Abstract
Esophageal squamous cell carcinoma (ESCC) is a common and aggressive cancer with limited responses to immunotherapy. High mobility group A1 (HMGA1), a chromatin remodeling protein, plays a key role in tumor progression, but its impact on anti-tumor immunity in ESCC remains unclear. Here we show that HMGA1 suppresses the stimulator of interferon genes (STING), inhibiting type I interferon secretion, downregulating interferon-stimulated genes, and impairing tumor-infiltrating lymphocyte (TIL) recruitment. HMGA1 inhibits STING transcription by competing with the coactivator CBP/p300 for binding to CREB. ESCCs from genetically modified mouse models with altered HMGA1 and STING expression exhibit varying TIL levels and sensitivity to STING agonists. Additionally, we design and synthesize a series of HMGA1 inhibitors, including a perylene-based nanoparticle, PDIC-DPC, which effectively inhibits HMGA1 and enhances TIL infiltration. Our findings identify HMGA1 as a critical immune checkpoint in ESCC and suggest that targeting HMGA1 could improve immunotherapy outcomes.
Similar content being viewed by others
Introduction
Esophageal cancer (ESCA), encompassing both esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), is one of the most prevalent gastrointestinal malignancies1,2. ESCC, in particular, is more common in Asia and Africa, where it accounts for approximately 90% of ESCA cases3. Patients with ESCC often present with advanced disease at diagnosis, resulting in a generally poor prognosis3,4. Despite significant advancements in targeted therapies and immunotherapy for various cancers, progress in these treatments for ESCA has been relatively slow4,5. In the past decade, only two targeted therapies for advanced EAC have emerged, and advancements in targeted therapy for ESCC have been minimal5. While ESCC may be more amenable to immune-based therapies compared to EAC5, the effectiveness of current immune checkpoint inhibitors, such as anti-PD-1 antibodies (e.g., camrelizumab, toripalimab, sintilimab, and tislelizumab), remains inconclusive6. These agents are being tested across various stages of ESCC in clinical trials, but definitive outcomes are still awaited6. Therefore, identifying new immunotherapy targets and strategies is crucial for improving the prognosis of ESCC patients.
High mobility group A1 (HMGA1) is a structural transcription factor that plays a crucial role in chromatin remodeling7,8,9,10. It binds to AT-rich regions in the minor grooves of DNA, inducing DNA bending that facilitates the binding of transcription factors and regulates gene expression7,11,12. This involvement in chromatin dynamics allows HMGA1 to participate in various cellular functions and processes. Notably, HMGA1 is often overexpressed in cancer cells and is associated with poor prognosis across multiple cancer types13,14,15,16.
The stimulator of interferon genes (STING) is a critical component of the innate immune response and a promising target for cancer immunotherapy17,18,19,20,21,22. STING is activated by cyclic GMP-AMP synthase (cGAS), which detects double-stranded DNA from pathogens or damaged host cells23,24. Upon recognition, cGAS produces the second messenger cGAMP, which activates STING and facilitates its translocation from the endoplasmic reticulum to the Golgi apparatus22,25. Activated STING then recruits and activates TANK-binding kinase 1 (TBK1) through phosphorylation. This, in turn, leads to the recruitment of interferon regulatory factor 3 (IRF3), which is phosphorylated by STING and TBK126,27,28. Phosphorylated IRF3 dimerizes and moves into the nucleus, which drives the transcription of type I interferons (T1IFNs) and interferon-stimulated genes (ISGs)29,30. The secretion of interferons (IFNs) and the expression of ISGs enhance the activation and recruitment of CD8+ T cells, thereby initiating and promoting anti-tumor immunity20,22,31,32. STING activation has been shown to propagate the cancer immunity cycle, reshape the tumor microenvironment, and ultimately contribute to tumor cell elimination, making STING agonists a promising strategy for cancer treatment33,34,35,36. Nonetheless, their effectiveness can be limited in some patients.
In this work, we show that depletion of HMGA1 leads to the upregulation of innate immune signaling factors and several key components in the cGAS/STING pathway. HMGA1 interference increases the transcription of STING, enhances the secretion of IFNs, stimulates the expression of ISGs, and consequently boosts T cell infiltration in the tumor microenvironment. This effect is negated in immune-deficient mice, highlighting the role of the immune system in this process. Notably, treatment with STING agonists in ESCC models—specifically in Hmga1flox/floxK14 (HMGA1 conditional knockout) and Hmga1KI/KIK14 (HMGA1 conditional knock-in) mice—reveals that overexpression of HMGA1 renders the tumors less responsive to these agonists. This suggests that ESCCs with high HMGA1 expression may be resistant to STING agonist therapies. Additionally, we synthesize and characterize a specific HMGA1 inhibitor, PDIC-DPC, based on the binding pocket of HMGA1. This perylene-based nanoparticle not only inhibits lung metastasis of ESCC cells and orthotopic ESCCs but also enhances T lymphocyte infiltration by suppressing HMGA1 expression. Overall, our findings propose a therapeutic strategy for ESCC, involving the inhibition of HMGA1 to upregulate STING and improve immune cell infiltration within the tumor microenvironment.
Results
HMGA1 Inhibits the Expression of ISGs in ESCC Cells
To investigate the role of HMGA1 in the tumorigenesis and development of ESCC, we first assessed HMGA1 expression in various human ESCC cell lines. HMGA1 expression was found to be significantly elevated in KYSE-30 and KYSE-510 cells, whereas its levels were comparatively lower in KYSE-70 and TE-13 cells (Supplementary Fig. 1a, b). To investigate its functional role, we generated HMGA1-knockdown KYSE-30 and KYSE-510 cells using two distinct shRNA duplexes (shHMGA1-30-1, shHMGA1-30-2, shHMGA1-510-1, and shHMGA1-510-2) (Supplementary Fig. 1c–f). Additionally, HMGA1 was overexpressed in KYSE-70 and TE-13 cells using a lentivirus-mediated V3 system (oeHMGA1-70 and oeHMGA1-TE13) (Supplementary Fig. 1g).
RNA sequencing was performed on shNC-30 (control) and shHMGA1-30-1 cells, and KEGG pathway analysis indicated an enrichment of immune regulation-related genes in the HMGA1-knockdown KYSE-30 cells (Fig. 1a and Supplementary Table 1). This suggests that HMGA1 may play a role in immune function regulation in ESCC.
a HMGA1 was knocked down by shHMGA1-1 in KYSE-30 cells, and RNA sequencing was performed on these cells. KEGG pathway analysis shows enrichment of genes involved in the immune system. b Heatmap showing the gene expression levels of inflammation-related cytokines and chemokines. c RNAs were extracted from KYSE-30 cells transfected with shNC, shHMGA1-1, and shHMGA1-2. After reverse transcription, qPCR was performed to detect the expression of interferon-stimulated genes (ISGs) and ISGs-related inflammatory factors. d Supernatants from KYSE-30 cells transfected with shNC, shHMGA1-1, and shHMGA1-2 were collected, and the contents of IFN-α, IFN-β, CXCL10, and CCL5 were measured using ELISA kits. e RNAs were extracted from KYSE-70 cells transduced with either an empty vector (EV) or an HMGA1 overexpression (oeHMGA1) construct. After reverse transcription, qPCR was performed to detect the expression of ISGs and ISGs-related inflammatory factors. f Supernatants from KYSE-70 cells transduced with either EV or oeHMGA1 were collected, and the contents of IFN-α, IFN-β, CXCL10, and CCL5 were measured using ELISA kits. ns, no statistical difference. KEGG: Kyoto Encyclopedia of Genes and Genomes. Data are presented as mean ± SD. Statistical significance was determined by two-tailed unpaired t-test (c–f). Sample sizes: (c, e) n = 4; (d, f) n = 3 independent experiments. Source data are provided in the Source Data file.
Analysis of the RNA sequencing data identified upregulation of several immune-stimulating cytokines following HMGA1 depletion (Fig. 1b and Supplementary Fig. 2). To validate these findings, real-time PCR (qPCR) was conducted on immune-related cytokine genes in HMGA1-manipulated cells. We observed increased expression of interferon-stimulated genes (ISGs), including CXCL10, CCL5, IFIT1, IFIT2, and IFIT3, in shHMGA1-30-2 (Fig. 1c) and shHMGA1-510-2 (Supplementary Fig. 3a) cells compared to control cells. Similarly, levels of IFN-α, IFN-β, CXCL10, and CCL5 were elevated in the supernatant of shHMGA1 cell cultures (Fig. 1d and Supplementary Fig. 3b). While some immune-related genes were upregulated in shHMGA1-30-1 cells (Fig. 1c, d), the changes did not reach statistical significance, likely due to incomplete HMGA1 knockdown.
Conversely, overexpression of HMGA1 in KYSE-70 and TE-13 cells led to reduced mRNA levels of ISGs and decreased contents of IFN-α, IFN-β, CXCL10, and CCL5 in the cell culture supernatant (Fig. 1e, f and Supplementary Fig. 3c, d). These results demonstrate that HMGA1 suppresses immune-responsive genes and reduces the expression of immune-stimulating cytokines in ESCC cells.
HMGA1 Promotes Tumor Growth by Inhibiting T Lymphocyte Infiltration in ESCCs
Chemokines such as CXCL10 and CCL5 are known to promote immune cell infiltration into tumors in response to immune stimuli37. To investigate whether HMGA1-mediated suppression of inflammatory cytokines and chemokines affects T lymphocyte infiltration, we manipulated HMGA1 expression in murine ESCC AKR cells and implanted these cells subcutaneously into C57BL/6 mice. This allowed us to assess tumor growth and T lymphocyte infiltration.
Knockdown of HMGA1 resulted in a significant reduction in tumor growth (Fig. 2a–c). Notably, HMGA1 knockdown also led to increased expression of CD3, CD8, and granzyme B (GzmB), markers of CD8+ T cells, both at the mRNA and protein levels (Fig. 2d, e and Supplementary Fig. 4a). To further characterize T cell infiltration, we performed immunohistochemistry (IHC) on the tumors. This analysis revealed a substantial increase in CD3+, CD8+, and GzmB+ cells in tumors with HMGA1 knockdown (Fig. 2f, g). Flow cytometry analysis of trypsinized tumors from HMGA1-knockdown AKR cells revealed a higher proportion of CD3+ and CD8+ T cells (Fig. 2h and Supplementary Fig. 4b). Moreover, tumor-infiltrating CD8+ T cells isolated from HMGA1-depleted AKR subcutaneous tumors showed significantly elevated expression of GzmB, indicating enhanced activation of these TILs (Fig. 2i).
a–i C57BL/6 mice were subcutaneously inoculated with shNC-AKR and shHMGA1-AKR cells. a Tumors on day 10. b Tumor volume. c Tumor weight. d Cd3, Cd8, and Gzmb mRNA levels in subcutaneous tumors. e The samples derive from the same experiment but different gels for HMGA1, CD3, CD8, GZMB and another for GAPDH were processed in parallel. GAPDH served as loading control. f, g Representative images (f) and quantification (g) of CD3, CD8, and GzmB from IHC staining of subcutaneous tumors. Scale bar: 20 μm. Data points were obtained by examining three distinct visual fields of each mouse tumor slice, with the median value calculated for each field. h Contents of CD3+ and CD8+ T cells in subcutaneous tumors by flow cytometry. i GzmB expression in CD8+ T cells was measured and quantified by flow cytometry. j–n Hmga1flox/floxK14, Hmga1flox/+K14, and Hmga1flox/flox mice were treated with 4NQO to induce ESCC. j Representative images of ESCC. The upper panel shows macroscopy of the esophagus, with circles indicating tumor locations. The bottom panel shows representative H&E staining of esophageal tissues. k Expression of Cd3, Cd8, and Gzmb in ESCCs measured by qPCR. l The samples derive from the same experiment, but different gels for HMGA1, CD3, CD8, GZMB and another for GAPDH were processed in parallel. m, n IHC staining and quantification of CD3, CD8, and GzmB in mouse ESCCs and adjacent esophagus tissue. Scale bar: 20 μm. We evaluated 6 mice in each group, examining the front, middle, and rear sections of each mouse’s esophagus. For each segment, three different visual fields were analyzed, and the median value was used. ns, no statistical difference. Data are presented as mean ± SD. Statistical significance was determined by two-tailed unpaired t-test (b–d, g–i, k, n). Sample sizes: (a–c, f, g) n = 8; (d, e, k, l) n = 3; (h, i) n = 5; (j, m, n) n = 6 independent mice. Image in e, f, i, j, l, m shows representative results from three independent experiments. Source data are provided in the Source Data file.
Conversely, overexpression of HMGA1 accelerated tumor growth (Supplementary Fig. 5a–c) and significantly reduced T cell infiltration into the tumors (Supplementary Fig. 5d–j). Taken together, our results suggest that HMGA1 promotes tumor growth by inhibiting the infiltration and activation of T lymphocytes in ESCC.
To further validate the negative correlation between HMGA1 and T cell infiltration, we generated an orthotopic ESCC model using various mouse genotypes: Hmga1flox/flox (wild-type control), Hmga1flox/+K14 (heterozygous), Hmga1flox/floxK14 (conditional knockout), Hmga1KI/KI (wild-type control for knock-in), and Hmga1KI/KIK14 (conditional knock-in). ESCC was induced by applying 4NQO for 5 months. Conditional knockout of HMGA1 significantly reduced the number of esophageal lesions, decreased esophageal weight, and improved the survival rate of mice (Supplementary Fig. 6a–c). H&E staining revealed typical ESCC in Hmga1flox/flox mice, whereas Hmga1flox/floxK14 mice (HMGA1 conditional knockout) predominantly displayed mild to moderate hyperplasia, with fewer instances of severe hyperplasia or in situ esophageal cancer (Fig. 2j and Supplementary Fig. 6d). Interestingly, compared to Hmga1flox/flox mice, Hmga1flox/+K14 mice exhibited a significantly reduced number of esophageal lesions, a markedly improved survival rate, and a notable alleviation in esophageal cancer malignancy (Supplementary Fig. 6a–d). In contrast, HMGA1 knock-in (Hmga1KI/KIK14) mice developed significantly larger ESCC tumors and severe hyperplasia after the same 4NQO treatment (Supplementary Fig. 7a; see Supplementary Fig. 26b for the percentage of pathological abnormalities).
A negative correlation between HMGA1 expression and the presence of CD3+, CD8+, and GzmB+ cells was confirmed by qPCR and Western blot analysis (Fig. 2k, l and Supplementary Fig. 7b, c). IHC staining demonstrated a marked increase in CD3+, CD8+, and GzmB+ T cells in the orthotopic ESCCs of Hmga1flox/floxK14 (KO) mice (Fig. 2m, n). Similarly, compared to those in Hmga1flox/flox mice, the expression levels of CD8 and GZMB in the orthotopic ESCCs of Hmga1flox/+K14 mice were markedly upregulated (Fig. 2m, n). In contrast, HMGA1 knock-in (Hmga1KI/KIK14) exacerbated tumor severity (Supplementary Fig. 7a) and significantly reduced T cell infiltration in ESCCs (Supplementary Fig. 7d, e).
To further corroborate the role of HMGA1 in suppressing T cell infiltration, we established mouse-derived, immunocompetent, subcutaneous allograft models using ESCCs from genetically engineered mouse models (GEMMs) of HMGA1. In syngeneic mice, knockout of HMGA1 (Hmga1flox/floxK14) significantly inhibited tumor growth and enhanced T cell infiltration into tumors (Supplementary Fig. 8). Conversely, HMGA1 knock-in promoted tumor growth and suppressed T cell infiltration (Supplementary Fig. 9). These results collectively demonstrate that HMGA1 inhibits T cell infiltration while promoting tumor growth.
To assess whether HMGA1 directly regulates ESCC proliferation, we manipulated HMGA1 expression in murine ESCC AKR cells and inoculated them subcutaneously into immunocompromised nude mice. Surprisingly, tumor size did not differ significantly between AKR cells with or without HMGA1 modulation (Supplementary Fig. 10a–d). This suggests that the expression of HMGA1 may not affect the growth of nude mouse xenograft tumors. Due to the limited sample size, further validation in larger-scale studies is necessary. To further investigate the impact of HMGA1 on the proliferation of ESCC, we also conducted an in vitro proliferation assay on murine ESCC cells. Results from the CCK8 assay showed that HMGA1 expression had no effect on the proliferation of AKR cells in vitro (Supplementary Fig. 10e, f). To rule out any tumor-intrinsic effects of HMGA1, we analyzed the PI3K/AKT/mTOR pathway and found that HMGA1 did not influence the activation of these signaling proteins in AKR cells (Supplementary Fig. 10g, h). Collectively, these findings exclude the possibility of tumor-intrinsic effects of HMGA1 in ESCC and reinforce its role in modulating anti-tumor immunity.
HMGA1 negatively regulates STING
To investigate how HMGA1 inhibits immune cell infiltration in ESCC, we analyzed immune-related genes using RNA-seq data from KYSE-30 cells with HMGA1 knockdown. STING expression was significantly upregulated in HMGA1-depleted cells (Fig. 3a). Further qPCR analysis of immune-related genes other than STING in the heatmap revealed that the expression of HMGA1 showed no significant negative correlation with the expression of other immune-related genes (Supplementary Fig. 11). We further examined the correlation between HMGA1 and STING at the transcriptional level in cancer patients by analyzing data from The Cancer Genome Atlas (TCGA). Consistent with our findings, a negative correlation between HMGA1 and STING expression was observed in the TCGA ESCC dataset (Fig. 3b).
a Heatmap showing the expression of genes related to innate immunity in control and shHMGA1-30-1 cells from the RNA-seq analysis. b A cohort of 82 ESCC patients (70 males and 12 females) from the TCGA database was analyzed to evaluate the correlation between HMGA1 and STING expression. The data were sourced directly from aggregated TCGA database entries and used for analysis without modification. c, d Levels of STING in HMGA1-knockdown KYSE-30 cells (c) and HMGA1-overexpressing KYSE-70 cells (d) were analyzed by qPCR. e, f Expression of STING protein in HMGA1-knockdown KYSE-30 cells (e) and HMGA1-overexpressing KYSE-70 cells (f). g Levels of Hmga1 and Sting in ESCCs from Hmga1flox/flox and Hmga1flox/floxK14 mice analyzed by qPCR. h The samples derive from the same experiment, but different gels for HMGA1, STING, and another for GAPDH were processed in parallel. GAPDH served as a loading control. i Levels of Hmga1 and Sting in ESCCs from Hmga1KI/KI and Hmga1KI/KIK14 mice analyzed by qPCR. j The samples derive from the same experiment, but different gels for HMGA1, STING, and another for GAPDH were processed in parallel. k, l Expression and quantification of HMGA1 and STING in the esophagus of Hmga1flox/flox and Hmga1flox/floxK14 mice (k) and Hmga1KI/KI and Hmga1KI/KIK14 mice (l). Mice were treated with 4NQO to induce ESCC. At the end of the induction, murine esophagus tissues were isolated, fixed, and stained via IHC. Representative images are presented in the left panels, and quantifications of IHC staining are shown in the right panels of k and l. Scale bar: 20 μm. TCGA: The Cancer Genome Atlas. Data are presented as mean ± SD. Statistical significance was determined by two-tailed unpaired t-test (c, d, g, i, k, l), or Pearson correlation coefficient (b). Sample sizes: (c, d) n = 4; (e, f) n = 3 independent experiments; (g–j) n = 3; (k, l) n = 6 independent mice. Image in e, f, h, j, k, and l shows representative results from three independent experiments. Source data are provided in the Source Data file.
To validate the correlation between HMGA1 and STING, we conducted qPCR and immunoblot analyses in cells with HMGA1 overexpression and knockdown. Consistent with our expectations, HMGA1 negatively regulated STING expression at both the transcriptional and translational levels (Fig. 3c–f and Supplementary Figs. 12, 13). In the 4NQO-induced orthotopic ESCC model, STING expression was significantly upregulated in the esophageal epithelia of Hmga1flox/floxK14 (KO) mice (Fig. 3g, h), while in HMGA1 knock-in (Hmga1KI/KIK14) mice, STING expression remained low in the esophageal epithelia and cancer tissues (Fig. 3i, j). Similarly, IHC staining confirmed the negative correlation between HMGA1 and STING in esophageal epithelia and cancer tissues in the GEMM model (Fig. 3k, l).
Taken together, our results demonstrate that HMGA1 negatively regulates STING expression, which may contribute to its role in suppressing immune cell infiltration in ESCC.
HMGA1 Inhibits the Activation of the STING Signaling Pathway
STING is a key receptor in innate immunity, activated by recognizing double-stranded DNA (dsDNA)23. To determine whether HMGA1 regulates the activation of the STING signaling pathway, we assessed the expression of phosphorylated STING (p-STING), TBK1 (p-TBK1), and IRF3 (p-IRF3) in HMGA1-knockdown KYSE-30 cells stimulated with poly(dA:dT), a dsDNA mimic. The results showed that HMGA1 knockdown markedly enhanced dsDNA-induced activation of STING signaling, as evidenced by increased phosphorylation of STING, TBK1, and IRF3 (Supplementary Fig. 14a, b).
Activation of the STING-TBK1-IRF3 axis leads to the release of interferon-β (IFN-β) and upregulation of interferon-stimulated genes (ISGs). IRF3 translocation to the nucleus is essential for the production of IFN-β in response to dsDNA stimulation. As expected, HMGA1 knockdown promoted nuclear translocation of IRF3 and increased IFNB expression (Supplementary Fig. 14c–e). Additionally, depletion of HMGA1 significantly upregulated dsDNA-induced expression of the chemokines CXCL10 and CCL5, both of which are induced by IFN-β and are known to promote T cell infiltration into tumors in response to immune stimulation (Supplementary Fig. 14e).
Conversely, enforced expression of HMGA1 in KYSE-70 cells inhibited poly(dA:dT)-triggered activation of the STING pathway (Supplementary Fig. 14f–j). Overexpression of HMGA1 suppressed dsDNA-induced phosphorylation of STING, TBK1, and IRF3 (Supplementary Fig. 14f, g), and also reduced nuclear translocation of IRF3 and expression of IFNB, CXCL10, and CCL5, regardless of poly(dA:dT) treatment (Supplementary Fig. 14h–j). Taken together, these findings indicate that HMGA1 inhibits dsDNA-stimulated activation of the STING signaling pathway, whereas HMGA1 depletion enhances STING pathway activation.
STING Mediates HMGA1-Deregulated Antitumor Immunity in ESCC
To determine the necessity of STING in HMGA1 deficiency-induced antitumor immunity, we knocked down STING in HMGA1-depleted KYSE-30 cells. Consistent with previous findings (Supplementary Fig. 14a, b), HMGA1 knockdown increased the phosphorylation of STING, TBK1, and IRF3 (Fig. 4a and Supplementary Fig. 15a–c). Depletion of STING abolished this HMGA1 knockdown-induced phosphorylation increase (Fig. 4a and Supplementary Fig. 15a–c). qPCR analysis revealed that STING knockdown also reversed the HMGA1 deficiency-induced upregulation of IFNB, CXCL10, and CCL5 (Fig. 4b and Supplementary Fig. 15d, e).
a Western blot analysis of p-STING, p-TBK1, and p-IRF3 in shNC-30 and shHMGA1-30-2 cells co-transfected with shSTING. The samples derive from the same experiment but different gels for HMGA1, STING, p-STING, p-TBK1, TBK1, p-IRF3, IRF3, and another for β-actin were processed in parallel. β-actin served as loading control. b qPCR analysis of inflammatory factors IFNB, CXCL10, and CCL5 in shNC-30 and shHMGA1-30-2 cells transfected with shSTING. c Western blot analysis of STING signaling in EV-70 and oeHMGA1-70 cells co-transfected with oeSTING. The samples derive from the same experiment but different gels for HMGA1, STING, p-STING, p-TBK1, TBK1, p-IRF3, IRF3, and another for β-actin were processed in parallel. β-actin served as loading control. d qPCR analysis of inflammatory factors IFNB, CXCL10, and CCL5 in EV-70 and oeHMGA1-70 cells co-transfected with oeSTING. e–g Depletion of STING abrogates HMGA1 knockout-induced activation of antitumor immunity and alleviation of ESCC tumorigenesis. Hmga1flox/flox, Hmga1flox/floxK14, Sting-/-, and Hmga1flox/floxK14Sting-/- mice were treated with 80 mg/L 4NQO for 5 months to induce ESCC. At the end of the induction, mice were sacrificed, and esophageal tissues were collected for the analysis. e Representative HE staining of esophageal tissues from the mice. Scale bar: 50 μm. f Pathological phenotypes of esophageal tissues in the mice. g Representative IHC staining of HMGA1, STING, CD3, CD8, and GzmB in esophageal tissues from the mice. Scale bar: 20 μm. Data are presented as mean ± SD. Statistical significance was determined by two-tailed unpaired t-test (b, d, g). Sample sizes: (a–d) n = 3 independent experiments; e–g n = 6 independent mice. Image in a, c, e, g shows representative results from three independent experiments. Source data are provided in the Source Data file.
Conversely, enforced expression of HMGA1 reduced the phosphorylation of STING, TBK1, and IRF3, which was restored by STING overexpression (Fig. 4c and Supplementary Fig. 16a, b). Furthermore, overexpression of STING reversed HMGA1-mediated suppression of IFNB, CXCL10, and CCL5 (Fig. 4d and Supplementary Fig. 16c).
To further investigate the role of STING in HMGA1-suppressed CD8+ T cell infiltration in ESCC (Fig. 2k–n), we generated an ESCC model in four groups of mice: Hmga1flox/flox (control, WT), Hmga1flox/floxK14 (HMGA1 conditional knockout), Sting-/-, and Hmga1flox/floxK14Sting-/- (HMGA1 and STING double knockout). ESCCs were induced using 4NQO for 5 months. HE staining showed that Hmga1flox/flox (WT) mice developed moderate to severe hyperplasia, as well as in situ ESCCs (Fig. 4e, f). In contrast, Hmga1flox/floxK14 (HMGA1 conditional knockout) mice exhibited mild to moderate hyperplasia with few instances of severe hyperplasia or in situ ESCCs (Fig. 4e, f). However, both Sting-/- and Hmga1flox/floxK14Sting-/- (double knockout) mice developed severe hyperplasia and invasive ESCCs following 4NQO treatment (Fig. 4e, f). qPCR analysis revealed significant upregulation of Ifnb, Cxcl10, and Ccl5 in esophageal tissues from HMGA1 knockout (Hmga1flox/floxK14) mice compared to WT (Hmga1flox/flox) mice (Supplementary Fig. 17). This upregulation was abolished in Hmga1flox/floxK14Sting-/- (double knockout) mice (Supplementary Fig. 17). IHC staining demonstrated extensive infiltration of CD3+, CD8+, and GzmB+ cells in esophageal tissues (mild to moderate hyperplasia) of HMGA1 knockout (Hmga1flox/floxK14) mice, which was significantly reduced in the double knockout mice (Hmga1flox/floxK14Sting-/-) (Fig. 4g).
Together, these findings suggest that HMGA1 depletion enhances the expression of interferon, ISGs, and downstream inflammatory cytokines, thereby promoting CD8+ T cell infiltration. STING is essential for the activation of antitumor immunity induced by HMGA1 deficiency.
HMGA1 inhibits the transcription of STING
To investigate how HMGA1 regulates STING, we first examined the DNA sensor cGAS, which functions upstream of STING and activates the STING-TBK1-IRF3 signaling axis to induce type I interferon production. Our analysis revealed that cGAS expression was unaffected by HMGA1 manipulation (Fig. 5a and Supplementary Fig. 18a–c). Consistently, cGAS did not mediate the regulation of STING by HMGA1 (Fig. 5a and Supplementary Fig. 18a–c). Since previous studies have shown that HMGA1 facilitates both the repair of oxidatively damaged mitochondrial DNA and non-homologous end joining (NHEJ) DNA repair38,39, we further explored whether HMGA1-induced suppression of STING activation is cGAS-dependent. We knocked down cGAS and observed that cGAS depletion inhibited STING signaling, regardless of HMGA1 expression (Supplementary Fig. 18d). These findings suggest that while HMGA1 may influence cGAS activation through DNA repair, its regulation of STING is likely independent of cGAS.
a Western blot of STING in shNC-30 and shHMGA1-30-2 cells transfected with sicGAS. The samples derive from the same experiment but different gels for HMGA1, STING, cGAS, and another for β-actin were processed in parallel. b Western blot to determine the half-life of STING. Data were quantified using ImageJ. c Interaction between HMGA1 and STING in KYSE-30 cells was determined by co-IP. d EV-30 and oeSTING-30 were transduced with shNC and shHMGA1. Expression of exogenous STING (tagged with FLAG) was detected by western blot. The samples derive from the same experiment but different gels for HMGA1, FLAG-STING, and another for β-actin were processed in parallel. e, f Luciferase reporter assay to detect the effect of HMGA1 silencing (e) or overexpression (f) on the luciferase activity of the STING promoter in ESCC cells. g, h pGL3-STING promoter construct P3 (-372 bp/+376 bp from TSS of STING) with wild-type or mutated CREB binding site was co-transfected with a CREB expression vector into ESCC cells with HMGA1 silencing (g) or overexpression (h). Luciferase activities were measured after 48 h. I ChIP-qPCR detecting HMGA1 binding to the STING promoter. j ChIP-qPCR detecting CREB binding efficiency to the STING promoter in the presence or absence of HMGA1. k, l Co-IP to detect the interaction between HMGA1 and CREB. m Levels of STING in shNC-30 and shHMGA1-30-2 cells treated with siNC or sip300 were determined by qPCR. n, o Co-IP detects that HMGA1 competes with p300 for binding to CREB in ESCC cells with HMGA1 knockdown (n) or overexpression (o). Data are presented as mean ± SD. Statistical significance was determined by two-tailed unpaired t-test (e–j, m). Sample sizes: (a–o) n = 3 independent experiments. Image in a–d, k, l, n, o shows representative results from three independent experiments. Source data are provided in the Source Data file.
To determine whether HMGA1 influences STING stability, we evaluated the half-life of STING in KYSE-30 cells with and without HMGA1 knockdown. The analysis revealed that the half-life of STING was approximately 2 h and remained unaffected by HMGA1 expression (Fig. 5b and Supplementary Fig. 19a). Additionally, co-immunoprecipitation (co-IP) assays showed no direct interaction between HMGA1 and STING (Fig. 5c and Supplementary Fig. 19b). We further transduced a STING-expressing vector driven by a CMV promoter into HMGA1-knocked down cells and observed that ectopically expressed STING was not influenced by HMGA1 depletion (Fig. 5d and Supplementary Fig. 19c). These data suggest that HMGA1 does not regulate STING stability at the protein level or interact directly with STING.
Given the role of HMGA1 as a structural transcription factor that regulates gene expression, we investigated whether it might inhibit STING transcription. RNA sequencing and subsequent validations confirmed that HMGA1 negatively regulates STING mRNA levels (Fig. 3c, d and Supplementary Fig. 13a–d). We cloned the human STING promoter sequence (-2002/ + 1049 relative to the transcription start site) into a firefly luciferase vector (pGL3-basic) (Supplementary Fig. 20a). We then divided the STING promoter sequence into four fragments: P1 (-2002 bp/-1112 bp), P2 (-1130 bp/-293 bp), P3 (-372 bp/+376 bp), and P4 ( + 273 bp/+1049 bp) (Supplementary Fig. 20a) and transfected these constructs into shNC and shHMGA1 cells. Luciferase assays showed that the P3 fragment (-372 bp/+376 bp) exhibited high luciferase activity, which was increased upon HMGA1 depletion and decreased upon HMGA1 overexpression (Fig. 5e, f).
Using the JASPAR database, we identified a binding site for the transcription factor CREB within the P3 fragment of the STING promoter. Previous studies have shown that CREB binds to the STING promoter and promotes its transcription40. We enforced the expression of CREB and found that it overcame HMGA1-mediated suppression of STING transcription, increasing luciferase activity (Fig. 5g, h). Mutation of the CREB binding site (mutant P3) abolished the effect of HMGA1 on STING transcription, and luciferase activity remained low, regardless of HMGA1 and CREB levels (Fig. 5g, h). Chromatin immunoprecipitation (ChIP)-qPCR assays confirmed that HMGA1 binds to the -172 bp/-1 bp region of the STING promoter within the P3 region (Fig. 5i and Supplementary Fig. 20b). Furthermore, ChIP assays using an anti-CREB antibody revealed that HMGA1 knockdown increased CREB binding to the STING promoter (Fig. 5j).
HMGA1 regulates transcription by binding to AT-rich regions of DNA through three AT hook domains7,12. Using a dual luciferase assay, we investigated the role of HMGA1’s AT-hook domains in regulating STING transcription (Supplementary Fig. 20c). Deletion of AT-hook I (21-31 aa) and AT-hook II (32-62 aa) reversed HMGA1-mediated suppression of STING promoter activity, while deletion of AT-hook III (63-89 aa) had no effect (Supplementary Fig. 20d). These results suggest that AT-hook I and AT-hook II are the main functional domains of HMGA1 in regulating STING transcription.
To further investigate the role of CREB in HMGA1-involved STING transcription, we examined its co-activators, CREB-binding protein (CBP) and p300, which are histone acetyltransferases that facilitate transcription by relaxing chromatin structure41,42. CBP/p300 interacts with CREB to fully activate CREB-mediated transcription43. Co-IP assays showed a physical interaction between HMGA1 and CREB (Fig. 5k, l). We hypothesized that HMGA1 competes with CBP/p300 for binding to CREB, thereby inhibiting STING transcription. To test this, we knocked down p300 using siRNA and measured STING transcription in the presence or absence of HMGA1. Depletion of p300 reduced STING transcriptional activity, regardless of HMGA1 expression (Fig. 5m and Supplementary Fig. 20e). Co-IP assays further confirmed that HMGA1 knockdown promoted the interaction between CREB and CBP/p300, while overexpression of HMGA1 reduced their binding (Fig. 5n, o). Together, these findings support our hypothesis that HMGA1 inhibits CREB-mediated transcription of STING by competing with CBP/p300 for binding to CREB.
Depletion of HMGA1 Enhances the Anti-Tumor Effect of STING Agonists
Our previous findings demonstrated that inhibiting HMGA1 promoted STING expression and activated anti-tumor immunity (Supplementary Fig. 14a–e). We speculated that high HMGA1 expression in tumor cells might weaken the efficacy of STING agonists in ESCCs. To test this, we used a syngeneic subcutaneous tumor model with murine AKR cells that had HMGA1 either knocked down or overexpressed in C57BL/6 mice. Mice were treated with the STING agonist 3'3’-cGAMP on days 4 and 7 (Fig. 6a). Tumors with HMGA1 knockdown exhibited significant growth inhibition following 3'3’-cGAMP treatment (Fig. 6b–d and Supplementary Fig. 21a). Chemokines CXCL10 and CCL5, along with the expression of CD3, CD8, and GzmB, were significantly elevated in HMGA1-silenced tumors treated with 3'3’-cGAMP (Supplementary Fig. 21b–e). Immunohistochemistry (IHC) further confirmed increased infiltration of CD3+/CD8+/GzmB+ cells in these tumors (Fig. 6e), a finding that was also supported by flow cytometry analysis (Supplementary Fig. 21f–h). In contrast, overexpression of HMGA1 diminished the anti-tumor effects of 3'3’-cGAMP and reduced CD3+/CD8+/GzmB+ cell infiltration in AKR tumors (Supplementary Fig. 22).
a–e Murine ESCC AKR cells with HMGA1 knocked down were subcutaneously transplanted into the front flank of C57BL/6 mice. Mice received intratumoral injections of 3'3’-cGAMP (10 μg per mouse) and Lipofectamine 2000 (Lip2000) or Lip2000 alone (control treatment) on days 4 and 7. a Schematic outline of 3'3’-cGAMP application in the treatment of subcutaneous ESCC tumors. The schematic diagram were created using Microsoft Office PowerPoint 2019. b Representative images of subcutaneous tumors. c Tumor volume. d Tumor weight. e Representative IHC images and quantitation of CD3, CD8, and GzmB in subcutaneous tumors of AKR cells in each group. Scale bar: 20 μm. f–h Hmga1flox/flox and Hmga1flox/floxK14 mice were treated with 80 mg/L 4NQO in drinking water for a total of 5 months. From the beginning of the third month, mice were injected intravenously (i.v.) with 10 μg of 3'3’-cGAMP and Lip2000 in 100 μL of PBS or Lip2000 alone with PBS (control treatment) every three days for 3 months. f Representative HE staining of esophagus from the mice. Scale bar: 50 μm. g Percentage of pathological alterations in esophageal tissues from the mice. h Representative IHC staining of CD3, CD8, and GzmB in esophageal tissues from the mice. Scale bar: 20 μm. Data are presented as mean ± SD. Statistical significance was determined by two-tailed unpaired t-test (c, d, e, h). Sample sizes: (c–e) n = 9 independent mice (shNC, shHMGA1, shNC+3'3’-cGAMP, and shHMGA1 + 3'3’-cGAMP); n = 5 independent mice (shNC+Lip2000, and shHMGA1+Lip2000); (f–h) n = 6 independent mice. Image in e, f, h shows representative results from three independent experiments. Source data are provided in the Source Data file.
To confirm these findings, we developed another syngeneic mouse allograft model using ESCC tumors from HMGA1 knockout (KO) mice induced with 4NQO. Similar to the AKR model, HMGA1 depletion enhanced the tumor suppressive effects of 3'3’-cGAMP (Supplementary Fig. 23). Collectively, our findings suggest that depleting HMGA1 activates cytotoxic T lymphocytes and inhibits tumor growth, improving the response to STING agonists.
We further tested the STING agonist DMXAA in a subcutaneous tumor model with HMGA1-knocked down AKR cells in C57BL/6 mice. DMXAA also significantly inhibited tumor growth, and CD3+/CD8+/GzmB+ cells were increased in HMGA1-silenced tumors (Supplementary Fig. 24).
In a final set of experiments, HMGA1 knockout and knock-in (KI) mice were treated with 4NQO for 5 months to induce murine ESCC. Mice were treated with 3'3’-cGAMP starting from the first day of the third month post-4NQO induction (Supplementary Fig. 25a). Knockout of HMGA1 significantly reduced tumor progression and increased inflammatory cytokines (Ifnb, Cxcl10, Ccl5) and T cell infiltration (CD3+/CD8+/GzmB+) in esophageal tissue, which were further enhanced by the treatment of 3'3’-cGAMP (Fig. 6f–h and Supplementary Fig. 25b, c). In stark contrast, HMGA1 knock-in mice showed only modest responses to 3'3’-cGAMP, with limited upregulation of cytokines and reduced T cell infiltration (Supplementary Fig. 26).
Together, these results indicate that HMGA1 reduces the anti-tumor efficacy of STING agonists. Depleting HMGA1 enhances the anti-tumor effects of STING agonists by promoting cytotoxic T cell activation and infiltration in tumors.
HMGA1 is Overexpressed in Human ESCCs and Negatively Correlates with STING Expression and T Lymphocyte Infiltration
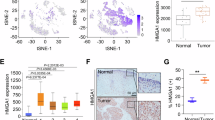
To investigate HMGA1 expression in human ESCCs, we first analyzed single-cell sequencing data from the GSE188900 dataset in the GEO database. We clustered cells in ESCC tissues into various cell types, including epithelial, immune, endothelial, and other tumor microenvironment cells, using classical markers (Fig. 7a). Cells from both adjacent normal and tumor tissues were visualized using t-distributed stochastic neighbor embedding (t-SNE) (Fig. 7b). The analysis revealed that HMGA1 was primarily expressed in malignant epithelial cells across all stages of ESCC (Fig. 7c–e and Supplementary Fig. 27), suggesting that HMGA1 plays a role from the early stages of the disease. In contrast, STING expression was notably low in the malignant epithelial cells of the ESCC clusters (Fig. 7e and Supplementary Fig. 27).
a, b tSNE map of esophageal cells from ESCC patients (scRNA-seq, GSE188900). c HMGA1 expression is higher in ESCCs than adjacent normal tissues. d HMGA1 levels across ESCA grades (TCGA). The data are presented as boxplots, which display the minimum, 25th percentile, median, 75th percentile, and maximum values. Normal: 8 males and 5 females; Grade 1: 14 males and 5 females; Grade 2: 68 males and 8 females; Grade 3: 42 males and 6 females. e HMGA1 is predominantly expressed in epithelial cells (tumors), while STING expression is low (GSE188900). f–k Human ESCCs and adjacent normal esophageal epithelia were obtained from 123 ESCC patients. f Representative IHC staining of HMGA1 and STING in human ESCCs and adjacent normal esophageal tissues (n = 123). Scale bar: 20 μm. g Percentage of HMGA1 and STING positive cells in ESCCs and adjacent normal esophageal tissues. At least 500 cells were counted in each sample. No or weak staining was counted as negative; moderate or strong staining was counted as positive (n = 123). h Correlation between HMGA1 and STING from IHC staining of ESCCs in the patient cluster (n = 123). i Serial pathological sections used for detection of HMGA1, STING, CD3, CD8, and GzmB by IHC staining (n = 6 independent experiments). Scale bar: 20 μm. j, k Percentage of STING, CD3, CD8, and GzmB positive cells in HMGA1-low and HMGA1-high expression ESCCs (j) and their correlation with HMGA1 expression (k). At least 500 cells were counted in each sample. No or weak staining was counted as negative (low) expression; moderate or strong staining was counted as positive (high) expression (n = 123). TCGA: The Cancer Genome Atlas; scRNA-seq: single cell RNA-seq. Data are presented as mean ± SD. Statistical significance was determined by two-tailed unpaired t-test (c, d, g, j, k). Image in (f, i) shows representative results from three independent experiments. Source data are provided in the Source Data file.
To further characterize HMGA1 expression in human ESCCs, we performed immunohistochemistry (IHC) on ESCC tissues. HMGA1 levels were low in adjacent normal esophageal tissues but markedly higher in malignant tissues (Fig. 7f, g), with 69.1% of ESCC cases showing strong HMGA1 positivity (Fig. 7h). In the same patient cohort, we assessed STING expression and found that it was predominantly located in stromal and epithelial cells, with lower levels in the malignant epithelial cells compared to adjacent normal tissues (Fig. 7f, g). Notably, high STING expression was associated with low HMGA1 levels, and vice versa (Fig. 7h), suggesting an inverse correlation between the two proteins in ESCC.
To validate the impact of HMGA1 on STING expression and T lymphocyte infiltration, we examined the presence of CD3+, CD8+, and GzmB+ T cells in human ESCC tissue sections of the same patient clusters. Tumors with high HMGA1 and low STING expression exhibited low T cell infiltration, whereas tumors with low HMGA1 and high STING showed marked T cell infiltration (Fig. 7i, j). Statistical analysis revealed a significant negative correlation between HMGA1 expression and STING levels, as well as with the number of CD3+, CD8+, and GzmB+ cells (Fig. 7k).
In conclusion, our findings reveal that HMGA1 is overexpressed in ESCC and is inversely correlated with STING expression and T lymphocyte infiltration, highlighting a potential mechanism through which HMGA1 promotes immune evasion in these tumors.
Synthesis and Characterization of the HMGA1 Inhibitor PDIC-DPC, Which Suppresses HMGA1 Expression and Promotes T Lymphocyte Infiltration
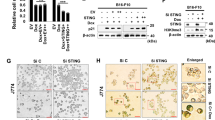
Given the high expression of HMGA1 in ESCC and the lack of specific inhibitors, developing HMGA1 inhibitors holds significant therapeutic potential for ESCC treatment. Perylene, a member of the polycyclic aromatic hydrocarbon (PAH) family, has a planar conjugated structure that can inhibit DNA replication by intercalating between base pairs, thus suppressing cell growth44,45,46. Based on its structural properties, we synthesized a series of perylene-based HMGA1 nano-inhibitors by modifying its backbone. After screening these derivatives, we identified PDIC-DPC, a nanomedicine with strong inhibitory activity against HMGA1 (Fig. 8a–d and Supplementary Fig. 28a, b).
a Chemical structure of PDIC-DPC drawn using ChemDraw. b A model showing the binding of PDIC-DPC to HMGA1. The mol2 structure file of PDIC-DPC was constructed using ChemDraw and OpenBabel, and molecular docking with HMGA1 was performed using Autodock 4. H-bonds between PDIC-DPC and HMGA1 are depicted as yellow dashed lines, with amino and oxygen atoms of PDIC-DPC forming H-bonds with Arg45 and Gly48 residues of HMGA1. c AKR cells were treated with different concentrations PDIC-DPC for 48 h. Cell survival was assessed using CCK8 assay, and the IC50 value was calculated using GraphPad Prism software 8. d AKR cells were treated with different concentrations PDIC-DPC for 10 h. HMGA1 and STING expression was analyzed by western blot. The samples derive from the same experiment but different gels for HMGA1, STING, and another for β-actin were processed in parallel. e–l Pulmonary metastasis model of AKR cells established by intravenous injection (i.v.) of AKR and oeHMGA1-AKR cells. Mice were treated with PBS or PDIC-DPC (2 mg/kg) by i.v. every 3 days. Lungs were harvested 16 days post-injection. e Body weight (BW) of mice. f Survival rate of mice. g, h Representative images of isolated lungs and HE staining of lung sections. Scale bar: 100 μm. i, j Number of metastatic nodules in the lungs. k, l Representative IHC staining of HMGA1, CD3, CD8, and GzmB in lung metastatic foci and quantification. At least 500 cells were counted per sample. No or weak staining was counted as negative (low) expression, while moderate or strong staining was counted as positive (high) expression. Scale bar: 50 μm. Data are presented as mean ± SD. Statistical significance was determined by two-tailed unpaired t-test (e, i, j, k, l) or Gehan-Breslow-Wilcoxon test (f). Sample sizes: (c, d) n = 3 independent experiments; (e, g–l) n = 5; (f) n = 6 independent mice. Image in d, g, h, k, l shows representative results from three independent experiments. Source data are provided in the Source Data file.
Molecular docking analysis revealed that the amino and oxygen atoms of PDIC-DPC formed hydrogen bonds with Arg45 and Gly48 residues of HMGA1 (Fig. 8a, b). In in vitro cell viability assays, the IC50 of PDIC-DPC against murine AKR cells and human KYSE-30 cells was 0.542 µmol/L and 0.709 µmol/L, respectively (Fig. 8c and Supplementary Fig. 28a). Treatment of AKR cells with increasing concentrations of PDIC-DPC resulted in a decrease in HMGA1 levels and a corresponding increase in STING expression (Fig. 8d and Supplementary Fig. 28b).
To evaluate the in vivo effects of PDIC-DPC, we established a pulmonary metastasis model by injecting AKR and HMGA1-overexpressing AKR cells into C57BL/6 mice and treated them with PBS or PDIC-DPC (2 mg/kg). PDIC-DPC treatment significantly prolonged survival and reduced lung metastasis in both models, without affecting the body weight of the mice (Fig. 8e–j). Immunohistochemistry (IHC) of lung tissue revealed decreased HMGA1 expression and increased infiltration of CD3+, CD8+, and GzmB+ T cells following PDIC-DPC treatment (Fig. 8k, l). Flow cytometry confirmed the increase in CD3+ and CD8+ T cells, as well as GzmB expression in tumor-infiltrating CD8+ T cells (Supplementary Fig. 29). Interestingly, PDIC-DPC did not affect the serum levels of CXCL10, CCL5, IFN-β, or IFN-γ, and there were no signs of systemic immune activation or colon inflammation (Supplementary Fig. 30).
In contrast, when mice injected with HMGA1-knockdown AKR cells were treated with PDIC-DPC, no significant changes in the body weight of the mice, tumor burden, T cell infiltration, or inflammatory markers were observed (Supplementary Fig. 31). These findings demonstrate that PDIC-DPC specifically targets HMGA1 to promote T lymphocyte infiltration and suppress lung metastasis in ESCC.
To explore the therapeutic potential of PDIC-DPC in primary ESCC, we generated an orthotopic ESCC model using Hmga1KI/KI (wild-type control) and Hmga1KI/KIK14 (HMGA1 conditional knock-in) mice, treated with 4NQO to induce ESCC over 5 months. Mice were administered PDIC-DPC starting from the first day of the second month post-4NQO induction (Supplementary Fig. 32a). 4NQO induction led to typical ESCC pathology, including hyperplasia and invasive carcinoma. HMGA1-knock-in mice displayed more aggressive pathology, but PDIC-DPC treatment reduced tumor burden, normalizing the pathology in both wild-type and HMGA1-knock-in mice (Supplementary Fig. 32b, c). IHC showed that PDIC-DPC significantly suppressed HMGA1 expression and enhanced T cell infiltration in esophageal tissues (Supplementary Fig. 32d, e).
In summary, PDIC-DPC is a potent HMGA1 inhibitor that suppresses malignant progression of ESCC by promoting anti-tumor immunity, specifically enhancing T lymphocyte infiltration in tumors and inhibiting metastasis.
Discussion
HMGA1 is a structural transcription factor that is highly expressed in a variety of cancers, including ESCC11,47,48,49. However, the molecular mechanisms by which HMGA1 promotes ESCC tumorigenesis and its potential role in targeted therapy remain largely uncharacterized. In this study, we conducted RNA-seq analysis of ESCC cells with HMGA1 knockdown and found that its downregulation led to increased expression of inflammatory cytokine genes and enhanced the enrichment of immune-related signaling pathways. Using an orthotopic ESCC model in HMGA1 knockout and knock-in mice, we demonstrated that conditional knockout of HMGA1 in esophageal epithelial cells inhibited tumorigenesis, delayed ESCC malignant progression, and promoted CD8+ T cell infiltration into the tumors (Fig. 2j–n). Conversely, knock-in of HMGA1 accelerated ESCC progression and significantly reduced CD8+ T cell infiltration (Supplementary Fig. 7). These findings underscore a critical role of HMGA1 in suppressing anti-tumor immunity in ESCC.
HMGA1 is known to participate in various biological processes, including transcriptional regulation7, tumorigenic transformation7,15,50, DNA damage repair51, and cell cycle control52,53. These functions are largely driven by HMGA1’s ability to alter chromatin structure and regulate gene expression7. CBP and p300, multifunctional transcriptional coactivators, regulate gene expression across mutiple cell processes54,55,56. These proteins interact with CREB to activate CREB-mediated transcription, and it has been shown that both CBP and p300 can acetylate HMGA157. Interestingly, CREB also promotes the transcription of STING40. Our results reveal that HMGA1 competes with CBP/p300 for binding to CREB, thereby inhibiting CREB-mediated STING transcription (Fig. 5k–o). This insight into STING regulation adds a layer of understanding and could inform STING-based immunotherapy strategies.
A key mechanism of innate immune detection in cancer is the activation of the STING signaling pathway, which bridges innate and adaptive immunity in tumors and holds great promise for cancer immunotherapy58,59,60,61. Activation of STING induces IFN-β secretion, which enhances tumor antigen presentation by dendritic cells, activates CD8+ T cells, recruits macrophages, and facilitates NK cell-mediated tumor killing58,62,63,64. Cancer cells may evade immune surveillance by silencing the STING pathway65,66,67. In this study, we observed markedly lower STING expression in ESCC tumors compared to adjacent normal tissues (Fig. 7f, g). Furthermore, we demonstrated that HMGA1 inhibited STING expression, reduced STING-mediated immune responses, suppressed CD8+ T cell infiltration, and promoted immune evasion in ESCC. Using mouse models, we established that STING is essential for HMGA1 function, as STING knockout abrogated the tumor-suppressive effects of HMGA1 depletion.
While this study focused primarily on the effects of HMGA1 on CD3+/CD8+/GzmB+ cells, future research should explore whether HMGA1 also impacts the infiltration of other immune cell types in ESCC. Additionally, since STING plays a role in antiviral immunity, HMGA1’s regulation of STING may also have implications for antiviral responses, a promising area for further study.
Preclinical studies suggest that STING agonists can activate type I interferon-dependent adaptive immune responses17,68,69. However, resistance to STING agonist monotherapy has been observed59,70,71,72. Our results showed that STING agonists such as 3'3’-cGAMP and DMXAA markedly suppressed tumor growth and increased CD8+ T cell infiltration in HMGA1-deficient tumors, but HMGA1-overexpressing tumors were relatively insensitive to STING agonists (Fig. 6 and Supplementary Figs. 21–26). These findings suggest that HMGA1 levels could serve as a predictive marker for the efficacy of STING agonist therapy in ESCC, with high HMGA1 expression indicating a potential lack of response.
In this study, we also synthesized and characterized a specific HMGA1 inhibitor, PDIC-DPC. In both the syngeneic ESCC pulmonary metastasis model and the 4NQO-induced orthotopic ESCC model, PDIC-DPC treatment resulted in significant reductions in HMGA1 expression, increased infiltration of CD3+/CD8+/GzmB+ cells, and inhibition of ESCC progression and lung metastasis. While we did not fully explore the specific mechanisms by which PDIC-DPC inhibits HMGA1 expression, our data suggest that the inhibitor may reduce HMGA1 stability. Further investigation into the precise mechanism of PDIC-DPC’s inhibition of HMGA1, and its potential for combination therapies with STING agonists and immune checkpoint inhibitors, could provide therapeutic strategies for ESCC.
In conclusion, our study demonstrates that HMGA1 inhibits STING transcription by competing with the transcriptional coactivators CBP/p300 for CREB binding. This leads to a decrease in STING-mediated immune signaling, reducing T lymphocyte infiltration in tumors and facilitating ESCC progression. Additionally, we identified PDIC-DPC as a potent HMGA1 inhibitor, offering a promising therapeutic approach for targeting HMGA1 in ESCC treatment (Supplementary Fig. 33).
Methods
Ethical approval and consent to participate
All animal experiments were conducted in accordance with the institutional guidelines, and the protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Henan University, China. The registration number is HUSOM2022-133. All animal protocols were conducted in accordance with institutional guidelines and the Laboratory Animal Center of Henan University. The study complies with ethical regulations for research involving animals and human subjects. The collection and use of clinical materials and human samples were approved by the Institutional Research Ethics Committee of the People’s Hospital of Anyang City. All of the patients have provided written informed consent.
Human ESCC tissue samples
ESCC tissue specimens, including tumor and adjacent normal tissues, were collected from patients diagnosed with ESCC in Beiguan District, Anyang City, Henan Province. The use of these tissues was approved by the Ethics Committee of Henan University. All of the patients have provided written informed consent.
Cell lines and reagents
The human ESCC cell lines KYSE-30, KYSE-70, KYSE-140, KYSE-510, TE-13, EC109, and EC9706 were obtained from the American Type Culture Collection (ATCC) and maintained in RPMI 1640 medium (Corning, 10,040-CVR), supplemented with 10% fetal bovine serum (FBS, Lonsera, S711-011S), under standard conditions (5% CO2, 37 °C). The mouse ESCC cell line AKR and human embryonic kidney 293 T (HEK293T) cells were obtained from Otwo Biotech (China) and cultured in Dulbecco’s Modified Eagle Medium (DMEM, Corning, 10-013-CV) with 10% FBS. To prevent mycoplasma contamination, a PCR-based method is employed to test the cells every two months. Briefly, after culturing the cells for three days, antibiotic-free culture supernatant is collected, and DNA is extracted using a silica column purification kit (Tiangen, China). PCR amplification is then performed according to the manufacturer’s protocol, and the products are analyzed by agarose gel electrophoresis. If no band is observed at 515-525 bp, the tested supernatant is considered free of mycoplasma and used for subsequent experiments.
Poly(dA:dT), Lipofectamine™ 2000, and 3'3’-cGAMP were purchased from Invitrogen. Cycloheximide (CHX) was procured from Sigma (Shanghai, China). 4-nitroquinoline N-oxide (4NQO) and 5,6-dimethylxanthenone-4-acetic acid (DMXAA) were acquired from Meilunbio (Dalian, China).
Subcutaneous and lung metastasis models of ESCC in mice
Experiments involving mice were housed in an animal barrier facility at Henan University. The housing conditions kept with suitable temperature (18-23 °C) and humidity (22-50%), a 12-h light/dark cycle, and adequate food and water provided ad libitum. For the subcutaneous tumor model, 5 × 10^6 control or HMGA1-modified AKR cells suspended in 100 μL of PBS were injected into the right flank of C57BL/6 (6–8 weeks old, n = 4 per sex), while 1 × 10^6 control or HMGA1-modified AKR cells suspended in 100 μL of PBS were administered to nude mice (6–8 weeks old, 4 males). All animals were sourced from the Animal Center of the National Science Council (Beijing, China). Tumor size was measured three times a week using a caliper. Tumor volume was calculated with the formula (L × W²) / 2, where L is the length and W is the width of the tumor. Euthanasia was performed either at the designated time point or when the tumor volume reached the permissible endpoint size (1.5 cm³). All animal experiments were conducted in accordance with institutional guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) of Henan University, China (registration number: HUSOM2022-133). All procedures were carried out in compliance with the standards of the Laboratory Animal Center of Henan University.
To establish the syngeneic lung metastasis model, 5 × 10^5 control or HMGA1-modified AKR cells were injected intravenously into C57BL/6 mice (6 - 8 weeks old, 5 males). The body weight and activity of mice were monitored every other day. After 4 days, therapeutic agents were administered to the mice. Since lung cancer in mice with lung metastases is not directly visible throughout their lifespan, the sacrifice time was determined based on weight loss and behavioral observations. Mice were euthanized if they lost 20% of their pre-experiment body weight, showed signs of severe debilitation, or were on the verge of death, such as being unable to move, and/or body condition scoring (BCS)73 reached 2.0.
Genetically engineered mouse model (GEMM) of HMGA1 and induction of ESCC in mice
Experiments involving mice were housed in an animal barrier facility at Henan University. The housing conditions kept with suitable temperature (18-23 °C) and humidity (22-50%), a 12-h alight/dark cycle, and adequate food and water provided ad libitum. Sting-/-, Hmga1flox/floxK14 conditional knock-out, and Hmga1KI/KIK14 conditional knock-in mice were C57BL/6 background and originally obtained from Cyagen (Suzhou, China) and maintained in the laboratory for crossbreeding. Hmga1flox/floxK14Sting-/- double knock-out mice were generated by crossbreeding Sting-/- and Hmga1flox/floxK14 mice in the laboratory. Control and genetically modified mice (6 - 8 weeks old, 3 males and 3 females) were exposed to 80 mg/L 4NQO in drinking water for 5 months to ESCCs. For mice treated with 3’3’-cGAMP, therapeutic interventions began on the first day of 3rd month following induction. The murine condition and body weight were monitored weekly72. Since 4NQO-induced in situ esophageal carcinoma is not directly visible throughout the lifespan of mice, the sacrifice time was determined based on weight loss and behavioral observations. Mice were euthanized if they lost 20% of their pre-experiment body weight, exhibited severe debilitation, or were on the verge of death, such as being unable to move. Euthanasia was also performed if their body condition scoring (BCS)73 reached 2.0.
Establishment of a syngeneic mouse allograft model with tumors originated from murine primary ESCCs
Experiments involving mice were housed in an animal barrier facility at Henan University. The housing conditions were kept with suitable temperature (18–23 °C) and humidity (22–50%), a 12-h light/dark cycle, and adequate food and water provided ad libitum. Hmga1flox/flox (control for KO, wild-type), Hmga1flox/floxK14 (HMGA1 conditional knockout), Hmga1KI/KI (control for KI, wild-type), and Hmga1KI/KIK14 (HMGA1 conditional knock-in) mice were treated with 80 mg/L 4NQO in drinking water for 5 months to induce ESCCs. After the induction period, the mice were sacrificed, and esophageal tumors were excised. The resected esophageal tumors were cut into 2–5 mm pieces. Each tumor fragment was implanted subcutaneously into the right flank of C57BL/6 mice (6–8 weeks old, 5 males). When the syngeneic allograft tumors reached approximately 500 mm³, the tumors were resected and transplanted into additional mice74,75. Mice bearing third-passage grafts were used to evaluate the effect of HMGA1 on tumor growth. Euthanasia was performed when the specified time point was reached or when the tumor volume reached the permissible endpoint size (1.5 cm³).
Therapeutics of the ESCC mice
3′3′-cGAMP was administered to mice via intratumoral or intravenous (i.v.) injection. For intratumoral injections, mice were randomly assigned to control or treatment groups. Each treatment mouse received 10 μg of 3′3′-cGAMP mixed with 3 μL of Lipofectamine 2000 in 100 μL PBS, which was injected directly into the tumor once every 3 days76. PBS containing Lipofectamine 2000 was used as the vehicle control. For treating orthotopic ESCC, 3′3′-cGAMP was administered through i.v. injection, where 10 μg of 3′3′-cGAMP and 3 μL of Lipofectamine 2000 in 100 μL PBS were injected intravenously once every 3 days, as detailed in the figure legends72. Additionally, DMXAA (25 mg/kg) dissolved in 100 μL PBS was administered intraperitoneally (i.p.) every other day.
For PDIC-PDC-mediated therapy in the lung metastasis model of ESCC, control and HMGA1-manipulated AKR cells were injected intravenously to establish the syngeneic mouse model, as described earlier. Four days after the tumor cell transplantation, 2 mg/kg of PDIC-PDC, dissolved in 100 μL PBS, was administered every 3 days until the conclusion of the experiment. In the treatment of mouse orthotopic ESCC, 2 mg/kg of PDIC-PDC, also dissolved in 100 μL PBS, was administered starting from the first day of the second month post-4NQO induction. This agent was i.v. injected every 10 days for a total of 4 months. Throughout the experiment, the health status and body weight of mice were monitored weekly.
Plasmid construction
HMGA1, STING, and CREB overexpression plasmids were constructed using the V3-HA-Flag vector. Human HMGA1, STING, and CREB genes were amplified by PCR from cDNA and cloned into the lentivirus vector, lentiCRISPR V3-Puro, with N-terminal HA and Flag tags using the restriction enzymes XhoI and EcoRI. To generate a STING promoter luciferase reporter plasmid, the promoter region of the STING gene (-2002/ + 1049 relative to the transcription start site) was amplified from human genomic DNA by PCR and sub-cloned into the MluI-XhoI sites of the pGL3-basic vector.
Lentiviral transduction
HEK293T cells were transfected with shHMGA1, HMGA1-HA-FLAG, STING-HA-FLAG, or empty vector along with the packaging vectors pSPAX2 and pMD2G in serum-free medium. After 8 h, the medium was replaced with DMEM supplemented with 10% FBS. Forty-eight hours post-transfection, the supernatants were collected and centrifuged for subsequent infection of target cells.
Small Interfering RNA (siRNA) Transfection
siRNAs targeting cGAS and STING were transfected using Lipofectamine™ 2000, and cells were incubated for 48 h before proceeding with functional assays. The sequences of siRNAs were listed in Supplementary Table 2. All siRNAs were synthesized by GenePharma (Shanghai, China).
Total RNA Extraction and RT-qPCR
Total RNA was extracted from cells or tissues using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. RNA was reverse-transcribed into cDNA using the PrimeScript™ RT reagent kit with gDNA Eraser (TaKaRa). Real-time PCR was performed on the Roche LightCycler 480 system using SYBR Premix Ex Taq (TaKaRa). The PCR primer sequences are listed in Supplementary Table 2. Amplification conditions were 95 °C for 30 sec, followed by 58 °C for 30 sec, and 72 °C for 30 sec for a total of 40 cycles.
Western blotting
Cells were washed with 1× PBS and lysed in RIPA buffer (Solarbio, China) supplemented with phosphatase and protease inhibitors (Roche). The lysates were incubated on ice for 30 min and then centrifuged at 12,000 × g for 15 min at 4 °C. The supernatant was mixed with loading buffer and denatured at 100 °C for 10 min. Whole-cell extracts (15 µg) were resolved by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, USA). Membranes were blocked with 5% BSA (Solarbio, China) in tris-buffered saline with 0.1% Tween-20 (TBST) for 1 h at room temperature, followed by overnight incubation with primary antibodies. After incubation with secondary anti-rabbit or anti-mouse antibodies for 1 h, the immune complexes were detected using a chemiluminescence method. The antibodies used in this study were listed in Supplementary Table 3.
Co-Immunoprecipitation (Co-IP)
Five hundred micrograms of cell lysates were incubated overnight at 4 °C with antibodies. The lysates were then incubated with pre-washed protein A/G beads (sc-2003, Santa Cruz) for 2 h at 4 °C. Immunoprecipitated complexes bound to the beads were washed five times with 1 mL of cold washing buffer, then analyzed by western blotting. The antibodies used in this study were listed in Supplementary Table 3.
RNA-seq analysis
Total RNA was extracted from shNC and shHMGA1-1 cells for RNA sequencing (RNA-seq) analysis. cDNA libraries were constructed and sequenced by Frasergen (Wuhan, China).
Immunohistochemistry (IHC)
Esophageal tissue samples from ESCC patients or mice were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at a thickness of 5 µm. The sections were deparaffinized using xylene and rehydrated through a graded ethanol series. Antigen retrieval was performed by microwave heating in 10 mM citrate buffer (pH 6.0) at high power for 7.5 min followed by medium power for another 7.5 min. Sections were cooled to room temperature, blocked with 3% BSA in PBST for 30 min, and incubated overnight at 4 °C with primary antibodies. After washing, sections were incubated with HRP-conjugated secondary antibodies for 30 min at room temperature. Staining was visualized using diaminobenzidine (DAB) as the chromogen, and sections were counterstained with hematoxylin for 3 min. Stained sections were examined using a Zeiss light microscope, and images were processed using Axiovision40 software. All tumor sections were reviewed by two independent pathologists. Quantifications were performed using ImageJ software. The percentage of positively stained cells was calculated for at least three slides per mouse, and results were statistically analyzed using GraphPad Prism 8. P-values were derived from Student’s t-test.
Flow cytometry
Mouse tumor tissues were excised, minced, and digested at 37 °C for 1 h in a solution containing 500 μL collagenase IV (2 mg/mL, Solarbio), 200 μL DNase I (5 mg/mL, Solarbio), and 300 μL cell culture medium. The digested tissues were filtered through a 70-μm cell strainer (Corning), washed with PBS, and treated with 1× RBC lysis buffer to eliminate red blood cells. Cells were stained anti-mouse CD3e-APC-Cy7 (clone 145-2C11, BD Pharmingen; Cat #550627), anti-mouse CD8a-FITC (clone 53-6.7, BD Pharmingen; Cat #553030), anti-mouse GzmB-PE (clone QA16A02, Biolegend; Cat #372207). Samples were analyzed using a BD LSRFortessa flow cytometer, and the data were processed using FlowJo software.
Immunofluorescence
shNC, shHMGA1, EV, and oeHMGA1 cells were stimulated with 1 μg/mL poly(dA:dT) for 12 h. Cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with PBS containing 0.2% Triton X-100 for 15 min, and blocked with 2% BSA for 1 h at room temperature. The cells were incubated overnight at 4 °C with anti-IRF3 antibody, followed by a 1-h incubation at room temperature with Alexa Fluor® 488-conjugated goat anti-rabbit secondary antibody (ab150077, Abcam; 1:1000). Cells were then stained with DAPI and observed using a Zeiss LSM 980 confocal microscope (Zeiss, Germany).
Dual Luciferase reporter assay
Cells were seeded in a 96-well plate at a density of 3,000 cells per well and transiently transfected with 0.1 µg of luciferase and renilla reporter plasmids per well. Forty-eight hours after transfection, luciferase activity was measured using the Duo-Lite Luciferase Assay System Kit (DD1205-01, Vazyme) according to the manufacturer’s protocol.
Chromatin immunoprecipitation (ChIP)-qPCR assay
Cells were grown to a density of approximately 1 × 107 and crosslinked with 1% paraformaldehyde at 37 °C for 10 min to fix protein-DNA interactions. Glycine (0.125 M) was added to neutralize the paraformaldehyde, and cells were washed twice with ice-cold PBS. The cell pellets were collected, resuspended in lysis buffer, and sonicated to fragment the nuclear membrane and genomic DNA. Immunoprecipitation of chromatin was performed using anti-HMGA1, anti-CREB, or control IgG. Following immunoprecipitation and washing, purified DNA was subjected to qPCR. The primer sequences used for amplifying the human STING promoter were listed in Supplementary Table 2.
Statistical analysis
All data are presented as the mean ± SD from at least three independent experiments or biological replicates. The individual data points represent biological replicates. For experiments requiring statistical analysis, each was repeated at least three times, yielding similar results. Statistical analyses were conducted using Prism 8 software (GraphPad Software). Comparisons between two groups were analyzed using an unpaired Student’s t-test. For correlation analysis, Pearson correlation coefficients were calculated if the data followed a normal distribution, determined by an appropriate normality test. If the normal distribution assumption was not met, Spearman correlation analysis was applied. Kaplan-Meier survival curves were analyzed using the Gehan-Breslow-Wilcoxon test to assess differences between groups. RNA-seq statistical analysis was performed using the DESeq2 package in R, applying a negative binomial distribution to calculate p-values. Results with p < 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA-seq data generated in this study have been deposited in the Gene Expression Omnibus (GEO) database [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE263439] under the accession number of GSE263439. Gene Expression Profiling Interactive Analysis was used for HMGA1 expression data. The single-cell sequencing data is deposited in GEO database [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE188900] under the accession number of GSE188900. All data supporting the current study are provided in the article, Supplementary Information, and Source data. Source data are provided with this paper.
References
Yang, Y. M., Hong, P., Xu, W. W., He, Q. Y. & Li, B. Advances in targeted therapy for esophageal cancer. Signal Transduct. Target Ther. 5, 229 (2020).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249 (2021).
Morgan, E. et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology 163, 649–658.e2 (2022).
Yang, H., Li, X. & Yang, W. Advances in targeted therapy and immunotherapy for esophageal cancer. Chin. Med J. (Engl.) 136, 1910–1922 (2023).
Rogers, J. E., Sewastjanow-Silva, M., Waters, R. E. & Ajani, J. A. Esophageal cancer: emerging therapeutics. Expert Opin. Ther. Targets 26, 107–117 (2022).
Li, N. & Sohal, D. Current state of the art: immunotherapy in esophageal cancer and gastroesophageal junction cancer. Cancer Immunol. Immunother. 72, 3939–3952 (2023).
Wang, L. et al. High mobility group A1 (HMGA1): structure, biological function, and therapeutic potential. Int J. Biol. Sci. 18, 4414–4431 (2022).
Vignali, R. & Marracci, S. HMGA genes and proteins in development and evolution. Int. J. Mol. Sci. 21, 654 (2020).
Olan, I. et al. HMGA1 orchestrates chromatin compartmentalization and sequesters genes into 3D networks coordinating senescence heterogeneity. Nat. Commun. 15, 6891 (2024).
Lau, R. I. et al. Gut microbiota in COVID-19: key microbial changes, potential mechanisms and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 1-15, 10.1038/s41575-022-00698-4 (2022).
Li, L. et al. HMGA1 chromatin regulators induce transcriptional networks involved in GATA2 and proliferation during MPN progression. Blood 139, 2797–2815 (2022).
Saitoh, Y. & Laemmli, U. K. Metaphase chromosome structure: bands arise from a differential folding path of the highly AT-rich scaffold. Cell 76, 609–622 (1994).
Li, Z. et al. HMGA1 augments palbociclib efficacy via PI3K/mTOR signaling in intrahepatic cholangiocarcinoma. Biomark. Res 11, 33 (2023).
Saed, L., Jeleń, A., Mirowski, M. & Sałagacka-Kubiak, A. Prognostic Significance of HMGA1 expression in lung cancer based on bioinformatics analysis. Int. J. Mol. Sci. 23, 6933 (2022).
Chia, L. et al. HMGA1 induces FGF19 to drive pancreatic carcinogenesis and stroma formation. J. Clin. Invest. 133, e151601 (2023).
Sgarra, R. et al. High Mobility Group A (HMGA): Chromatin Nodes Controlled by a Knotty miRNA Network. Int. J. Mol. Sci. 21, 717 (2020).
Tani, T. et al. TREX1 inactivation unleashes cancer Cell STING-interferon signaling and promotes antitumor immunity. Cancer Discov. 14, 752–765 (2024).
Lv, H. et al. TET2-mediated tumor cGAS triggers endothelial STING activation to regulate vasculature remodeling and anti-tumor immunity in liver cancer. Nat. Commun. 15, 6 (2024).
Lanng, K. R. B., Lauridsen, E. L. & Jakobsen, M. R. The balance of STING signaling orchestrates immunity in cancer. Nat. Immunol. 25, 1144–1157 (2024).
Maxwell, M. B. et al. ARID1A suppresses R-loop-mediated STING-type I interferon pathway activation of anti-tumor immunity. Cell 187, 3390–3408.e19 (2024).
Samson, N. & Ablasser, A. The cGAS-STING pathway and cancer. Nat. Cancer 3, 1452–1463 (2022).
Chin, E. N., Sulpizio, A. & Lairson, L. L. Targeting STING to promote antitumor immunity. Trends Cell Biol. 33, 189–203 (2023).
Dvorkin, S., Cambier, S., Volkman, H. E. & Stetson, D. B. New frontiers in the cGAS-STING intracellular DNA-sensing pathway. Immunity 57, 718–730 (2024).
Decout, A., Katz, J. D., Venkatraman, S. & Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 21, 548–569 (2021).
Chen, C. & Xu, P. Cellular functions of cGAS-STING signaling. Trends Cell Biol. 33, 630–648 (2023).
Zhang, C. et al. Structural basis of STING binding with and phosphorylation by TBK1. Nature 567, 394–398 (2019).
Zhao, B. et al. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature 569, 718–722 (2019).
Liu, S. et al. The mechanism of STING autoinhibition and activation. Mol. Cell 83, 1502–1518.e10 (2023).
Kwon, J. & Bakhoum, S. F. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov. 10, 26–39 (2020).
Ghosh, M., Saha, S., Li, J., Montrose, D. C. & Martinez, L. A. p53 engages the cGAS/STING cytosolic DNA sensing pathway for tumor suppression. Mol. Cell 83, 266–280.e6 (2023).
Li, W. et al. cGAS-STING-mediated DNA sensing maintains CD8(+) T cell stemness and promotes antitumor T cell therapy. Sci. Transl. Med. 12, eaay9013 (2020).
Chen, T. et al. NSUN2 is a glucose sensor suppressing cGAS/STING to maintain tumorigenesis and immunotherapy resistance. Cell Metab. 35, 1782–1798.e8 (2023).
Liu, Z. et al. cGAS-STING signaling in the tumor microenvironment. Cancer Lett. 577, 216409 (2023).
Huang, R. et al. Targeting STING for cancer immunotherapy: From mechanisms to translation. Int Immunopharmacol. 113, 109304 (2022).
Wang, J., Meng, F. & Yeo, Y. Delivery of STING agonists for cancer immunotherapy. Curr. Opin. Biotechnol. 87, 103105 (2024).
Pan, X. et al. Strategies involving STING pathway activation for cancer immunotherapy: Mechanism and agonists. Biochem Pharm. 213, 115596 (2023).
Chen, G., Zheng, D., Zhou, Y., Du, S. & Zeng, Z. Olaparib enhances radiation-induced systemic anti-tumor effects via activating STING-chemokine signaling in hepatocellular carcinoma. Cancer Lett. 582, 216507 (2024).
Mao, L. et al. HMGA1 levels influence mitochondrial function and mitochondrial DNA repair efficiency. Mol. Cell Biol. 29, 5426–5440 (2009).
Pellarin, I. et al. The architectural chromatin factor high mobility group A1 enhances DNA ligase IV activity influencing DNA repair. PLoS One 11, e0164258 (2016).
Chen, J. H. et al. Long non-coding RNA MALAT1 targeting STING transcription promotes bronchopulmonary dysplasia through regulation of CREB. J. Cell Mol. Med 24, 10478–10492 (2020).
Kikuchi, M. et al. Epigenetic mechanisms to propagate histone acetylation by p300/CBP. Nat. Commun. 14, 4103 (2023).
Salutari, I. & Caflisch, A. Dynamics of the histone acetyltransferase lysine-rich loop in the catalytic core of the CREB-binding protein. J. Chem. Inf. Model 62, 1014–1024 (2022).
Wang, D. et al. FOXO1 inhibition prevents renal ischemia-reperfusion injury via cAMP-response element binding protein/PPAR-γ coactivator-1α-mediated mitochondrial biogenesis. Br. J. Pharm. 177, 432–448 (2020).
Liu, Z. et al. Regulating twisted skeleton to construct organ-specific perylene for intensive cancer chemotherapy. Angew. Chem. Int Ed. Engl. 60, 16215–16223 (2021).
Zhou, Y. et al. Selective killing of cancer cells by nonplanar aromatic hydrocarbon-induced DNA damage. Adv. Sci. (Weinh.) 6, 1901341 (2019).
Zhao, X. et al. Sulfonated Perylene as Three-in-One STING agonist for cancer chemo-immunotherapy. Angew. Chem. Int Ed. Engl. 63, e202318799 (2024).
Toyozumi, T. et al. Fra-1 regulates the expression of HMGA1, which is associated with a poor prognosis in human esophageal squamous cell carcinoma. Ann. Surg. Oncol. 24, 3446–3455 (2017).
Yang, J. Y. et al. HMGA1 drives chemoresistance in esophageal squamous cell carcinoma by suppressing ferroptosis. Cell Death Dis. 15, 158 (2024).
Liu, M. J. et al. HMGA1 promotes the progression of esophageal squamous cell carcinoma by elevating TKT-mediated upregulation of pentose phosphate pathway. Cell Death Dis. 15, 541 (2024).
Resar, L., Chia, L. & Xian, L. Lessons from the Crypt: HMGA1-amping up Wnt for stem cells and tumor progression. Cancer Res 78, 1890–1897 (2018).
Charbonnel, C. et al. The linker histone GH1-HMGA1 is involved in telomere stability and DNA damage repair. Plant Physiol. 177, 311–327 (2018).
Petrosino, S. et al. HMGA1 regulates the expression of replication-dependent histone genes and cell-cycle in breast cancer cells. Int. J. Mol. Sci. 24, 594 (2022).
Fu, F. et al. HMGA1 exacerbates tumor growth through regulating the cell cycle and accelerates migration/invasion via targeting miR-221/222 in cervical cancer. Cell Death Dis. 9, 594 (2018).
Bose, D. A. et al. RNA binding to CBP stimulates histone acetylation and transcription. Cell 168, 135–149.e22 (2017).
Narita, T. et al. Enhancers are activated by p300/CBP activity-dependent PIC assembly, RNAPII recruitment, and pause release. Mol. Cell 81, 2166–2182.e6 (2021).
Chen, Q. et al. Histone acetyltransferases CBP/p300 in tumorigenesis and CBP/p300 inhibitors as promising novel anticancer agents. Theranostics 12, 4935–4948 (2022).
Munshi, N. et al. Coordination of a transcriptional switch by HMGI(Y) acetylation. Science 293, 1133–1136 (2001).
Woo, S. R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014).
Wang, R., Hussain, A., Guo, Q. & Ma, M. cGAS-STING at the crossroads in cancer therapy. Crit. Rev. Oncol. Hematol. 193, 104194 (2024).
Wang, X. et al. DNA sensing via the cGAS/STING pathway activates the immunoproteasome and adaptive T-cell immunity. Embo j. 42, e110597 (2023).
Gu, Z. et al. Enhancing anti-tumor immunity through liposomal oxaliplatin and localized immunotherapy via STING activation. J. Control Release 357, 531–544 (2023).
Vanpouille-Box, C., Demaria, S., Formenti, S. C. & Galluzzi, L. Cytosolic DNA sensing in organismal tumor control. Cancer Cell 34, 361–378 (2018).
Knelson, E. H. et al. Activation of tumor-cell STING Primes NK-Cell therapy. Cancer Immunol. Res 10, 947–961 (2022).
Lam, K. C. et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 184, 5338–5356.e21 (2021).
Li, J. et al. Metastasis and immune evasion from extracellular cGAMP hydrolysis. Cancer Discov. 11, 1212–1227 (2021).
Meng, F. et al. Induced phase separation of mutant NF2 imprisons the cGAS-STING machinery to abrogate antitumor immunity. Mol. Cell 81, 4147–4164.e7 (2021).
Zou, S. S. et al. Intrinsic strategies for the evasion of cGAS-STING signaling-mediated immune surveillance in human cancer: How therapy can overcome them. Pharm. Res 166, 105514 (2021).
Amouzegar, A., Chelvanambi, M., Filderman, J. N., Storkus, W. J. & Luke, J. J. STING agonists as cancer therapeutics. Cancers (Basel) 13, 2695 (2021).
Kong, X. et al. STING as an emerging therapeutic target for drug discovery: Perspectives from the global patent landscape. J. Adv. Res 44, 119–133 (2023).
Li, A. et al. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J. Hematol. Oncol. 12, 35 (2019).
Nguyen, D. C. et al. Mannosylated STING agonist drugamers for dendritic cell-mediated cancer immunotherapy. ACS Cent. Sci. 10, 666–675 (2024).
Li, S. et al. STING-induced regulatory B cells compromise NK function in cancer immunity. Nature 610, 373–380 (2022).
Ullman-Culleré, M. H. & Foltz, C. J. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim. Sci. 49, 319–323 (1999).
Karamboulas, C., Meens, J. & Ailles, L. Establishment and use of patient-derived xenograft models for drug testing in head and neck squamous cell carcinoma. STAR Protoc. 1, 100024 (2020).
Liu, J. et al. Long noncoding RNA AGPG regulates PFKFB3-mediated tumor glycolytic reprogramming. Nat. Commun. 11, 1507 (2020).
Demaria, O. et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl Acad. Sci. USA 112, 15408–15413 (2015).
Acknowledgements
The authors thank the Xu lab team for their helpful discussions and technical support. This research was financially supported by the National Key Research and Development Program of China (2023YFE0109800 to Z.X. and 2021YFA1101000 to R.C.), the National Natural Science Foundation of China (Nos. 82020108024 to Z.X. and 82400658 to Y.J.), and the China Postdoctoral Science Foundation (No. 2022M721014 to Y.J.). Correspondence should be addressed to Zhi-Xiang Xu or Yong-Ping Jian first.
Author information
Authors and Affiliations
Contributions
K.H. and J.G. designed the study, conducted the experiments, and wrote the manuscript. A.Z., M.L., J.Y., X.L., Z.Y., J.L., D.W., H.L., L.Z., and F.G. performed the experiments. Y.H. synthesized the HMGA1 inhibitors and participated in the characterization of PDIC-DPC. A.Z. and Q.D. participated in the research design, analyzed the data, and edited the manuscript. Y.J., R.C., and Z.X. contributed to the conception of the study and provided manuscript writing assistance and revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Manabu Muto, Walter Storkus, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, KY., Zhao, A., Guo, JR. et al. High mobility group A1 (HMGA1) promotes esophageal squamous cell carcinoma progression by inhibiting STING-mediated anti-tumor immunity. Nat Commun 16, 5098 (2025). https://doi.org/10.1038/s41467-025-60221-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60221-6