Abstract
Nasopharyngeal carcinoma (NPC), a malignancy highly prevalent in East and Southeast Asia, is primarily treated with radiotherapy (RT). However, hypoxia-induced radioresistance presents a significant challenge. Nanozymes, nanomaterials with catalase-like activity, have emerged as a promising strategy for radiosensitization by converting elevated hydrogen peroxide in the tumor microenvironment into oxygen. Despite their potential, effectively targeting hypoxic lesions has been difficult. Here, we identify transferrin receptor 1 (TfR1) as an upregulated target in NPC, with its expression levels positively correlated with hypoxia. Human heavy-chain ferritin, a specific ligand of TfR1, selectively recognizes hypoxic NPC lesions in preclinical models. Based on these findings, we design a hypoxia-targeted nanozyme by loading platinum nanoparticles into ferritin. This nanozyme exhibits enhanced catalase-like activity and effectively alleviates tumor hypoxia in NPC xenografts. When combined with RT, a single injection of the nanozyme significantly inhibits tumor growth and prolongs mouse survival, outperforming sodium glycididazole, a clinically used radiosensitizer. In summary, our findings highlight TfR1 as an accessible cell surface target in hypoxic NPC lesions. The nanozyme targeting TfR1 holds promise for enhancing the therapeutic effectiveness of RT in NPC through an in situ oxygen-generation mechanism.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy arising from the nasopharyngeal mucosal lining, particularly prevalent in southern China with an age-standardized rate of 3.0 per 100,0001. Radiotherapy (RT) is the primary treatment for NPC due to its complex anatomical structure. Although advances in RT techniques have greatly improved the survival of NPC patients2,3, those in the advanced stage still face a grim prognosis4. Moreover, over 70% of patients are already in an advanced stage at their first visit5. Local recurrence and distant metastasis are the primary causes of treatment failure in NPC patients6,7, resulting in a median overall survival (OS) of only 20 months8. Treatments such as re-irradiation and concurrent chemotherapy can enhance control over relapse or metastasis but may exacerbate complications, resulting in limited improvements in the overall life quality of patients9,10. Therefore, it remains imperative to develop effective strategies to enhance RT effectiveness and reduce complications in NPC treatment.
The highly hypoxic tumor microenvironment (TME) has been widely reported as a major factor promoting RT resistance as well as recurrence and metastasis in solid tumors. Despite the well-vascularized structure of the nasopharynx, clinical studies reveal that hypoxia is present in 100% of primary lesions and 58% of cervical nodal metastases of NPC, with hypoxic levels 2 to 3 times higher than normal tissue11. Adequate oxygenation is a predictor of better treatment response to RT, while highly hypoxic tumors are associated with a higher risk of recurrence and metastasis12,13. During RT, oxygen (O2) promotes radiation damage by rapidly reacting with damaged DNA sites to form peroxides. Conversely, under hypoxic conditions, tumor cells become radioresistant by reversing radiation damage through DNA repair enzymes and thiol compounds14. The hypoxic TME also promotes NPC progression by inducing the expression of hypoxia-related proteins, contributing to anti-apoptosis, metastasis, and angiogenesis15,16. Clinical trials of NPC should therefore prioritize RT-combined therapies that overcome hypoxic resistance.
Direct approaches to increase tumor oxygenation involve hyperbaric oxygen inhalation and the use of oxygen-carrying agents17. However, lacking tumor specificity, these strategies have limited efficiency in alleviating hypoxia and may result in severe side effects such as barotrauma and hyperoxemia18. Another extensively studied approach involves the use of nitroimidazole compounds, acting as oxygen mimetics to augment RT efficacy. Early-developed nitroimidazole derivatives like misonidazole suffered from severe dose-limiting neurotoxicity, resulting in unsatisfactory clinical trial outcomes19. Some low-toxicity nitroimidazole compounds were subsequently developed for radiosensitization in cancer treatment, such as doramapimod and nimorazole20,21,22. Despite significant progress made with various strategies and the initiation of clinical trials involving certain radiosensitizers, the outcomes have not fully met the substantial clinical demand. There remains a gap between expectations and realized effectiveness in the clinical application of radiosensitizers.
The application of nanozymes, nanomaterials with catalase-like activity, for converting endogenic elevated hydrogen peroxide (H2O2) in TME into O2, is emerging as a novel approach to alleviate hypoxia23,24. This strategy offers precise and sustained O2 supply in tumor tissues, circumventing issues like uncontrolled O2 release, rapid depletion, or potential toxicity, making it more in line with clinical needs. Especially, nanozymes composed of high-Z elements such as platinum (Pt), gold (Au), and palladium (Pd), not only alleviate hypoxia but also possess strong absorption capabilities for ionizing radiation, making them promising radiosensitizer candidates25,26,27. However, successful hypoxia alleviation necessitates the development of highly active nanozymes with robust targeting approaches, given that the target H2O2 substrate is confined to hypoxic lesions and has a relatively limited generation rate (up to 0.5 nmol/104 cells/hour)28. Unlike small molecules that can freely diffuse into cells, nanozymes, being larger entities, rely on accessible cell surface targets to accumulate at desired lesions29. However, our understanding of the cell surface features of hypoxic tumors remains elusive, impeding the development of effective nanozyme targeting strategies.
In this work, we perform hypoxia scoring on NPC cohorts sourced from the Gene Expression Omnibus (GEO) database. Our analysis reveals a significant hypoxic state in NPC. Of note, we identify transferrin receptor 1 (TfR1) as a reliable cell surface target for hypoxic lesions in NPC patients through the analysis of clinical samples. We then create a hypoxia-targeting nanozyme (Pt-HFn) by loading platinum nanoparticles (PtNPs) into cage-shaped human heavy-chain ferritin (HFn), a specific ligand of TfR130,31,32. This nanozyme accumulate in NPC hypoxic lesions and alleviate hypoxia through its enhanced catalase-like activity. Through systematic validation in both cell line-derived xenograft (CDX) and patient-derived xenograft (PDX) NPC models, we demonstrate that this nanozyme in combination with RT outperforms the efficacy of either RT alone or RT combined with free PtNPs or a clinical radiosensitizer, while minimal adverse effects are observed.
Results
Identification of TfR1 as a cell surface target of hypoxic NPC
To analyze the hypoxic status of NPC, we first focused on three established hypoxia signatures relevant to head and neck cancers (15-gene33, 26-gene34, 99-gene35; Supplementary Table S1). These genes indicative of hypoxia exhibited predominantly higher expression levels in NPC tumors than in normal samples across three NPC cohorts (GSE1245236, GSE5381937, GSE6121838) from the GEO database (Supplementary Fig. S1). In all three cohorts, tumors consistently displayed significantly elevated hypoxia scores compared to normal samples (Supplementary Fig. S2), suggesting that NPC is strongly associated with hypoxia.
Cell surface proteins (CSPs) are vital targets for drug development, especially for drugs that cannot readily penetrate cell membranes. We attempted to identify the CSPs overexpressed in NPC and tightly associated with hypoxia from a total of 1442 CSPs listed in the Cell Surface Protein Atlas (CSPA) database (Fig. 1a). Our differential gene expression (DGE) analysis revealed that 36 CSPs in GSE12452, 109 CSPs in GSE53819, and 111 CSPs in GSE61218 were significantly upregulated in tumor samples (Supplementary Fig. S3). The intersection of these datasets identified 11 CSPs that consistently exhibited overexpression in all three cohorts (Fig. 1b). To identify hypoxia-related CSPs, we analyzed the GSE10234939 cohort consisting of 113 NPC tumor samples. Using hypoxia scores based on the 15-gene, 26-gene and 99-gene hypoxia signatures, 50, 75 and 97 CSPs were identified respectively as upregulated in highly hypoxic tumors in this cohort (Fig. 1b and Supplementary Fig. S4). The intersection of these datasets revealed 38 CSPs that consistently exhibited hypoxia-upregulated under all three signatures (Fig. 1b and Supplementary Fig. S4). Among these CSPs, RCN1 and TFRC stood out for being both overexpressed in tumors and upregulated under hypoxic conditions (Fig. 1b). RCN1 encodes reticulocalbin-1, a calcium-binding protein primarily located in the lumen of the endoplasmic reticulum, though it has been identified as a CSP on human endothelial and prostate cancer cell lines40. TFRC encodes TfR1, a cell surface transmembrane glycoprotein that mediates iron absorption and transport after binding to transferrin41. TfR1 is reported to be highly expressed on the surface of various tumors including NPC32,42. Therefore, compared to reticulocalbin-1, TfR1 is likely a more suitable cell surface target for nanozymes targeting hypoxic lesions in NPC.
a Schematic representation of CSPs upregulated in hypoxic NPC identified through DGE analysis among a total of 1442 CSPs. b Left: Venn diagram illustrating the intersection of CSPs upregulated in tumor samples of the three cohorts: GSE12452 (log2(fold change) >1 and P < 0.01), GSE53819 (log2(fold change) >1 and P < 0.01), and GSE61218 (log2(fold change) >1 and P < 0.01). Right: Venn diagram illustrating the intersection of CSPs that are upregulated in tumor samples with high hypoxia scores of the GSE102349 cohort (log2(fold change) >10 and P < 0.01). c Distribution of TfR1 mRNA abundance in tumor and normal samples from different cohorts: GSE12452 (normal, n = 10; tumor, n = 31), GSE53819 (normal, n = 18; tumor, n = 18), and GSE61218 (normal, n = 6; tumor, n = 10). d Distribution of TFRC mRNA abundance in tumor samples from the GSE102349 cohort (n = 113) with high or low hypoxia scores. e Representative images of IHC staining showing TfR1 protein expression in clinical primary NPC tissues and NNE tissues collected from multiple patients. Scale bar, 50 μm. f Distribution of TfR1 IHC staining scores in NPC (n = 174) and NNE (n = 98) tissues. g Distribution of TfR1 IHC staining scores in NPC and matched adjacent non-cancerous tissues (n = 117). h IF staining analysis in NPC tissues depicting the co-expression of HIF-1α and TfR1. Scale bar, 50 μm. i Spearman correlation between HIF-1α expression and TfR1 expression based on h (n = 40 random regions from different tissues). The box plots in c, d, and f show the median ±1 quartile, with whiskers extending to the minimum or maximum values within 1.5 times the interquartile range from the box boundaries. Mann–Whitney test in c, d, and f and Wilcoxon matched-pairs signed rank test in g were used to calculate P values. Source data are provided as a Source Data file.
Across the three NPC cohorts (GSE12452, GSE53819, GSE61218), the mRNA abundance of TFRC in tumors was significantly higher than in normal samples (Fig. 1c). In the GSE102349 cohort, tumors with high hypoxia scores exhibited significantly higher TFRC mRNA levels than tumors with low hypoxia scores (Fig. 1d), and TFRC mRNA expression showed a significant correlation with hypoxia scores (Supplementary Fig. S5), demonstrating its robustness as a hypoxia-related biomarker. We further validated the expression of TFRC using published transcriptome-wide gene expression profiles from microdissected NPC tumors, dysplasia, and normal controls43. A progressive increase in TfR1 levels was observed from normal epithelium to tumor (Supplementary Fig. S6a). Specifically, TfR1 expression was significantly higher in tumors compared to normal nasopharyngeal epithelium, normal-adjacent tissue, and the microenvironment. Additionally, the three hypoxia signatures (15-gene, 26-gene, 29-gene) were applied to classify the tumors into high and low hypoxia groups. Across all scoring methods, TfR1 expression was higher in the high hypoxia group compared to the low hypoxia group (Supplementary Fig. S6b). These findings confirm that TfR1 is upregulated in NPC tumors relative to normal tissues and is positively correlated with hypoxic conditions. Moreover, using spatial transcriptomics data of NPC from the GEO database (GSE20031044), we analyzed the spatial co-localization of TFRC expression and hypoxic lesions. Each spatial spot was scored with the three hypoxia signatures (15-gene, 26-gene, 29-gene), revealing consistent hypoxic regions in the NPC tissues of two patients, indicating hypoxia heterogeneity within NPC tissues (Supplementary Fig. S7). The spatial expression analysis demonstrated that regions with high TFRC expression co-localized with highly hypoxic areas, and TFRC expression levels were significantly correlated with the degree of hypoxia. These findings confirm a spatial correlation between TFRC expression and hypoxic areas within NPC tissues.
We then analyzed TfR1 protein levels in clinical NPC tissues. TfR1 exhibited strong cell surface expression in 174 NPC cases but remarkably weaker expression in 98 cases of non-cancerous nasopharyngeal epithelium (NNE) tissues (Fig. 1e, f). Moreover, TfR1 expression in NPC tissues was significantly higher than in paired adjacent non-cancerous (ANC) tissues (Fig. 1g). The area under the ROC curve (AUC) based on IHC staining score yielded a value of 0.869 (Supplementary Fig. S8a), indicating that TfR1 has potential as a diagnostic marker for NPC. However, there was no significant difference in OS between individuals with high and low TfR1 protein levels (Supplementary Fig. S8b), suggesting that TfR1 is not a prognostic biomarker for NPC. As well, none of the clinical characteristics, including gender, age, TNM stages, tumor sizes, and tumor numbers, showed a significant correlation with TfR1 (Supplementary Table S2). To confirm the correlation between TfR1 and hypoxia, we analyzed the co-localization of TfR1 with hypoxia-inducible factor 1α (HIF-1α) in clinical NPC tissues. The results showed that TfR1 expression was significantly higher in HIF-1α-positive regions compared to HIF-1α-negative regions (Fig. 1h) and exhibited a positive correlation with the expression levels of HIF-1α (Fig. 1i). Additional analyses on recurrent NPC samples indicated no significant difference in the expression levels of HIF-1α and TfR1 between primary and recurrent NPC tissues (Supplementary Fig. S9). Collectively, these findings suggested that TfR1 is an overexpressed and hypoxia-upregulated CSP in NPC.
HFn specifically targets hypoxic lesions in NPC
HFn, known as a ligand for TfR1, has a cage-like protein structure and is used as a nanocarrier to deliver chemotherapeutic drugs45. Given the overexpression of TfR1 in hypoxic NPC, we validated whether HFn could specifically recognize hypoxic lesions in NPC. First, Cy5.5-labeled HFn was used for IF staining analysis on NPC tissue slices derived from C17 PDX and C666-1 CDX models. The results revealed that Cy5.5-HFn exhibited stronger fluorescent signals in HIF-1α-positive regions than in HIF-1α-negative regions (Fig. 2a, b, and Supplementary Fig. S10a, b), indicating its stronger binding to hypoxic lesions. Subsequently, we intravenously injected Cy5.5-HFn into C17 PDX and C666-1 CDX mouse models to assess its in vivo targeting capability towards NPC (Fig. 2c and Supplementary Fig. S10c). In vivo fluorescence imaging showed a visible accumulation of Cy5.5-HFn in the livers and tumors in both models. Over the 48-h post-injection period, the fluorescence signal of Cy5.5-HFn increased in tumors over time, while gradually decreasing in livers after rapid accumulation within the first hour (Fig. 2d, e, and Supplementary Fig. S10d, e). Ex vivo fluorescence imaging at 48 h indicated significantly higher fluorescence signal intensity of Cy5.5-HFn at the tumor compared to other organs except the liver (Fig. 2f and Supplementary Fig. S10f). In parallel, pimonidazole (Pimo), a hypoxia fluorescence probe, was co-injected at 11 h after Cy5.5-HFn injection to trace hypoxic lesions in C17 PDX tumors. IF analysis revealed that Cy5.5-HFn accumulated in the vascular regions of the xenografts. In non-vascular areas, the average signal intensity of Cy5.5-HFn was significantly higher in hypoxic lesions than in normoxic lesions (Fig. 2g, h). These findings suggest that HFn can specifically target NPC tissues and preferentially accumulate in hypoxic lesions.
a IF analysis of NPC tissue from C17 PDX models showing the co-localization of Cy5.5-HFn with HIF-1α. Scale bar, 50 μm. b Distribution of Cy5.5-HFn staining intensity in hypoxic and normoxic regions of NPC tissue based on a (n = 8 randomly selected fields from different tissues). c Schematic representation of the intravenous (i.v.) injection of Cy5.5-HFn into C17 PDX mouse model. d In vivo fluorescence imaging of C17 PDX model mice at different time points before and after the injection of Cy5.5-HFn. e Quantitative analysis of Cy5.5-HFn signal in the livers and tumors based on d (n = 3 mice). f Distribution of Cy5.5-HFn signal intensity in major organs and tumors at 48 h post-injection (n = 3 mice). Inset images show ex vivo fluorescence images. g Co-localization IF analysis of Cy5.5-HFn with Pimo-tracked hypoxic regions in C17 PDX tumor tissue at 12 h post-injection. Scale bar, 50 μm. h Distribution of average fluorescence signals of Cy5.5-HFn in vascular regions and hypoxic and normoxic areas of non-vascular regions, based on g (n = 6 randomly selected fields from the same tissue). Data are shown as mean ± SEM. Paired, two-tailed Student’s t test in b and repeated-measures one-way ANOVA with Geisser-Greenhouse correction and with Tukey’s multiple comparisons test in h were used to calculate P values. Source data are provided as a Source Data file.
Synthesis and characterization of Pt-HFn catalase-like nanozyme
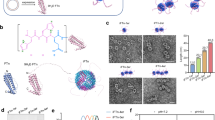
PtNPs, known as high-Z nanozymes with catalase-like properties, can absorb radiation and facilitate the conversion of H2O2 into O225,26. Based on the efficient targeting of NPC hypoxic lesions by HFn, we postulated that Pt-HFn, where PtNPs are coated by HFn, could potentially enhance the sensitivity of NPC to RT. A series of Ptx-HFn (x = input ratio, 100, 400, 700, 1000) was synthesized by mineralizing Pt within the cavity of HFn using a one-step reduction method (Supplementary Fig. S11a). The color of Ptx-HFn turned visible dark brown as the Pt input ratio reached 400 and intensified with higher Pt input (Supplementary Fig. S11b). The UV-visible absorption of Ptx-HFn gradually shifted upwards with increasing Pt input from 100 to 1000, while Pt100-HFn exhibited no significant difference from HFn (Supplementary Fig. S11c). The X-ray diffraction (XRD) patterns of Pt400-HFn, Pt700-HFn, and Pt1000-HFn exhibited typical Pt peaks that gradually became more prominent with the increase of Pt input (Supplementary Fig. S11d). X-ray photoelectron spectroscopy (XPS) analysis showed that both Pt0 and Pt2+ were present in Pt400-HFn and samples with higher Pt input, while only Pt2+ was present in Pt100-HFn (Supplementary Fig. S11e). These results indicate that Pt existed as Pt2+ in HFn at low (<100) Pt input. As the input increased from 100 to 400, Pt-core began to form and grew with further input up to 1000. The Pt loadings in Ptx-HFn (x = 100, 400, 700, 1000) were measured to be 9.70 ± 1.37, 177.70 ± 8.64, 266.98 ± 51.83, and 371.71 ± 18.55, respectively (Supplementary Fig. S11f). In comparison to HFn, the protein bands of Ptx-HFn in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) remained consistent (Supplementary Fig. S12a), while the bands shifted slightly downward in the native-PAGE with increasing Pt loading (Supplementary Fig. S12b). The peak shapes of the circular dichroism (CD) spectra (Supplementary Fig. S12c) and Fourier transform infrared (FT-IR) spectra (Supplementary Fig. S12d) of Ptx-HFn samples are all consistent with HFn, indicating that the secondary structure and surface groups of their protein shell have not been changed. Transmission electron microscopy (TEM) revealed the presence of the cage-shaped protein shell and the well-dispersed Pt-core in Pt1000-HFn, with respective diameters of 11.69 ± 0.78 nm and 4.39 ± 0.97 nm (Fig. 3a, b). High-resolution TEM revealed an atomic lattice arrangement of Pt-core with predominant (200) and (111) crystal faces (Fig. 3c), consistent with the XRD results. The atomic mass and atomic number percentages of Pt in Pt1000-HFn were 10.34% and 0.83%, respectively, as measured by energy-dispersive X-ray spectroscopy (EDS) (Fig. 3d). Taken together, platinum nanoparticles were successfully synthesized within the cavity of HFn.
a Left: TEM image of Pt-HFn under negative staining conditions showing the morphology of the HFn shell. Right: Size distribution of HFn based on TEM analysis. Scale bar, 10 nm. b Left: TEM image of Pt-HFn under non-negative staining conditions showing the morphology of the Pt-core. Right: Size distribution of the Pt-core based on TEM analysis. Scale bar, 10 nm. c High-resolution TEM image of the Pt-core in Pt-HFn, with insets displaying atomic-level resolution images of selected areas and their corresponding fast Fourier transform (FFT) patterns. Scale bar, 2 nm. d TEM-EDS pattern of Pt-HFn, inset displaying the mass and atomic percentages of various elements present in the sample. e Upper: Schematic illustration of Pt-HFn catalyzing the decomposition of H2O2 to produce O2, mimicking catalase activity. Lower: Photographs of O2 bubble generation in H2O2 solutions for each group. f Changes in absorbance at 240 nm indicate the reduction of H2O2 content in H2O2 solutions for each group (n = 4 independent experiments). g Changes in dissolved oxygen content show the increase in O2 levels in H2O2 solutions for each group (n = 3 independent experiments). Data are shown as mean ± SEM. Source data are provided as a Source Data file.
In the H2O2 solution, visible gas bubbles gradually increased with higher Pt loading in Ptx-HFn (Fig. 3e). In parallel, the decrease of H2O2 content (Fig. 3f) and the increase of dissolved O2 levels (Fig. 3g) followed a similar trend. These results collectively indicate that Pt400-HFn, Pt700-HFn, and Pt1000-HFn exhibited a sequential increase in catalase-like activity, while HFn and Pt100-HFn showed negligible activity. To investigate the role of the HFn shell in the catalytic activity of Pt-HFn (specifically Pt1000-HFn), we digested the shell to isolate the Pt-core. After pepsin treatment, no protein signals were detected on SDS-PAGE gel (Supplementary Fig. S13a) or in TEM images (Supplementary Fig. S13b), confirming the effective removal of the HFn shell. The de-shelled Pt-core appeared as aggregated nanoparticles (Fig. S13c, d) with atomic mass and atomic number percentages of Pt being 76.61% and 23.91%, respectively (Supplementary Fig. S13e). We found that the catalytic activity of intact Pt-HFn (kcat/KM = 102.76 M−1 s−1) was significantly higher than that of the de-shelled Pt-core (kcat/KM = 31.01 M−1 s−1) and a synthesized unmodified PtNPs (kcat/KM = 76.4 M−1 s−1) (Supplementary Figs. S14, S15 and Supplementary Table S3). These findings indicate the essential role of the HFn shell in maintaining the homogeneity and catalytic activity of Pt-HFn.
Unlike natural catalase, which lost activity above 60 °C and at extreme pH levels (pH <6 or pH >10), the activity of Pt-HFn increased with rising temperature and pH, indicating better tolerance to these conditions (Supplementary Fig. S16). Moreover, Pt-HFn remained stable and retained over 75% of its catalytic activity after 15 months of storage at 4 °C in PBS, with no insoluble precipitates or obvious protein degradation (Supplementary Fig. S17). PtNPs are reported to adsorb serum proteins in the blood, forming a protein corona that reduces their catalytic activity46. Our results showed that after incubation with mouse serum, PtNPs absorbed many serum proteins and retained only 46.44% of their activity (Supplementary Fig. S18). In contrast, Pt-HFn absorbed few proteins and retained 83.98% of its catalase-like activity. This indicates that the ferritin shell protected the Pt nanozyme from interacting with serum proteins, preserving its catalytic properties. Moreover, ELISA analysis demonstrated that Pt-HFn retained specific binding to TfR1 (Supplementary Fig. S19). These results suggest that Pt-HFn offers good stability, biocompatibility, and TfR1-targeting ability.
Pt-HFn alleviates hypoxia in NPC models
To evaluate the potential of Pt-HFn in targeting hypoxic regions and alleviating hypoxia in NPC, we administered Pt-HFn intravenously into C666-1 CDX-bearing mice. Pt was primarily distributed in the liver and spleen at 6 h after injection and gradually decreased over time. In contrast, within the tumor, Pt content continued to rise for 48 h (Fig. 4a), likely due to the enhanced permeability and retention (EPR) effect, which traps Pt-HFn as collapsed lymphatic vessels prevent its exit47,48. Photoacoustic imaging (PAI) showed a significant increase in intratumoral hemoglobin oxygen saturation (sO2) at 12 h (Fig. 4b, c), accompanied by a notable reduction in HIF-1α expression in tumor tissues (Fig. 4d, e). Moreover, 18F-FDG PET/CT indicated enhanced tumor metabolic activity 10 days after Pt-HFn injection, indicating a profound alteration in the hypoxic TME (Supplementary Fig. S20). In parallel studies using C17 PDX-bearing mice, imaging mass cytometry (IMC) showed sustained Pt accumulation in xenografts for 72 h (Fig. 4f, g), while IF revealed a marked reduction in hypoxic areas (Fig. 4h, i). These results demonstrate that Pt-HFn, acting as a catalase-like nanozyme, effectively ameliorated the hypoxic TEM in both the NPC CDX and PDX models.
a Distribution of Pt content in major organs and tumors of C666-1 CDX mice (n = 3 mice). b PAI shows changes in intratumoral sO2. Scale bar, 2 mm. c Quantitative analysis of intratumoral sO2 based on b (n = 3 mice). d IF analysis of HIF-1α expression levels. Scale bar, 50 μm. e Quantitative assessment of the positive area of HIF-1α based on d (n = 3 mice). f Distribution of Pt inside tumors of C17 PDX mice. Scale bar, 100 μm. g Quantitative analysis of Pt content based on f (n = 4 randomly selected fields from different tissues). h IF analysis of hypoxic regions in C17 PDX xenografts traced by Pimo. Scale bar, 100 μm. i Quantitative assessment of hypoxic area based on h (n = 3 mice). j Distribution of Pt content in major organs and tumors of C17 PDX mice (n = 3 mice). k IHC analysis of hypoxic regions traced by Pimo in C17 PDX xenografts. Scale bar, 200 μm. l Quantitative assessment of hypoxic area based on k (n = 3 mice). m IHC analysis of hypoxic regions traced by Pimo in C17 PDX tumors with different sizes (100, 300, 500 mm3). Scale bar, 200 μm. n Quantitative assessment of hypoxic area based on m (n = 3 mice). o IHC analysis of hypoxic regions traced by Pimo in C17 PDX tumors at different time points (1 day, 3 days, 5 days) after injection of Pt-HFn. Scale bar, 200 μm. p Quantitative assessment of hypoxic area based on o (n = 3 mice). Data are shown as mean ± SEM. Repeated-measures one-way ANOVA with Dunnett’s multiple comparisons test in c, unpaired, two-tailed Student’s t test in e and j, ordinary one-way ANOVA with Dunnett’s multiple comparisons test in g, ordinary one-way ANOVA with Tukey’s multiple comparisons test in a, i and l, and ordinary two-way ANOVA with Sidak’s multiple comparisons test in n and p were used to calculate P values. Source data are provided as a Source Data file.
We compared Pt-HFn and PtNPs in terms of tumor targeting and hypoxia alleviating. In C17 PDX model mice, Pt-HFn achieved a tenfold higher Pt concentration in tumors (6.76 %ID/g) than PtNPs (0.67 %ID/g) (Fig. 4j), demonstrating superior tumor targeting. Conversely, PtNPs accumulated more in the lungs (166.13 %ID/g), while Pt-HFn had significantly reduced lung distribution (3.08 %ID/g), indicating improved biodistribution. As a result, Pt-HFn was more effective than PtNPs in reducing tumor hypoxia (Fig. 4k, l). We further analyzed the hypoxia alleviation effect of Pt-HFn in tumors of different sizes (100, 300, 500 mm3). Despite variations in tumor volume, the proportion of hypoxic regions remained consistent (Fig. 4m, n). Pt-HFn was most effective in alleviating hypoxia in smaller tumors (100 mm3), while its effect was more limited in larger tumors (500 mm3). This may be attributed to the slightly lower distribution of Pt-HFn in larger tumors at the same dosage (Supplementary Fig. S21), likely due to differences in tumor density and vascular permeability. To assess the long-term activity of Pt-HFn, we measured Pt distribution and hypoxia levels in tumors of C17 PDX-bearing mice at different time points (1 day, 3 days, 5 days) post-injection. Pt remained detectable in the tumors at all three time points, with a slight decrease on day 5 (Supplementary Fig. S22), likely due to redistribution to other tissues or dilution from tumor growth. The most significant reduction in tumor hypoxia was observed on day 1, with effects persisting but gradually diminishing by day 5 (Fig. 4o, p).
Pt-HFn sensitizes NPC to RT in vitro and in vivo
To validate the radiosensitivity of NPC cells under normoxic and hypoxic conditions, as well as the radiosensitizing capability of Pt-HFn, we conducted cellular experiments using the C666-1 cell line. Compared to normal nasopharyngeal epithelial NP69 cells, C666-1 cells exhibited significantly higher baseline mRNA and protein levels of TfR1 (Supplementary Fig. S23), consistent with previous reports of elevated TfR1 expression in most NPC cell lines42. Upon exposure to 24 h of CoCl2-induced hypoxia, the mRNA levels of TfR1 further increased in C666-1 cells, while TfR1 protein levels remained consistently high (Supplementary Fig. S23). In contrast, both mRNA and protein levels of TfR1 in NP69 cells were significantly upregulated under hypoxic conditions. These results confirm that TfR1 is upregulated in NPC cells and correlates positively with hypoxia.
Under normoxic conditions, a single 6 Gy irradiation dose reduced the proportion of proliferating cells from 53.4% to 44.4%, while the combination of Pt-HFn and 6 Gy irradiation further decreased this proportion to 33.8% (Supplementary Fig. S24a, b). This suggests that Pt-HFn enhances the radiosensitivity of normoxic cells, likely through platinum-mediated energy deposition49. Under hypoxic conditions, the proliferation of cells was notably lower, with only 17.8% in the PBS-treated group (Fig. 5a, b). Interestingly, treatment with Pt-HFn alone increased the proportion of proliferating cells to 33.4%, likely due to its catalase-like activity decomposing H2O2 to produce O2 which alleviates hypoxia and reduces the oxidative stress. For hypoxic cells, 6 Gy irradiation alone exhibited little effect on proliferation (17.5%), indicating greater radiation resistance compared to normoxic cells. However, the combination of Pt-HFn and 6 Gy irradiation significantly reduced proliferation to 4.0%, highlighting its potent radiosensitizing effect.
a Fluorescence imaging for EdU and Hoechst 33342 labeling of six groups of cells. The cells in indicated groups were treated with PBS, HFn or HFn-Pt (Pt, 5 μg/mL) in the presence of 200 μM CoCl2 and 25 μM H2O2, for 12 h, followed by irradiation with or without 6 Gy RT. Scale bar, 50 μm. b Quantitative analysis of EdU-positive cells based on a (n = 6 independent experiments). c Fluorescence imaging for γ-H2AX and DAPI labeling of six groups of cells. Scale bar, 50 μm. d Quantitative analysis of γ-H2AX fluorescence intensity in cells based on c (PBS, n = 152 cells; HFn, n = 182 cells; Pt-HFn, n = 112 cells; PBS + RT, n = 129 cells; HFn + RT, n = 135 cells; Pt-HFn + RT, n = 140 cells). e The proportion of cells in the G1 phase and G2 + S phase in each group (n = 4 independent experiments). f Colony formation analysis of C666-1 cells in six groups using crystal violet staining. g Quantification of the number of colonies (n = 6 independent experiments). Data are shown as mean ± SEM. Ordinary one-way ANOVA with Dunnett’s multiple comparisons test in b and g, and Kruskal–Wallis with Dunn’s multiple comparisons test in d were used to calculate P values. Source data are provided as a Source Data file.
Moreover, we analyzed DNA damage in cells by detecting γ-H2AX. The results showed that 6 Gy irradiation increased DNA damage in C666-1 cells, and Pt-HFn significantly enhanced radiation-induced DNA damage under both normoxic and hypoxic conditions (Fig. 5c, d, and Supplementary Fig. S24c, d). Additionally, Pt-HFn increased radiation-induced cell cycle arrest, leading to more cells being retained in the G2/M phase (Fig. 5e and Supplementary Fig. S25). Colony formation assays under hypoxia confirmed that Pt-HFn alone did not affect long-term (14 days) cell proliferation (Supplementary Fig. S26a, b). However, while 2 Gy irradiation partially inhibited colony formation, its combination with Pt-HFn significantly enhanced this inhibition (Fig. 5f, g and Supplementary Fig. S26c, d). These findings demonstrated that Pt-HFn enhanced proliferation inhibition, DNA damage, and cell cycle arrest in C666-1 cells under both normoxic and hypoxic conditions. This enhancement can be attributed to its catalase-like activity that alleviates hypoxia and the radiation deposition characteristics of Pt.
To evaluate the radiosensitizing potential of Pt-HFn, we analyzed the therapeutic response of the C666-1 CDX model to localized RT (6 Gy) in combination with Pt-HFn (Supplementary Fig. S27a). Mice treated with RT alone exhibited delayed tumor growth, whereas the combination of RT and Pt-HFn resulted in more pronounced tumor suppression (Supplementary Fig. S27b, c). On the third day post-treatment, significant apoptosis was detected in the tumors of the Pt-HFn + RT group, while tumors subjected to RT alone exhibited only modest apoptosis (Supplementary Fig. S28). By the treatment endpoint on day 17, all mice in the non-RT or RT-alone groups had been defined as deceased (tumor volume exceeding 1000 mm3), whereas 7 out of 9 mice in the Pt-HFn + RT group were still alive (Supplementary Fig. S27d).
We then conducted in vivo experiments on C17 PDX mice to compare the radiosensitizing effects of PtNPs and Pt-HFn with localized radiotherapy (6 Gy) (Fig. 6a). The results showed that the combination of PtNPs and RT provided only a modest delay in tumor growth compared to RT alone, while the combination of Pt-HFn and RT exhibited a significant tumor-suppressive effect (Fig. 6b, c). By day 20 of treatment, the survival rate in the Pt-HFn + RT group was 71.4%, while mice in all other groups had been defined as deceased (Fig. 6d). These results indicate that Pt-HFn enhances the effects of radiotherapy more effectively than PtNPs, consistent with its improved tumor distribution and ability to alleviate hypoxia (Fig. 4j–l).
a Schematic diagram of the single-dose RT (6 Gy) treatment of mice inoculated with C17 PDX tumor subcutaneously on the right flank (PtNPs + RT, n = 6 mice; other group, n = 7 mice). b Average and c individual tumor growth curves in groups undergoing the treatment regimen depicted in a. d Kaplan–Meier survival curves of control and RT (6 Gy)-treated C17 PDX mice with or without Pt-HFn. e Schematic diagram of the single-dose RT (6 Gy) treatment of C17 PDX mice inoculated with C17 tumor subcutaneously on the right flank (n = 7 mice). f Average and g individual tumor growth curves in groups receiving the treatment regimen depicted in e. h Kaplan–Meier survival curves of control and RT (6 Gy)-treated C17 PDX mice with Pt-HFn, HFn, or CMNa. i Schematic diagram illustrating the fractionated RT (2 Gy × 5) treatment of C17 PDX-bearing mice (n = 5 mice). j Average and k individual tumor growth curves in groups receiving the treatment regimen depicted in i. l Kaplan–Meier survival curves of control and RT (2 Gy × 5)-treated C17 PDX-bearing mice receiving Pt-HFn, HFn, or CMNa. Data are shown as mean ± SEM. Ordinary two-way ANOVA with Tukey’s multiple comparisons test in b, f, and j and log-rank (Mantel–Cox) test in d, h, and l were used to calculate P values. Source data are provided as a Source Data file.
A parallel validation was performed on the C17 PDX model with the clinical radiosensitizer sodium glycididazole (CMNa) serving as a control (Fig. 6e). In comparison to the RT alone group, tumor growth was attenuated in the CMNa + RT group (Fig. 6f, g, and Supplementary Fig. S29). In contrast, Pt-HFn exhibited a more significant enhancement of RT-induced tumor suppression than CMNa, while HFn did not show a radiosensitizing effect. On the third day post-treatment, tumors in the Pt-HFn + RT group exhibited higher Caspase-3 expression and lower Ki-67 expression compared to the RT alone group, but with no significant difference compared to the CMNa + RT group (Supplementary Fig. S30). Pt-HFn significantly improved the prognosis of mice treated with RT, increasing the median survival time from 16 days to 24 days (Fig. 6h). In comparison, the combination of CMNa or HFn with RT did not extend survival, with a median survival time of 16 days and 14 days, respectively.
We also validated the inhibitory effects of Pt-HFn in combination with fractionated RT (2 Gy × 5) on C17 PDX xenografts (Fig. 6i). All mice subjected to fractionated RT displayed slowed tumor growth (Fig. 6j, k). However, only the Pt-HFn + RT group exhibited enhanced tumor suppression, while CMNa and HFn did not demonstrate a sensitizing effect with fractionated RT. All mice in the control groups were defined as deceased on the 18th day post-treatment, while 2 out of 5 mice in the Pt-HFn + RT group were still alive on the 22nd day post-treatment (Fig. 6l). These results suggest that Pt-HFn offers a more durable and robust enhancement of fractionated RT compared to CMNa, likely due to its extended tumor distribution and sustained hypoxia relief (Fig. 4o, p).
In all in vivo treatment experiments, Pt-HFn, whether combined with RT or used alone, did not cause significant changes in body weight, major organs, or blood biochemistry of treated mice (Supplementary Figs. S31–S35). Moreover, Pt-HFn did not induce notable hemolysis (Supplementary Fig. S36), indicating good blood compatibility. To assess long-term effects, Pt-HFn was administered intravenously to healthy 6-week-old male BALB/c mice, with platinum content in major organs measured over time, along with pathological analysis. After one month, significant levels persisted in the liver and spleen. While platinum levels in other organs decreased to very low levels by four months and showed some reduction in the liver, they remained high in both the liver and spleen, indicating slow degradation and metabolism of Pt-HFn (Supplementary Fig. S37a). Despite this, no signs of toxicity were detected, as reflected by stable blood biochemical markers and normal histopathological findings (Supplementary Fig. S37b, c), indicating that Pt-HFn has good biocompatibility and potential for long-term safety. These results highlight the radiosensitizing effect of Pt-HFn on NPC alongside its favorable biosafety profile.
Discussion
The current lack of effective drugs for alleviating tumor hypoxia limits the outcomes of RT for solid tumors, including NPC. Drugs designed to alleviate hypoxia must selectively target hypoxic lesions, which renders conventional drug targets ineffective. Consequently, identifying specific and reliable targets for hypoxic lesions is crucial. Our analysis of NPC clinical data reveals that TfR1 is overexpressed in tumors and strongly associated with hypoxia, highlighting its potential as a therapeutic target in hypoxic NPC.
TfR1 is a crucial transporter for cellular iron uptake, and its overexpression in hypoxic NPC tissues enhances iron absorption in cancer cells. Iron is an essential cofactor for various enzymes, crucial for ATP generation, antioxidant function, and DNA repair50. Hypoxic cancer cells require higher iron levels for invasive proliferation and maintaining redox balance51. Previous studies suggest that TfR1 is a downstream target of HIF-152,53,54, which activates TfR1 expression under hypoxic conditions, facilitating iron uptake to help cells adapt and improve oxygen utilization55. While the exact mechanism of TfR1 overexpression in hypoxic NPC cells requires further investigation, targeting TfR1 may enable iron-depletion therapeutic strategies using iron chelators like deferoxamine56, deferiprone57, and deferasirox58, or silencing TfR1 with monoclonal antibodies59 and siRNA42,60 to limit iron availability for NPC cells.
Excess intracellular iron is associated with cancer progression but can also induce oxidative stress and ferroptosis61. Studies have shown that ferroptosis-sensitive cells exhibit elevated levels of TfR162, and inhibiting iron uptake by silencing TFRC impairs erastin-induced ferroptosis63. Therefore, TfR1 overexpression may increase the sensitivity of hypoxic NPC to ferroptosis. Future strategies could target TfR1 to deliver ferroptosis inducers like metformin64 and sulfasalazine65, enhancing the oxidative capacity of iron ions to promote cancer cell death. Additionally, since hypoxic cancer cells can develop resistance to ferroptosis through HIF-1α activation66, targeting TfR1 to alleviate hypoxia may further enhance ferroptosis sensitivity in NPC.
Moreover, TfR1 has been commonly targeted in the development of lysosome-targeting chimeras (LYTACs), a class of drugs designed for the targeted degradation of extracellular and membrane-bound proteins through the lysosomal pathway67. Our research indicates that TfR1 may be a suitable target for LYTACs development in hypoxic NPC cells due to its high expression compared to other CSPs. Additionally, the screening strategy for CSPs used in our study not only assists in identifying targets for nanozymes and nanomedicines but may also facilitate the screening of specific surface targets for LYTACs in the future, representing a promising area for exploration.
One prominent advantage of TfR1 as a target lies in its ligand, HFn, which is a versatile cage-shaped drug carrier capable of accommodating numerous small molecules or metal atoms68. Based on the specific binding to TfR1, HFn shows an enhanced targeting towards hypoxic lesions. This discovery promotes HFn as a superior carrier for tumor hypoxia-responsive drugs. Based on these findings, we designed a hypoxia-targeted RT sensitizer using HFn as a carrier to precisely deliver Pt nanozyme. Of note, HFn can be easily purified through heat treatment and loaded with Pt through a one-step reduction process, making large-scale production of Pt-HFn feasible. The Pt-HFn nanomedicine efficiently enriched in NPC hypoxic lesions, improving the TME and making NPC more sensitive to RT. HFn effectively shielded Pt nanozymes from self-aggregation or adverse interactions with biological components in the body, preventing any potentially deleterious effects. Interestingly, Pt nanozymes encapsulated within HFn demonstrate unexpectedly enhanced catalase-like activity, which may be attributed to the improved particle dispersity or potential synergistic effects between the protein shell and the metal core. The catalytic role of protein components should therefore be considered in studying the catalytic mechanisms of ferritin-coated nanozymes, which has been previously overlooked69.
Pt-HFn demonstrates superior synergistic inhibition of tumor growth in combination with RT compared to CMNa, suggesting the potential of this hypoxia-targeted nanozyme to replace existing radiosensitizing drugs. The enhancement in the therapeutic effect of Pt-HFn may be attributed to several factors. In addition to its enhanced hypoxia targeting capability, Pt nanozymes possess both radiation absorption and catalase-like activities, thereby leading to more efficient utilization of radiation. Moreover, it is worth investigating whether the catalase-like catalytic activity of Pt-HFn is enhanced upon radiation excitation, as observed in some nanozymes70,71. We found that CMNa, although exhibiting a positive therapeutic effect in single-dose radiation (6 Gy), lost its effectiveness in fractionated radiation (2 Gy × 5). In contrast, Pt-HFn significantly enhanced the efficacy of both single-dose radiation (6 Gy) and fractionated radiation (2 Gy × 5), highlighting its broader potential application in clinical RT sensitization.
The efficacy of this radiosensitizer in treating recurrent NPC is worthy of exploration, given that recurrent NPC retains the original characteristics of high TfR1 expression and hypoxia. However, studies indicate that the occurrence of hypoxia in cervical lymph node metastases (58%) is lower than in primary NPC lesions (100%)11, suggesting that improving hypoxia may not directly benefit metastatic NPC. Furthermore, since our approach involves local irradiation, it may not provide apparent advantages for distant metastatic sites. Nevertheless, enhancing local control through hypoxia alleviation could help reduce residual lesions, thereby lowering the risk of recurrence and metastasis. To facilitate successful clinical translation, extensive evaluation of the long-term toxicity of Pt-HFn is essential. In addition, the optimal dosage and treatment frequency of Pt-HFn in combination with RT require further investigation. Moreover, the exploration of combination treatment strategies with chemotherapy and immunotherapy holds promise for future research.
Methods
Ethical regulations
All research complied with the relevant ethical regulations. All animal experiments were conducted following the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) at the Fifth Affiliated Hospital of Sun Yat-sen University (animal protocol No. # 00040). Mice were housed in groups of five per cage in a specific pathogen-free facility with controlled temperature (22–26 °C) and humidity (40–70%). The animals were kept on a 12-h light/dark cycle. The ethical committee established the following endpoint criteria for animal experiments: a 10% decrease in body weight, a tumor with the longest diameter exceeding 2 cm, or a tumor volume exceeding 2000 mm3. Animals were promptly euthanized using CO2 gas either upon reaching predetermined endpoints or at the conclusion of the experiment.
NPC hypoxia scoring
Four independent datasets of gene expression for NPC were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/): GSE12452 with mRNA microarray data from 31 patient tumor samples and 10 normal tissue samples; GSE53819 with mRNA microarray data from 18 patient tumor samples and 18 normal tissue samples; GSE61218 with mRNA microarray data from 10 patient tumor samples and 6 normal tissue samples; GSE102349 with mRNA sequencing data from 113 patient tumor samples. Gene annotation and quality control were performed on the downloaded raw data.
Each GSE12452, GSE53819, GSE61218 datasets were divided into tumor and normal groups, and the differences in hypoxia scores between these groups were compared. Firstly, three previously reported hypoxia signature gene sets for head and neck cancer were selected. These three gene sets contained 1533, 2634, and 9935 hypoxia indicator genes, respectively. For GSE12452, GSE53819, and GSE61218 data, DGE analysis was performed using the limma package in R. Hypoxia indicator genes from the hypoxia signature gene set were selected, and their differential expression in tumor and normal tissues was visualized using volcano plots and heatmaps. For each hypoxia signature gene set, hypoxia scoring was conducted by the following method: for each gene in the hypoxia signature gene set, if its expression in the target sample was higher than the mean expression of that gene across all samples, the sample received a hypoxia score of +1. Conversely, the sample received a hypoxia score of −1. The scores from all genes in the hypoxia signature gene set were summed to obtain the hypoxia score for that sample. To avoid errors from Simpson’s paradox, hypoxia scoring was independently performed using the three separately reported hypoxia signature gene sets for each sample.
Identification of CSP biomarkers in hypoxic tumors
To identify CSP biomarkers in hypoxic tissues of NPC, we obtained information on human CSPs from the CSPA database (https://wlab.ethz.ch/cspa/). After removing duplicates from 1492 records, we gathered gene names for 1442 distinct CSPs.
Firstly, we identified CSPs showing significantly elevated expression in tumor tissues. For GSE12452, GSE53819, and GSE61218 cohorts, samples were divided into tumor and normal groups. DGE analysis was conducted using the limma package in R and CSPs upregulated in tumors were identified based on criteria of log2(fold change) >1 and P < 0.01.
Then, we identified CSPs positively correlated with hypoxia in tumor tissues. Integrating expression and hypoxia score data, tumor samples within each dataset were classified into high and low hypoxia score groups based on the median hypoxia score. By performing DGE analysis using the limma package in R, CSPs upregulated in high hypoxia score tumors of the GSE102439 cohort were identified based on criteria of log2(fold change) >10 and P < 0.01.
Spearman’s correlation analysis in R was performed to determine the correlation between the expression of individual genes (e.g., TFRC) with hypoxia scores.
IHC staining assay of TfR1 in clinical NPC tissues
For IHC analysis, a tissue microarray (n = 140) containing 126 NPC tissues (5 cases were damaged during IHC) and 14 NNE tissues was purchased from Shanghai OUTDO Biotech Co., Ltd (Shanghai, China; Cat no: NPC1401). In addition, 82 newly diagnosed and untreated NPC primary tumor biopsies, 53 recurrent NPC biopsies and 84 NNE tissue samples were obtained from the Department of Otolaryngology-Head and Neck Surgery, First Affiliated Hospital of Guangxi Medical University (Nanning, China). All patients provided informed consent, and the study received approval from the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (approval number: 2018-KY-165).
Detection of TfR1 protein expression was conducted using the Universal PV kit (PV-6000, ZSGB-BIO, Beijing, China). Tissue sections were incubated with anti-TfR1 antibodies (Sigma, catalog no. HPA028598, 1:400) and anti-HIF-1α antibodies (CST, catalog no. 36169S, 1:800). The peroxidase reaction was visualized with 3,3′-diaminobenzidine (DAB) reagent (ZSGB-BIO, catalog no. ZLI-9018) for 2 min, followed by counterstaining with hematoxylin. Images were captured using a microscope (Axiocam 208 color, Carl Zeiss AG, Germany). Two pathologists, blinded to the sample information, independently evaluated the IHC results. The intensity of TfR1 staining was scored and graded as previously described72. IHC data were analyzed using SPSS 26.0 software (SPSS Inc., Chicago, IL, USA).
IF staining assay of HIF-1α and TfR1 in clinical NPC tissues
For the analysis of HIF-1α and TfR1 expression in NPC, three NPC tissues were collected from the Pathology Department of the Fifth Affiliated Hospital of Sun Yat-sen University (Zhuhai, China). This study received approval from the Medical Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University (approval number: K129-1). A standard IF procedure was used to process the paraffin-embedded tissue sections. Deparaffinization and rehydration were carried out, followed by antigen retrieval through microwave heating combined with high-pressure cooking at Tris-EDTA buffer (pH 9.0) for 10 min. Subsequently, the sections were rinsed with PBS and treated with 3% H2O2. Tissue blocking was performed using 10% goat serum (ZSGB-BIO, catalog no. ZLI-9022) at room temperature for 60 min. Two consecutive tissue sections (3 μm thickness each) were then incubated separately with anti-HIF-1α (CST, catalog no. 36169S, 1:100) and anti-TfR1 (Sigma-Aldrich, catalog no. HPA028598-100UL, 1:300) antibodies overnight at 4 °C, followed by three washes with PBST. Subsequently, secondary antibodies, goat anti-rabbit AF555 (Bioss, catalog no. Bs-0295G-AF555, 1:100) for HIF-1α and goat anti-rabbit AF488 (Bioss, catalog no. Bs-0295G-AF488, 1:100) for TfR1, were applied at room temperature for 1 h, followed by three PBST washes and mounting with a DAPI-containing anti-quenching mounting medium. Fluorescent images were captured using a Pannoramic 250 Flash II digital scanner (3DHISTECH Inc., Budapest, Hungary), and analyzed using ImageJ 1.51j8 software.
Expression and purification of HFn
The cDNA sequence of HFn was inserted into the pET30a plasmid at the Nde I/BamH I restriction sites and synthesized by GenScript Biotechnology Co., Ltd. The synthesized expression vector of HFn was transformed into E. coli BL21 (DE3) strain and expressed in kanamycin-resistant LB liquid medium. Bacterial cells were collected and resuspended in 20 mM Tris-HCl buffer (pH 8.0), then subjected to high-pressure homogenization to release the HFn protein. The supernatant post-homogenization was collected, and the protein was heat-treated at 72 °C for 15 min to precipitate thermolabile impurities. Subsequently, the crude protein solution underwent purification sequentially through a Q-Sepharose Fast Flow anion exchange column (GE Healthcare, catalog no. 17051001) and a Hiload 16/600 superdex 200 pg size exclusion column (GE Healthcare, catalog no. 28989335) to obtain the purified HFn protein.
Synthesis of Pt-HFn
HFn (50 mg) was mixed with 546, 2184, 3822, 5460, or 10920 μL of K2PtCl6 (17.975 mM) separately, maintaining molar ratios of HFn to K2PtCl6 at 1:100, 1:400, 1:700, 1:1000, respectively. The mixtures were brought to 50 mL using 50 mM Tris-HCl buffer (pH 8.0) and incubated at room temperature for 1 h. Subsequently, 1 mL of NaBH4 (0.1 M) was rapidly added, followed by gentle agitation for 10 min. The reaction was then left at room temperature for 3 h. The sample was then centrifuged at 1400 × g for 10 min and the supernatant was filtered through a 0.22 μm filter to remove insoluble synthetic products. A G25 desalting column (GE Healthcare, catalog no. 17003301) was employed to remove any salts. The desalted sample was then purified using a Hiload 16/600 superdex 200 pg size exclusion column (GE Healthcare, catalog no. 28989335) to obtain purified Pt-HFn samples. The protein concentration of the synthesized Pt-HFn was quantified using a BCA assay kit (Pierce, catalog no. 23225), while the Pt concentration was quantified by dissolving the sample in a HNO3-H2O2 system and measuring it using Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Agilent 7800 ce). The loading capacity was calculated based on the measured molar concentrations of Pt and the HFn protein. Unless otherwise stated, the concentration of Pt-HFn in this paper refers to the protein concentration of HFn.
Structural characterization of Pt-HFn
The structure of Pt-HFn was thoroughly characterized using various advanced techniques. Morphological characterization was performed using a field emission transmission electron microscope (FEI Talos F200X). Pt-HFn samples (HFn, 1 mg/mL) were placed on a support grid and negatively stained with 2% uranyl acetate, and the morphology of the protein shell was then imaged. Furthermore, at the same concentration, Pt-HFn samples were imaged without negative staining, allowing observation of the Pt core within Pt-HFn. Elemental composition analysis was performed via an integrated X-ray energy-dispersive spectrometer (EDS, Super-X) housed in the TEM. Statistical analysis of particle size distribution was conducted using Nano Measurer 1.2 software, while the crystal lattice of the Pt core was analyzed using Digital Micrograph software.
In addition, circular dichroism (CD) spectroscopy and UV-visible spectroscopy were used for detailed studies. CD spectra were performed using a Chirascan Plus spectrometer scanning Pt-HFn (HFn, 0.1 mg/mL) in 20 mM Tris-HCl (pH 8.0) buffer from 260 nm to 200 nm with 1 nm accuracy. The UV-visible spectrum of Pt-HFn (0.33 mg/mL) in 20 mM Tris-HCl (pH 8.0) buffer was measured using a HITACHI U-3900 spectrophotometer in the range of 900 nm to 200 nm, with a scanning accuracy of 1 nm.
In addition, Pt-HFn samples were freeze-dried and underwent a series of careful analyses. X-ray photoelectron spectroscopy (XPS) of samples is analyzed using precise monochromatic Al Kα radiation assisted by the Thermo ESCALAB 250 Xi spectrometer to gain insight into surface composition. X-ray diffraction (XRD) analysis was performed using Cu Kλ radiation in the range of 10–90°(2θ) on a D8Advance X-ray diffractometer (Bruker-AXS, Germany) to reveal the crystal structure. Fourier transform infrared spectroscopy (FT-IR) analysis was performed on a Nicolet iS10 infrared spectrometer in the 400–4000 cm−1 wavenumber range, with a spectral resolution of 4 cm−1 and a signal-to-noise ratio of 50,000:1 and a scan iteration of 32 times to promote a deep understanding of molecular vibrations.
Removal of the ferritin shell from Pt-HFn
5 mL Pt-HFn (1 mg/mL) dispersed glycine-HCl buffer (0.05 M, pH 2.1) was subjected to enzymatic digestion by adding 10 μL of pepsin (2 mg/mL). The mixture was incubated at 37 °C for 60 min, then centrifuged at 1400 × g for 5 min to remove the supernatant. The Pt-core precipitate was washed twice with 5 mL of glycine-HCl buffer (0.05 M, pH 2.1) and finally resuspended in 5 mL of PBS for a single wash and resuspension.
H2O2 decomposition assay
Pt-HFn and H2O2 solution were separately diluted using phosphate buffer (50 mM, pH 7.0). The decomposition of H2O2 catalyzed by Pt-HFn was measured using a UV-Vis spectrophotometer (Hitachi U-3900). For the assay, 2 mL of Pt-HFn samples (50 µg/mL; Pt, 5.95 µg/mL) were mixed with 1 mL of H2O2 solution (30 mM) in a cuvette. The absorbance changes of the reaction system at 240 nm were continuously measured at 20 °C over 5 min.
O2 generation assay
On one hand, 10 µL of Pt-HFn samples (HFn, 1 mg/mL) were dispersed in 1 mL of 100 mM H2O2 solution, and the generation of bubbles was observed after 5 min at room temperature. On the other hand, 100 µL of Pt-HFn samples (1 mg/mL; Pt 119 µg/mL) were mixed with 5 mL H2O2 solution (1 mM) diluted in phosphate buffer (50 mM, pH 7.0) under gentle stirring. The dissolved O2 concentration within the solution was detected using an oxygen electrode on a multi-parameter analyzer (HQ 30D, HACH) for 5 min.
Enzymatic kinetic analysis
The enzymatic kinetics of Pt-HFn, Pt-core, and PtNPs samples were analyzed by measuring the O2 generation rate at varying H2O2 concentrations (0–75 mM). 15 µL of the test sample (Pt, 147 µg/mL) were mixed with 5 mL of H2O2 solution (0–75 mM) diluted in phosphate buffer (50 mM, pH 7.0) under gentle stirring. The dissolved O2 concentration was detected using an oxygen electrode on a multi-parameter analyzer (HQ 30D, HACH) for 2 min. The Michaelis-Menten equation was then used to calculate the enzymatic kinetic parameters.
In the equation, Vmax represents the maximum reaction rate when the nanozyme is saturated by H2O2. [S] denotes the H2O2 concentration. The KM value signifies the [S] at which the enzyme-catalyzed reaction reaches half of Vmax. A higher KM value indicates a weaker affinity between the nanozyme and the substrate H2O2. Taking the H2O2 concentration ([S]) as the x-axis and the reaction rate (v) as the y-axis, a kinetic curve was plotted to determine the kinetic parameters KM and Vmax using GraphPad Prism 8.0 software. The kcat value was determined by dividing Vmax by the molar concentration of Pt ([E])
Protein corona analysis
Fresh blood (1 mL) from healthy BALB/c mice was collected and allowed to rest for 12 h to obtain serum. We then mixed 0.1 mL of PtNPs or Pt-HFn (Pt, 1 mg/mL) with 0.1 mL of serum and incubated the mixture at 37 °C for 1 h. The incubated solution was diluted to 1 mL with PBS and centrifuged at 12,400 × g for 30 min at 4 °C. The precipitate was resuspended in PBS and washed three times using the same centrifugation method. The final precipitate was used for SDS-PAGE and catalase-like activity analysis.
ELISA assay
HFn, Pt-HFn (experimental groups) or BSA (negative control group) at a final concentration of 10 µg/mL were prepared using 0.2 M carbonate buffer (pH 9.0). Sample solution (100 µL) from each group was added to separate wells in a 96-well plate and incubated at 4 °C in a humid environment for 12 h. Afterward, the plate was washed three times with PBS and twice with PBST. Subsequently, 300 µL of 5% skimmed milk was added to each well and incubated at 37 °C for 2 h. After further washing, each well was incubated with 100 µL of TfR1 solution (SinoBiological, catalog no. 11020-H07H, 2 µg/mL) at 37 °C for 2 h. After another round of washing, each well was incubated with 100 µL of anti-TfR1 antibody (SinoBiological, catalog no. 11020-MM02, 1 µg/mL) at 37 °C for 2 h. Following additional washes, 100 µL of HRP-conjugated anti-mouse IgG (GE Healthcare, catalog no. NA931, 1:5000) was added and incubated at 37 °C for 1 h. Finally, 100 µL of one-component TMB chromogenic substrate (InnoReagents, catalog no. TMB-S-004) was added to each well. Absorbance at 652 nm was immediately measured using an ELISA reader (SpectraMax M4), recording absorbance values every minute for 60 min. The data obtained were analyzed using GraphPad Prism 8.0 software to interpret the colorimetric results.
Cell lines
The human nasopharyngeal carcinoma C666-1 cell line was purchased from FuHeng Cell Center (Shanghai, China, catalog no. FH1098). C666-1 NPC cells were cultured in RPMI-1640 medium (Gibco, catalog no. C11875500BT) supplemented with 10% fetal bovine serum (Moregate Biotech, catalog no. FBSF) and 1% penicillin-streptomycin (Gibco, catalog no. 15140122). The human normal nasopharyngeal epithelial NP69 cell line73 was properly stored in the laboratory and cultured in Defined Keratinocyte-SFM (K-SFM) medium (Gibco, 10785012). The cell lines were authenticated by short tandem repeat (STR) profiling and verified to be mycoplasma negative routinely. The cultures were maintained at 37 °C with 5% CO2.
Quantitative Real-Time PCR analysis
NP69 and C666-1 cells were cultured in 6-well plates until reaching approximately 80% confluence. The cells were exposed to 200 μM CoCl2 or left untreated for 24 h. For qRT-PCR analysis, TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract Total RNA, and Prime Script RT kit (Invitrogen, Carlsbad, CA, USA) was used to synthesize cDNA. The Quant Studio 7 Flex Real-time PCR system was used to detect mRNA expression levels of TFRC. The experimental setup consisted of a 10 μL reaction volume with the following components: 5 μL Power SYBR Green PCR Master Mix, 0.4 μL each of Forward and Reverse primers (5 μM each), 2 μL cDNA and 2.2 μL ddH2O. The amplification program began with 5 min of denaturation at 95 °C, followed by 40 cycles of 15 s at 95 °C and 30 s at 60 °C. The mRNA transcription levels of TFRC were calculated using the ΔΔCt method. The sequences of the primers used were as follows: (TFRC-Forward: 5′-AGCAAAGTCTGGCGTGATCA-3′, TFRC-Reverse: 5′-AACAGCTTTTCTGCAGCAGC-3′, ACTB-Forward: 5′-AGAGCTACGAGCTGCCTGAC-3′, ACTB-Reverse: 5′-AGCACTGTGTTGGCGTACAG-3′).
Western blot
NP69 and C666-1 cells were cultured in 6-well plates until approximately 80% confluence was achieved. The cells were then exposed to 200 μM CoCl2 or left untreated for 24 h. Following treatment, the cells were lysed with RIPA buffer containing protease and phosphatase inhibitors. Protein concentrations were determined using the BCA Protein Assay Kit (Thermo, catalog no.23227). Equal amounts of protein (15 μg) from each sample were loaded onto an SDS-PAGE gel, separated by electrophoresis, and transferred to a PVDF membrane. The membrane was incubated with 5% non-fat milk in TBST (Tris-buffered saline containing 0.1% Tween-20) for 1 h at room temperature. After blocking, the membrane was incubated with primary anti-TfR1 antibodies (Sigma, catalog no. HPA028598, 1:1000) overnight at 4 °C. The following day, the membrane was washed three times with TBST and incubated with an HRP-conjugated secondary antibody (Abclonal, catalog no.AS014, 1:5000) for 1 h at room temperature. After additional washes with TBST, protein bands were detected using an enhanced chemiluminescence (ECL) detection system (Affinibody, AIWB-006), and images were captured with a chemiluminescence imager (Tanon, 4600SF). The intensity of protein bands was quantified using ImageJ 1.51j8 software, and the relative protein expression levels were normalized to an internal loading control of β-actin.
Colony formation assay
C666-1 cells were cultured at 37 °C. For the Pt-HFn treatment groups, cells were exposed to media containing Pt-HFn at varying concentrations (Pt, 0, 1.25, 2.5, 5, and 10 μg/mL) along with 200 μM CoCl2 and 25 μM H2O2 for 24 h. For the X-ray groups, cells were irradiated with different X-ray doses (0, 2, 4, 6, and 8 Gy) and incubated in media containing 200 μM CoCl2 and 25 μM H2O2 for 24 h. After treatment, cells were seeded in 6-well plates at 5000 cells per well, with the medium refreshed every 3 days. Colony growth was terminated after 14 days, and cells were washed with PBS, fixed in 4% PFA for 15 min, and subjected to crystal violet staining (Beyotime, catalog no. C0121). Surviving colonies were counted.
For additional experiments, cells were treated with PBS, HFn, or Pt-HFn (Pt, 5 μg/mL) with 200 μM CoCl2 and 25 μM H2O2 for 12 h, followed by 2 Gy irradiation or no irradiation. After 14 days of culturing, colonies were fixed and stained as described above.
Cell proliferation assay
C666-1 cells (1 × 105) were plated in 12-well plates and cultured at 37 °C for 24 h. The cells were then treated with PBS, HFn, or Pt-HFn (Pt, 5 μg/mL) with or without 200 μM CoCl2 and 25 μM H2O2 for 12 h, followed by 6 Gy irradiation or no irradiation. After treatment, cells were incubated for an additional 24 h. Cell proliferation was assessed using the EdU Cell Proliferation Kit (Beyotime, catalog no. C0071S) according to the manufacturer’s protocol.
DNA damage detection
For γ-H2AX immunofluorescence staining, C666-1 cells (1 × 105) were inoculated in 12-well plates and cultured at 37 °C for 24 h and then treated with PBS, HFn, Pt-HFn (Pt, 5 μg/mL), with or without 200 μM CoCl2, 25 μM H2O2, for 12 h followed by irradiation with or without 6 Gy RT. Then, the cells were washed with RPMI-1640 medium three times before a 15-min fixation with 4% PFA. After removing PFA by washing with PBS, cells were permeabilized with 0.3% Triton X-100 at room temperature for 20 min. Subsequently, after 1 h of blocking with goat serum (ZSGB-BIO, catalog no. ZLI-9022), cells were incubated overnight at 4 °C with anti-phospho-histone γ-H2AX rabbit monoclonal antibody (CST, catalog no.9718 T, 1:400). Then, the cells were further incubated with goat anti-rabbit-AF555 (Bioss, catalog no. Bs-0295G-AF555, 1:100) secondary antibody at room temperature in the dark for 1 h. After washing with PBS three times, the cells were treated with a sealing solution containing a DAPI anti-fluorescence quenching agent. Fluorescent images were captured using fluorescence microscopy (Olympus, BX53) and quantitatively analyzed using ImageJ 1.51j8 software.
Cell cycle assay
C666-1 cells (1 × 105) were plated in 12-well plates and cultured at 37 °C for 24 h. Cells were then treated with PBS, HFn, or Pt-HFn (Pt, 5 μg/mL) with or without 200 μM CoCl2 and 25 μM H2O2 for 12 h, followed by irradiation with or without 6 Gy. The cells were then cultured for an additional 24 h. To prepare for analysis, the cells were detached using EDTA-free trypsin and centrifuged at 100 × g for 3 min. The collected cells were fixed in 70% pre-cooled ethanol overnight at 4 °C. After removing the ethanol, the cells were washed with PBS. Subsequently, 500 μL of PI solution (Beyotime, catalog no. C1052) was added, and the cells were incubated at 37 °C for 30 min. Fluorescence was measured at an excitation wavelength of 488 nm by flow cytometry (CytoFLEX LX, Beckman Coulter, IN, USA), and the data were analyzed using Modfit LT software (V4.1.7, Verity Software House, Topsham, ME, USA).
C666-1 CDX model establishment
Four-week-old SPF-grade BALB/c nude male mice were purchased from SiPeiFu (Beijing) Biotechnology Co., Ltd. To establish the C666-1 CDX model, 100 μL of the cell suspension (5 × 107 cells/mL) was implanted subcutaneously into the right rear flank of BLAB/c nude male mice.
C17 PDX model establishment
Patient-derived EBV-positive xenografted tumor C1774 was a gift from Prof. Pierre Busson, Institut Gustave Roussy, France. C17 has been passaged subcutaneously and perpetually propagated in nude mice75. All experiments described herein were performed using C17 PDX from passage 27. This study has been approved by the Animal Ethics Committee of Guangxi Medical University.
When the tumor reaches a “plateau phase,” characterized by a stabilized growth trend or surface ulceration, euthanize the mice humanely and carefully extract the tumors. Remove the capsule and necrotic central tissue, retaining the compact tissue, and cut the tumor tissue into approximately 1–2 mm3 fragments using scissors. Place these fragments into a six-well plate and wash them three times with PBS. Subsequently, place the tumor tissue in RPMI-1640 cell culture medium containing antibiotics (10% FBS, 1% antibiotics), wash it three times, and then transplant it into new mice. To establish the C17 PDX model, male BALB/c nu mice (4–5 weeks old) were anesthetized using 1.5% isoflurane. The skin was disinfected with iodine, and an incision of approximately 0.5 centimeters was made on the dorsal area near the hind limbs of the mice. Using ophthalmic forceps, a “tunnel” of approximately 1–1.5 cm in length was created between the skin and muscle, directed towards the right hind limb, where the tissue was implanted at the transplantation site (subcutaneous area of the right rear flank). The incision was closed with wound clips, and the skin was further disinfected with iodine.
IF staining assay of Cy5.5-HFn on C666-1 CDX and C17 PDX NPC tissue
Paraffin sections of C666-1 CDX and C17 PDX xenografts were subjected to deparaffinization and rehydration. Antigen retrieval was carried out by high-pressure cooking in Tris-EDTA buffer (pH 9.0) for 10 min. The sections were then incubated with goat serum (ZSGB-BIO, catalog no. ZLI-9022) at room temperature for 60 min. Two adjacent sections were taken, one incubated with anti-HIF-1α (CST, catalog no. 36169S, 1:100), and the other incubated with anti-TfR1 (Sigma-Aldrich, catalog no. HPA028598-100UL, 1:300) + Cy5.5-HFn (11.6 μg/mL; Cy5.5, 28.6 nM). Incubation was carried out overnight at 4 °C, followed by three washes with PBST. The sections were then separately treated with secondary antibody goat anti-rabbit AF555 (Bioss, catalog no. Bs-0295G-AF555, 1:100) for HIF-1α staining and goat anti-rabbit AF488 (Bioss, catalog no. Bs-0295G-AF488, 1:100) for TfR1 staining, incubated at room temperature for 1 h. Following three PBST washes, the sections were mounted with a DAPI-containing anti-quenching mounting medium. Fluorescence images were captured using a Pannoramic 250 Flash II digital scanner (3DHISTECH Inc., Budapest, Hungary) and quantitatively analyzed using ImageJ 1.51j8 software.
In vivo fluorescence imaging of Cy5.5-HFn
C666-1 CDX-bearing or C17 PDX-bearing mice (n = 3) with average tumor volume up to 1000 mm3 were anesthetized using 1.5% isoflurane inhalation. Subsequently, an intravenous injection of 75 μL Cy5.5-HFn (5.8 mg/mL; Cy5.5, 14.3 μM) was administered. Maintaining the 1.5% isoflurane anesthesia, the mice were placed in the IVIS Lumina Series III imaging system (PerkinElmer, USA) for live fluorescence imaging at pre-injection and post-injection time points of 1, 6, 12, 24, 36, and 48 h. The imaging parameters were set as follows: Field of view: C (10 cm), Subject height: 1.5 cm, Exposure time: 1 s, Binning: 2, F/Stop: 8. After live imaging, under 2% isoflurane anesthesia, eyeball extraction was performed for blood collection. Subsequently, the mice were euthanatized and various organs including the brain, heart, lungs, liver, spleen, pancreas, kidneys, small intestine, muscles, bones, and tumor tissues were collected for ex vivo imaging. Following ex vivo imaging, tumor tissues were fixed in 4% paraformaldehyde (PFA), processed for dehydration, paraffin embedding, and sectioned (3-4 μm). After obtaining fluorescence images, fluorescence signals were quantitatively analyzed using the Living Image software provided by the imaging system.
Validation of Cy5.5-HFn in vivo targeting hypoxic NPC in C17 PDX tissue
C17 PDX-bearing mice (n = 3) with average tumor volume up to 700 mm3 were received an intravenous injection of 125 μL Cy5.5-HFn (5.8 mg/mL; Cy5.5, 14.3 μM) under 1.5% isoflurane inhalation anesthesia. To trace hypoxic tissue regions, at 11 h post-injection, a 50 μL intraperitoneal injection of pimonidazole (30 mg/mL) was administered. At 12 h after Cy5.5-HFn injection, the mice were euthanized, and the tumor tissues were immediately embedded in the O.C.T compound (SAKURA, catalog no. 4583) and placed at −80 °C for 2 h before being sectioned into frozen sections (30 μm). The sections were then incubated at room temperature for 1 h with anti-CD31 (BD, catalog no. BD553370, 1:200) and anti-pimonidazole (Hypoxyprobe, catalog no. PAb2627AP, 1:50). After washing with PBS, the sections were stained with secondary antibody donkey anti-rat-Cy3 (Jackson, catalog no. 712-165-150, 1:100) for CD31 and donkey anti-rabbit-AF488 (Jackson, catalog no. 711-545-152, 1:100) for pimonidazole. Incubation was done at room temperature in the dark for 30 min. The sections were then mounted with an anti-fade mounting medium containing DAPI. Fluorescence images were immediately captured using an EVOS M7000 inverted fluorescence microscope (Thermo Fisher Scientific).
Detection of oxygenation level in C666-1 CDX tumors treated with Pt-HFn
When the volume of the C666-1 CDX tumors reached 300 mm3 (n = 4), mice were anesthetized using 1.5% isoflurane inhalation, followed by the intravenous injection via the tail vein of 125 μL PBS or Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL). Photoacoustic images at the tumor site were collected using a high-frequency small-animal photoacoustic multimodal imaging system (Vevo LAZR-X, VisualSonics, Fujifilm, Japan) at pre-injection and post-injection time points of 6, 12, 24, 36, and 48 h. The system was operated in photoacoustic oxygenation mode to calculate the oxygenation level within the tumor.
IF staining assay of HIF-1α in C666-1 CDX tumors treated with Pt-HFn
After the average volume of C666-1 CDX tumors reached 300 mm3, the mice were randomly assigned to two groups (n = 3) and injected intravenously with 125 μL PBS or Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL). At 24 h post-injection, tumor tissues were harvested and fixed in 4% PFA. The fixed tissue was dehydrated, transparentized, embedded in paraffin, and sectioned into 4 μm thick slices by the third-party service company Seville Biotechnology Co., Ltd. according to standard procedures. Before performing the IF staining, the paraffin sections underwent deparaffinization and rehydration. Heat-induced epitope retrieval was performed in Tris-EDTA buffer (pH 9.0) under high pressure for 10 min. The sections were then incubated with goat serum (ZSGB-BIO, catalog no. ZLI-9022) at room temperature for 60 min for blocking. Following two washes with PBST, a biotin-avidin blocking system was employed to the sections: PBST was discarded, and a drop of avidin-blocking buffer (Vector, catalog no.30010) was added, incubating at room temperature for 15 min, followed by two PBST washes. Another drop of biotin-blocking buffer (Vector, catalog no. 30011) was added, incubated for 15 min at room temperature, and washed twice with PBST. The sections were incubated at 4 °C overnight with rabbit anti-HIF-1α (CST, catalog no. 36169S, 1:100) and IB4 (Vector, catalog no. B-1205-0.5, 1:300). After three PBST washes, goat anti-rabbit-AF555 (Bioss, catalog no. Bs-0295G-AF555, 1:100) secondary antibody was used for HIF-1α staining, and Fluorescein AvidinD (Vector, catalog no. A-2001-5, 1:200) was used for IB4 staining at room temperature in the dark for 1 h. The sections were washed three times with PBST and then mounted with an anti-fade mounting medium containing DAPI. Fluorescent images were captured using a Pannoramic 250 Flash II digital scanner (3DHISTECH Inc., Budapest, Hungary) and quantitatively analyzed using ImageJ 1.51j8 software.
Evaluating metabolic changes using 18F-FDG PET/CT Imaging
A total of 12 C666-1 CDX-bearing mice were intravenously injected with 125 μL of PBS or Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL). Twelve hours and 10 days later, the 0-30 min dynamic PET/CT (Mediso nano PET/CT 82 s, Hungary) scans were conducted by intravenously injected with approximately 300 μCi of 18F-FDG under 1.5% isoflurane to maintain spontaneous breathing during imaging. Before imaging, mice were fasted for 8 h while having access to water. PET data were acquired in list mode and reconstructed into 27-time frames (10 × 3 s, 3 × 10 s, 4 × 60 s, 10 × 150 s). CT scanning parameters included a voltage of 50 kVp, a current of 980 μA, a pitch of 1.0, with a rotation time of 0.3 s, using a binning of 1:4, and a reconstruction layer thickness of 0.3 mm. PET scanning parameters included 3D acquisition, a coincidence mode of 1–5, a matrix of 102 × 102, and a single field of view (FOV) of 98.5 mm. Dynamic PET data were analyzed using Carimas 2.10 software developed by the Turku PET Centre in Finland. Tumor volumes of interest (VOIs) were delineated to obtain time-activity curves (TACs).
Tissue mass spectrometry analysis of Pt deposition in C17 PDX tumors
Tissue sections from mice with average tumor volumes reaching 100–300 mm3 at 12, 24, and 72 h post-injection of Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL, 125 μL) or 12 h post-injection of HFn (9.27 mg/mL, 125 μL) were obtained for analysis. Following a standard procedure, the tissue sections underwent rehydration, baking, deparaffinization, and rehydration. Subsequently, the distribution of Pt was assessed using the Hyperion Imaging Mass Cytometry System (Helios-Hyperion, Fluidigm). The system allowed for the detection of Pt distribution within the tissues, and localization was analyzed based on corresponding hematoxylin and eosin (H&E) staining.
Biodistribution analysis
C666-1 CDX-bearing and C17 PDX-bearing mice were used to study the biodistribution of Pt-HFn. Mice with tumors grown to ~300 mm3 were intravenously injected with Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL). To evaluate distribution over time, three groups of C666-1 CDX-bearing mice (n = 3) were respectively sacrificed at 6, 24, and 48 h post-injection, while three groups of C17 PDX-bearing mice (n = 3) were respectively sacrificed at 1, 3, and 5 days post-injection. Additionally, to study the effect of tumor size, C17 PDX-bearing mice with tumors of ~100, ~300, and ~500 mm3 (n = 3) were injected and sacrificed 24 h later. Moreover, to compare Pt-HFn with PtNPs, two groups of C17 PDX-bearing mice (n = 3) were injected with Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL) or PtNPs (Pt, 1.0 mg/mL) and sacrificed at 24 h post-injection. For all groups, the heart, liver, spleen, lungs, kidneys, brain, and tumor tissues were collected and weighed. The tissues were then homogenized and digested in aqua regia at 90 °C for 2 days. The digested solution was deacidified by evaporating to 1 mL, diluted to 10 mL with 5% HCl, and centrifuged. Pt concentrations in the supernatant were measured using ICP-MS (NexION 2000), and the Pt content in each organ was calculated.
Detection of hypoxia in C17 PDX tumors after Pt-HFn treatment
C17 PDX-bearing mice with tumor volumes reaching 100–300 mm3 were stratified and randomly divided into three groups (n = 3). Following anesthesia with 1.5% isoflurane inhalation, mice received intravenous injections of 125 μL PBS, HFn (9.27 mg/mL), or Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL). At 23 h post-injection, mice were intraperitoneally injected with 60 mg/kg pimonidazole. After an additional 1 hour period, mice were euthanized, and tumor tissues were collected and embedded in O.C.T compounds for freezing. Frozen tissue sections were prepared using a cryostat at −20 °C, sectioning the tissues layer-by-layer from the edge to the center with a thickness of 30 μm. The sections were washed three times with PBS, then fixed with pre-cooled acetone at 4 °C for 15 min. After three rinses with PBS, the sections were blocked with 10% normal donkey serum (Jackson, catalog no. 017-000-121) at room temperature for 60 min. Subsequently, the sections were incubated overnight at 4 °C with anti-CD31 (BD, catalog no. BD553370 1:200) and anti-pimonidazole (Hypoxyprobe, catalog no. PAb2627AP, 1:50), followed by three washes with PBS. Staining was performed by using donkey anti-rat-Cy3 (Jackson, catalog no. 712-165-150, 1:100) secondary antibody for CD31 and donkey anti-rabbit-AF488 (Jackson, catalog no. 711-545-152, 1:100) for pimonidazole, incubating at room temperature in the dark for 1 h. After three PBS washes, the sections were mounted with an anti-fade mounting medium containing DAPI. Fluorescent images were captured using fluorescence microscopy (Olympus, BX53) and quantitatively analyzed using ImageJ 1.51j8 software.
Hypoxia in tumor tissues was also quantified by IHC. To assess the long-term effect of Pt-HFn on tumor hypoxia, eighteen C17 PDX-bearing mice with tumors around 300 mm3 were randomly divided into six groups (n = 3). Each mouse received intravenous injections of 125 μL PBS or Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL). After 1, 3, or 5 days, the mice were intraperitoneally injected with 60 mg/kg pimonidazole and euthanized after one additional hour. Tumor tissues were collected and fixed with 4% PFA and sent for paraffin sectioning at Seville Biotechnology Co., Ltd. Furthermore, to explore the effect of Pt-HFn on tumors of varying sizes, mice bearing C17 PDX tumors of approximately 100, 300, and 500 mm3 (n = 3) were intravenously injected with 125 μL PBS or Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL). After 23 h, pimonidazole was administered at 60 mg/kg intraperitoneally, and tumors were collected after one additional hour and fixed with 4% PFA for paraffin sections. Moreover, to compare the hypoxia-alleviating effects of Pt-HFn and PtNPs, nine mice bearing C17 PDX tumors of approximately 300 mm³ were randomly assigned to three groups (n = 3). Each mouse was intravenously injected with 125 μL PBS, Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL), or PtNPs (Pt, 1.0 mg/mL). After 23 h, the mice received pimonidazole intraperitoneally at 60 mg/kg, and after an additional 1 hour, the tumors were collected, fixed with 4% PFA, and sent for paraffin sectioning.
For the IHC analysis, the paraffin sections were dried in a 65 °C oven for 2 h, dewaxed twice with xylene preheated to 37 °C for 10 min each time, and hydrated with a gradient of ethanol and distilled water. Antigen retrieval was performed using citric acid buffer (pH 6.0) at 100 °C for 2.5 min under high pressure, followed by cooling to room temperature. The sections were then treated with 3% H2O2 for 15 min and blocked with 5% goat serum for 1 h. Next, the primary antibody anti-pimonidazole (Hypoxyprobe, catalog no. PAb2627AP, 1:500) was added and incubated overnight at 4 °C. Following PBS washes, an HRP-labeled secondary antibody was applied and incubated at 37 °C for 30 min. DAB staining was performed for 10 min, followed by counterstaining with hematoxylin for 20 s, differentiation with hydrochloric acid and alcohol, and bluing with tap water. The sections were then dehydrated using a gradient of ethanol, cleared with xylene for 10 min, and sealed with neutral resin for analysis. IHC images were captured using a digital pathology slide scanner (Leica, CS2) and quantitatively analyzed using ImageJ 1.51j8 software.
Single-dose RT (6 Gy) experiment on C666-1 CDX-bearing mice
Once the tumor volume reached approximately 100 mm3, C666-1 CDX-bearing mice were assigned to four different groups: untreated, Pt-HFn, RT, and Pt-HFn + RT (n = 9). Following anesthesia using 1.5% isoflurane inhalation, mice were intravenously injected with 125 μL PBS or Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL). At 12 h post-injection, under 1% pentobarbital sodium solution anesthesia (100 μL intraperitoneally), mice underwent local RT treatment on the subcutaneous tumor site of the right rear flank or remained untreated. A single-dose RT (6 Gy) was performed using a linear accelerator (Synergy, Elekta, Sweden) with a multi-leaf collimator to ensure effective conformation of the radiation to the tumor. Mice were monitored for changes in body weight every two days, and tumor volume was measured using a caliper. Growth curves for body weight and tumor volume were plotted. The experiment was concluded upon reaching the endpoint criteria (10% decrease in body weight, tumor longest diameter exceeding 2 cm, or tumor volume exceeding 2000 mm3), and survival curves for the mice were generated by taking the mice with tumor volume exceeding 1000 mm3 as dead. At the experimental endpoint, blood was collected via eyeball extraction after the mice were anesthetized with 2% isoflurane. Following euthanasia, tumor tissues and vital organs (heart, liver, spleen, lungs, kidneys) were harvested and fixed in 4% PFA. The collected serum and fixed tissues were sent to a third-party service company, Seville Biotechnology Co., Ltd., for H&E staining and assessment of liver and kidney functions.
Single-dose (6 Gy) and fractionated RT (2 Gy × 5) experiments on C17 PDX-bearing mice
C17 PDX-bearing mice, with an average tumor volume ranging from 100 to 300 mm3, were randomly divided into seven groups: untreated, HFn, Pt-HFn, RT, HFn + RT, CMNa + RT, Pt-HFn + RT (single-dose RT, n = 7; fractionated RT, n = 5). A volume of 125 μL of PBS, HFn (9.27 mg/mL), or Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL) was injected intravenously into mice 12 h before RT under 1.5% isoflurane anesthesia. CMNa was administered intraperitoneally 1 h before RT (125 μL, 43.568 mg/mL, calculated based on human dosage and adjusted for animal surface area and weight). Single local external X-ray radiation of 6 Gy was used to treat the tumors. Fractionated RT experiments follow similar groupings and experimental procedures, except that the RT was performed with a dose of 2 Gy once a day for 5 consecutive times. Experimental endpoint observations, growth curves for both weight and tumors, survival analysis, tissue processing, and toxicity assessments were conducted following the same experimental methods as for the C666-1 CDX treatment.
TUNEL staining analysis
C666-1 CDX-bearing mice with an average tumor volume of 300 mm3 were randomly divided into four groups: untreated, Pt-HFn, RT, and Pt-HFn + RT (n = 3). Under 1.5% isoflurane anesthesia, mice received an intravenous injection of 125 μL PBS or Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL). At 12 h post-injection, under 1% pentobarbital sodium solution anesthesia (100 μL intraperitoneally), mice underwent a single local RT or remained untreated, with a single-dose radiation of 6 Gy. After 72 h post-radiation, tumor tissues were collected and fixed in 4% PFA. The fixed tissues were sent to a third-party service company, Seville Biotechnology Co., Ltd., for standard procedures including dehydration, clarification, paraffin embedding, and sectioning into 3-4 μm thick slices. A terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was carried out using a TMR (red) TUNEL cell apoptosis detection kit (Servicebio, catalog no. G1502-50T) to analyze apoptotic cell death.
Tumor tissue proliferation and apoptosis staining assay
C17 PDX-bearing mice with an average tumor volume growth between 100 and 300 mm3 were randomly divided into seven groups: untreated, HFn, Pt-HFn, RT, HFn + RT, CMNa + RT, Pt-HFn + RT (n = 3). 125 μL of PBS, HFn (9.27 mg/mL), or Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL) were intravenously injected 12 h before irradiation. CMNa (217.84 mg/kg) was injected intraperitoneally 1 h before RT. A single local external radiation dose of 6 Gy was administered to treat the tumors. At 72 h post-RT, mice were euthanized, and tumor tissues were fixed in 4% PFA. Fixed tissues were sent to a third-party service company, Seville Biotechnology Co., Ltd., for standard procedures including dehydration, clarification, paraffin embedding, and sectioning into 4 μm thick sections. For tissue staining, adjacent sections were incubated separately with anti-Ki67 (ThermoFisher, catalog no. 14-5698-82, 1: 100) and anti-Cleaved Caspase 3 (CST, catalog no. 9661, 1: 400), overnight at 4 °C. The sections were washed three times with PBS and then incubated with secondary antibody goat anti-rat-HRP (Jackson, catalog no. 112-035-143, 1: 500) for Ki67 staining and goat anti-rabbit-HRP (Invitrogen, catalog no. 31460, 1: 500) for Cleaved Caspase 3 staining, incubated at room temperature for 1 h. Images were captured using a Pannoramic 250 Flash II digital scanner (3DHISTECH Inc., Budapest, Hungary) and quantitatively analyzed using ImageJ 1.51j8 software.
Long-term biosafety analysis
Healthy 6-week-old male BALB/c mice were intravenously injected with 125 μL of Pt-HFn (9.27 mg/mL; Pt, 1.0 mg/mL). The mice were maintained under standard conditions, and their health was monitored. At 1 month or 4 months post-injection, the mice were euthanized, and blood samples were collected for serum analysis. The heart, liver, spleen, lungs, kidneys, and brain were harvested and fixed in 4% PFA. The collected serum and fixed tissues were sent to a third-party service company, Seville Biotechnology Co., Ltd., for H&E staining and blood biochemistry analysis to assess liver and kidney functions. Additionally, at each time point, three mice were randomly selected, and their hearts, livers, spleens, lungs, kidneys, brains, and tumor tissues were collected and weighed. The tissues were homogenized and digested in aqua regia at 90 °C for 2 days. The digested solution was deacidified by reducing the volume to 1 mL, diluted to 10 mL with 5% HCl, and centrifuged. Pt concentrations in the supernatant were measured using ICP-MS (NexION 2000), and the Pt content in each organ was calculated.
Hemolysis assay
Whole blood (about 1 mL) was collected from BALB/c mice into tubes containing anticoagulants. The collected blood was mixed with 1 mL of 0.9% saline solution and centrifuged at 500 × g for 5 min to separate the supernatant. The supernatant was discarded, and 2 mL of 0.9% saline solution was added followed by centrifugation at 500 × g for 5 min. This procedure was repeated 4 times until the supernatant appeared colorless and transparent. The precipitated red blood cells were then dispersed in 0.9% saline to obtain a suspension. The red blood cell suspension was treated with Pt-HFn at final concentrations between 3.125 and 400 μg/mL. The negative control consisted of a red blood cell suspension diluted with 0.9% saline solution, whereas the positive control was a red blood cell suspension diluted with distilled water. The tubes were gently mixed and incubated at room temperature for 4 h. The samples were then centrifuged at 14,500 × g to separate blood cells from the supernatant. The presence of hemoglobin in the supernatant was analyzed by spectrophotometric absorbance at 541 nm, and the hemolysis rate (HR%) was calculated using the following formula.
Where Asa, Apc, and Anc represent the absorbance at 541 nm of the sample, positive control, and negative control, respectively.
Statistics and reproducibility
GraphPad Prism 8.0 software was used for statistical analysis in this study. Two-tailed Student’s t test or Mann–Whitney test was used to analyze differences between two groups. Analysis of variance (ANOVA) or Kruskal–Wallis test was used to assess differences between more than two groups depending on the experimental design. P values of multiple comparisons were adjusted by using Tukey’s, Dunnett’s, Dunn’s or Sidak’s corrections. Survival was analyzed using the Kaplan–Meier curve method with the log-rank test for comparison. Spearman correlation analysis was performed to assess the strength of the relationship between two variables. P < 0.05 was considered statistically significant. All quantitative data are means ± SEM. Experimental details (sample sizes, experimental replicates, and statistical tests) are provided in the figure legends and methods section. All in vivo studies were randomized into groups but unblinded. Sample sizes and replicates were determined based on previous studies and the format and scope of each experiment, as specified in the figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The NPC bulk RNA-seq data used in this study are publicly available in the GEO database under accession codes GSE1245236, GSE5381937, GSE6121838, and GSE10234939. The spatial sequencing data of primary NPC tissues are available in the GEO database under accession code GSE20031044. Transcriptome-wide gene expression profiles from microdissected NPC tissues are sourced from a previously published study43 [https://doi.org/10.1126/sciadv.abh2445]. Additional data are provided within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper.
References
Chen, Y.-P. et al. Nasopharyngeal carcinoma. Lancet 394, 64–80 (2019).
Sun, X. et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: An analysis of survival and treatment toxicities. Radiother. Oncol. 110, 398–403 (2014).
Au, K. H. et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study). Oral Oncol. 77, 16–21 (2018).
Ou, X. et al. Treatment outcomes and late toxicities of 869 patients with nasopharyngeal carcinoma treated with definitive intensity modulated radiation therapy: new insight into the value of total dose of cisplatin and radiation boost. Oncotarget 6, 38381–38397 (2015).
Skinner, D. W., Van Hasselt, C. A. & Tsao, S. Y. Nasopharyngeal carcinoma: Modes of presentation. Ann. Otol. Rhinol. Laryngol. 100, 544–551 (1991).
Lee, A. W. M., Ma, B. B. Y., Ng, W. T. & Chan, A. T. C. Management of nasopharyngeal carcinoma: Current practice and future perspective. J. Clin. Oncol. 33, 3356–3364 (2015).
Mao, Y.-P. et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin. J. Cancer 35, 103 (2016).
Jin, Y. et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J. Cancer Res. Clin. Oncol. 138, 1717–1725 (2012).
Tian, Y.-M. et al. Long-term outcome and pattern of failure for patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Head Neck 41, 1246–1252 (2019).
Takiar, V. et al. Disease control and toxicity outcomes for T4 carcinoma of the nasopharynx treated with intensity-modulated radiotherapy. Head Neck 38, E925–E933 (2016).
Yeh, S.-H. et al. Fluorine-18 fluoromisonidazole tumour to muscle retention ratio for the detection of hypoxia in nasopharyngeal carcinoma. Eur. J. Nucl. Med. 23, 1378–1383 (1996).
Nordsmark, M., Overgaard, M. & Overgaard, J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother. Oncol. 41, 31–39 (1996).
Nordsmark, M. et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 77, 18–24 (2005).
Lomax, M. E., Folkes, L. K. & O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 25, 578–585 (2013).
Shan, Y. et al. Hypoxia-induced matrix metalloproteinase-13 expression in exosomes from nasopharyngeal carcinoma enhances metastases. Cell Death Dis. 9, 382 (2018).
Hui, E. P. et al. Coexpression of hypoxia-inducible factors 1α and 2α, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin. Cancer Res. 8, 2595–2604 (2002).
Singleton, D. C., Macann, A. & Wilson, W. R. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Rev. Clin. Oncol. 18, 751–772 (2021).
Ortega, M. A. et al. A general overview on the hyperbaric oxygen therapy: Applications, mechanisms and translational opportunities. Medicina 57, 864 (2021).
Simpson, J. R. et al. Radiation therapy alone or combined with misonidazole in the treatment of locally advanced non-oat cell lung cancer: Report of an rtog prospective randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 16, 1483–1491 (1989).
Nishimura, Y. et al. Phase I/II trial of sequential chemoradiotherapy using a novel hypoxic cell radiosensitizer, doranidazole (PR-350), in patients with locally advanced non–small-cell lung cancer (WJTOG-0002). Int. J. Radiat. Oncol. Biol. Phys. 69, 786–792 (2007).
Overgaard, J. et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother. Oncol. 46, 135–146 (1998).
Thomson, D. et al. NIMRAD-a phase III trial to investigate the use of nimorazole hypoxia modification with intensity-modulated radiotherapy in head and neck cancer. Clin. Oncol. 26, 344–347 (2014).
Sahu, A., Kwon, I. & Tae, G. Improving cancer therapy through the nanomaterials-assisted alleviation of hypoxia. Biomaterials 228, 119578 (2020).
Xu, D., Wu, L., Yao, H. & Zhao, L. Catalase-like nanozymes: Classification, catalytic mechanisms, and their applications. Small 18, 2203400 (2022).
Yang, S. et al. Au-Pt nanoparticle formulation as a radiosensitizer for radiotherapy with dual effects. Int. J. Nanomed. 16, 239–248 (2021).
Li, Y. et al. Porous platinum nanoparticles as a high-Z and oxygen generating nanozyme for enhanced radiotherapy in vivo. Biomaterials 197, 12–19 (2019).
Yang, Y. et al. NIR-II driven plasmon-enhanced catalysis for a timely supply of oxygen to overcome hypoxia-induced radiotherapy tolerance. Angew. Chem. Int. Ed. 58, 15069–15075 (2019).
Szatrowski, T. P. & Nathan, C. F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 51, 794–798 (1991).
Mailänder, V. & Landfester, K. Interaction of nanoparticles with cells. Biomacromolecules 10, 2379–2400 (2009).
Li, L. et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. 107, 3505–3510 (2010).
Montemiglio, L. C. et al. Cryo-EM structure of the human ferritin–transferrin receptor 1 complex. Nat. Commun. 10, 1121 (2019).
Fan, K. et al. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 7, 459–464 (2012).
Toustrup, K. et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 71, 5923–5931 (2011).
Eustace, A. et al. A 26-gene hypoxia signature predicts benefit from hypoxia-modifying therapy in laryngeal cancer but not bladder cancer. Clin. Cancer Res. 19, 4879–4888 (2013).
Winter, S. C. et al. Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer Res. 67, 3441–3449 (2007).
Sengupta, S. et al. Genome-Wide Expression Profiling Reveals EBV-Associated Inhibition of MHC Class I Expression in Nasopharyngeal Carcinoma. Cancer Res. 66, 7999–8006 (2006).
Bao, Y. et al. Urokinase-type plasminogen activator receptor signaling is critical in nasopharyngeal carcinoma cell growth and metastasis. Cell Cycle 13, 1958–1969 (2014).
Fan, C. et al. Construction of a lncRNA–mRNA Co-Expression Network for Nasopharyngeal Carcinoma. Front. Oncol. 12, 809760 (2022).
Zhang, L. et al. Genomic Analysis of Nasopharyngeal Carcinoma Reveals TME-Based Subtypes. Mol. Cancer Res. 15, 1722–1732 (2017).
Cooper, C. R. et al. Novel surface expression of reticulocalbin 1 on bone endothelial cells and human prostate cancer cells is regulated by TNF-α. J. Cell. Biochem. 104, 2298–2309 (2008).
Galy, B., Conrad, M. & Muckenthaler, M. Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 25, 133–155 (2024).
Feng, G. et al. Knockdown of TFRC suppressed the progression of nasopharyngeal carcinoma by downregulating the PI3K/Akt/mTOR pathway. Cancer Cell Int. 23, 185 (2023).
Tay, J. K. et al. The microdissected gene expression landscape of nasopharyngeal cancer reveals vulnerabilities in FGF and noncanonical NF-κB signaling. Sci. Adv. 8, eabh2445 (2022).
Gong, L. et al. Nasopharyngeal carcinoma cells promote regulatory T cell development and suppressive activity via CD70-CD27 interaction. Nat. Commun. 14, 1912 (2023).
He, J., Fan, K. & Yan, X. Ferritin drug carrier (FDC) for tumor targeting therapy. J. Controlled Rel. 311-312, 288–300 (2019).
Tarricone, G. et al. Catalytic Bioswitch of Platinum Nanozymes: Mechanistic Insights of Reactive Oxygen Species Scavenging in the Neurovascular Unit. Nano Lett. 23, 4660–4668 (2023).
Padera, T. P. et al. Lymphatic Metastasis in the Absence of Functional Intratumor Lymphatics. Science 296, 1883–1886 (2002).
Maeda, H., Nakamura, H. & Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Delivery Rev. 65, 71–79 (2013).
Porcel, E. et al. Platinum nanoparticles: a promising material for future cancer therapy? Nanotechnology 21, 085103 (2010).
Katsarou, A. & Pantopoulos, K. Basics and principles of cellular and systemic iron homeostasis. Mol. Aspects Med. 75, 100866 (2020).
Liu, S., Cao, X., Wang, D. & Zhu, H. Iron metabolism: State of the art in hypoxic cancer cell biology. Arch. Biochem. Biophys. 723, 109199 (2022).
Lok, C. N. & Ponka, P. Identification of a hypoxia response element in the transferrin receptor gene. J. Biol. Chem. 274, 24147–24152 (1999).
Tacchini, L., Gammella, E., De Ponti, C., Recalcati, S. & Cairo, G. Role of HIF-1 and NF-κB transcription factors in the modulation of transferrin receptor by inflammatory and anti-inflammatory signals. J. Biol. Chem. 283, 20674–20686 (2008).
Tacchini, L., Bianchi, L., Bernelli-Zazzera, A. & Cairo, G. Transferrin receptor induction by hypoxia: HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J. Biol. Chem. 274, 24142–24146 (1999).
Yang, L. et al. Hypoxic preconditioning increases iron transport rate in astrocytes. Biochim. Biophys. Acta Mol. Basis Dis. 1822, 500–508 (2012).
Komoto, K. et al. Iron chelation cancer therapy using hydrophilic block copolymers conjugated with deferoxamine. Cancer Sci 112, 410–421 (2021).
Fiorillo, M., Tóth, F., Brindisi, M., Sotgia, F. & Lisanti, M. P. Deferiprone (DFP) Targets Cancer Stem Cell (CSC) Propagation by Inhibiting Mitochondrial Metabolism and Inducing ROS Production. Cells 9, 1529 (2020).
Narusaka, T. et al. Nanog is a promising chemoresistant stemness marker and therapeutic target by iron chelators for esophageal cancer. Int. J. Cancer 149, 347–357 (2021).
Neiveyans, M. et al. A recycling anti-transferrin receptor-1 monoclonal antibody as an efficient therapy for erythroleukemia through target up-regulation and antibody-dependent cytotoxic effector functions. mAbs 11, 593–605 (2019).
Campisi, A. et al. Gene Silencing of Transferrin-1 Receptor as a Potential Therapeutic Target for Human Follicular and Anaplastic Thyroid Cancer. Mol. Ther. Oncolytics 16, 197–206 (2020).
Li, J. et al. Ferroptosis: past, present and future. Cell Death Dis. 11, 88 (2020).
Yang, W. S. & Stockwell, B. R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 15, 234–245 (2008).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 59, 298–308 (2015).
Yang, J. et al. Metformin induces Ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J. Exp. Clin. Cancer Res. 40, 206 (2021).
Yu, H. et al. Sulfasalazine‑induced ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the transferrin receptor. Oncol. Rep. 42, 826–838 (2019).
Yang, M. et al. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci. Adv. 5, eaaw2238 (2019).
Su, L.-Y. et al. Anti-tumor immunotherapy using engineered bacterial outer membrane vesicles fused to lysosome-targeting chimeras mediated by transferrin receptor. Cell Chem. Biol. 31, 1219–1230.e1215 (2024).
Jutz, G., van Rijn, P., Santos Miranda, B. & Böker, A. Ferritin: A versatile building block for bionanotechnology. Chem. Rev. 115, 1653–1701 (2015).
Jiang, B., Fang, L., Wu, K., Yan, X. & Fan, K. Ferritins as natural and artificial nanozymes for theranostics. Theranostics 10, 687–706 (2020).
Zhu, X. et al. Engineering single-atom iron nanozymes with radiation-enhanced self-cascade catalysis and self-supplied H2O2 for radio-enzymatic therapy. ACS Nano 16, 18849–18862 (2022).
Zhou, J. et al. Coordination-driven self-assembly strategy-activated Cu single-atom nanozymes for catalytic tumor-specific therapy. J. Am. Chem. Soc. 145, 4279–4293 (2023).
Lu, Y. et al. Epigenetic Inactivation of Acetyl-CoA Acetyltransferase 1 Promotes the Proliferation and Metastasis in Nasopharyngeal Carcinoma by Blocking Ketogenesis. Front. Oncol. 11, 667673 (2021).
Tsao, S. W. et al. Establishment of two immortalized nasopharyngeal epithelial cell lines using SV40 large T and HPV16E6/E7 viral oncogenes. Biochim. Biophys. Acta Mol. Cell Res. 1590, 150–158 (2002).
Busson, P. et al. Establishment and characterization of three transplantable EBV-containing nasopharyngeal carcinomas. Int. J. Cancer 42, 599–606 (1988).
Mrizak, D. et al. Effect of Nasopharyngeal Carcinoma-Derived Exosomes on Human Regulatory T Cells. JNCI J. Natl. Cancer Inst. 107, 363 (2014).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 82122037, K.F.; 81930050, X.Y.; 22121003, X.Y.; U23A20686, K.F.; 32301164, R.Z.), National Key Research and Development Program of China (No. 2021YFC2102900, K.F.), CAS Project for Young Scientists in Basic Research (YSBR-089, K.F.), and Sanming Project of Medicine in Shenzhen (NO. SZSM202211022, X.Y.).
Author information
Authors and Affiliations
Contributions
K.F. and R.Z. conceptualized and designed the study. R.Z. analyzed the mRNA expression data of NPC cohorts and performed hypoxia score analysis. X.Z. and J.Z. performed IHC analysis of clinical NPC samples. R.Z. designed and synthesized Pt-HFn materials and characterized their physiochemical properties and catalytic activity. H.Z. and Z.X.Z. assisted with material synthesis and catalytic activity measurements. R.Z. and Y.S. designed the in vitro and in vivo experimental protocol. J.L. assisted in cell experiments. R.Z. and Y.S. constructed the animal model and performed in vivo experiments. H.J., H.D. and G.N. assisted in animal model construction. S.G. assisted with the in vivo radiotherapy experiments. R.Z. wrote the draft and revised the manuscript. Y.S. and X.Z. assisted in writing the draft. K.F., X.Y., Y.W., and Z.Z. supervised all stages of the study. All authors discussed the manuscript and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yiqiao Hu, Joel Schick and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, R., Shen, Y., Zhou, X. et al. Hypoxia-tropic delivery of nanozymes targeting transferrin receptor 1 for nasopharyngeal carcinoma radiotherapy sensitization. Nat Commun 16, 890 (2025). https://doi.org/10.1038/s41467-025-56134-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56134-z