changes in bat ecology. Nature 613, 340–344 (2023).
Gibb, R. et al. The anthropogenic fingerprint on emerging infectious diseases. Preprint at bioRxiv https://doi.org/10.1101/2024.05.22.24307684 (2024).
Eskew, E. A. et al. Reservoir displacement by an invasive rodent reduces Lassa virus zoonotic spillover risk. Nat. Commun. 15, 3589 (2024).
Skinner, E. B., Glidden, C. K., MacDonald, A. J. & Mordecai, E. A. Human footprint is associated with shifts in the assemblages of major vector-borne diseases. Nat. Sustain. 6, 652–661 (2023).
Morand, S., McIntyre, K. M. & Baylis, M. Domesticated animals and human infectious diseases of zoonotic origins: domestication time matters. Infect. Genet. Evol. 24, 76–81 (2014).
Greenspoon, L. et al. The global biomass of wild mammals. Proc. Natl. Acad. Sci. USA 120, e2204892120 (2023).
Johnson, C. K. et al. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci. Rep. 5, 14830 (2015).
Jones, B. A. et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 110, 8399–8404 (2013).
Hayek, M. N. The infectious disease trap of animal agriculture. Sci. Adv. 8, eadd6681 (2022).
Jori, F., Hernandez-Jover, M., Magouras, I., Dürr, S. & Brookes, V. J. Wildlife–livestock interactions in animal production systems: what are the biosecurity and health implications? Animal Front. 11, 8–19 (2021).
Mora, C. et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Change 12, 869–875 (2022).
Mordecai, E. A. et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 22, 1690–1708 (2019).
Carlson, C. J., Carleton, T. A., Odoulami, R. C. & Trisos, C. H. The historical fingerprint and future impact of climate change on childhood malaria in Africa. Preprint at bioRxiv https://doi.org/10.1101/2023.07.16.23292713 (2023).
Childs, M. L., Lyberger, K., Harris, M., Burke, M. & Mordecai, E. A. Climate warming is expanding dengue burden in the Americas and Asia. Preprint at medRxiv https://doi.org/10.1101/2024.01.08.24301015 (2024).
Carlson, C. J., Bannon, E., Mendenhall, E., Newfield, T. & Bansal, S. Rapid range shifts in African Anopheles mosquitoes over the last century. Biol. Lett. 19, 20220365 (2023).
Kraemer, M. U. G. et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 4, 854–863 (2019).
Eisen, R. J. & Eisen, L. Evaluation of the association between climate warming and the spread and proliferation of Ixodes scapularis in northern states in the eastern United States. Ticks Tick-borne Dis. 15, 102286 (2024).
McCoy, K. D. et al. Climate change in the Arctic: testing the poleward expansion of ticks and tick-borne diseases. Glob. Change Biol. 29, 1729–1740 (2023).
VanWormer, E. et al. Viral emergence in marine mammals in the North Pacific may be linked to Arctic sea ice reduction. Sci. Rep. 9, 15569 (2019).
Morales-Castilla, I. et al. Forecasting parasite sharing under climate change. Phil. Trans. R. Soc. Lond. B 376, 20200360 (2021).
Carlson, C. J. et al. Climate change increases cross-species viral transmission risk. Nature 607, 555–562 (2022).
Trisos, C. H., Merow, C. & Pigot, A. L. The projected timing of abrupt ecological disruption from climate change. Nature 580, 496–501 (2020).
Pigot, A. L., Merow, C., Wilson, A. & Trisos, C. H. Abrupt expansion of climate change risks for species globally. Nat. Ecol. Evol. 7, 1060–1071 (2023).
Worobey, M. et al. The Huanan seafood wholesale market in Wuhan was the early epicenter of the COVID-19 pandemic. Science 377, 951–959 (2022).
Guan, Y. et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302, 276–278 (2003).
Wang, M. et al. SARS-CoV infection in a restaurant from palm civet. Emerg. Infect. Dis. 11, 1860–1865 (2005).
Xiao, K. et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature 583, 286–289 (2020).
Hughes, L. J., Morton, O., Scheffers, B. R. & Edwards, D. P. The ecological drivers and consequences of wildlife trade. Biol. Rev. Camb. Phil. Soc. 98, 775–791 (2023).
Huong, N. Q. et al. Coronavirus testing indicates transmission risk increases along wildlife supply chains for human consumption in Viet Nam, 2013–2014. PLoS ONE 15, e0237129 (2020).
Anthony, S. J. et al. Global patterns in coronavirus diversity. Virus Evol. 3, vex012 (2017).
He, W.-T. et al. Virome characterization of game animals in China reveals a spectrum of emerging pathogens. Cell 185, 1117–1129.e8 (2022).
Brook, C. E. et al. Population trends for two Malagasy fruit bats. Biol. Conserv. 234, 165–171 (2019).
Ripple, W. J. et al. Bushmeat hunting and extinction risk to the world’s mammals. R. Soc. Open. Sci. 3, 160498 (2016).
Roy, H. E. et al. (eds) Thematic Assessment Report on Invasive Alien Species and Their Control (IPBES Secretariat, 2023).
Bryant, J. E., Holmes, E. C. & Barrett, A. D. T. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 3, e75 (2007).
Mnzava, A., Monroe, A. C. & Okumu, F. Anopheles stephensi in Africa requires a more integrated response. Malar. J. 21, 156 (2022).
Ryan, S. J. et al. Mapping current and future thermal limits to suitability for malaria transmission by the invasive mosquito Anopheles stephensi. Malar. J. 22, 104 (2023).
Rohr, J. R. et al. A planetary health innovation for disease, food and water challenges in Africa. Nature 619, 782–787 (2023).
Weets, C. M. & Katz, R. Global approaches to tackling antimicrobial resistance: a comprehensive analysis of water, sanitation and hygiene policies. BMJ Glob. Health 9, e013855 (2024).
Bürgmann, H. et al. Water and sanitation: an essential battlefront in the war on antimicrobial resistance. FEMS Microbiol. Ecol. 94, https://doi.org/10.1093/femsec/fiy101 (2018).
Orozovic, G., Orozovic, K., Lennerstrand, J. & Olsen, B. Detection of resistance mutations to antivirals oseltamivir and zanamivir in avian influenza A viruses isolated from wild birds. PLoS ONE 6, e16028 (2011).
Rosi, E. J., Fick, J. B. & Han, B. A. Are animal disease reservoirs at risk of human antiviral exposure? Environ. Sci. Technol. Lett. 10, 439–445 (2023).
Ostfeld, R. S. & Keesing, F. Biodiversity and disease risk: the case of Lyme disease. Conserv. Biol. 14, 722–728 (2000).
Couper, L. I., MacDonald, A. J. & Mordecai, E. A. Impact of prior and projected climate change on US Lyme disease incidence. Glob. Change Biol. 27, 738–754 (2021).
Plowright, R. K. et al. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. Biol. Sci. 278, 3703–3712 (2011).
Becker, D. J., Eby, P., Madden, W., Peel, A. J. & Plowright, R. K. Ecological conditions predict the intensity of Hendra virus excretion over space and time from bat reservoir hosts. Ecol. Lett. 26, 23–36 (2023).
Baranowski, K., Faust, C. L., Eby, P. & Bharti, N. Quantifying the impacts of Australian bushfires on native forests and gray-headed flying foxes. Glob. Ecol. Conserv. 27, e01566 (2021).
Mo, M. et al. Estimating flying-fox mortality associated with abandonments of pups and extreme heat events during the austral summer of 2019–20. Pac. Conserv. Biol. 28, 124–139 (2021).
Plaza, P. I., Gamarra-Toledo, V., Euguí, J. R. & Lambertucci, S. A. Recent changes in patterns of mammal infection with highly pathogenic avian influenza A(H5N1) virus worldwide. Emerg. Infect. Dis. 30, 444–452 (2024).
Guinat, C. et al. Bayesian phylodynamics reveals the transmission dynamics of avian influenza A(H7N9) virus at the human–live bird market interface in China. Proc. Natl Acad. Sci. USA 120, e2215610120 (2023).
Offeddu, V., Cowling, B. J. & Peiris, J. S. M. Interventions in live poultry markets for the control of avian influenza: a systematic review. One Health 2, 55–64 (2016).
Yin, S. et al. Habitat loss exacerbates pathogen spread: an agent-based model of avian influenza infection in migratory waterfowl. PLoS Comput. Biol. 18, e1009577 (2022).
Vandegrift, K. J., Sokolow, S. H., Daszak, P. & Kilpatrick, A. M. Ecology of avian influenza viruses in a changing world. Ann. NY Acad. Sci. 1195, 113–128 (2010).
Prosser, D. J., Teitelbaum, C. S., Yin, S., Hill, N. J. & Xiao, X. Climate change impacts on bird migration and highly pathogenic avian influenza. Nat. Microbiol. 8, 2223–2225 (2023).
Crits-Christoph, A. et al. Genetic tracing of market wildlife and viruses at the epicenter of the COVID-19 pandemic. Cell 187, 5468–5482.e11 (2024).
Pekar, J. E. et al. The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2. Science 377, 960–966 (2022).
Cui, J., Li, F. & Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 17, 181–192 (2019).
Lednicky, J. A. et al. Independent infections of porcine deltacoronavirus among Haitian children. Nature 600, 133–137 (2021).
Vlasova, A. N. et al. Novel canine coronavirus isolated from a hospitalized patient with pneumonia in East Malaysia. Clin. Infect. Dis. 74, 446–454 (2022).
Rulli, M. C., D’Odorico, P., Galli, N. & Hayman, D. T. S. Land-use change and the livestock revolution increase the risk of zoonotic coronavirus transmission from rhinolophid bats. Nat. Food 2, 409–416 (2021).
Beyer, R. M., Manica, A. & Mora, C. Shifts in global bat diversity suggest a possible role of climate change in the emergence of SARS-CoV-1 and SARS-CoV-2. Sci. Total. Environ. 767, 145413 (2021).
Warmuth, V. M., Metzler, D. & Zamora-Gutierrez, V. Human disturbance increases coronavirus prevalence in bats. Sci. Adv. 9, eadd0688 (2023).
Meyer, M. et al. Bat species assemblage predicts coronavirus prevalence. Nat. Commun. 15, 2887 (2024).
Busch, J. & Engelmann, J. Cost-effectiveness of reducing emissions from tropical deforestation, 2016–2050. Environ. Res. Lett. 13, 015001 (2017).
Jones, I. J. et al. Improving rural health care reduces illegal logging and conserves carbon in a tropical forest. Proc. Natl. Acad. Sci. USA 117, 28515–28524 (2020).
Rockström, J. et al. The planetary commons: a new paradigm for safeguarding Earth-regulating systems in the anthropocene. Proc. Natl. Acad. Sci. USA 121, e2301531121 (2024).
Kashwan, P., Biermann, F., Gupta, A. & Okereke, C. Planetary justice: prioritizing the poor in earth system governance. Earth Syst. Gov. 6, 100075 (2020).
Albery, G. F. et al. The science of the host–virus network. Nat. Microbiol. 6, 1483–1492 (2021).
Tseng, K. K. et al. Viral genomic features predict orthopoxvirus reservoir hosts. Preprint at bioRxiv https://doi.org/10.1101/2023.10.26.564211 (2023).
Fischhoff, I. R., Castellanos, A. A., Rodrigues, J. P. G. L. M., Varsani, A. & Han, B. A. Predicting the zoonotic capacity of mammals to transmit SARS-CoV-2. Proc. R. Soc. B 288, 20211651 (2021).
Betke, B. A., Gottdenker, N. L., Meyers, L. A. & Becker, D. J. Ecological and evolutionary characteristics of anthropogenic roosting ability in bats of the world. iScience 27, 110369 (2024).
Pruvot, M. et al. WildHealthNet: supporting the development of sustainable wildlife health surveillance networks in Southeast Asia. Sci. Total. Environ. 863, 160748 (2023).
Stevens, T. et al. A minimum data standard for wildlife disease studies. Preprint at EcoEvoRxiv https://doi.org/10.32942/x2tw4j (2024).
Richards, B. J., Miller, K. J. & White, C. L. WHISPers—Providing Situational Awareness of Wildlife Disease Threats to the Nation—A Fact Sheet for the Biosurveillance Community (USGS, 2022).
Geoghegan, J. L. et al. Virological sampling of inaccessible wildlife with drones. Viruses 10, 300 (2018).
Sievers, B. L. et al. Smart markets: harnessing the potential of new technologies for endemic and emerging infectious disease surveillance in traditional food markets. J. Virol. 98, e0168323 (2024).
Wägele, J. W. et al. Towards a multisensor station for automated biodiversity monitoring. Basic. Appl. Ecol. 59, 105–138 (2022).
Ärje, J. et al. Automatic image‐based identification and biomass estimation of invertebrates. Methods Ecol. Evol. 11, 922–931 (2020).
Baird, D. J. & Hajibabaei, M. Biomonitoring 2.0: a new paradigm in ecosystem assessment made possible by next-generation DNA sequencing. Mol. Ecol. 21, 2039–2044 (2012).
Heberling, J. M., Miller, J. T., Noesgaard, D., Weingart, S. B. & Schigel, D. Data integration enables global biodiversity synthesis. Proc. Natl Acad. Sci. USA 118, e2018093118 (2021).
Li, S. L. et al. Mapping environmental suitability of Haemagogus and Sabethes spp. mosquitoes to understand sylvatic transmission risk of yellow fever virus in Brazil. PLoS Negl. Trop. Dis. 16, e0010019 (2022).
Gippet, J. M. W., Bates, O. K., Moulin, J. & Bertelsmeier, C. The global risk of infectious disease emergence from giant land snail invasion and pet trade. Parasit. Vectors 16, 363 (2023).
Simons, D., Attfield, L. A., Jones, K. E., Watson-Jones, D. & Kock, R. Rodent trapping studies as an overlooked information source for understanding endemic and novel zoonotic spillover. PLoS Negl. Trop. Dis. 17, e0010772 (2023).
Redding, D. W., Gibb, R. & Jones, K. E. Ecological impacts of climate change will transform public health priorities for zoonotic and vector-borne disease. Preprint at bioRxiv https://doi.org/10.1101/2024.02.09.24302575 (2024).
García-Peña, G. E. et al. Land-use change and rodent-borne diseases: hazards on the shared socioeconomic pathways. Phil. Trans. R. Soc. Lond. B 376, 20200362 (2021).
Astorga, F. et al. Biodiversity data supports research on human infectious diseases: global trends, challenges, and opportunities. One Health 16, 100484 (2023).
Edmunds, S. C. et al. Publishing data to support the fight against human vector-borne diseases. Gigascience 11, giac114 (2022).
Shimabukuro, P. et al. Bridging biodiversity and health: the Global Biodiversity Information Facility’s initiative on open data on vectors of human diseases. GigaByte 2024, gigabyte117 (2024).
Južnič-Zonta, Ž. et al. Mosquito Alert: leveraging citizen science to create a GBIF mosquito occurrence dataset. GigaByte 2022, gigabyte54 (2022).
Carney, R. M. et al. Integrating global citizen science platforms to enable next-generation surveillance of invasive and vector mosquitoes. Insects 13, 675 (2022).
Rund, S. S. C., Moise, I. K., Beier, J. C. & Martinez, M. E. Rescuing troves of hidden ecological data to tackle emerging mosquito-borne diseases. J. Am. Mosq. Control. Assoc. 35, 75–83 (2019).
DiEuliis, D., Johnson, K. R., Morse, S. S. & Schindel, D. E. Specimen collections should have a much bigger role in infectious disease research and response. Proc. Natl. Acad. Sci. USA 113, 4–7 (2016).
Thompson, C. W. et al. Preserve a voucher specimen! The critical need for integrating natural history collections in infectious disease studies. mBio 12, e02698-20 (2021).
Colella, J. P. et al. Leveraging natural history biorepositories as a global, decentralized, pathogen surveillance network. PLoS Pathog. 17, e1009583 (2021).
Wood, C. L. & Vanhove, M. P. M. Is the world wormier than it used to be? We’ll never know without natural history collections. J. Anim. Ecol. 92, 250–262 (2023).
Bell, K. C., Carlson, C. J. & Phillips, A. J. Parasite collections: overlooked resources for integrative research and conservation. Trends Parasitol. 34, 637–639 (2018).
Hämmerle, M. et al. Screening great ape museum specimens for DNA viruses. Sci. Rep. 14, 29806 (2024).
Speer, K. A. et al. A comparative study of RNA yields from museum specimens, including an optimized protocol for extracting RNA from formalin-fixed specimens. Front. Ecol. Evol. 10, 953131 (2022).
Martinez, S. et al. Living safely with bats: lessons in developing and sharing a global One Health educational resource. Glob. Health Sci. Pract. 10, e2200106 (2022).
Amman, B. R. et al. Marburgvirus resurgence in Kitaka Mine bat population after extermination attempts, Uganda. Emerg. Infect. Dis. 20, 1761–1764 (2014).
Viana, M. et al. Effects of culling vampire bats on the spatial spread and spillover of rabies virus. Sci. Adv. 9, eadd7437 (2023).
Jenkins, H. E. et al. Effects of culling on spatial associations of Mycobacterium bovis infections in badgers and cattle. J. Appl. Ecol. 44, 897–908 (2007).
Hopkins, S. R. et al. Evidence gaps and diversity among potential win–win solutions for conservation and human infectious disease control. Lancet Planet. Health 6, e694–e705 (2022).
Reaser, J. K., Witt, A., Tabor, G. M., Hudson, P. J. & Plowright, R. K. Ecological countermeasures for preventing zoonotic disease outbreaks: when ecological restoration is a human health imperative. Restor. Ecol. 29, e13357 (2021).
Prist, P. R. et al. Promoting landscapes with a low zoonotic disease risk through forest restoration: the need for comprehensive guidelines. J. Appl. Ecol. 60, 1510–1521 (2023).
Gallagher, M. R. et al. Can restoration of fire-dependent ecosystems reduce ticks and tick-borne disease prevalence in the eastern United States? Ecol. Appl. 32, e2637 (2022).
Pattanayak, S. K., Kramer, R. A. & Vincent, J. R. Ecosystem change and human health: implementation economics and policy. Phil. Trans. R. Soc. Lond. B 372, 20160130 (2017).
Hopkins, S. R. et al. How to identify win–win interventions that benefit human health and conservation. Nat. Sustain. 4, 298–304 (2020).
Trisos, C. H. et al. Mosquito net fishing exemplifies conflict among Sustainable Development Goals. Nat. Sustain. 2, 5–7 (2019).
Bonwitt, J. et al. Unintended consequences of the ‘bushmeat ban’ in West Africa during the 2013–2016 Ebola virus disease epidemic. Soc. Sci. Med. 200, 166–173 (2018).
Li, Y. et al. Closure of live bird markets leads to the spread of H7N9 influenza in China. PLoS ONE 13, e0208884 (2018).
Quammen, D. Spillover: Animal Infections and the Next Human Pandemic (W. W. Norton, 2012).
Osofsky, S. A., Lieberman, S., Walzer, C., Lee, H. L. & Neme, L. A. An immediate way to lower pandemic risk: (not) seizing the low-hanging fruit (bat). Lancet Planet. Health 7, e518–e526 (2023).
Dzingirai, V. et al. Structural drivers of vulnerability to zoonotic disease in Africa. Phil. Trans. R. Soc. Lond. B 372, 20160169 (2017).
Magalhães, A. R. et al. Neglected tropical diseases risk correlates with poverty and early ecosystem destruction. Infect. Dis. Poverty 12, 32 (2023).
Sokolow, S. H. et al. Ecological and socioeconomic factors associated with the human burden of environmentally mediated pathogens: a global analysis. Lancet Planet. Health 6, e870–e879 (2022).
Farmer, P. Social inequalities and emerging infectious diseases. Emerg. Infect. Dis. 2, 259–269 (1996).
Fiorella, K. J. et al. Human health alters the sustainability of fishing practices in east Africa. Proc. Natl. Acad. Sci. USA 114, 4171–4176 (2017).
Bonds, M. H., Keenan, D. C., Rohani, P. & Sachs, J. D. Poverty trap formed by the ecology of infectious diseases. Proc. Biol. Sci. 277, 1185–1192 (2010).
Worsley-Tonks, K. E. L. et al. Strengthening global health security by improving disease surveillance in remote rural areas of low-income and middle-income countries. Lancet Glob. Health 10, e579–e584 (2022).
Wenham, C. et al. Global health security and universal health coverage: from a marriage of convenience to a strategic, effective partnership. BMJ Glob. Health 4, e001145 (2019).
Moore, M. et al. Core components of infectious disease outbreak response. SSM - Health Syst. 3, 100030 (2024).
Pike, J., Bogich, T., Elwood, S., Finnoff, D. C. & Daszak, P. Economic optimization of a global strategy to address the pandemic threat. Proc. Natl. Acad. Sci. USA 111, 18519–18523 (2014).
Galaz, V. et al. Financial influence on global risks of zoonotic emerging and re-emerging diseases: an integrative analysis. Lancet Planet. Health 7, e951–e962 (2023).
Hoffman, S. J. et al. International treaties have mostly failed to produce their intended effects. Proc. Natl. Acad. Sci. USA 119, e2122854119 (2022).
Liew, J. H. et al. International socioeconomic inequality drives trade patterns in the global wildlife market. Sci. Adv. 7, eabf7679 (2021).
Gallo-Cajiao, E. et al. Global governance for pandemic prevention and the wildlife trade. Lancet Planet. Health 7, e336–e345 (2023).
Heckley, A. M., Lock, L. R. & Becker, D. J. A meta‐analysis exploring associations between habitat degradation and Neotropical bat virus prevalence and seroprevalence. Ecography 2024, e07041 (2024).
Cohen, L. E., Fagre, A. C., Chen, B., Carlson, C. J. & Becker, D. J. Coronavirus sampling and surveillance in bats from 1996–2019: a systematic review and meta-analysis. Nat. Microbiol. 8, 1176–1186 (2023).
Vicente-Santos, A. et al. Serum proteomics reveals a tolerant immune phenotype across multiple pathogen taxa in wild vampire bats. Front. Immunol. 14, 1281732 (2023).
Albery, G. F., Sweeny, A. R., Becker, D. J. & Bansal, S. Fine‐scale spatial patterns of wildlife disease are common and understudied. Funct. Ecol. 36, 214–225 (2022).
Civitello, D. J. et al. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl. Acad. Sci. USA 112, 8667–8671 (2015).
Di Bari, C. et al. The global burden of neglected zoonotic diseases: current state of evidence. One Health 17, 100595 (2023).
Benavides, J. A. et al. Defining new pathways to manage the ongoing emergence of bat rabies in Latin America. Viruses 12, 1002 (2020).
Standley, C. J., Fogarty, A. S., Miller, L. N. & Sorrell, E. M. One Health systems assessments for sustainable capacity strengthening to control priority zoonotic diseases within and between countries. Risk Manag. Healthc. Policy 16, 2497–2504 (2023).
Ceballos, G. et al. Accelerated modern human-induced species losses: entering the sixth mass extinction. Sci. Adv. 1, e1400253 (2015).
Van Kerkhove, M. D., Ryan, M. J. & Ghebreyesus, T. A. Preparing for ‘Disease X’. Science 374, 377 (2021).
Morse, S. S. et al. Prediction and prevention of the next pandemic zoonosis. Lancet 380, 1956–1965 (2012).
Wolfe, N. D., Daszak, P., Kilpatrick, A. M. & Burke, D. S. Bushmeat hunting, deforestation, and prediction of zoonoses emergence. Emerg. Infect. Dis. 11, 1822–1827 (2005).
Wolfe, N. D. et al. Naturally acquired simian retrovirus infections in central African hunters. Lancet 363, 932–937 (2004).
Dai, L. et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell 182, 722–733.e11 (2020).
Nachbagauer, R. et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 27, 106–114 (2021).
Gibb, R. et al. Data proliferation, reconciliation, and synthesis in viral ecology. Bioscience 71, 1148–1156 (2021).
Stephens, P. R. et al. Global Mammal Parasite Database version 2.0. Ecology 98, 1476 (2017).
Bensch, S., Hellgren, O. & Pérez-Tris, J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 9, 1353–1358 (2009).
Luis, A. D. et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. Biol. Sci. 280, 20122753 (2013).
Heckley, A. M. & Becker, D. J. Tropical bat ectoparasitism in continuous versus fragmented forests: a gap analysis and preliminary meta-analysis. Ecol. Evol. 13, e9784 (2023).
Murray, M. H. et al. City sicker? A meta‐analysis of wildlife health and urbanization. Front. Ecol. Environ. 17, 575–583 (2019).
Fanelli, D. Negative results are disappearing from most disciplines and countries. Scientometrics 90, 891–904 (2012).
Winands-Kalkuhl, S. & Holm-Müller, K. Bilateral vs. multilateral? On the economics and politics of a global mechanism for genetic resource use. J. Nat. Resour. Policy Res. 7, 305–322 (2015).
Deplazes-Zemp, A. et al. The Nagoya protocol could backfire on the Global South. Nat. Ecol. Evol. 2, 917–919 (2018).
Lajaunie, C. & Morand, S. Nagoya protocol and infectious diseases: hindrance or opportunity? Front. Public. Health 8, 238 (2020).
Laird, S. et al. Rethink the expansion of access and benefit sharing. Science 367, 1200–1202 (2020).
Richter, C. D. European Patent Office grants controversial patent protecting virus: lessons from the Middle East respiratory syndrome coronavirus outbreak. Nat. Biotechnol. 39, 287–290 (2021).
Moore, S., Hill, E. M., Dyson, L., Tildesley, M. J. & Keeling, M. J. Retrospectively modeling the effects of increased global vaccine sharing on the COVID-19 pandemic. Nat. Med. 28, 2416–2423 (2022).
Watson, O. J. et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect. Dis. 22, 1293–1302 (2022).
Sedyaningsih, E. R., Isfandari, S., Soendoro, T. & Supari, S. F. Towards mutual trust, transparency and equity in virus sharing mechanism: the avian influenza case of Indonesia. Ann. Acad. Med. Singap. 37, 482–488 (2008).
Carlson, C. J. et al. Save lives in the next pandemic: ensure vaccine equity now. Nature 626, 952–953 (2024).
Carlson, C. et al. Engineering data equity: the LISTEN principles. Preprint at SSRN https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5022896 (2024).
Acknowledgements
The authors thank S. Hopkins, F. Keesing, R. Ostfeld, A. Phelan, Z. O’Donoghue, A. Sweeny, L. Damodaran and S. Seifert for helpful conversations and edits during the preparation of the manuscript. This work was supported by an National Science Foundation (NSF) Biology Integration Institute grant (DBI 2213854) to the Verena programme (viralemergence.org). C.J.C. was additionally supported by the NSF (SES 2314520).
Author information
Authors and Affiliations
Contributions
C.J.C., C.B.B., D.J.B., C.A.W. and T.P. researched data for the article. All authors contributed substantially to discussion of the content, wrote the article, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.J.C. has previously received funding support from the Coalition for Epidemic Preparedness Innovations; has been a consultant for the US Department of State on Global Health issues; and receives funding from the Carnegie Corporation of New York and the US National Science Foundation for research related to the Pandemic Agreement. D.J.B. is a member of the Lancet-PPATS Commission on Prevention of Viral Spillover. The authors declare no other competing interests.
Peer review
Peer review information
Nature Reviews Biodiversity thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
GBIF: www.gbif.org
One Health High-Level Expert Panel: https://www.who.int/groups/one-health-high-level-expert-panel
One Health Joint Plan of Action (2022-2026): https://www.who.int/publications/i/item/9789240059139
The Pathogen Harmonized Observatory (PHAROS) database: www.pharos.viralemergence.org
Verena: www.viralemergence.org
Supplementary information
Glossary
- Arbovirus
-
A shorthand for arthropod-borne (such as mosquito-borne or tick-borne) virus.
- Emerging infectious disease
-
An infectious disease that has recently undergone an expansion of host range, geographic range, impact or even just attention from scientific research or public health; this term is subjective, and is frequently used interchangeably with the terms zoonotic, vector-borne and environmentally transmitted diseases.
- Epidemic
-
An infectious disease outbreak in humans, with a significant duration, size or impact.
- Epizootic
-
An infectious disease outbreak in non-human animals, with a significant duration, size or impact.
- Host competence
-
The ability of a host to amplify and transmit infection to another susceptible host or vector.
- Macroparasite
-
Parasites that can be observed with the naked eye, such as ticks, fleas and some worms; the term is sometimes used interchangeably with the term parasites.
- Microparasite
-
Microscopic parasites such as bacteria, viruses and some worms (for example, schistosomes); the term is sometimes used interchangeably with the term pathogens.
- One Health
-
A principle that emphasizes the connections between human health, animal health and the environment, as well as the importance of solutions that benefit all three.
- Pandemic
-
A high-impact global outbreak of a human infectious disease. This term is subjective; some sources use the term to capture any global outbreak, and others limit their definition based on the degree of circulation or mortality (see Supplementary Note 3 for all criteria we use).
- Panzootic
-
A high-impact global outbreak of an infectious disease of animals.
- Parasite
-
An organism that exists in an adversarial symbiotic relationship with a host.
- Parasitoid
-
An organism that must kill a host to complete its life cycle (for example, braconid wasps).
- Pathogen
-
An infectious microorganism that causes disease in a host.
- Reservoir
-
Competent hosts that sustain pathogen transmission at the population or community level.
- Spillback
-
Human-to-animal pathogen transmission (also called reverse zoonosis).
- Spillover
-
Animal-to-human pathogen transmission.
- Synanthropic
-
Living alongside humans (for example, in cities or human-built structures).
- Vector-borne disease
-
A disease caused by a pathogen that is transmitted by an arthropod vector, such as a mosquito, tick or flea.
- Zoonotic disease
-
A human infectious disease caused by a pathogen of animal origin.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carlson, C.J., Brookson, C.B., Becker, D.J. et al. Pathogens and planetary change. Nat. Rev. Biodivers. 1, 32–49 (2025). https://doi.org/10.1038/s44358-024-00005-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44358-024-00005-w