Abstract
Osteosarcoma (OS) is a rapidly progressive primary malignant bone tumor that occurs in children and adolescents aged between 15 and 19 years and adults aged over 60 years. As alternative splicing (AS) changes caused by abnormal splicing factors contribute to tumor progression, gene expression and AS analyses were performed on 44 osteosarcoma patients to create a genome-wide co-expression network of RNA-binding proteins (RBPs), AS events, and AS genes. A gain- or loss-of-function osteosarcoma cell model was established, and an interactive network analysis and enrichment analysis were performed. Karyopherin Subunit Alpha 2 (KPNA2) negatively correlated with patient survival. KPNA2 transports splicing factor Y-box Binding Protein 1 (YBX1) into the nucleus and YBX1 accelerates the degradation of the ATP-dependent RNA helicase DDX3X (DDX3X) through the nonsense-mediated decay (NMD) pathway to promote intron retention of the DDX3X gene, thus reducing DDX3X protein levels. KPNA2/YBX1 axis regulates the stability of DDX3X mRNA and cell cycle progression. KPNA2/YBX1/DDX3X axis might be potential targets for inhibiting disease progression and improving OS patient survival. It integrates AS control of DDX3X into the progression of OS and represents a potential prognostic biomarker and therapeutic target for OS therapy.
Similar content being viewed by others
Introduction
Osteosarcoma (OS) is a common primary malignant bone tumor can occur both in children and adults [1, 2]. The combination of neoadjuvant chemotherapy and surgery in patients with nonmetastatic osteosarcoma has a greater than 60% long term overall survival [3]. Nevertheless, the survival rates of patients with OS with distant metastases or local recurrence have remained unchanged for the last three decades, with 5-year event-free survival rates of ~20% [4, 5]. Owing to the malignancy and unfavorable prognosis associated with osteosarcoma, there is an urgent need for further comprehensive research on its pathophysiology.
Alternative splicing (AS) of pre-mRNAs is an important post-transcriptional regulatory mechanism that regulates mRNA stability, diversity, and ultimately influences protein expression [6]. AS, serving as a bridge between mRNA and protein, also plays a crucial role in forming the basis of tumor heterogeneity [7]. During normal physiological processes, over 95% of mammalian genes undergo AS, which results in the generation of multiple mRNAs [8].
AS is mainly executed by the spliceosome mechanism, including skipped exon (SE), intron retention (IR), mutually exclusive exon (MXE), alternative 5’splice site (A5SS), and the alternative 3’splice site (A3SS) that is regulated by splicing factors [9]. The change in splicing factor expression results in alterations in AS patterns, thereby affecting the mechanisms driving tumor cell proliferation, invasion, treatment resistance, and metastasis, though little work has been done in OS [9]. A previous study reported that the splicing factor GEMIN5 significantly inhibits the migratory rate of mouse OS cells [10]. An effective biomarker of p53 attenuation in OS and a prognostic indicator for patients with OS is the ratio of the two transcripts of the HDMX gene, HDMX-S and HDMX-FL [11]. The existence of irregular splicing factors or AS patterns observed in OS indicates the potential contribution of AS to the development of OS progression [12, 13].
This study combined RNA sequencing analysis of OS tissues and bioinformatics analysis of differential AS events to establish a co-expression network of RNA-binding proteins (RBPs) with AS. Subsequently, the expression of KPNA2 in OS, its clinical relevance, biological function. and the mechanism of dependent regulation of splicing factor YBX1 were elucidated. With regard to AS control, multiple AS events influenced by YBX1 were identified. The dependence of the KPNA2/YBX1 axis on the IR of DDX3X was determined using RNA-seq and motif analysis. YBX1 promoted the proliferation, migration, invasion and cell cycle progression of OS cells by regulating DDX3X expression. In summary, the findings highlighted the role of the KPNA2/YBX1/DDX3X axis in OS progression.
Method
Differentially expressed gene (DEG) and alternative splicing analysis
The RNA sequencing data of 23 primary osteosarcomas, 8 metastatic osteosarcomas, and 3 normal tissues were derived from the NCBI Gene Expression Omnibus database coded as GSE87624. The data files were downloaded from the Sequence Read Archive (SRA). SRA Run files were converted to fastq format using the NCBI SRA Tool fastq-dump. Raw reads were trimmed using the FASTX-Toolkit (v.0.0.13, http://hannonlab.cshl.edu/fastx_toolkit/). Clean reads were evaluated using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastQC). Clean reads were aligned to the human genome using HISAT2 software [14]. Uniquely mapped reads were used to calculate the read number and fragments per kilobase of exon per million fragments mapped (FPKM) for each gene. The expression levels of genes were evaluated using FPKM. DEseq2 was used to analyze the differential genes, and the differential genes were screened according to the condition of fold change (FC) ≥ 2 or ≤0.5 and false discovery rate ≤0.05. The expression profiles of differentially expressed RBPs (DERBPs) were filtered out of all DEGs according to a catalog of 2141 RBPs retrieved from four previous reports [15,16,17,18]. Regulatory alternative splicing events (RASEs) were defined and quantified using the Splice sites Usage Variation Analysis (SUVA) pipeline [19]. Splicing in each group was analyzed. The read proportion of SUVA AS events (pSAR) was calculated for each AS event.
Co-expression analysis
Co-expression analysis was performed for all DERBP and RASE (pSAR ≥ 50%). The Pearson correlation coefficient between DERBP and RASE was calculated, and DERBP-RASE relationship pairs satisfying an absolute value of correlation coefficient ≥ 0.8 and a P ≤ 0.01 were screened.
Single cell sequencing data analysis
Single-cell RNA sequencing (scRNA-seq) data from primary and metastatic OS tissues of patients were obtained from the NCBI Gene Expression Omnibus database, coded as GSE152048 [20]. According to the Seurat package process, cells with fewer than 300 genes were filtered out first, and then the data were integrated. After removing red blood cells or cells in which mitochondrial genes were greated than 20%, the cells were clustered, and dimensionality was reduced. Cluster annotations were performed based on the marker and PanglaoDB (https://panglaodb.se/index.html) website information on OS cells, as previously described. Finally, KPNA2, Ki-67, OS stemness markers, and OS metastatic markers were displayed using the DimPlot function.
Cell lines and chemicals
Human OS cell lines (MNNG/HOS, HOS, MG-63, and U2OS) and a normal osteoblast cell line (hFOB1.19) were purchased from Wuhan Pricella Biotechnology Co., Ltd. (Wuhan, China). All cell lines were authenticated periodically by STR profiling. Mycoplasma contamination was tested using MycoBlue Mycoplasma Detector (#D101-01, Vazyme, China) every month. MNNG/HOS, HOS, and MG63 cells were cultured in MEM (Gibco) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin-streptomycin (Gibco). U2OS cells were cultured in McCoy’s 5A medium (Gibco) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Gibco). hFOB1.19 cells were maintained in DMEM/F12 (Gibco, USA) medium with 10% fetal bovine serum and 0.3 mg/ml G418 (Gibco, USA). All the cell lines were maintained at 37 °C with 5% CO2 in a humidified incubator. NMDI14 (#HY-111374) was purchased from Med Chem Express and used at 5 μM. Actinomycin D (#HY-17559, Med Chem Express) was used at 5 μg/ml.
Overexpression or knockdown of genes
Short hairpin RNA (shRNA) lentiviral particles (titer > 1 × 108 TU/mL) against KPNA2, YBX1, and DDX3X, or the negative control (Table S1), were purchased from Hanbio Tech (Shanghai, China) and cloned into the pHBLV-U6-MCS-CMV-ZsGreen-PGK-Puro lentiviral vector. Human KPNA2 (NM_001320611.1) and YBX1 (NM_004559.5) full-length overexpressing lentiviral particles (titer > 1 × 108 TU/mL) were purchased from Hanbio Tech (Shanghai, China) using the HBLV-ZsGreen-PURO vector. Puromycin (#HY-K1057, Med Chem Express) was used to screen and establish stable cell lines.
Colony formation assay
In 6-well plates, cells were seeded at a density of 800 cells/well. The medium was replaced weekly. Following a 2-week period of culturing, the samples were fixed with 4% paraformaldehyde solution for 10 min, followed by staining with 0.1% crystal violet solution for 15 min. The colonies with a cell count >20 were enumerated.
Migration and invasion assay
Following the manufacturer’s instructions, a volume of 200 μL of 1% serum medium containing 5 × 104 cells was introduced into the upper chamber of the Transwell chamber for migration determination or the Transwell chamber pre-coated with Matrigel (Corning Inc., USA) for invasion determination. Subsequently, 1000 μL of complete medium was added to the lower chamber and incubated for 24 h. Samples were treated with 4% paraformaldehyde solution for 15 min, followed by simultaneous staining with 0.1% crystal violet solution. The images were acquired using an Olympus microscope (Evident Corporation, Tokyo, Japan).
Polymerase chain reaction
Total RNA was extracted using the FastPure Cell/Tissue Total RNA Isolation Kit V2 according to the manufacturer’s instructions (#RC112-01, Vazyme Biotech Co., Ltd., Nanjing, China). Nuclear RNA was extracted using an NE-PER Kit (#78833, Thermo Fisher Scientific Inc.) according to the manufacturer’s instructions. RNA quality was evaluated by measuring the optical density (OD) at 260 nm/280 nm. A total of 3 μg RNA was used for the synthesis of cDNA using the HiScript III 1st Strand cDNA Synthesis Kit (#R312-01, Vazyme Biotech Co., Ltd., Nanjing, China). Quantitative reverse transcription PCR (qRT-PCR) was performed using a Bio-Rad CFX Connect instrument. The calculation of the fold change in gene expression was performed using the 2−ΔΔCt method and normalized to GAPDH. RT-PCR was performed as previously described to detect AS events. The PCR primer sequences for these genes are listed in Table S1.
Western blot
The ethics council at Tongji Medical College in China conducted an evaluation to assess the rationality of utilizing human tissues, specifically, eight pairs of matched OS and adjacent non-tumor tissues. Written informed consent was obtained from all patients or their guardians. Tumor tissues or cells were lysed using RIPA lysis buffer (NCM Biotech, China) supplemented with 1% phosphatase and protease inhibitors. Nuclear and cytoplasmic proteins were extracted using a Nuclear and Cytoplasmic Extraction Kit (#CW0199S; CoWin Biotech, China). The samples underwent sonication using an ultrasonicator (Branson, USA), followed by centrifugation at 12,000 rpm per minute for 15 min at a temperature of 4 °C. The resulting supernatant was collected. BCA protein assay kit (NCM Biotech, China) was used to evaluate the protein content of the lysate. Proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred onto a polyvinylidene fluoride (PVDF) membrane (#03010040001, Roche). The PVDF membrane was rinsed with 5% skim milk for a duration of 1 h at room temperature, followed by overnight incubation at a temperature of 4 °C. Subsequently, the membrane using 1X TBST and incubated with the secondary antibody for 1 h at ambient temperature. ECL kit (NCM Biotech, China) was used to develop the immunoreactive strips. The results of each blot were examined using the ImageJ software (National Institutes of Health, USA). The primary antibodies are as follows: KPNA2 Rabbit mAb (ab289858, 1:1000 dilution, abcam), Histone H3 (ab1791, 1:1000 dilution, abcam), YBX1 (A7704), MEK1 (A4868), DDX3X (A5637), CDK2 (A0294), AKT1 (A17909), and p21 (A1904) (1:1000 dilution, ABclonal Technology Co., Ltd.). GAPDH (A19056) antibody dilution ratio was 1:10000. The corresponding anti-mouse or rabbit horseradish peroxidase-conjugated secondary antibody (1:10000 dilution, ABclonal Technology Co., Ltd.) were used. Protein expression in each sample was standardized to that of GAPDH.
Co-immunoprecipitation
Cells were lysed in RIPA lysis buffer (Solarbio Life Sciences, China) supplemented with protease inhibitor cocktail (Solarbio Life Sciences, China) for 20 min at 4 °C. The lysates were clarified by centrifugation at 12,000 rpm for 15 min at 4 °C, and then incubated with KPNA2 antibody for overnight at 4 °C followed by mixing with protein A/G magnetic beads. The reaction and samples were then subjected to western blot analysis.
Immunofluorescence and immunohistochemistry
The cells were cultured on coverslips, fixed with 4% paraformaldehyde, blocked with 5% goat serum albumin for 1 h, and incubated to YBX1 (A7704, 1:200 dilution, ABclonal) antibodies at 4 °C overnight. After treatment with Cy3 Goat Anti-Rabbit IgG (AS007, 1:1000 dilution, ABclonal), the coverslips were stained with 4’,6-diamidino-2-phenylindole (DAPI, Sigma, D9542). Images were captured using an Olympus fluorescence microscope (Evidence Corporation) and A1Si laser confocal system (Nikon).
Different doses of xylene and alcohol were used to remove wax from paraffin sections. The cells were incubated with 3% H2O2 at room temperature for 30 min and washed with water. Then, the antigen was recovered using 2 min microwaved EDTA solution and incubated with goat serum and the primary antibody (KPNA2, ab289858, 1:200; ki67, Proteintech for IHC, 1:2000) overnight at 4 °C, washed with 1X PBS five times, and incubated with the DAKO secondary antibody at room temperature for 40 min. The sections were washed three timeswith1X PBS and incubated with DAB for 5 min. Subsequently, the sections were washed once more with 1X PBS and re-stained with hematoxylin for 10 min. Sections were imaged with an Olympus microscope.
Flow cytometry assay
The cell suspension for flow cytometry was prepared by trypsin digestion and resuspension in 1X PBS. The cells were then immersed in 1 ml of 75% cold ethanol for 1 h. The cells underwent three rounds of cold PBS washing and were thereafter immersed in 100 μL 1X PBS supplemented with 0.5 mL of PI/RNase Staining Buffer (#F10797, Thermos Fisher Scientific Inc.) to prevent exposure to light at room temperature for 15 min. A flow test was conducted during a 24-h timeframe following 50 μg/ml PI staining. Cell cycle distribution was assessed by flow cytometry (BD Biosciences). Data analysis was conducted using FlowJo software.
RNA immunoprecipitation
The RNA immunoprecipitation (RIP) experiments were performed according to the instructions of the RIP kit (Bersin Bio, Bes5101). Briefly, OS cells cultured on a 10-cm plate underwent three rounds of cold PBS washing, following which the cell lysate was acquired using RIPA. 5 μg of YBX1 antibody was added and mixed with Protein A/G magnetic beads in immunoprecipitation buffer for 2 h, followed by overnight rotation at 4 °C. The non-specific binding was subsequently eliminated three times using a 400 μL elution buffer. RNA was extracted using TRIzol, and the number of introns binding to DDX3X was determined by qRT-PCR and compared with the input group. IgG was used as a negative control. The primers used are listed in Table S1.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP)-qPCR assay was performed according to the manufacturer’s protocol (BersinBio, Bes5001). Briefly, 1 × 107 OS cells were collected and PBS containing 1% formaldehyde was added for cross-linking. After eliminating the supernatant from the samples, the cell debris mixture was sonicated to cleave the DNA fragments to 300–1000 bp. The corresponding antibodies and magnetic beads were then used for immunoprecipitation. After washing three times, samples were placed at 65 °C for 6 h to uncrosslink. Finally, the DNA was precipitated, and qPCR experiment was performed. Promoter primers used for DDX3X are listed in Supplementary Table S1.
RNA sequencing
Total RNA was extracted from U2OS and MNNG/HOS cells using Trizol. The RNA was further purified using two phenol-chloroform treatments, and treated with RQ1 DNase (#M6101, Promega, Madison, WI, USA) to remove the DNA. The quality and quantity of purified RNA were determined by measuring the absorbance at 260 and 280 nm using a NanoDrop spectrophotometer (Thermo Fisher Scientific Inc.). RNA integrity was verified by 1.0% agarose gel electrophoresis. RNA-seq assays were performed by Wuhan Ruixing Biotechnology Co., Ltd. (http://www.rxbio.cc). For each sample, 1 μg of total RNA was used for directional RNA-seq library preparation by VAHTS Universal V8 RNA-seq Library Prep Kit for Illumina (#NR605, Vazyme, China) for RNA-seq library preparation. The mRNAs were captured using VAHTS mRNA capture beads (#N401; Vazyme, China). Fragmented RNAs was converted into double-stranded cDNA. Following end repair and A tailing, the DNAs were ligated to VAHTS RNA Multiplex Oligos Set 1 for Illumina (#N323, Vazyme, China), the ligated products were amplified, purified, quantified, and stored at −80 °C before sequencing. The strand marked with dUTP (the 2nd cDNA strand) was not amplified, which allowed strand-specific sequencing. For high-throughput sequencing, libraries were prepared according to the manufacturer’s instructions and applied to the Illumina Novaseq 6000 system for 150 nt paired-end sequencing. To ensure high-quality AS analysis, we performed RNA-seq with higher sequencing depth to reliably detect splicing events and support subsequent analyses. Raw data can be accessed via the NCBI Gene Expression Omnibus database (GSE263410).
Tumor xenografts
Female BALB/c nude mice aged 4–5 weeks were randomly assigned to treatment groups. No blinding was performed, but standardized protocols were followed to minimize bias. The Experimental Animal Ethics Committee of Huazhong University of Science and Technology approved this procedure. MNNG/HOS cell were subcutaneously injected into the hypodermis of 6-week-old female nude mice (5 per in each group). The size and volume of the tumors were measured with calipers every 2 days. When the tumor volume reached 1000 mm3, the mice were euthanized, and the animals were treated strictly in accordance with the American Veterinary Medical Association guidelines. After collecting the tumor, the growth curve of the tumor was drawn, weighed, and immunohistochemical analysis was performed.
Statistical analysis
Each experiment was conducted with a minimum of three independent replicates (n). All data consist of the mean and standard deviation (SD) of a minimum of three distinct experiments. Statistical analyses were conducted using the GraphPad Prism 8 software (GradPad Software, Inc.). ImageJ software (National Institutes of Health, USA) was used to measure the intensity of immunofluorescence and immunohistochemistry. The survival differences were evaluated using the log-rank test. Differences in cancer cell or tissue characteristics were statistically evaluated using Student’s t-test, Mann–Whitney U test, and analysis of variance (ANOVA) [21]. The study employed statistical analysis techniques, namely one- or two-sided paired Student’s t-test for single comparisons and one- or two-way ANOVA with a post-hoc test for multiple comparisons. Statistical significance was set at P < 0.05 [21].
Results
Identification of abnormal alternative splicing patterns in osteosarcoma tissue
To investigate alternative splicing events (RASEs) in OS, the GSE87624 mRNA-seq was evaluated [22]. This dataset contained 44 patients with osteosarcoma (24 primary osteosarcoma, 9 metastatic OS, and 11 unknown) and 3 normal bone tissues. After data quality control, 23 primary osteosarcoma, eight metastatic osteosarcoma, and three normal bone tissue samples were included in the analysis (two outlier samples were deleted, namely, primary_7: SRR4343927-32 and metastatic_6: SRR4344043-44). Alternative 5’ splice site (alt5p) events were the most common RASEs among the three groups, followed by the alternative 3’ splice site (alt3p), intron retention events (ir), and multiple event types (olp, including alt5p, alt3p, and ir), and finally the event containing alt5p and alt3p (Fig. 1A). The total number of AS events detected was shown in Fig. S1A, and KEGG enrichment result of RASEs was shown in Fig. S1B. The proportion of SUVA AS events (pSAR) was calculated based on the two transcripts involved in the splicing event to all transcripts using the read ratio. The higher the pSAR, the more dominant the event in gene transcription. After counting the median pSAR values of the differential AS events, the pSAR values of most differential AS events was found to be greater than 50% (Fig. 1B). PCA was performed on the splicing ratio values of events with a pSAR ≥ 50%, and from the normal, primary osteosarcoma, and metastatic osteosarcoma groups (Fig. 1C). The heat map shows the ratio values of AS events with pSAR ≥ 50% (Fig. 1D). GO functional enrichment analysis was performed on genes associated with AS with pSAR ≥ 50%, and biological processes such as mitosis, G2/M phase transition of the cell cycle, regulation of catalytic activity, and cell division were enriched (Fig. 1E). The results of KEGG pathway enrichment analysis were shown in Fig. 1B. The above-mentioned AS with pSAR ≥ 50% were analyzed overlapping in the primary vs. normal group and metastatic vs normal group, and it was found that 493 RASEs play a dominant role specifically in primary vs normal, and 174 RASEs played a dominant role in both primary vs. normal and metastatic vs. normal comparisons (Fig. 1F). Gene expression in OS was shown in Fragments Per Kilobase of transcript per Million mapped reads (FPKM) (Table S2). All identified RASEs from three types of OS samples were shown in Table S3. The top ten biological process GO enrichment analyses of overlapping regulated AS genes (RASGs) and primary vs. normal RASGs were shown (Fig. S2A). Representative RASEs (MACF1, DNM2, SEMA3F, HNRNPL, and LEF1) in the primary vs. normal group and RASEs (TUSC3, TERF1, and FES) in the overlap group were shown (Fig. S2B, C). KEGG pathway enrichment analysis was performed for overlapping RASGs, primary vs. normal RASGs (Fig. S3A, B). These results suggest the presence of abnormal AS patterns in osteosarcoma.
A An analysis of the statistical characteristics of regulated AS events (RASEs) in osteosarcoma (P ≤ 0.05). B The statistical analysis determined the median proportion of SUVA AS ratio (pSAR) values associated with differential splicing events. C Principal component analysis (PCA) analysis established on RASEs of pSAR ≥ 50%. D Heatmap presented the ratio value of RASEs in three types of tissues (pSAR ≥ 50%). E The top 10 biological process GO enrichment analyses of regulated AS genes (pSAR ≥ 50%). The color scale displayed the row-scaled significance. F Venn diagram showed the overlap of RASEs between the primary vs. normal group and the metastatic vs. normal group.
Co-expression network between differential RBPs and regulated AS genes in osteosarcoma
RNA-binding proteins (RBPs) refer to a class of proteins associated with RNA regulation and metabolism. Therefore, the potential relationship between DERBPs and AS in the unique RASE pattern of OS and constructed a co-expression network was examined. The overlap of the RBPs with DEGs and identified 71 DERBPs is shown in Fig. 2A and Table S4. The heat map shows the FPKM expression levels of the 71 DERBPs (Fig. 2B). GO functional analysis of DERBPs indicated that the cell cycle, cell division, and signal transduction were enriched (Fig. 2C). Next, the correlation between the expression of 71 DERBPs and the ratio value of RASE with pSAR ≥ 50% was analyzed to construct a co-expression network. It was found that 25 DERBPs co-expressed a total of 106 RASE (|correlation coefficient| ≥ 0.8, RASE ≤ 0.01) (Fig. 2D and Table S5). Representative DERBPs in the co-expression network (KPNA2, LEPR, ADD3, F11R, EXOSC7, and STRBP) FPKM values were shown (Fig. 2E and S4A). Representative splicing ratios in the co-expression network of clualt5p174302 MCM7, clualt5p196175 GSN, cluir62713 PLK1, and cluir60246 POLG were shown (Fig. 2F). The overall survival curve of DERBPs in TCGA-SARC database was plotted using a KM plotter (Fig. S4B). The top 10 most enriched KEGG pathways are illustrated for DERBPs (Fig. S4C). The data reveal a significant relationship between RBPs and AS in OS, suggesting a mechanism for controlling AS events and facilitating the development of OS.
A Venn diagram showed the overlap of genes between human RBP genes and differential expression genes (DEGs) associated with osteosarcoma. B Hierarchical clustering heatmap showed the FPKM of the differentially expressed RBPs (DERBPs) in three types of tissues. C The top 10 enriched GO terms (biological process) were illustrated for DERBPs co-expression RASEs (pSAR ≥ 50%) genes among different groups. D Co-expression network showed the important pathways of RASEs regulated by DERBPs. E Box plot showed the FPKM value of three representative DERBPs (LEPR, ADD3, and KPNA2). F Boxplot showed the splicing ratios of clualt5p174302 MCM7, clualt5p196175 GSN, cluir62713 PLK1, and cluir60246 POLG. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
KPNA2 is upregulated in osteosarcoma and associated with poor prognosis
To identify the main RBP that regulates AS events in OS, DERBPs were detected in the co-expression network using qRT-PCR in osteosarcoma cell lines (U2OS and MNNG/HOS) and found that the expression of KPNA2 was the highest (Fig. 3A). Previous studies have also found that paraffin-embedded surgical specimens show that there is high KPNA2 expression in OS. The OS cohort GSE21257 showed that patients with high KPNA2 expression had a decreased overall survival (log-rank P = 0.04) (Fig. 3B). Similarly, the TCGA-SARC cohort suggested that high KPNA2 expression predicted poor overall suvial (log-rank P = 0.059) (Fig. 3C). Compared to the normal human osteoblast cell line hFOB1.19, the protein levels of KPNA2 in many OS cell lines (U2OS, MG63, HOS, and MNNG/HOS) were higher (Fig. 3D). Similarly, western blotting showed that the protein expression of KPNA2 in OS tissues was higher than that in adjacent normal tissues (P = 0.001) (Fig. 3E, F). mRNA levels demonstrated the same trend (Fig. 3G). To further explore whether KPNA2 was specifically highly expressed in OS subsets, seven single-cell datasets of primary osteosarcoma were analyzed (Fig. 3H). The expression of KPNA2 was mainly concentrated in OS cells (Fig. 3I). The distribution of cell subsets with high KPNA2 expression was similar to that of the cell proliferation marker Ki-67, suggesting that KPNA2 is mainly concentrated in OS cells and may regulate cell proliferation (Fig. 3J). In the scRNA-seq data from two patients with lung metastatic OS, KPNA2 was predominantly enriched in the OS cell subpopulation and exhibited a distribution pattern similar to Ki-67 (Fig. S5A). Furthermore, the relationship between KPNA2 and OS stemness markers as well as metastasis-related markers was also revealed. In metastatic OS, KPNA2 showed partial co-expression with PTN, CD99, PLK1, PBK, and PRC1 (Fig. S5B, C). In primary OS, KPNA2 exhibited partial overlap with the distribution of marker genes such as CD24, PTN, EZR, CD99, PLK1, PBK, and PRC1 (Fig. S5D, E). KPNA2 immunohistochemical (IHC) staining was performed on three paired OS patients (Fig. 3K, L). This demonstrates that the expression of KPNA2 is up-regulated in osteosarcoma and may be developed as a biomarker to predict the prognosis of patients with osteosarcoma.
A The qRT-PCR results of the co-expression network of DERBPs in osteosarcoma cell lines were shown in the bar plot. B, C Kaplan-Meier survival analysis of overall survival in patients, separately in two independent cohorts (the GSE21257 osteosarcoma cohort and the TCGA-SARC cohort). D Western blot assay showed KPNA2 levels in osteoblast cell line (hFOB1.19) and osteosarcoma cell lines (U2OS, MG63, HOS, MNNG/HOS). E, F Western blotting analysis detected the protein levels of KPNA2 from osteosarcoma specimens and corresponding adjacent non-tumor tissue (NAT) (n = 8). G The mRNA expression levels of KPNA2 in sarcoma. H The uniform manifold approximation and projection (UMAP) plot of the 15 identified main cell types in 7 primary osteosarcoma lesions. I, J Feature plots of the KPNA2 and Ki-67 genes in the scRNA dataset. The color legend showed the expression levels of the genes. K, L IHC analysis was used to assess the protein levels of KPNA2 in parried osteosarcoma tissue. *P ≤ 0.05, ****P ≤ 0.0001. Log-rank test was used for survival comparison in (B, C). Two-tailed paired t tests for (F, G, L). Data were presented as mean ± SD (error bars) and representative of at least three independent experiments in (A, D–G, K–L).
KPNA2 promotes osteosarcoma progression in vitro and in vivo through splicing factor YBX1
KPNA2 is a member of the alpha protein family of nuclear transporters, acts as a junction protein, and plays a crucial role in facilitating the transport of proteins from the cytoplasm to the nucleus [23,24,25]. Enrichment of the KPNA2-binding protein profile suggested that KPNA2 was associated with the spliceosome, cell cycle, and mRNA splicing pathways (Fig. S6A, B). Subsequently, 17 RBPs overlapped with primary vs. normal group DEGs, human RBPs, and KPNA2 bound proteins, and according to the expression level, YBX1 ranked first (Fig. 4A and Table S6). To explore the influence of KPNA2 and YBX1 in OS, a lentivirus was constructed to knockdown the genes and verified the knockdown efficiency (Fig. 4B). Binding between endogenous KPNA2 and YBX1 was confirmed in U2OS, MG63, and MNNG/HOS cells (Fig. 4C). Subsequent colony formation assays showed that KPNA2 or YBX1 inhibition significantly decreased the proliferation of OS cells in vitro, whereas YBX1 overexpression partially reversed the decrease in cell proliferation induced by KPNA2 inhibition (Fig. 4D–F). Migration and invasion assays demonstrated that KPNA2 inhibition or YBX1 inhibition significantly reduced the migration and invasion abilities of OS cells in vitro, whereas the overexpression of YBX1 partially reversed the decrease in tumor cell migration and invasion caused by KPNA2 inhibition (Fig. 4G, H). The results of the in vivo modeling of tumor growth curve and tumor mass in vitro indicated that knockdown of KPNA2 or YBX1 significantly inhibited tumor growth, but overexpression of YBX1 partially reversed this effect (Fig. 4I–L). Similarly, knockdown of KPNA2 or YBX1 reduced the number of Ki-67-positive cells, but overexpression of YBX1 partially reversed this effect (Fig. 4K–M). In summary, KPNA2 promotes osteosarcoma tumor cell progression in vivo and in vitro through the splicing factor YBX1.
A Venn diagram showed the overlap of genes between DEGs in the primary vs. normal group, human RBPs, and KPNA2 binding proteins ranked by expression level in osteosarcoma tissue. B The U2OS and MNNG/HOS osteosarcoma cells underwent infection with the specified shRNAs. Cells were collected for Western blotting analysis after puromycin screening. C Analysis of white blood cells (WCL) or immunoprecipitation (IP) from U2OS, MG63, and MNNG/HOS cells using Western blotting. D–F Colony formation assays detected cell proliferation of U2OS and MNNG/HOS cells (n = 4). G, H Transwell assays demonstrated the migration and invasion capabilities of U2OS and MNNG/HOS cells (n = 4). The scale bar was 100 µm. I The tumor growth curve (n = 5). J MNNG/HOS cells were subcutaneously into the nude mice for xenografts assay (n = 5). K Ki-67 staining of the tumor. The scale bar was 100 µm. L Excised tumor mass as indicated (n = 5). M The bar chart showed the quantitative results of Ki-67. Data were shown as the mean ± standard deviation of at least three independent experiments. ns no statistical significance; *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. One-way analysis of variance (ANOVA) was used to compare the difference in (E, F, H, I, L, M). Data were presented as mean ± SD (error bars) and representative of at least three independent experiments in (B–M).
KPNA2 facilitates the nuclear import of YBX1
AS is a cellular process that normally occurs in the nucleus. To investigate the potential impact of KPNA2 on the subcellular distribution of the splicing factor YBX1, the subcellular ___location of YBX1 in cells with either KPNA2 knockdown or overexpression was determined. Immunofluorescence (IF) analysis revealed that depletion of KPNA2 led to the retention of YBX1 in the cytoplasm (Fig. 5A, B). In contrast, KPNA2 overexpression significantly increased the proportion of nuclear YBX1 (Fig. 5A, B). Western blot results of nuclear/cytoplasmic separation also confirmed this trend (Fig. 5C, D). Western blot analysis showed that the protein level of YBX1 decreased in cells with KPNA2 depleted by lentiviral-mediated knockdown., while the protein level of YBX1 increased in cells when KPNA2 was overexpressed (Fig. 5E). Furthermore, confocal microscopy observations further confirmed the crucial role of KPNA2 in facilitating YBX1 nuclear import. KPNA2 knockdown resulted in significant cytoplasmic retention of YBX1, whereas KPNA2 overexpression markedly promoted its nuclear translocation (Fig. 5F, G). These results suggested that KPNA2 promotes the entry of YBX1 into the nucleus and affects the expression of YBX1.
A, B The subcellular distribution of YBX1 in the control transfected cells, KPNA2 knockdown, and KPNA2 overexpression by nuclear/cytoplasmic fractionation via immunofluorescence. The scale bar was 100 µm. C, D Western blotting showed YBX1 levels in different cellular components. The cytoplasmic control employed was MEK1/2, while the nuclear control utilized was Histone H3. E Expressions of YBX1 in the U2OS cells knockdown or overexpressed of KPNA2 were assessed by Western blotting. F, G Confocal microscopy was used to observe the distribution of YBX1 in the cytoplasm and nucleus across the control group, KPNA2 knockdown group, and KPNA2 overexpression group. The scale bar was 10 µm. ns no statistical significance; *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Two-tailed paired t tests for (B, G). Data were presented as mean ± SD (error bars) and representative of at least three independent experiments in (A–G).
Landscape of the YBX1-affected AS and gene expression in osteosarcoma cells
To screen for AS events involved in YBX1 regulation in OS, high-throughput RNA sequencing (RNA-Seq) was performed on the WT and KD cell lines of MNNG/HOS and U2OS (Fig. S7A, B). After YBX1 knockdown, significant DEGs were observed in the two OS cell lines (Fig. S7C and Tables S9, S10). U2OS exhibited 188 upregulated genes and 132 downregulated genes (Fig. S7D). Similarly, MNNG/HOS had 1410 genes that were upregulated and 893 downregulated genes (Fig. S7E). Additionally, several novel AS events were identified (Fig. S7F). Based on the fact that each sample was generated from high-quality RNA-seq reads, there were 2566 and 3204 YBX1-affected AS events identified in U2OS and MNNG/HOS cells, respectively, most of which belonged to the IR category (Fig. 6A). The analysis of AS events revealed no statistically significant differences between the upregulated (activation) and downregulated (repression) events (Fig. 6B). YBX1 regulated various AS events, and the results indicated that U2OS and MNNG/HOS cells exhibited multiple common events, with the majority falling into the IR category (Fig. 6C and Table S7). Furthermore, enrichment analysis after YBX1 knockdown showed that RNA splicing, cell cycle, cell growth, and nonsense-mediated decay (NMD) were significantly enriched (Fig. 6D and Table S8). U2OS and MNNG/HOS cell lines were analyzed by gene expression analysis after YBX1 knockdown (Tables S9 and S10). RNA-seq analysis also showed that, compared to the WT group, certain cell cycle-related genes were downregulated, and some NMD-and RNA degradation-related genes were upregulated in U2OS and MNNG/HOS cells with YBX1 depletion, which reaffirmed the role of YBX1 in OS progression (Fig. 6E). The protein-protein interaction (PPI) network map, generated by identifying the DEGs overlapped in U2OS and MNNG/HOS cell lines, revealed multiple sets of genes associated with the cell cycle, NMD, and splicing factors (Fig. S7G). To validate the precision of the AS RNA-seq results, nine YBX1-affected AS events overlapped in U2OS and MNNG/HOS cells was analyzed, supporting the involvement of YBX1 in IR and ES events (Fig. 6F).
A YBX1-affected AS events in U2OS (left) and MNNG/HOS (right) cell lines. The AS events were categorized into five distinct groups, namely exon skipping (ES), intron retention (IR), alternative 5′ splice site (A5SS), alternative 3′ splice site (A3SS), and mutually exclusive exon (MXE). B In each category, the relative proportion of AS events that are positively (activated) or negatively (repressed) by YBX1 was investigated. C Venn diagram showed overlapping AS events in each category of the activation/repression groups between U2OS and MNNG/HOS cell lines. D GO enrichment terms for DEGs following YBX1 knockdown. Fisher’s exact P values, which have been log10 transformed, were graphed for each functional term that has been enriched. E Heatmap illustrated the differential expression of genes in osteosarcoma cells that were transfected with a control lentivirus and YBX1 shRNA (n = 3 for each group). NMD was the abbreviation of nonsense-mediated decay (NMD). F The validation of AS events impacted by YBX1 via RT-PCR. Representative images from three independent experiments were shown. The schematic representation of the structure of each PCR product was depicted on the right side. Alternative exons and introns affected by YBX1 were painted red or blue. Each gel was accompanied by the percentage of total products that were exon or intron inclusion products (in%). Data were presented as representative of three independent experiments in (F).
KPNA2/YBX1 axis regulates DDX3X intron retention and RNA stability
Many IR transcripts are degraded through the NMD pathway because of premature termination codons (PTCs) inside the intron region [26,27,28,29]. To investigate the AS events that are directly controlled by YBX1, through the analysis of the YBX1 cross-linking immunoprecipitation (CLIP)-seq data set (PRJNA860283 in NCBI), 12 significant YBX1-bound motif sequences were identified (Fig. S8A). By searching for PTCs associated with the upstream open reading frame, the potential YBX1-binding motif sequence and PTC were found in intron 17 of DDX3X (Fig. 7A). Furthermore, the RIP assay demonstrated significant binding capacity between YBX1 and the 5’ splice site region of intron 17 of DDX3X (Fig. 7B). As YBX1 was previously reported to be involved in transcriptional regulation, no significant difference in the ChIP experiments of the DDX3X promoter as found, suggesting that YBX1 does not interact with the DDX3X promoter (Fig. 7C). YBX1 knockdown significantly reduced the retention of intron 17 of DDX3X, while simultaneously increasing the number of exons, as determined by qRT-PCR on nuclear RNA (Fig. 7D). Elevated levels of YBX1 and lower levels of DDX3X were indicative of worse overall survival in OS (Fig. S8B, C). Therefore, it was hypothesized that YBX1 could accelerate the degradation of the DDX3X mRNA NMD pathway by promoting the retention of intron 17 of the DDX3X gene. Using NMDI14, an NMD inhibitor, NMDI14 did not increase the relative level of IR of the DDX3X gene after YBX1 inhibition, but this effect was partially reversed by the overexpression of YBX1 (Fig. 7E). Moreover, the stability assay performed with mRNA treated with actinomycin D revealed that the suppression of KPNA2 or YBX1 increased the stability of DDX3X mRNA, leading to an increase in its half-life, but this effect was reversed by overexpression of YBX1 (Fig. 7F). Western blotting verified that the protein level of DDX3X increased after knockdown of KPNA2 or YBX1, but this effect could be partially reversed by overexpression of YBX1 (Fig. 7G). Thus, the results demonstrated that YBX1 interacts with an intron of DDX3X, and that the KPNA2/YBX1 axis regulates DDX3XIR and RNA stability.
A Sashimi plots of two osteosarcoma cell lines showed that DDX3X intron retention (IR) decreased after YBX1 knockdown. The DDX3X IR introduced premature termination codons (PTCs) that induce nonsense-mediated mRNA decay. The IR ratios and YBX1 binding motif were indicated. B The IgG and YBX1 groups were subjected to RIP followed by qPCR (RIP-qPCR) analysis to target DDX3X intron 17 (n = 3). C The interaction of YBX1 protein and DDX3X promoter was identified using the ChIP-qPCR assay and assessed by relative enrichment to input (n = 3). D qRT-PCR for DDX3X spliced and IR transcripts, following nuclear fractionation of U2OS cell lysates after YBX1 knockdown (n = 3). E qRT-PCR for DDX3X IR/mRNA transcript level with or without NMD inhibitor (NMDI14) treatment regulated by KPNA2/YBX1 axis (n = 3). F The half-live time (t1/2) of DDX3X mRNA was assessed after Actinomycin D (ActD) treatment regulated by KPNA2/YBX1 axis (n = 3). G Western blotting showed the DDX3X protein levels in U2OS and MNNG/HOS cell lines regulated by the KPNA2/YBX1 axis (left panel), with quantification of DDX3X expression normalized to GAPDH shown in the right panel (n = 3). ns no statistical significance; *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Two-tailed paired t tests for (A). One-way analysis of variance (ANOVA) was used to compare the difference in (B–E, G). Data were presented as mean ± SD (error bars) and representative of three independent experiments in (A–G).
YBX1 promotes osteosarcoma progression in vitro and in vivo through DDX3X
DDX3X facilitated the interaction between SP1 and the p21 promoter, leading to increased transcription of p21 [30, 31]. Knockdown of DDX3X significantly increased the proliferation of the osteosarcoma cell lines U2OS and MNNG/HOS, while knockdown of YBX1 significantly rescued this effect (Fig. 8A–C). Migration and invasion experiments showed that DDX3X deletion significantly increased the migration and invasion abilities of OS cells in vitro, while simultaneous inhibition of YBX1 partially reversed the improvement in tumor cell migration and invasion caused by DDX3X inhibition (Fig. 8D, E). In addition, a xenograft tumor assay was used to assess the in vivo growth potential of the tumor cells. The results of the tumor growth curve and tumor mass in vitro suggested that knocking down DDX3X could significantly promote tumor growth, but knocking down YBX1 simultaneously could partially reverse this effect (Fig. 8F, G and Fig. 8I). Similarly, the knockdown of DDX3X increased the number of Ki-67-positive cells, but the knockdown of YBX1 partially reversed this effect (Fig. 8H, J). Cell cycle analysis revealed that the knockdown of YBX1 partially reversed the acceleration of the cell cycle processed by DDX3X (Fig. 8K). Western blotting showed that the levels of the cell cycle suppressor protein p21 decreased and the levels of AKT1 and CDK2 increased after DDX3X knockdown, which could be partially reversed by YBX1 knockdown (Fig. 8L). In summary, YBX1 promotes the progression of OS cells in vivo and in vitro through the tumor suppressor gene DDX3X.
A–C Colony formation assays detected cell proliferation of U2OS and MNNG/HOS cells (n = 4). D, E Transwell assays demonstrated the migration and invasion capabilities of U2OS and MNNG/HOS cells. The scale bar was 100 µm. F The tumor growth curve (n = 5). G MNNG/HOS cells were subcutaneously into the nude mice for xenografts assay (n = 5). H Ki-67 staining of the tumor. The scale bar was 100 µm. I Excised tumor mass as indicated (n = 5). J The bar chart showed the quantitative results of Ki-67. K Flow cytometry results that shDDX3X reversed the effect of shYBX1 on G0/G1 cell cycle in osteosarcoma cells (n = 3). L Western blotting analysis of osteosarcoma tumor cells (U2OS and MNNG/HOS) after transfected with indicated lentiviral particles. Data were shown as the mean ± standard deviation of at least three independent experiments. ns no statistical significance; *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. One-way analysis of variance (ANOVA) was used to compare the difference in (B, C, E, F, I, J). Data were presented as mean ± SD (error bars) and representative of at least three independent experiments in (A–L).
Discussion
A comprehensive study of RNA-seq data from primary OS, metastatic OS, and normal tissues identified 174 regulated AS events that were both highly significant and related. RASEs are essentially concentrated in biological pathways, such as the cell cycle, cell division, and regulation of protein binding. Subsequently, a co-expression network was constructed of RBPs and RASEs. Of the 71 DERBPs, 25 expressed 106 RASEs. The main enrichments for these 25 RBPs were endocytosis, extracellular matrix organization, cell division, and the cell cycle in biological pathways. The study shows that RBPs are closely related to abnormal AS during the development of OS. Previous studies have shown that the RBP ALKBH5-mediated alteration of SOCS3 m6A inhibits OS proliferation by blocking the STAT3 pathway [32]. The competitive binding of STARD13 to miRNAs by the RBP protein PUM2 inhibits OS growth [33]. RBPs also have the potential to influence OS development through their effects on mRNA localization, mRNA stability, and ceRNA mechanisms [34] Nevertheless, there is an absence of involvement of RBP-mediated AS in OS, thereby impacting the progression of osteosarcoma tumorigenesis.
KPNA2, known as importin α-1, has been found to exhibit oncogenic functions in 15 types of cancers, including non-small cell lung cancer, breast cancer, and hepatocellular carcinoma [24]. KPNA2 is significantly upregulated in gliomas, promoting tumor proliferation and invasiveness via the facilitation of c-myc and E2F1 nuclear translocation, glucose uptake, glycolytic enzyme activity, and lactic acid generation [35]. Increased KPNA2 expression is correlated with decreased disease-free survival in patients with breast cancer [36]. Upregulation of KPNA2 improves motion, growth, and attachment to the extracellular matrix and decreases programmed cell death in breast cancer cells [37]. The transcription factor IRF2 significantly inhibits the proliferation of osteosarcoma cells by inhibiting the transcription of KPNA2 [38]. KPNA2 molecules transport numerous cancer-promoting proteins into the nucleus through nuclear pore complexes and play a role in tumorigenesis, cell differentiation, and apoptosis [39]. Most studies focus on tissues, while the potential of blood KPNA2 levels as diagnostic, prognostic, or therapeutic biomarkers in OS remains unexplored. We propose that the potential of KPNA2 as an OS biomarker should be validated through large-scale patient cohort studies. However, whether KPNA2 affects the localization of splicing factors in cells remains unknown, as fundamentally shapes the pathological state of cancer cells [40, 41]. The results of this study highlight the strong correlation between elevated KPNA2 levels and poor prognosis in individuals diagnosed with OS [23]. KPNA2 affects the proliferation, migration, and invasion of osteosarcomas by regulating the splicing factor YBX1. This reveals a new pathway through which KPNA2 promotes the progression of OS. Future research into inhibitors targeting the KPNA2/YBX1 axis may identify novel therapeutic strategies for OS treatment.
YBX1 plays a role in various RNA-dependent processes such as mRNA transcription, AS, mRNA packaging, and the control of mRNA stability and translation [42]. Knockdown of YBX1 hinders early embryonic development and affects mRNA decay and AS [27]. YBX1 plays a role in hematological cancers by maintaining intron 12 of MKNK1, which leads to a decrease in MKNK1 protein levels, inhibits ERK signaling pathway activity, and ultimately restores cell sensitivity to JAK inhibitors [28]. YBX1 also promotes senescence of bone marrow cells by affecting the variable splicing of exons of genes related to osteogenic differentiation, providing a potential target for the treatment of senile osteoporosis [43]. YBX1 promotes the proliferation, migration, and invasion of OS cells by upregulating VEGF165 transcription and inducing angiogenesis in OS tumors [44]. Nevertheless, there is a deficiency in the characteristic AS pattern of the entire YBX1 gene in OS. This study found that YBX1 mainly affects variable splicing of the IR type in OS and is closely related to mRNA degradation, splicing factors, and the cell cycle via knockdown of YBX1 in two OS cell lines. More importantly, 12 significant motif sequences were identified in which YBX1 directly binds to mRNA and found that YBX1 mediates the retention of intron 17 of the DDX3X mRNA that directly leads to a decrease in DDX3X protein levels. Consistent with previous studies, YBX1, a trans-acting factor regulating AS, mainly affects the IR events of mRNA [28]. This is the first study to show that YBX1 promotes tumor progression through AS regulation in osteosarcoma.
Several recent studies have focused on targeting KPNA2 or YBX1. Clinical trials specifically targeting KPNA2 remain lacking, possibly due to the presence of multiple nuclear import proteins, which has limited the development of KPNA2 inhibitors. Additionally, the small-molecule inhibitor SU056, targeting YBX1, has been reported to effectively impede disease progression in acute myeloid leukemia cell lines and patient-derived xenograft (PDX) models [45]. Similarly, SU056 has been shown to independently suppress tumor progression in ovarian cancer while synergizing with paclitaxel, with no observed hepatotoxicity [46]. YBX1 has also been identified as an immunogenic target in the STEMVAC vaccine, which has completed a Phase 1 clinical trial in breast cancer, demonstrating a favorable immune response. A Phase 2 trial is currently underway to assess its therapeutic potential in advanced triple-negative breast cancer [47]. Furthermore, a Phase 2 clinical trial of STEMVAC is ongoing in patients with non-small cell lung cancer, though results have not yet been published (NCT05242965). Overall, as an emerging therapeutic target, YBX1 remains at the forefront of small-molecule inhibitor development and clinical application, warranting further investigation. The development of small-molecule drugs targeting the KPNA2/YBX1 axis may serve as a valuable addition to the currently limited treatment options for OS. Although our study remains at the preclinical stage, it provides a potential direction for future clinical interventions.DDX3X is associated with KRAS, CDK2, p21, and others to maintain chromosome stability and promote correct chromosome separation suggesting that DDX3X may have a tumor suppressor role in osteosarcoma [4, 30, 48, 49]. DDX3X also promotes hnRNPK arginine methylation and induces apoptosis in OS [50]. In addition, DDX3X has a cancer-promoting function in Ewing’s sarcoma, gallbladder cancer, and breast cancer, probably because it is involved in different biological processes; however, further research is needed [51,52,53]. The oncogenic or tumor-suppressive role of DDX3X may be influenced by factors such as cell type, tumor microenvironment, and upstream regulatory networks. In U2OS and SAOS2 OS cell lines, DDX3X mRNA expression levels are relatively high, whereas its protein levels remain low. In contrast, PDX models exhibit elevated DDX3X protein levels [52]. This discrepancy may reflect post-transcriptional regulatory mechanisms, including differences in RNA stability, translation efficiency, or protein degradation pathways. Since PDX models more closely recapitulate the in vivo tumor microenvironment of patients, they may alter the activity of the KPNA2/YBX1 axis, thereby influencing both the RNA and protein levels of DDX3X. These functional differences underscore the critical impact of experimental conditions, cellular context, and tumor microenvironment on research outcomes, highlighting the necessity of considering these factors in DDX3X-related studies. Our results demonstrated that YBX1 stimulates the degradation of DDX3X by influencing IR, a process that NMDI14 can inhibit. Knockdown of DDX3X in OS leads to a decrease in p21 level and an increase in AKT1 and CDK2 levels to promote cell cycle progression. Changes in DDX3X mRNA and protein levels are regulated by the KPNA2/YBX1 axis. In summary, the function of DDX3X in tumors is dynamic and context-dependent, influenced by distinct biological backgrounds and experimental factors. Future studies are needed to further elucidate the mechanisms regulating DDX3X in different contexts.
Conclusions
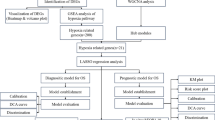
Abnormal protein transfer disrupts cellular homeostasis and promotes cancer progression. Various cancers highly express KPNA2, a nuclear receptor associated tumor progression. However, few studies have investigated the abnormal transport of splicing factors during OS. The study found that KPNA2 is highly expressed in OS and is associated with a poor clinical prognosis. KPNA2 is a promising prognostic marker for OS. In addition, KPNA2 regulates the nuclear translocation of the splicing factor YBX1. RNA sequencing showed that YBX1 widely affected AS in OS. YBX1 promotes IR events and decreased the mRNA stability and protein level of DDX3X by binding to intron 17 of DDX3X. DDX3X also participates in OS cell cycle progression and plays an anti-cancer role in OS. In summary, the results identified the KPNA2/YBX1/DDX3X axis as a regulator of OS progression and provided new insights into the clinical treatment of OS (Fig. 9).
Data availability
The RNA-seq data of two osteosarcoma cell lines, U2OS and MNNG/HOS, following the knockdown of YBX1 were uploaded and assigned the identifier GSE263410 in NCBI. The datasets utilized and/or examined in the present study can be obtained from the corresponding author (ZS, [email protected]) upon reasonable request. Osteosarcoma tissue sequencing data were derived from GEO (accession number GSE87624). Single-cell data for osteosarcoma were obtained from GEO (entry number: GSE152048). The YBX1 clip-seq for motif analysis was obtained from NCBI (accession number PRJNA860283).
References
Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029–35.
Whelan JS, Davis LE. Osteosarcoma, chondrosarcoma, and chordoma. J Clin Oncol. 2018;36:188–93.
Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21:vii320–5.
Beird HC, Bielack SS, Flanagan AM, Gill J, Heymann D, Janeway KA, et al. Osteosarcoma. Nat Rev Dis Prim. 2022;8:77.
Meltzer PS, Helman LJ. New horizons in the treatment of osteosarcoma. N Engl J Med. 2021;385:2066–76.
Ge Y, Porse BT. The functional consequences of intron retention: alternative splicing coupled to NMD as a regulator of gene expression. BioEssays. 2014;36:236–43.
Öther-Gee Pohl S, Myant KB. Alternative RNA splicing in tumour heterogeneity, plasticity and therapy. Dis Models Mech. 2022;15:dmm049233.
Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–63.
Zhang Y, Qian J, Gu C, Yang Y. Alternative splicing and cancer: a systematic review. Signal Transduct Target Ther. 2021;6:78.
Lee JH, Horak CE, Khanna C, Meng Z, Yu LR, Veenstra TD, et al. Alterations in Gemin5 expression contribute to alternative mRNA splicing patterns and tumor cell motility. Cancer Res. 2008;68:639–44.
Lenos K, Grawenda AM, Lodder K, Kuijjer ML, Teunisse AF, Repapi E, et al. Alternate splicing of the p53 inhibitor HDMX offers a superior prognostic biomarker than p53 mutation in human cancer. Cancer Res. 2012;72:4074–84.
Dai Z, Sun Y, Maihemuti M, Jiang R. Genome-wide identification of alternative splicing and splicing regulated in immune infiltration in osteosarcoma patients. Front Genet. 2023;14:1051192.
Venkataramany AS, Schieffer KM, Lee K, Cottrell CE, Wang PY, Mardis ER, et al. Alternative RNA splicing defects in pediatric cancers: new insights in tumorigenesis and potential therapeutic vulnerabilities. Ann Oncol. 2022;33:578–92.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat methods. 2015;12:357–60.
Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–406.
Castello A, Fischer B, Frese CK, Horos R, Alleaume AM, Foehr S, et al. Comprehensive identification of RNA-binding domains in human cells. Mol cell. 2016;63:696–710.
Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet. 2014;15:829–45.
Hentze MW, Castello A, Schwarzl T, Preiss T. A brave new world of RNA-binding proteins. Nat Rev Mol Cell Biol. 2018;19:327–41.
Cheng C, Liu L, Bao Y, Yi J, Quan W, Xue Y, et al. SUVA: splicing site usage variation analysis from RNA-seq data reveals highly conserved complex splicing biomarkers in liver cancer. RNA Biol. 2021;18:157–71.
Zhou Y, Yang D, Yang Q, Lv X, Huang W, Zhou Z, et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat Commun. 2020;11:6322.
Schlenker E. Tips and tricks for successful application of statistical methods to biological data. Methods Mol Biol. 2016;1366:271–85.
Scott MC, Temiz NA, Sarver AE, LaRue RS, Rathe SK, Varshney J, et al. Comparative transcriptome analysis quantifies immune cell transcript levels, metastatic progression, and survival in osteosarcoma. Cancer Res. 2018;78:326–37.
Jiang L, Liu J, Wei Q, Wang Y. KPNA2 expression is a potential marker for differential diagnosis between osteosarcomas and other malignant bone tumor mimics. Diagn Pathol. 2020;15:135.
Han Y, Wang X. The emerging roles of KPNA2 in cancer. Life Sci. 2020;241:117140.
Zheng S, Li X, Deng T, Liu R, Bai J, Zuo T, et al. KPNA2 promotes renal cell carcinoma proliferation and metastasis via NPM. J Cell Mol Med. 2021;25:9255–67.
Sureau A, Gattoni R, Dooghe Y, Stévenin J, Soret J. SC35 autoregulates its expression by promoting splicing events that destabilize its mRNAs. EMBO J. 2001;20:1785–96.
Deng M, Chen B, Liu Z, Wan Y, Li D, Yang Y, et al. YBX1 mediates alternative splicing and maternal mRNA decay during pre-implantation development. Cell Biosci. 2022;12:12.
Jayavelu AK, Schnöder TM, Perner F, Herzog C, Meiler A, Krishnamoorthy G, et al. Splicing factor YBX1 mediates persistence of JAK2-mutated neoplasms. Nature. 2020;588:157–63.
Pervouchine D, Popov Y, Berry A, Borsari B, Frankish A, Guigó R. Integrative transcriptomic analysis suggests new autoregulatory splicing events coupled with nonsense-mediated mRNA decay. Nucleic acids Res. 2019;47:5293–306.
Mo J, Liang H, Su C, Li P, Chen J, Zhang B. DDX3X: structure, physiologic functions and cancer. Mol cancer. 2021;20:38.
Zhang H, Mañán-Mejías PM, Miles HN, Putnam AA, MacGillivray LR, Ricke WA. DDX3X and stress granules: emerging players in cancer and drug resistance. Cancers. 2024;16:1131.
Yang Z, Cai Z, Yang C, Luo Z, Bao X. ALKBH5 regulates STAT3 activity to affect the proliferation and tumorigenicity of osteosarcoma via an m6A-YTHDF2-dependent manner. EBioMedicine. 2022;80:104019.
Hu R, Zhu X, Chen C, Xu R, Li Y, Xu W. RNA-binding protein PUM2 suppresses osteosarcoma progression via partly and competitively binding to STARD13 3’UTR with miRNAs. Cell Prolif. 2018;51:e12508.
Que Z, Yang K, Wang N, Li S, Li T. Functional Role of RBP in osteosarcoma: regulatory mechanism and clinical therapy. Anal Cell Pathol. 2023;2023:9849719.
Li J, Liu Q, Liu Z, Xia Q, Zhang Z, Zhang R, et al. KPNA2 promotes metabolic reprogramming in glioblastomas by regulation of c-myc. J Exp Clin Cancer Res. 2018;37:194.
Dankof A, Fritzsche FR, Dahl E, Pahl S, Wild P, Dietel M, et al. KPNA2 protein expression in invasive breast carcinoma and matched peritumoral ductal carcinoma in situ. Virchows Arch. 2007;451:877–81.
Noetzel E, Rose M, Bornemann J, Gajewski M, Knüchel R, Dahl E. Nuclear transport receptor karyopherin-α2 promotes malignant breast cancer phenotypes in vitro. Oncogene. 2012;31:2101–14.
Xia S, Ma Y. IRF2 Destabilizes oncogenic KPNA2 to modulate the development of osteosarcoma. J Oncol. 2022;2022:9973519.
Christiansen A, Dyrskjøt L. The functional role of the novel biomarker karyopherin α 2 (KPNA2) in cancer. Cancer Lett. 2013;331:18–23.
Marasco LE, Kornblihtt AR. The physiology of alternative splicing. Nat Rev Mol Cell Biol. 2023;24:242–54.
Bonnal SC, López-Oreja I, Valcárcel J. Roles and mechanisms of alternative splicing in cancer - implications for care. Nat Rev Clin Oncol. 2020;17:457–74.
Lyabin DN, Eliseeva IA, Ovchinnikov LP. YB-1 protein: functions and regulation. Wiley Interdiscip Rev RNA. 2014;5:95–110.
Xiao Y, Cai GP, Feng X, Li YJ, Guo WH, Guo Q, et al. Splicing factor YBX1 regulates bone marrow stromal cell fate during aging. EMBO J. 2023;42:e111762.
Quan B, Li Z, Yang H, Li S, Yan X, Wang Y. The splicing factor YBX1 promotes the progression of osteosarcoma by upregulating VEGF(165) and downregulating VEGF(165b). Heliyon. 2023;9:e18706.
Schnoeder TM, Perner F, Jayavelu AK, Mao L, Zhang Q, Hsu C-J, et al. Pre-Clinical investigation of a novel small molecule inhibitor targeting YBX1 in AML. Blood. 2022;140:491–2.
Tailor D, Resendez A, Garcia-Marques FJ, Pandrala M, Going CC, Bermudez A, et al. Y box binding protein 1 inhibition as a targeted therapy for ovarian cancer. Cell Chem Biol. 2021;28:1206–20.e6.
Dinh NTM, Nguyen TM, Park MK, Lee CH. Y-Box Binding Protein 1: Unraveling the Multifaceted Role in Cancer Development and Therapeutic Potential. Int J Mol Sci. 2024;25:717.
Pek JW, Kai T. DEAD-box RNA helicase Belle/DDX3 and the RNA interference pathway promote mitotic chromosome segregation. Proc Natl Acad Sci USA. 2011;108:12007–12.
Patmore DM, Jassim A, Nathan E, Gilbertson RJ, Tahan D, Hoffmann N, et al. DDX3X suppresses the susceptibility of hindbrain lineages to medulloblastoma. Dev cell. 2020;54:455–70.e5.
Chen CC, Yang JH, Fu SL, Lin WJ, Lin CH. Arginine methylation of hnRNPK inhibits the DDX3-hnRNPK interaction to play an anti-apoptosis role in osteosarcoma cells. Int J Mol Sci. 2021;22:9764.
Botlagunta M, Vesuna F, Mironchik Y, Raman A, Lisok A, Winnard P Jr., et al. Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene. 2008;27:3912–22.
Wilky BA, Kim C, McCarty G, Montgomery EA, Kammers K, DeVine LR, et al. RNA helicase DDX3: a novel therapeutic target in Ewing sarcoma. Oncogene. 2016;35:2574–83.
Miao X, Yang ZL, Xiong L, Zou Q, Yuan Y, Li J, et al. Nectin-2 and DDX3 are biomarkers for metastasis and poor prognosis of squamous cell/adenosquamous carcinomas and adenocarcinoma of gallbladder. Int J Clin Exp Pathol. 2013;6:179–90.
Funding
The study was financed by The National Natural Science Foundation of China (No. 82272709 and No. 82072979). LC was financed by the Fundamental Research Funds for the Central Universities and the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
Li Cao, Ke Jia, Van Tine BA, and Yihan Yu contributed equally to data collection, analysis and writing of the manuscript. Yizhong Peng, Xuanzuo Chen, Qing Pan, Wenbo Yang, Zhicai Zhang, Wei Wu, and Zengwu Shao contributed to study design and writing of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
LC, KJ, YY, YP, XC, QP, WY, ZZ, ZS, and WW report no conflicts of interest. VT BA reports research grants from Polaris, Pfizer, Merck, Tracon Pharma, GlaxoSmithKline; consulting fees from Bayer, Cytokinetics Inc., Deciphera Pharmaceuticals, Daiichi Sankyo, EcoR1, Advenchen, Putnam, Salarius Pharmaceuticals, Boxer Capital LLC, Acuta Capital Partners LLC, Aadi Biosciences, Race Oncology Limited, Hinge Bio, Inc., and Kronos Bio, Inc.; honoraria for educational talks from Iteration Therapeutics, Inc. and Total Health Conference; fees for participation in Data/Safety Advisory Boards for Apexigen, Daiichi Sankyo, Epizyme, Bayer, PTC Therapeutics, Aadi Biosciences, Boehringer Ingelheim, Agenus, Regeneron Pharmaceuticals, Advenchen, and Curtis; and speaking fees from Caris, Janssen, Lilly, Target Oncology, Bionest Partners, and Intellisphere LLC.
Ethical approval
The research was carried out in accordance with the principles outlined in the Declaration of Helsinki. The Animal Use and Care Committees at Tongji Medical College, Huazhong University of Science and Technology approved approval for the research (IACUC Number: [2023] 3790).
Consent for publication
All subjects signed a written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cao, L., Jia, K., Van Tine, B. et al. KPNA2 promotes osteosarcoma progression by regulating the alternative splicing of DDX3X mediated by YBX1. Oncogene 44, 2186–2200 (2025). https://doi.org/10.1038/s41388-025-03375-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-025-03375-3