Abstract
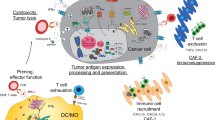
The tumour microenvironment is programmed by cancer cells and substantially influences anti-tumour immune responses1,2. Within the tumour microenvironment, CD8+ T cells undergo full effector differentiation and acquire cytotoxic anti-tumour functions in specialized niches3,4,5,6,7. Although interactions with type 1 conventional dendritic cells have been implicated in this process3,4,5,8,9,10, the underlying cellular players and molecular mechanisms remain incompletely understood. Here we show that inflammatory monocytes can adopt a pivotal role in intratumoral T cell stimulation. These cells express Cxcl9, Cxcl10 and Il15, but in contrast to type 1 conventional dendritic cells, which cross-present antigens, inflammatory monocytes obtain and present peptide–major histocompatibility complex class I complexes from tumour cells through ‘cross-dressing’. Hyperactivation of MAPK signalling in cancer cells hampers this process by coordinately blunting the production of type I interferon (IFN-I) cytokines and inducing the secretion of prostaglandin E2 (PGE2), which impairs the inflammatory monocyte state and intratumoral T cell stimulation. Enhancing IFN-I cytokine production and blocking PGE2 secretion restores this process and re-sensitizes tumours to T cell-mediated immunity. Together, our work uncovers a central role of inflammatory monocytes in intratumoral T cell stimulation, elucidates how oncogenic signalling disrupts T cell responses through counter-regulation of PGE2 and IFN-I, and proposes rational combination therapies to enhance immunotherapies.
Similar content being viewed by others
Main
Although T cell responses are often suppressed in the tumour microenvironment (TME) by inhibitory signals, emerging data suggest that the TME also has a pivotal role in supporting T cell function1,11. The activation and differentiation of CD8+ T cells was thought to occur primarily in the lymph node. However, recent findings indicate that after reaching the tumour, antigen-committed memory or effector T cells require further restimulation to expand, differentiate and effectively control tumour growth3,4,8,11,12. This process is thought to take place in discrete niches within the TME, where T cells spatially organize with myeloid cells3,4,5,6,7, in particular activated CCR7– type 1 conventional dendritic cells (cDC1s) and conventional dendritic cells (cDCs) in a stimulatory CCR7+ state3,8,9,10,13,14. Such multicellular hubs have increasingly been linked to positive outcomes in immunotherapy, thereby underscoring their therapeutic relevance4,5. The growing recognition of T cell restimulation within the TME has prompted the addition of ‘the TME subcycle’ as a new step in the cancer immunity cycle11. However, the processes that facilitate restimulation of primed CD8+ T cells within the TME remain incompletely understood.
To identify mechanisms of T cell restimulation in the tumour, we capitalized on matched pairs of tumour models that were derived by exposing targeted therapy-naive (NTT) BrafV600E-driven melanoma models to MAPK pathway inhibitors (BRAF inhibitor (BRAFi), and BRAFi and MEK inhibitor (BRAFi/MEKi)) until they acquired resistance (RTT). We previously demonstrated that although NTT tumours are susceptible to eradication through immune checkpoint blockade (ICB) and adoptive T cell transfer (ACT), RTT tumours harbour an immune-evasive TME that renders them cross-resistant to immunotherapies15. Prospective clinical studies16,17,18 have confirmed that resistance to targeted therapy jeopardizes subsequent responses to immunotherapy. Here using these models, we show that inflammatory monocytes facilitate the intratumoral expansion of primed T cells even in the absence of cDC1s. We provide mechanistic insights into their mode of action, identify cancer-cell-derived cues that disrupt this process and propose mechanism-based therapies that can reinstate anti-tumour immunity.
Myeloid polarization underlies immune escape
We established NTT tumours and RTT tumours of the YUMM1.7 mouse melanoma model (BrafV600E, Pten–/–Cdkn2a–/–) that express the model antigen ovalbumin (OVA) in Rag2–/– mice and performed ACT by intravenously injecting activated tumour antigen-specific CD8+ T cells (OT-1Luc) (Fig. 1a). As previously shown15, in NTT tumours, T cells infiltrated and controlled tumour growth, whereas RTT tumours were resistant (Fig. 1b and Extended Data Fig. 1a). Notably, OT-1Luc T cells effectively killed NTT and RTT tumour cells in vitro (Extended Data Fig. 1b), which implicated a role for the TME in mediating resistance to T cell killing in vivo. To define the composition and transcriptional states of the immune cells within the TME of NTT tumours and RTT tumours, we performed single-cell RNA sequencing (scRNA-seq) of CD45+ immune cells before ACT and 72 h after ACT. The immune landscape was markedly different between NTT and RTT YUMM1.7OVA tumours. Specifically, there was a reduction in total CD45+ abundance in RTT tumours and prominent differences within the myeloid cell compartment (Fig. 1c,d and Extended Data Fig. 1c–e). Monocytes, the most abundant immune population in the TME of NTT tumours, were strongly reduced in RTT tumours (Fig. 1c,d and Extended Data Fig. 1d). This population expressed typical monocyte markers (Ly6c2, C5ar1 and Fcgr1), low levels of macrophage markers (Adgre and Apoe) and lacked classical cDC markers (Cd24a, Flt3, Dpp4 and Zbtb46) (Extended Data Fig. 1e and Supplementary Table 1). Moreover, in RTT tumours, cDCs (Zbtb46+, Flt3+ and Cd24+), including cDC1s and activated CCR7+ DCs (often referred to as mregDCs)19,20,21,22, were severely reduced and in a dysfunctional state (Fig. 1c,d and Extended Data Fig. 1d–f). Furthermore, immunosuppressive tumour-associated macrophages (TAMs)21,23,24 (for example, Spp1+ and Ctsk+) and cycling TAMs were increased in RTT tumours compared with NTT tumours. We observed similar repolarization of the myeloid compartment in the YUMM3.3 (BrafV600E, Cdkn2a–/–) model, in which RTT tumours failed to respond to ICB (that is, anti-PD-1 and anti-CTLA-4 agents)15 (Extended Data Fig. 1g–j). These results highlight the conserved regulation and functional importance of the myeloid TME across models and immunotherapies.
a, Schematic of subcutaneous injection of YUMM1.7OVA NTT cells or RTT cells in Rag2–/– mice and OT-1Luc ACT through intravenous (i.v.) injection. b, Left, ACT responses of NTT and RTT tumours (n = 5 mice per group). Right, representative bioluminescence imaging (BLI) pictures of T cells 96 h after ACT; key indicates radiance (photons per second that leave a square centimeter of tissue and radiate into a solid angle of one steradian). c, Uniform manifold approximation and projection (UMAP) maps of scRNA-seq of CD45+ cells in NTT and RTT tumours 72 h after ACT (n = 4 tumours pooled per group). d, Relative cell frequencies from scRNA-seq. e, Representative multiparameter IF images of YUMM1.7OVA NTT and RTT tumours 48 h after ACT (n = 3 tumours per group). Scale bar, 400 µm (zoom-ins, 50 µm and 10 µm). f, Relative T cell frequency and distance to the next immune cell in NTT tumours (n = 3 tumours per group). g, Representative IF image of Nur77–GFP OT-1 cells in YUMM1.7OVA NTT and RTT tumours 48 h after ACT (n = 3 tumours per group). Scale bar, 10 µm. Arrows indicate Nur77+ OT-1 cells. h, Representative IF image of YUMM1.7OVA NTT and RTT tumours 72 h after ACT. Dashed lines depict the tumour border (n = 2 tumours per group). Scale bar, 500 µm. i, Left, quantification of T cells by BLI over time after intratumoral (i.t.) ACT (mean ± s.e.m., n = 4 mice per group). Two-way analysis of variance (ANOVA) with Sidak’s multiple comparisons test. Right, representative BLI image 96 h after ACT (key as in b). j, T cell states by flow cytometry 120 h after i.t. ACT (mean ± s.e.m., n = 5 NTT, n = 4 RTT tumours). k, Effector memory T cell signature29 on scRNA-seq of T cells (n = 14 NTT, n = 18 RTT pooled tumours). l, Left, representative BLI images of T cells in NTT and contralateral (CL) RTT tumours after i.t. ACT (key as in b). Right, growth curves (mean ± s.e.m., n = 6 mice per group). Arrows in b and l indicate day of ACT.
T cells are restimulated in permissive TMEs
Recent studies have shown that activated CD8+ T cells require additional stimuli from intratumoral myeloid cells to acquire full effector functions and to sustain a T cell response3,6,10,12. To examine this idea further, we used multiparameter immunofluorescence (IF) microscopy for discriminatory myeloid cell markers to determine the main interaction partner (or partners) of tumour-infiltrating T cells (Fig. 1e and Extended Data Fig. 2a–c). In NTT tumours, tumour-specific CD8+ T cells were in close proximity to cDC1s and monocytes and were often organized in multicellular clusters at the tumour margin (Fig. 1f and Extended Data Fig. 2a–c). Notably, tumour-infiltrating T cells interacted with monocytes more commonly than with cDC1s, which is possibly due to the high abundance of monocytes in the TME (Fig. 1f and Extended Data Fig. 2a,b). Within these hubs, T cells stained positive for Nur77, a marker indicative of recent T cell receptor (TCR) signalling, which indicated not only proximity but also direct antigen-specific stimulation by the interacting myeloid cells (Fig. 1g and Extended Data Fig. 2d,e). Within 72 h after ACT, T cells in NTT tumours permeated the entire tumour parenchyma, which was not abolished by blocking T cell egress from the lymph node with FTY720. This result suggested the occurrence of local expansion (Fig. 1h and Extended Data Fig. 2f). By contrast, RTT tumours did not contain such hubs, and T cells remained confined to the periphery. Moreover, they rarely interacted with the few cDCs and monocytes present, and Nur77+ T cells were strongly reduced (Fig. 1g and Extended Data Fig. 2d,e).
To investigate T cell functionality within TMEs, we injected the same amount of activated OT-1Luc T cells intratumorally into NTT tumours and RTT tumours (Fig. 1i). In contrast to RTT tumours, CD8+ T cells rapidly underwent a proliferative burst and expanded in NTT tumours (Fig. 1i and Extended Data Fig. 2g). Five days after intratumoral injection, we observed a significant reduction in differentiated CD8+ T cells (TCF1–PD1+TIM3+) in RTT tumours compared with NTT tumours. By contrast, in both conditions, a small fraction (about 5%) of all T cells remained in a stem-cell-like state (TCF1+PD1+TIM3–), which is required for expansion of the T cell effector pool12,25,26,27,28 (Fig. 1j and Extended Data Fig. 2h,i). In NTT tumours, but not in RTT tumours, T cells acquired features of effector memory T cells29, and the majority displayed upregulated expression of the T cell effector marker CXCR6 (ref. 30) (Fig. 1k, Extended Data Fig. 2j,k and Supplementary Table 2). Altogether, these findings suggest that multicellular hubs that contain cDCs and, unexpectedly, a substantial fraction of monocytes, are associated with T cell expansion. Moreover, T cell proliferation and effector differentiation is facilitated only within the TME of NTT tumours but not of RTT tumours.
Local licensing of systemic immunity
To examine whether an immune-permissive TME can act as a reservoir for T cell restimulation, we established NTT tumours and RTT tumours in opposite flanks within the same mouse and performed intravenous ACT. T cells expanded in NTT tumours and, at later time points, infiltrated contralateral RTT tumours and transiently controlled their growth (Extended Data Fig. 2l–o). Of note, we did not observe differences in the myeloid composition of RTT tumours in the presence of a contralateral NTT tumour (Extended Data Fig. 2p). Treatment with FTY720 only marginally reduced T cell infiltration into contralateral RTT tumours, but still resulted in tumour control. This result suggests that T cells may traffic partially through the lymph node and directly through the circulation (Extended Data Fig. 2q,r). To further confirm that restimulated T cells traffic between tumours, we directly injected activated OT-1Luc T cells into one tumour and evaluated T cell infiltration in contralateral tumours. T cells introduced into a NTT tumour demonstrated the capacity to expand locally and infiltrate contralateral tumours, irrespective of whether these were NTT tumours or RTT tumours. Contralateral NTT tumours fully regressed, and even contralateral RTT tumours were temporarily controlled (Fig. 1l and Extended Data Fig. 2s–u). Notably, T cells directly injected into RTT tumours initially failed to expand but trafficked to contralateral NTT tumours, where they expanded and controlled tumour growth and, eventually, re-infiltrated RTT tumours (Extended Data Fig. 2t,u). Collectively, our data indicate that after initial priming of T cells, restimulation and subsequent effector functions are strongly dictated by the characteristics of the TME. We conclude that T cells are capable of trafficking between tumours and that T cell restimulation in an immune-permissive TME can facilitate the control of distant, resistant tumours.
Immunostimulatory role of monocytes
Activated cDC1s and CCR7+ cDCs have long been implicated in anti-tumour immunity13,31,32,33,34,35 and, recently, in intratumoral CD8+ T cell restimulation3,4,8,9. Consistent with that, cDC1 vaccination in RTT tumours restored T cell infiltration and led to transient tumour control (Extended Data Fig. 3a–c). Notably, when NTT cells were injected into Rag2–/–Batf3–/– mice, which lack functional cDC1s, and ACT was performed, T cells still infiltrated, expanded and controlled NTT tumours (Fig. 2a). In the absence of functional cDC1s, multiparameter IF showed retained immune hubs, with monocytes clustering together with T cells in NTT tumours but not in RTT tumours (Extended Data Fig. 3d–f). Furthermore, in NTT tumours grown in Zbtb46–DTR bone marrow chimeras, in which all cDC subsets are depleted, T cells were effectively restimulated and expanded (Extended Data Fig. 3g–i).
a, Left, ACT response of YUMM1.7OVA NTT tumours in Rag2–/– mice (n = 4 mice) and Batf3–/–Rag2–/– (n = 5 mice). Right, representative BLI image of T cells 96 h after ACT (key as in Fig. 1b). b, Gene expression in individual clusters from scRNA-seq from Fig. 1c. c, Scoring of an ISG signature37 on our scRNA-seq data from Fig. 1c. Mod., module. d, Regulon specificity score of monocytes calculated using SCENIC in NTT tumours. e, Left, representative histograms depicting CFSE intensity. Right, quantification of T cell proliferation after 72 h of co-culture of naive CFSE-labelled T cells and inflammatory (Ly6A+) or non-inflammatory monocytes (Ly6A–) isolated from NTT tumours in Rag2–/– mice (n = 4 tumours). Two-tailed unpaired Student’s t-test. f, Left, schematic of injection of YUMM1.7OVA NTT CTRL or B2m KO cells in BALB/c mice. Right, representative histograms depicting H2-Kb levels. g, Left, schematic of injection of NTT CTRL or NTT B2m KO tumours in Rag2–/– mice and Batf3–l–Rag2–/– mice. Middle, quantification of T cell infiltration by BLI. Right, representative BLI image of T cell infiltration 96 h after ACT (n = 5 NTT in Rag2–/– and n = 6 mice for other groups) (key as in Fig. 1b). One-way ANOVA with Tukey’s multiple comparisons test. h, Left, UMAP of human inflammatory macrophages from a melanoma scRNA-seq myeloid dataset23. Right, enrichment score (ES) values of the inflammatory monocyte gene signature for each cell cluster. i, Top, representative field of view (FOV) of human metastatic melanoma (n = 2 of 72 FOVs) analysed by CosMx spatial transcriptomics profiling. Bottom, Pearson’s correlation values between cell types across FOVs (n = 72) were determined and displayed as a heatmap (n = 34 melanoma samples). The white arrow depicts activated CD8+ T cells, the black arrow depicts CXCL9+CXCL10+C1QC+ macrophages, and the red arrow CXCL9+CXCL10+ macrophages and DCs. Mac, macrophage. Bar graphs depict the mean ± s.e.m.
Next, we analysed monocytes for expression of genes linked to T cell stimulation. Monocytes expressed increased levels of the antigen presentation machinery (Psmb8, Psmb9, major histocompatibility complex class I (MHCI) and MHCII) and Il15, which mediates T cell survival and effector differentiation3. Cxcl9 and Cxcl10, which are essential for T cell recruitment and linked to positive immunotherapy responses33, were also expressed at high levels (Fig. 2b). These monocytes did not score positively for an established monocyte-derived DC signature21, and a large subset of these monocytes also expressed high levels of interferon-stimulated genes (ISGs), which identified them as inflammatory monocytes (Figs. 1c and 2b and Extended Data Fig. 3j). These inflammatory monocytes expressed the interferon-induced surface marker Ly6A (Extended Data Fig. 1e), a result consistent with analyses of human and mouse tumours and viral infection models, which showed that myeloid cells transition to an inflammatory state after IFN-I signalling23,36,37,38,39,40. As expected, previously defined ISG signatures (Supplementary Table 3) scored the highest in the monocyte and inflammatory monocyte cluster in NTT tumours (Fig. 2c and Extended Data Fig. 3k). Single-cell regulatory network inference and clustering (SCENIC) analyses predicted transcriptional activity of Irf9, Irf7, Irf2 and Stat2, major effectors of interferon signalling, specifically in inflammatory monocytes (Fig. 2d). Altogether, these data suggest that even in the absence of cDCs, monocytes are capable of mediating the expansion of tumour-specific CD8+ T cells and promoting anti-tumour immunity.
MHCI-dressed monocytes stimulate T cells
Given that T cells close to monocytes within immune hubs expressed Nur77, indicative of TCR stimulation (Fig. 1g and Extended Data Fig. 3l), we assessed whether monocytes are able to present tumour antigens. H2-Kb-SIINFEKL staining revealed that inflammatory monocytes, but not their non-inflammatory counterparts, displayed tumour-derived antigens on MHCI (Extended Data Fig. 3m). Moreover, they induced naive T cell activation and proliferation ex vivo, as measured by CFSE dilution (Fig. 2e). However, inflammatory monocytes do not express genes involved in cross-presentation (Clec9a and Wdfy4)14 (Fig. 2b). This prompted us to investigate whether inflammatory monocytes can acquire and display antigens through the direct transfer of intact peptide–MHCI (pMHCI) complexes from adjacent cells, a process called MHCI cross-dressing37,41,42.
To investigate whether monocytes are able to cross-dress, we established NTT tumours, which are derived from C57BL/6 mice and therefore express H2-Kb, into MHCI haplotype-mismatched (H2-Kd) BALB/c mice and examined cancer-cell-derived H2-Kb expression on myeloid subsets. Only inflammatory monocytes (Ly6A+) harboured cancer-cell-derived H2-Kb and were capable of activating naive T cells ex vivo (Fig. 2f and Extended Data Fig. 3n,o). Notably, in NTT tumours harbouring a knockout (KO) of β2-microglobulin (NTT B2m KO), no H2-Kb signal was detected on BALB/c inflammatory monocytes. This result demonstrated that pMHCI complexes on inflammatory monocytes are sourced from cancer cells (Fig. 2f and Extended Data Fig. 3n). To test the relative contribution of cross-presenting cDC1s and cross-dressed inflammatory monocytes to T cell restimulation, we injected NTT and NTT B2m KO tumours, which abolishes the ability of inflammatory monocytes to present tumour antigens through MHCI cross-dressing, into Rag2–/– mice and Rag2–/–Batf3–/– mice and performed ACT (Fig. 2g). When pMHCI cross-dressing on monocytes was intact, T cells expanded in NTT tumours, even in the absence of cDC1s. Conversely, when pMHCI cross-dressing on inflammatory monocytes was abolished (B2m KO tumours) but cDC1s were present, T cells were also able to expand, which reflected the established capacity of cDC1s to restimulate CD8+ T cells through classical cross-presentation. Only when both cDC1s and cross-dressed inflammatory monocytes were absent (B2m KO tumours engrafted into Rag2–/–Batf3–/– mice) did T cells fail to become restimulated (Fig. 2g). Collectively, our data highlight that pMHCI cross-dressing by inflammatory monocytes, together with stimulatory cytokine expression, underlies their ability to promote restimulation of primed CD8+ T cells in the TME.
ISG+ macrophages in human melanoma
We scored our mouse inflammatory monocyte signature in human myeloid scRNA-seq datasets of melanoma and non-small cell lung cancer (NSCLC)23,43. This analysis showed that ISG+ (CXCL9+CXCL10+) macrophages and CD16+ monocytes are the analogous inflammatory populations across human cancers (Fig. 2h, Extended Data Fig. 4a–c and Supplementary Table 2). Notably, analysing the spatial distribution of T cells with immune cells in human melanoma samples revealed that activated CD8+ T cells preferentially co-localized with CXCL9+CXCL10+ inflammatory macrophages and cDCs in immune hubs. By contrast, regions that lacked CXCL9+CXCL10+ macrophages were devoid of T cells (Fig. 2i).
Cancer cells produce PGE2 and dampen IFN-I responses
To identify factors derived from NTT and RTT cells that determine the intratumoral immune landscape pivotal for T cell restimulation, we isolated cancer cells from tumours and performed RNA-seq. Pathway enrichment analysis of differentially regulated genes revealed upregulation of the prostaglandin synthesis pathway and downregulation of IFN-I signalling in RTT cells as the top differential pathways compared with NTT cells (Fig. 3a and Supplementary Table 3). Metabolomics analysis identified PGE2 as the most enriched eicosanoid in RTT tumours (Extended Data Fig. 5a). PGE2 can limit cDC1-mediated support of CD8+ T cells9,34,44, but its impact on inflammatory monocytes and on their ability to promote T cell restimulation is unclear.
a, Pathway enrichment analysis of differential gene expression in cancer cells isolated from YUMM1.7OVA NTT tumours and RTT tumours (n = 3 tumours per group; Supplementary Table 3). Adjusted P values were computed using the Benjamini–Hochberg correction. b, PGE2 ELISA of YUMM1.7OVA tumours in Rag2–/– mice (n = 10 NTT, n = 11 RTT CTRL, n = 7 RTT Ptgs1/2 KO over 2 independent experiments). c, Top, representative BLI image of T cells 96 h after ACT (key as in Fig. 1b). Bottom, BLI quantification (n = 4 mice NTT, n = 6 mice RTT CTRL and RTT Ptgs1/2 KO). d, Left, response of YUMM1.7OVA tumours in Rag2–/– mice to ACT (n = 7 mice per group). Right, YUMM3.3 in C57BL/6 mice (n = 5 mice per group). e, IFNβ ELISA of supernatants from YUMM1.7OVA NTT and RTT cells (n = 5 replicates per group over 2 independent experiments). f, Top, representative BLI images of T cells in YUMM1.7OVA RTT CTRL and RTT IRF3/7 tumours in Rag2–l– mice 96 h after ACT (key as in Fig. 1b). Bottom, BLI quantification (n = 6 mice per group). g, Left, response of YUMM1.7OVA tumours in Rag2–/– mice to ACT (n = 4 mice RTT CTRL, n = 5 mice RTT IRF3/7). Right, YUMM3.3 in C57BL/6 mice (n = 6 mice per group). h, Response to ACT of YUMM1.7OVA RTT Ptgs2 KO (left) and RTT IRF3/7 tumours (right) in Rag2–/– (n = 4 mice per group) and Batf3–/–Rag2–/– mice (n = 5 mice per group). i, PGE2 and IFNβ ELISAs of NTT and RTT A375 human melanoma (n = 2 replicates per group for PGE2, n = 3 tumours per group for IFNβ). Arrows in d, g and h indicate day of ACT. Bar graphs depict the mean ± s.e.m. Statistical analysis was performed with a two-tailed unpaired Student’s t-test (e,f,i) or one-way ANOVA with Tukey’s multiple comparisons test (b,c).
To understand the role of cancer-cell-derived PGE2 in immune evasion, we ablated PGE2 production through cyclooxygenase-1 (COX1; encoded by Ptgs1) and COX2 (encoded by Ptgs2) KO in RTT cells and engrafted them into Rag2–/– mice. RTT tumours deficient in Ptgs1 and Ptgs2 (Ptgs1/2) displayed reduced PGE2 levels that were comparable to those of NTT tumours (Fig. 3b) and increased T cell infiltration (Fig. 3c). Notably, Ptgs1/2 deletion fully re-sensitized RTT tumours to ACT and grew unperturbed without ACT (Fig. 3d and Extended Data Fig. 5b). Genetic ablation of only Ptgs2 also led to re-sensitization of RTT tumours to ACT (Extended Data Fig. 5c–e). Similarly, Ptgs2 deletion in the YUMM3.3 RTT model resulted in tumour rejection in immunocompetent C57BL/6 mice in a T cell-dependent manner, even without ICB treatment (Fig. 3d and Extended Data Fig. 5f,g). Similar effects of Ptgs2 inactivation have been reported in other mouse models34,44,45,46, which highlights the role of PGE2 in the TME as a strong modulator of T cell responses.
Given the effects of PGE2, we wondered whether the transcriptional downregulation of the IFN-I program in RTT cells (Fig. 3a) was merely a reflection of low interferon levels in a PGE2-induced immunosuppressive TME or an independent driver of immune evasion. IFNβ was significantly reduced in RTT tumour lysates compared with NTT tumour lysates (Extended Data Fig. 5h), with RTT cells producing less IFNβ in vitro. This result implied that there is cancer-cell-intrinsic regulation of IFN-I cytokine production without microenvironmental cues (Fig. 3e). Upstream regulator analysis (Ingenuity) predicted that these transcriptional changes stemmed from decreased activity of the transcription factors IRF3 and IRF7 (IRF3/7), which are important regulators of interferon production (Extended Data Fig. 5i).
To test whether re-establishment of a functional IFN-I pathway in RTT cancer cells is sufficient to restore responses to ACT, we overexpressed IRF3/7 in RTT cells and established tumours in Rag2–/– mice. This model showed restored IFN-I cytokine levels in the TME (Extended Data Fig. 5h) and increased T cell infiltration, which in turn led to full tumour control after ACT (Fig. 3f,g and Extended Data Fig. 5j). YUMM3.3 RTT tumours also harboured lower IFNβ levels than their NTT counterparts (Extended Data Fig. 5k), and after re-establishment of an interferon response and injection in C57BL/6 mice, the tumours were controlled (Fig. 3g). Moreover, similar to NTT tumours, YUMM1.7OVA RTT tumours with Ptgs2 KO or IRF3/7 overexpression were controlled in Rag2–/–Batf3–/– mice, which lack cDC1s (Fig. 3h). Collectively, these findings suggest that RTT cancer cells establish an immune-evasive TME by increasing PGE2 and simultaneously reducing IFN-I cytokine production. Reverting either of these events re-sensitizes tumours to killing by activated T cells, even in the absence of cDC1s (Fig. 3h).
MAPK signalling regulates PGE2 and IFN-I responses
We recently showed that cross-resistance between targeted therapy (BRAFi/MEKi) and immunotherapy is driven by reactivated oncogenic RAF–MEK–ERK signalling15. To explore whether the hyperactivated MAPK pathway in RTT tumours is the common regulator of both immune-evasive programs, we inhibited the MAPK pathway in cancer cells in vitro and in vivo. This strategy induced the expression of ISGs and reduced COX2 levels (Extended Data Fig. 5l–n), a result consistent with previous reports44,47. We examined whether PGE2 could be the cause of the dampened IFN-I program in RTT cancer cells. However, Ptgs2 deletion did not restore ISG expression and it did not increase IFN-I cytokine levels in the TME (Extended Data Fig. 5o,p). Similarly, IRF3/7 overexpression in RTT tumours did not attenuate PGE2 production (Extended Data Fig. 5q).
To address the human relevance of these findings, we assessed PGE2 and IFN-I cytokine production in matched pairs of the human RAFi-sensitive (NTT) and RAFi-resistant (RTT) melanoma cell lines A375, M249 and LOX48. We consistently found an increased production of PGE2 in targeted-therapy-resistant cells, together with a decrease in IFN-I responses. This result was also confirmed in the KRAS-driven NSCLC cell line NCI-H358 after it acquired resistance to a targeted KRAS inhibitor (Fig. 3i and Extended Data Fig. 5r). COX2 levels were regulated by the MAPK pathway in the A375 cell line after BRAF inhibition or after NRAS overexpression (Extended Data Fig. 5s). Collectively, these studies indicate a common regulatory module driven by oncogenic MAPK signalling that upregulates PGE2 levels and downregulates IFN-I responses in cancer cells to drive immune evasion.
PGE2 and IFN-I responses instruct myeloid polarization
We next wanted to understand how genetic ablation of PGE2 synthesis or restoration of IFN-I responses in RTT cancer cells reinstates an immune-permissive TME rich in inflammatory monocytes. We performed scRNA-seq and flow cytometry analyses of CD45+ cells from YUMM1.7OVA NTT tumours, RTT control (CTRL) tumours, RTT Ptgs1/2 KO tumours and RTT IRF3/7 overexpressing tumours 72 h after ACT (Fig. 4a and Extended Data Fig. 6a–e). The most substantial TME changes after deletion of Ptgs1/2 or overexpression of IRF3/7 were in the monocyte and macrophage (MoMac) compartment. In RTT IRF3/7 tumours, inflammatory monocytes were the predominant population, together with a TAM cluster with enhanced stimulatory functions (TAM H2-Ab1) that was absent in RTT tumours (Fig. 4a and Extended Data Fig. 6d). In RTT Ptgs1/2 KO tumours, we observed an increase in both monocytes and inflammatory monocytes, with a reduction in immunosuppressive TAMs (Spp1+, C1q+ and Ctsk+) and an increase in H2-Ab1 TAMs. After PGE2 reduction or IRF3/7 overexpression, cDC1 abundance and functionality, as well as CCR7+ DCs, were significantly increased and antigen presentation capacity was enhanced (Fig. 4a and Extended Data Fig. 6c–f). Last, NK cells were also rescued in RTT Ptgs1/2 KO tumours and in IRF3/7 overexpressing tumours, but NK cell depletion in Ptgs2 KO tumours did not significantly change the infiltration of cDCs and T cells or overall tumour control in our models (Extended Data Fig. 6g–j).
a, UMAP of scRNA-seq of CD45+ cells in YUMM1.7OVA RTT Ptgs1/2 KO and RTT IRF3/7 tumours 72 h after ACT (n = 3 tumours pooled per group). See Fig. 1c for cell cluster annotation. b, Scoring of the TME-COX and TME-IRF3/7 signatures (Supplementary Table 4) in myeloid fractions of responder (n = 6) and non-responder (n = 7) patients before TIL infusion43. c, Scoring of an ISG signature37 in the scRNA-seq from a. d, Flow cytometry quantification of myeloid populations normalized to the CD45+ fraction from YUMM1.7OVA NTT tumours in Rag2–/– mice treated with anti-IFNAR1 or anti-IFNγ (n = 5 tumours per group, except n = 4 in anti-IFNAR1 + ACT and ant-IFNγ + ACT). e, RNA velocity of the MoMac compartment from RTT Ptgs1/2 KO tumours. f, Representative contour plots of Ly6C+ monocytes depicting Ly6A expression 72 h after i.t. transfer into NTT and RTT tumours in Rag2–/– mice (n = 5 tumours per group). g, Left, BLI quantification of T cells 96 h after ACT of YUMM1.7OVA RTT cells into Cd11ccre-Ptger2–/–Ptger4fl/fl mice (EP2/EP4 KO) or Cd11ccre (CTRL) mice (n = 7 mice per CTRL group and n = 8 mice per EP2/EP4 KO group); two-tailed Mann–Whitney U-test. Right, representative BLI image (key as in Fig. 1b). h, Representative contour plots of Ly6C+ monocytes depicting expression of Ly6A 48 h after treatment with CM from cancer cells with or without a COX1/2i (indomethacin) and with or without anti-ΙFNAR1 or isotype (n = 3 biological replicates). i, Heatmap of scaled ISG expression in BMDCs exposed to CM from NTT or RTT cells and with or without a COX1/2i in the presence of anti-ΙFNAR1 or isotype (n = 4 technical replicates) measured by RT–qPCR. j, Heatmap of scaled ISG expression in BMDCs treated with IFNβ and PGE2 measured by RT–qPCR (n = 4 technical replicates). Bar graphs depict the mean ± s.e.m.
To investigate the distinctive features of PGE2 depletion or IFN-I response reinstatement in patients with cancer, we established a signature of the top upregulated genes in immune cells after Ptgs1/2 KO (TME-COX signature, n = 26) and the top upregulated genes after IRF3/7 overexpression (TME-IRF3/7 signature, n = 40; Supplementary Table 4). Both signatures were strongly correlated with a gene expression signature of CD8+ T cell infiltration and were highly predictive of survival in patients with melanoma treated with ICB49 (Extended Data Fig. 7a,b). Furthermore, both signatures were enriched in a group of patients who responded to subsequent tumour-infiltrating lymphocyte (TIL) therapy43 (Fig. 4b and Extended Data Fig. 7c). From these results, we conclude that PGE2 depletion and IFN-I cytokine increase is associated with an immune-permissive state of the TME and better response to therapy in preclinical models and in patients.
IFN-I and IFNγ drive the inflammatory state
The TME of NTT tumours, RTT Ptgs1/2 KO tumours and RTT IRF3/7 tumours share an inflammatory state, and monocytes are the most abundant cell type in this transcriptional state (Fig. 4c). After ACT, inflammatory monocytes strongly expanded, especially in NTT tumours and RTT Ptgs2 KO tumours, which suggested that both cancer-cell-derived IFN-I cytokines and T cell-derived IFNγ play a part in inducing the inflammatory state (Extended Data Fig. 6b). Indeed, looking at the most variable genes in the immune compartment of the TME before and after ACT, a core IFNγ response gene set (for example, Cxcl9, Gbp2 and Slamf7) was strongly increased in NTT tumours. Independent of the effects of T cell transfer, a set of genes characteristic of an IFNα response (for example, Irf7, Isg15 and Cxcl10) was higher in NTT TMEs than in RTT TMEs, which suggested that both IFN-I and IFN-II cytokines modulate an inflammatory TME in NTT tumours (Extended Data Fig. 7d,e).
To assess how interferons modulate the inflammatory TME and monocytes, we selectively blocked IFN-I and IFN-II cytokines before and after ACT. Blocking IFN-I signalling before ACT prevented the formation of inflammatory monocytes. Specifically, low levels of IFN-I cytokines produced by RTT Ptgs2 KO cells were sufficient for inducing an inflammatory state. After ACT, IFNγ depletion substantially reduced (NTT) or completely abolished (RTT Ptgs2 KO) inflammatory monocytes (Fig. 4d and Extended Data Fig. 7f). In NTT tumours that produce high levels of IFN-I cytokines, the combinatorial depletion of IFN-I and IFN-II cytokines was necessary to fully abolish inflammatory monocytes after ACT (Fig. 4d). These data indicate that monocytes initially rely on tumour-derived IFN-I cytokines to transition to an inflammatory state, which shifts towards IFNγ when T cells infiltrate the TME.
The expansion of inflammatory monocytes after T cell transfer is coupled with a reduction in macrophages, which suggests that there is a shift in myeloid differentiation towards inflammatory monocytes at the expense of TAM maturation (Extended Data Fig. 6b). To further understand myeloid differentiation trajectories, we performed RNA velocity analysis of the MoMac compartment in NTT tumours, RTT tumours and RTT Ptgs1/2 KO tumours. The analysis predicted that a common monocyte precursor population (monocyte 1 cluster) can give rise to both TAMs and inflammatory monocytes (Fig. 4e and Extended Data Fig. 7g). This results suggests that in RTT tumours, high PGE2 and low IFN-I cytokine levels drive the differentiation of monocytes into suppressive macrophages, whereas in NTT tumours, monocytes are maintained, with a subset acquiring the inflammatory state. Indeed, treatment of Ly6C+ monocytes with PGE2 in vitro promoted their differentiation towards F4/80+ macrophages (Extended Data Fig. 7h). In the absence of PGE2 (RTT Ptgs1/2 KO), TAMs were reduced and monocytes were increased, with a fraction becoming inflammatory (Fig. 4a). In RTT IRF3/7 tumours, despite high levels of PGE2, the increased levels of IFN-I cytokines mediated inflammatory monocyte formation (Fig. 4a). To further analyse monocyte differentiation trajectories in vivo, we intratumorally injected bone-marrow-derived Ly6C+ monocytes. In NTT tumours, 70–80% of these became inflammatory compared with only 10–20% in RTT tumours (Fig. 4f and Extended Data Fig. 7i). Thus, both cancer cells and T cells shape the immune status of the TME by driving the differentiation of monocytes and their inflammatory state in therapy-responsive tumours.
PGE2 impairs the inflammatory state
To rule out that high levels of PGE2 in the TME directly impair T cell function30,50, we knocked out the PGE2 receptors EP2 (encoded by Ptger2) and EP4 (encoded by Ptger4) in OT-1 T cells, followed by their intravenous injection into RTT tumour-bearing mice. This strategy had modest effects and did not achieve tumour control (Extended Data Fig. 7j). We then examined effects of PGE2 on the myeloid compartment using Cd11ccre(Itgax-cre)Ptger2–/–Ptger4fl/fl mice, in which EP2 and EP4 are selectively ablated in CD11c+ cells. We observed improved T cell infiltration, and RTT tumours were controlled in a CD8-dependent manner in both the YUMM1.7 and YUMM3.3 models (Fig. 4g, Extended Data Fig. 7k and Extended Data Fig. 8a). In line with these data, inflammatory monocytes and cDC2s, along with cDC1s, were increased in Cd11ccre(Itgax-cre)Ptger2–/–Ptger4fl/fl mice compared with control mice (Extended Data Fig. 8b). These data suggest that disruption of PGE2 signalling, either by inhibiting PGE2 production in cancer cells or by blocking downstream signalling in myeloid cells, is sufficient to restore the inflammatory state in myeloid cells and subsequent T cell function.
Given that IFN-I cytokines and PGE2 represent distinct biological classes of mediators, we investigated their individual and combined effects on the inflammatory state of myeloid cells. We exposed mouse Ly6C+ monocytes and bone-marrow-derived DCs (BMDCs) to conditioned medium (CM) from NTT cells, RTT cells and RTT IRF3/7 cells. CM from NTT cells or RTT IRF3/7 cells, but not RTT cells, induced an inflammatory state in BMDCs and monocytes (Fig. 4h,i and Extended Data Fig. 8c–e). This inflammatory response was blocked by anti-IFNAR1 treatment. Using CM of RTT cells treated with a COX1 and COX2 inhibitor (COX1/2i) also increased the inflammatory state, which fully depended on the marginal remaining IFN-I cytokines produced by RTT cells (Fig. 4h,i and Extended Data Fig. 8c–e). Furthermore, inhibiting the MAPK pathway with a MEKi in RTT cells during conditioning of the medium also resulted in an increase in ISG expression in BMDCs, thereby highlighting the role of oncogenic MAPK signalling in regulating PGE2 and IFN-I cytokine production (Extended Data Fig. 8f). Exposure of human monocytes to CM from NTT cells or RTT cells of various human melanoma and NSCLC cell lines, with or without COX1/2i, demonstrated effects similar to those in mouse monocytes. This result provides support for a role of counter-regulated PGE2 and IFN-I cytokines in myeloid dysfunction in human cancers (Extended Data Fig. 8g). Finally, low IFNβ levels were sufficient to induce ISG expression and the inflammatory marker AXL in BMDCs, and adding PGE2 significantly impaired their response to IFN-I cytokines, thereby hampering the acquisition of an inflammatory state (Fig. 4j and Extended Data Fig. 8h).
Pharmacological targeting of PGE2 and IFN-I
Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit PGE2 production by COX enzymes and are commonly used for pain management in patients with cancer. Retrospective studies51,52 and our own meta-analysis revealed that NSAID co-medication significantly improves overall response rates to ICB in melanoma and in patients with NSCLC (odds ratio = 1.68, 95% confidence interval (CI) = 1.16–2.42, P = 0.006), as well as progression-free survival compared with patients receiving only ICB (Fig. 5a, Extended Data Fig. 9a–d and Supplementary Tables 5 and 6). Nevertheless, these benefits did not translate into durable responses or long-term survival (Extended Data Fig. 9c). To examine how COX2 inhibition affects the immune TME, we treated RTT tumour-bearing Rag2–/– mice with celecoxib, adoptively transferred T cells intravenously and performed scRNA-seq (Fig. 5b). In line with findings in other models53, COX2 inhibition significantly increased the total amount of cDCs, including cDC1s and CCR7+ DCs, monocytes and their inflammatory state and immunostimulatory TAMs while reducing suppressive TAMs (Fig. 5b,c and Extended Data Fig. 10a–c). Continuous COX2 inhibition with celecoxib and etoricoxib increased CD8+ T cell infiltration and led to shrinkage of RTT tumours in combination with ACT (Extended Data Fig. 10d). Notably, when COX2i treatment was stopped, tumours rapidly relapsed, which suggested that continuous TME remodelling is required for T cell restimulation (Extended Data Fig. 10d).
a, Forest plot of pooled odds ratios and 95% CIs across clinical studies for overall response rates in patients receiving ICB with or without NSAID co-medication (n = 722 patients over 8 independent cohorts; Extended Data Fig. 9a and Supplementary Table 5). Statistical analysis was performed with a random effects model and data are presented as the mean ± 95% CI. b, UMAP of scRNA-seq of CD45+ cells of RTT CTRL and COX2i-treated YUMM1.7OVA RTT tumours 72 h after ACT (n = 3 tumours pooled per condition). See Fig. 1c for cell cluster annotation. c, Relative frequency of cell types across conditions as assessed by scRNA-seq. d, Left, treatment schedule of YUMM1.7OVA RTT tumours in Rag2–/– mice with celecoxib (COX2i) in combination with FLT3L or 5-AZA. Right, BLI quantification. Top, representative BLI images 72 h after ACT for vehicle (n = 8 mice), COX2i (n = 9 mice), COX2i + 5-AZA (n = 6 mice) and COX2i + FLT3L (n = 8 mice) groups (key as in Fig. 1b). Bar graphs depict the mean ± s.e.m. One-way ANOVA with Tukey’s multiple comparisons test. e, Left, Survival of Rag2–/– mice bearing YUMM1.7OVA RTT tumours treated with ACT and vehicle (n = 8 mice), COX2i (n = 9 mice), COX2i + 5-AZA (n = 7 mice) or COX2i + FLT3L (n = 8 mice). Right, survival of C57BL/6 mice bearing YUMM3.3 RTT tumours treated with an anti-PD1 and anti-CTLA4 with vehicle (n = 6 mice), COX2i (n = 8 mice), COX2i + FLT3L (n = 9 mice) or COX2i + 5-AZA (n = 9 mice). Log-rank Mantel–Cox test.
To further enhance the effects of COX2 inhibition, we explored different mechanism-based drug combinations aimed at inducing IFN-I responses or expanding antigen-presenting cells. To investigate whether the induction of IFN-I responses in RTT tumours could synergize with COX2 inhibition, we used 5-azacitidine (5-AZA), a clinically used DNA methyltransferase inhibitor that induces IFN-I responses54. Treatment with 5-AZA and its combination with a COX2i led to strong repolarization of the immune TME, increasing inflammatory monocytes and T cell infiltration that in turn led to tumour regression in all Rag2–/– mice receiving ACT (Fig. 5d and Extended Data Fig. 10e–h). Similar to COX2 inhibition, short-term treatment with 5-AZA only led to transient tumour control, which further underlined the need for a continuous stimulatory TME (Extended Data Fig. 10h).
Next, we combined ACT with a COX2i and FLT3L, a cytokine that promotes the expansion of cDCs32,55. This strategy increased inflammatory hubs (Extended Data Fig. 10i), improved T cell expansion and led to more durable responses compared with the COX2i alone (Fig. 5d,e and Extended Data Fig. 10h). Of note, FLT3L treatment alone did not lead to significant tumour control (Extended Data Fig. 10h). After 130 days, 3 out of 9 mice remained alive in the COX2i group, 4 out of 8 mice in the COX2i + FLT3L group and 2 out of 7 in the COX2i + 5-AZA group (Fig. 5e). Reinjection of YUMM1.7OVA RTT cells in long-term tumour-free mice did not form tumours, which indicated the presence of immune memory and T cell recall (Extended Data Fig. 11a). The benefit of combining a COX2i with FLT3L or 5-AZA was even more substantial in the YUMM3.3 RTT model, in which COX2i + ICB had modest effects, but the addition of FLT3L or 5-AZA induced tumour control and significantly improved survival (Fig. 5e and Extended Data Fig. 11b–e). Similar benefits of these combination therapies were observed in several KRAS-driven models, including the colorectal cancer model CT-26, the NSCLC model KPAR and the PDAC model EPP2 (Extended Data Fig. 11f–n). Altogether, our results demonstrate that an immune-evasive TME orchestrated by cancer-cell-derived PGE2 and low IFN-I cytokines levels can be pharmacologically targeted using rational therapy combinations (Extended Data Fig. 11o).
Discussion
Although the lymph node has long been recognized as a crucial environment for determining CD8+ T cell function, findings by us and others assign a complementary role to the TME3,4,5,6,24. In this study, we showed that inflammatory monocytes, which in human tumours correspond to CXCL9+CXCL10+ macrophages, drive T cell restimulation in the TME. We demonstrated that PGE2 and IFN-I responses, controlled by oncogenic MAPK signalling in cancer cells, disrupt this process. These findings provide mechanistic insights into the recent discoveries that CXCL10+ macrophages56, often present in immune hubs together with CD8+ T cells in patients5, and CXCL9:SPP1 macrophage polarity24 are predictive of responses to ICB.
We showed that inflammatory monocytes exhibit immunostimulatory capacities, as evidenced by their expression of CXCR3 ligands (Cxcl9 and Cxcl10), which recruit and position T cells, and IL-15, which promotes the expansion and survival of the effector pool3. Unlike cDC1s, which cross-present antigens, inflammatory monocytes obtain intact pMHCI complexes from tumour cells through cross-dressing. Both inflammatory monocytes and cDC1s facilitated intratumoral T cell restimulation in our models. In future studies, it will be important to dissect these seemingly redundant functions of cDC1s and inflammatory monocytes and macrophages, and the contribution of other cell types, such as cDC2s37 and CD4+ T cells4,57,58. This information will help determine whether the signals provided by these cells, including different modes of TCR engagement (cross-presentation versus cross-dressing), enhance the effectiveness of intratumoral CD8+ T cell restimulation or differentially affect T cell function, as has been shown for a subset of macrophages that promote T cell exhaustion by providing a suboptimal TCR trigger59. The plasticity, high abundance and short half-life of monocytes and macrophages render them promising therapeutic targets for boosting the efficacy of immunotherapies, in particular in tumours in which functional intratumoral cDC1s are limited, a common feature of immune-evasive tumours14,34. Ultimately, it will be pivotal to understand whether T cell restimulation within the TME is strictly required across tumour entities and/or modes of immunotherapy.
We showed that patients with NSCLC and patients with metastatic melanoma receiving ICB and NSAIDs concomitantly had improved therapy responses and progression-free survival51,52. However, the use of NSAIDs did not translate into long-term benefit, which is possibly due to incomplete inhibition of PGE2 production or discontinuation of treatment, which, in our preclinical models led to rapid tumour regrowth. We propose mechanism-based interventions that combine immunotherapy with suppression of PGE2 levels through a COX2i and increase in IFN-I responses or cDC function through 5-AZA or FLT3L administration, respectively. Given that combining these clinically approved agents may cause toxicity and/or chronic IFN-I signalling resulting in T cell exhaustion60, further optimization in terms of drug timing and sequence will be required before use in patients. Previous studies have shown that PGE2 limits inflammatory gene expression in infection models61. A thorough understanding of the pathways that underlie this process in intratumoral myeloid cells has the potential to reveal new therapeutic targets for counteracting immune evasion.
Methods
Cell lines
YUMM1.7 and YUMM3.3 mouse melanoma62 cell lines (obtained from M. Bosenberg, Yale University) were cultured in Dulbecco’s modified Eagle’s medium (DMEM)–F12 produced in-house. A375, M249 (ref. 63) (obtained from J. Massague, MSKCC), KPAR64 (obtained from J. Downward, Francis Crick Institute) and EPP2 (ref. 65) (obtained from J. Zuber, IMP) cell lines were cultured in DMEM (Gibco). LOX48 (obtained from J. Massague, MSKCC), CT-26 (ref. 66) and NCI-H358 cell lines were purchased from the American Type Culture Collection and cultured in RPMI-1640 (Gibco). The NCI-H358 RTT derivative was generated by culturing NCI-H358 parental cells in the presence of 1 μM KRAS inhibitor (Amgen) for 90 days until cells became resistant. YUMM1.7OVA clones and all NTT and RTT derivatives were generated as previously described15. RTT BRAFi-resistant cancer cells (YUMM1.7 and YUMM3.3 model) and all genetically engineered derivatives were cultured continuously in 100 nM dabrafenib (Selleckchem). MEKi-resistant cancer cells were cultured continuously in 10 nM trametinib (Selleckchem). Human NTT and RTT melanoma cell line derivatives (A375, M249 and LOX) were generated as previously described48, and RTT cells were maintained in culture on 1 µM vemurafenib (LC-Labs). HEK-293T cells were purchased from Takara (Lenti-X 293T, 632180) and cultured in DMEM high-glucose produced in-house. BMDCs were cultured according to an adapted version of a previously described protocol67. In brief, for the first 6–7 days, cells were cultured at a density of 1 × 106 cells per ml. On day 4, fresh medium was added to minimize cell death. After that, cells were either seeded for assays or counted and re-seeded at a density of 300,000 cells per ml. BMDCs were cultured in full T cell medium supplemented with 200 ng ml–1 FLT3L-Ig (BioXcell) and 5 ng ml–1 GM-CSF (in-house produced). Bone-marrow-derived Ly6C+ monocytes were cultured in DMEM medium (Gibco). Human MONO-MAC-1 (obtained from J. Zuber, IMP) and BLaER-1 (ref. 68) (obtained from M. Gaidt, IMP) cell lines were cultured in RPMI-1640 (Gibco). All media for cell lines were supplemented with 10% FBS, 2 mM l-glutamine (Gibco) and 100 IU ml–1 penicillin–streptomycin (Thermo Fisher). BLaER-1 and NCI-H358 cells were additionally supplemented with 1× sodium pyruvate. CD8+ T cells were cultured in full T cell medium containing RPMI-1640 supplemented with 10% FBS, 2 mM l-glutamine and 100 IU ml–1 penicillin–streptomycin, 1× sodium pyruvate (Gibco), 1× non-essential amino acids (Gibco), 20 mM HEPES (produced in-house) and 0.05 mM β-mercaptoethanol (Millipore). All cells were cultured at 37 °C and 5% CO2. Cells were routinely tested negative for mycoplasma contamination. STR Profiling was performed in-house for the YUMM1.7, YUMM3.3, EPP2 and KPAR cell lines. Moreover, sensitivity to MAPK inhibitors was confirmed for A375, M249 and LOX (BRAFi), CT-26 (MEKi) and for NCI-H358 (KRAS inhibitor).
Animal experiments and ethics
All mice were bred and housed in pathogen-free conditions with a housing temperature of 22 ± 1 °C, 55 ± 5% humidity and a photoperiod of 14 h of light and 10 h of dark. Within each experiment, age-matched and sex-matched groups were used. B6.129S(C)-Batf3tm1Kmm/J (Batf3–/–) mice, B6(Cg)-Zbtb46tm1(HBEGF)Mnz/J (zDC-DTR) mice, B6.Cg-Tg(Itgax-cre)1-1Reiz/J (Cd11c-cre) mice and NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were purchased from Jackson Laboratories. B6.Cg-Rag2tm1.1Cgn/J Ly5.2 (Rag2–/–), BALB/c and C57BL/6J mice were obtained from the Vienna Biocenter in-house breeding facility. ItgaxcrePtger2−/−Ptger4fl/fl mice were provided by J. Boettcher (TUM, Munich). For Rag2–/–Batf3–/– strain generation, Batf3–/– mice were crossed to Rag2–/– mice, and homozygous offspring (Rag2–/– × Batf3–/–) were confirmed by genotyping and used in subsequent experiments to evaluate the lack of cDC1s in the context of ACT. For Rag2–/– zDC-DTR strain generation, zDC-DTR mice were crossed to Rag2–/– mice and homozygous offspring were confirmed by genotyping and used in subsequent experiments to evaluate the effects of DC depletion. For ACT experiments and injection of YUMM1.7OVA cell lines, Rag2–/– mice were used. For the injection of YUMM3.3, KPAR and EPP2 cell lines, C57BL/6 mice were used. For the injection of the CT-26 cell line, BALB/c mice were used. For the generation of BMDCs and Ly6C+ monocytes, bones (femurs and tibias) were collected from in-house-bred C57BL/6 mice. For all above strains, mice were used between 6 and 12 weeks old. For OT-1Luc CD8+ T cell isolation, 6–24-week-old OT-1Luc Thy1.1 mice69 were used. All mouse experiments were performed according to our licence approved by the Austrian Ministry (GZ: MA58-2260492-2022-22; GZ: 340118/2017/25; BMBWF-66.015/0009-V/3b/2019; GZ: 801161/2018/17; and GZ: 2021-0.524.218 and their amendments). Mice were euthanized when the humane end point was reached (for example, weight loss > 20%, signs of distress and pain), when tumours displayed signs of continuous necrosis or when tumours reached the maximum allowed tumour volume of 1,500 mm3.
Tumour cell injections
For subcutaneous injections, mice were anaesthetized with 2–4% isoflurane. For the YUMM1.7OVA model and all its derivatives, 0.5–1 × 106 YUMM1.7OVA cancer cells were subcutaneously injected into the flank of each mouse in a volume of 50 µl. For contralateral experiments, alternating flanks were used for the injection of NTT and RTT cells to avoid preferential growth biases. For the YUMM3.3 model, 0.3–1 × 106 cells were subcutaneously injected in a volume of 50 µl. For the CT-26 model, 0.25 × 106 cells were subcutaneously injected in 50 µl. For the KPAR model 0.35 × 106 cells were subcutaneously injected in 50 µl. For the EPP2Luc cell line derivative, orthotopic injections were performed as previously described65. In brief, surgeries were performed under isoflurane (2–4%) anaesthesia on a heated plate. A small incision on the upper left quadrant of the shaved abdomen was made and the was spleen identified. After externalization of the pancreas, 1 × 106 cells were intrapancreatically injected. Organs were re-situated, and the peritoneum closed with a resorbable 6-0 Vicryl suture, followed by skin closure with sterile wound clips. Animals received intraperitoneal (i.p.) injections of 5 mg kg–1 carprofen pre-emptively and every 12–48 h after surgery. The health status of mice was monitored daily, and the tumour burden was assessed by BLI. All cell lines were resuspended in PBS mixed 1:1 with Matrigel (Corning) in the final injection volume. Subcutaneous tumours were monitored by calliper measurements every 2–4 days, and tumour volume was calculated according to the following formula: volume = (D × d2)/2, in which D and d are the long and short tumour diameters, respectively.
Isolation and activation of naive OT-1Luc CD8+ T cells
Spleen and lymph nodes were isolated from OT-1Luc mice, and red blood cell lysis was performed with ammonium–chloride–potassium lysis buffer (Thermo Fisher) according to the manufacturer’s protocol. T cell isolation was performed using a Magnisort mouse CD8+ naive T cell enrichment kit (Thermo Fisher) according to the manufacturer’s protocol. T cells were activated for the first 24 h by seeding them on a plate coated with 2 µg ml–1 anti-CD3 (145-2C11, eBioscience) overnight, and adding 1 µg ml–1 anti-CD28 (37.51, eBioscience) and 20 ng ml–1 carrier-free IL-2 (BioLegend). T cells were expanded for approximately 6–7 days in the presence of IL-2 and maintained daily at a concentration of 1 × 106 cells per ml in fresh T cell medium.
ACT, intratumoral injection and BLI
Unless otherwise specified, when tumours reached a volume of 100–150 mm3, 4 × 106 in vitro-activated OT-1Luc CD8+ T cells were i.v. injected into mice in a volume of 100 µl PBS. For i.t. injections, 4 × 106 in vitro-activated OT-1Luc CD8+ T cells were injected in a volume of 50 µl PBS. For measuring T cell infiltration by BLI, d-luciferin (150 mg kg–1, Goldbio) was injected retro-orbitally or by tail vein injection into anaesthetized mice, and mice were imaged with an IVIS machine (Caliper Life Sciences) and analysed using Living Image software (v.4.4; Caliper Life Sciences). In NTT tumours, T cell recruitment to the tumour is detectable by BLI within 24–48 h. This initial recruitment is followed by a phase of T cell expansion, with peak BLI signals between 96 and 120 h. Hence, we depict 96 h post-ACT images (unless otherwise specified in figure legends) as a suitable time point to assess T cell expansion in immune-permissive TMEs.
In vivo treatments
For treatment with ICB, mice were i.p. injected with anti-PD1 (clone RMP1-14, BioXcell) and anti-CTLA4 (clone 9D9, BioXcell) in 100 µl of PBS when tumours reached a volume of 150–200 mm3 (usually between 6 and 8 days after injection). The YUMM3.3 model was treated with 200 µg anti-PD1/anti-CTLA4, the CT-26 model with 100 µg anti-PD1, and the EPP2 model with 100 µg anti-PD1. ICB treatment was administered every 3 days and continued for at least for 3 weeks, as indicated in the figure legends. Control mice were treated with an isotype control antibody (rat IgG2a anti-trinitrophenol, clone 2A3, BioXcell, and mouse IgG2b, clone MPC-11, BioXcell). For COX2i treatment, celecoxib (LC Laboratories) was reconstituted in a 60:40 (DMSO to PEG400, dH2O) mixture as previously described53. Etoricoxib (Sellekchem) was dissolved first in a small volume of DMSO and then in 1% sodium carboxymethyl cellulose. COX2i was given by oral gavage every day (30 mg kg–1) in a volume of 200 µl. For both COX2i regiments (celecoxib and etoricoxib), the treatment was started at day 3 after injection, when tumours were palpable, and continued every day until the termination of the experiment. 5-AZA (Sigma-Aldrich) was reconstituted in DMSO to a stock concentration of 10 mg ml–1 and further diluted in PBS for in vivo treatments and given as i.p. injections (1 mg kg–1) in 100–250 µl every 3 days, as previously described54. For NK cell depletion, 200 µg anti-NK1.1 (clone PK136, BioXcell) was administered every 3 days through i.p. injections, starting at day 1 after tumor induction. NK cell depletion was confirmed by flow cytometry. For blocking T cell egress from the lymph node, mice were given an i.p. injection of 20 µg per mouse of FTY720 (Sigma) in 100 µl saline. Treatment was started on the day of T cell transfer and administered for 5–7 consecutive days. Control mice received saline injection. FLT3L (recombinant FLT3L-Ig, hum/hum, BioXCell) treatment (30 µg per mouse in 100 µl PBS i.p.) was started at day 3 after injection and administered every day for 9 consecutive days. In vivo IFNAR blockade was performed with InVivoMab anti-mouse IFNAR-1 (clone MAR1-5A3, BioXcell) and was administered i.p. (200 µg per mouse) in 100 µl. For IFNγ, the neutralizing anti-mouse IFNγ monoclonal antibody was used (clone XMG1.2, BioXcell). Treatment was started on the day of tumour engraftment and administered every 3 days. InVivoMab IgG1 isotype control (BioXCell) was used as the control. For experiments in which CD8 depletion was performed, mice were treated with 50 µg anti-CD8 (clone 2.43, in-house produced), whereas control mice were treated with isotype control (rat IgG2b anti-keyhole limpet haemocyanin, clone LTF-2) starting the day before tumour engraftment and then every 3 days.
DC vaccination with BMDCs
BMDCs were cultured with FLT3L and GM-CSF as described above. At day 10–12 after isolation, DCs were activated overnight with polyI:C (5 µg ml–1, Invitrogen), pulsed with recombinant SIINFEKL peptide (5 µg ml–1, Genscript) and sorted by FACS on the basis of alive MHCII+CD103+CD11c+ cells. Next, 1 × 106 cells in a volume of 50 µl PBS were i.t. injected. Control mice received 50 µl PBS. For DC vaccinations, 2 doses of i.t. injections were administered on day 4 and day 6 after tumour engraftment.
In vivo depletion of DCs with diphtheria toxin
For generation of bone marrow chimeras, Rag2–/– Ly5.1 mice were preconditioned (2×5 Gy), before transferring back 10 × 106 bone marrow cells by i.v. injection. As donor mice, Rag2–/– Ly5.2 zDC-DTR mice were used. After 8 weeks of reconstitution, mice were used for experiments. NTT cells were injected, and DCs were depleted by injecting 25 µg kg–1 of body weight of diphtheria toxin (Sigma-Aldrich) i.p. in PBS, starting on the day of tumour engraftment and then every 3 days for 3–4 doses. Reconstitution efficiency and depletion of intratumoral DCs was confirmed by flow cytometry.
Lentivirus generation and cell transduction
Lenti-X (HEK-293T) cells were transfected with 4,000 ng of the plasmid of interest, 2,000 ng of VSV-G plasmid and 1,000 ng of PAX2 plasmid using polyethylenimine (Avantor). Virus-containing supernatant was collected 24 h and 48 h after transfection and subsequently filtered through a 0.45 µm filter. The cell lines of interest were transduced with the collected virus mixed with 8 µg ml–1 polybrene (Merck).
Generation of CRISPR–Cas9 KO and overexpression cell lines
Doxycycline-inducible Cas9 (iCas9) clones from parental cell lines were generated to allow inducible expression of Cas9. sgRNAs were chosen on the basis of the best VBC score70 (Supplementary Table 7) and were cloned into a vector containing a puromycin selection marker and mCherry or eGFP (hU6-sgRNA–PuroR–mCherry/eGFP). sgRNAs targeting the ROSA26 locus were used as controls for KO cell lines. After transduction, cells were selected with puromycin (5–8 µg ml–1) for 5 days. All sgRNA sequences are provided in Supplementary Table 7. For the generation of single-cell-derived clonal cell lines, cells were FACS sorted on the basis of the fluorescent marker on the sgRNA backbone, at 1 cell per well into 96-well plates. To avoid immunogenicity caused by antibiotic selection markers or fluorophores in the YUMM3.3 model, we transiently transfected the cell lines with an all-in-one vector containing Cas9, the sgRNA of interest and eGFP (U6-IT-EF1As-Cas9-P2A-eGFP). For transient transfection, 7,000 ng of the plasmid with polyethylenimine was used, and single-cell clones were established. For IRF3/7 overexpression, synthesized cDNA sequences were ordered from Twist Biosciences and cloned into two different expression vectors with distinctive selection/fluorescent markers (SFFV-IRF3–mCherry and SFFV-IRF7–PuroR). After transduction, cells were selected with puromycin (5–8 µg ml–1 for 5 days) and bulk FACS-sorted on the basis of mCherry expression. The same cell line engineered with an empty vector containing an mCherry and a puromycin resistance cassette was used as a control. KO and overexpression of the target proteins was confirmed by genotyping, western blotting or quantitative PCR with reverse transcription (RT–qPCR). For the YUMM1.7 and YUMM3.3 Ptgs2 KO cell lines, single-cell-derived clonal cell lines were generated, and several were tested in vivo for growth kinetics.
EP2 and EP4 KO in T cells
sgRNAs targeting the Ptger2 and Ptger4 mouse genes were designed according to the VBC score70 and cloned into a dual hU6-sgRNA-mU6-sgRNA-EF1α-mCherry-PuroR backbone (Supplementary Table 7). As a control, we used a sgRNA targeting a gene desert in chromosome 1. The lentiviral vector was produced as described above. T cells were isolated from Cas9–OT-1 mice, which were a gift from J. Zuber (IMP), as described above. Twelve hours after CD3/CD28 activation, T cells were spin-infected with the lentiviral vector containing the sgRNAs in a 1:1 ratio for 1 h at 32 °C and 800g. At 12 h after infection, T cells were removed from the activation plate, washed with PBS and cultured in the presence of 20 ng ml–1 IL-2. Selection with puromycin was performed 30 h after viral transduction. Before ACT, mCherry levels were assessed, and KO was confirmed by functional in vitro assays.
Flow cytometry and cell sorting
For flow-cytometry-based characterization of the TME, tumours were isolated between day 7 and 11 after injection, cut into pieces and digested for 1.5 h at 37 °C with collagenase A (1 mg ml–1, Roche) and DNAse (20 µg ml–1, Worthington) in unsupplemented RPMI-1640 medium. Digested tumours were strained through a 70 µm filter and resuspended in FACS buffer (0.5% BSA and 2 mM EDTA in PBS). Fc-block was performed with anti-CD16/32 (clone 2.4G2, Pharmingen) for 10 min at 4 °C to avoid Fc-specific antibody capture, and staining for cell surface markers was performed for 30 min at 4 °C. For intracellular staining, a Foxp3 Transcription Factor staining kit was used (eBioscience). Live/dead exclusion was performed by staining with the fixable viability dye eFluor780 (1:1,000, eBioscience). DCs were defined in most experiments as MHCII+CD11c+CD24+ out of alive CD45+ cells. cDC1s were identified as CD103+CD11b– out of the total DCs, cDC2s as CD103–CD11b+ and inflammatory cDC2 as CD103–CD11b+AXL+. AXL was previously described to identify inflammatory cDC2s37. Monocytes were defined as Ly6C+CD11b+F4/80–, and inflammatory monocytes were identified as monocytes that were Ly6A+. Ly6A was previously described to identify monocytes expressing high levels of ISGs38. Macrophages were defined as Ly6C–F4/80+Cd11b+. Acquisition of the samples was performed using a BD LSR Fortessa machine (BD Biosciences) with FACS Diva software (v.9.0.1), and analysis was conducted using FlowJo software (v.10.8 or newer). For cell sorting, a BD Aria cell sorter (BD Biosciences) with FACS Diva software (v.9.0.1) was used.
Antibodies for flow cytometry
The following antibodies (all anti-mouse) were used for flow cytometry stainings (target (clone, catalogue number, manufacturer, dilution)): AXL PE-Cy7 (MAXL8DS, 25-1084-82, eBioscience, 1:200); CD103 PerCP/cyanine5.5 (2E7, 121415, BioLegend, 1:100); CD103 PE (2E7, BioLegend, 121405, 1:100); CD11b APC (M1/70,17-0112-81, eBioscience, 1:200); CD11b PerCP/cyanine5.5 (M1/70, 101229, BioLegend, 1:200); CD11c BV605 (HL3, 563057, BD Pharmigen, 1:100); CD11c FITC (N418, 117305, BioLegend, 1:100); CD24 BV510 (M1/69, 101831, BioLegend, 1:100); CD24 FITC (M1/69, 11-0242-82, eBioscience, 1:100); CD279/PD-1 BV785 (29F.1A12, 135225, BioLegend, 1:200); CD279/PD-1 FITC (29F.1A12, 135213, BioLegend, 1:200); CD40 APC (3/23, 124611, BioLegend, 1:200); CD45 BV711 (30-F11, 103147, BioLegend, 1:500); CD45 FITC (30-F11, 103107, BioLegend, 1:500); CD86 BV510 (GL-1, 105039, BioLegend, 1:100); CD3 BV605 (17A2, 564009, BD Horizon, 1:100); CD3 AF647 (17A2, 100209, BD Horizon, 1:100); CD3 AF488 (17A2, 100212, BD Horizon, 1:100); CD8a eFluor 450 (53-6.7, 48-0081-80, eBioscience, 1:100); CD8a AF647 (53-6.7, 128041, BioLegend, 1:100); MHCI (H-2Kb) APC (AF6-88.5.5.3, 17-5958-82, Bioscience, 1:200); MHCI (H-2Kb) PE (AF6-88.5.5.3, 17-5958-80, Bioscience, 1:200); MHCII (I-A/I-E) eFluor450 (M5/114.15.2, 48-5321-80, eBioscience, 1:200); MHCII (I-A/I-E) APC (M5/114.15.2, 107613, BioLegend, 1:200); NK-1.1 BV711 (PK136, 108745, BioLegend, 1:100); TCF1 PE (S33-966, 564217, BD Pharmigen, 1:50); TIM3 BV711 (RMT3-23, 119727, BioLegend, 1:100); CD88 PE (20/70, 135805, BioLegend, 1;100); Ly-6A/E (Sca-1) FITC (D7, 108105, BioLegend, 1:100); SIINFEKL-HK2B PE (25-D1.16, 12-5743-81, Invitrogen, 1:100); F4/80 PE (BM8, B123110, BioLegend, 1:200); and rat IgG1, K Isotype control PE (R3-34, 5546, BD Pharmigen). Further information is provided in Supplementary Table 8.
RNA extraction of cancer cells sorted from tumours, in vitro cell lines and myeloid cells
Tumours were surgically removed between days 10 and 12 after injection. The tissue was processed as described above, and cancer cells were isolated by flow cytometry on the basis of alive, CD45– cells and a fluorescent marker. For cancer cell lines and in vitro assays with myeloid cells, cells were washed with PBS and snap-frozen in liquid nitrogen and kept at −70 °C until further processing. RNA was extracted using a magnetic bead-based RNA extraction protocol (in-house produced). In brief, cells were lysed and incubated with beads together with DNase I (NEB) followed by magnetic isolation. RNA was purified by further elution with nuclease-free water.
RT–qPCR
Reverse transcription was performed for cDNA formation with 1 µg of RNA per sample utilizing a LunaScript RT SuperMix kit (NEB) according to the manufacturer’s instructions. RT–qPCR was performed with 10 ng cDNA per sample either with Luna Universal qPCR master mix (NEB) or an in-house produced MTD qPCR Dye 2× HS master mix according to the manufacturer’s protocol. Each sample included four technical replicates. The RT–qPCR reaction was carried out in a Bio-Rad CFX384 real‐time cycler and contained 1 min of initial denaturation (95 °C) and 45 annealing cycles lasting 15 s at 95 °C and 30 s at 60 °C. The analysis of gene expression levels was determined by the quantification cycles (Cq). Internal controls and the housekeeping gene GAPDH were used to correct for differences in sample quality and to normalize expression values. qPCR primer pair sequences are listed in Supplementary Table 7.
In vitro assays with BMDCs
For cancer cell CM experiments, supernatants (in full T cell medium) from confluent cancer cells were collected after 48 h, filtered through a 45 µm filter and frozen at −70 °C until further use. Full T cell medium was supplemented with 20 µM of the COX1/2i indomethacin (Selleckchem) or 5 nM of the MEKi trametinib (Selleckchem) for the evaluation of MAPK and COX1/2 activity before media conditioning. BMDCs were differentiated as described above and collected at day 6. Next, 0.5–1 × 106 cells were seeded in triplicate in a 12-well plate in CM and treated with 10 µg ml–1 InVivoMab anti-mouse IFNAR-1 antibody or InVivoMab IgG1 isotype control (BioXCell). Cells were cultured for 24 h, collected and processed for flow cytometry analysis or RNA extraction. For treatment with PGE2 and IFNβ, cells were collected at day 6 and seeded at a concentration of 0.5–1 × 106 cells per ml. Cells were treated for 24–48 h with recombinant PGE2 (100 ng ml–1, Sigma-Aldrich) and recombinant mouse/human IFNβ (R&D Systems) at the concentrations indicated in the corresponding figures. Same volumes of acetone and PBS were used as a control for PGE2 and IFNβ, respectively.
Isolation of bone-marrow-derived Ly6C+ monocytes for intratumoral injection and in vitro assays
Ly6C+ monocytes were directly isolated from the bone marrow of CD45.1+ C57BL/6 mice using a monocyte isolation kit (Miltenyi Biotec) following the manufacturer’s instructions. For intratumoral monocyte transfer, 1 × 106 monocytes were i.t. injected into NTT and RTT tumours established in CD45.2+ Rag2–/– mice. Tumours were isolated for FACS analysis 72 h after intratumoral transfer. For in vitro assays to assess effects of PGE2, Ly6C+ monocytes were seeded at a density of 1 × 106 cells per ml and cultured in recombinant IL-4 and GM-CSF (both produced in-house) and exposed to 200 ng ml–1 PGE2 or vehicle for 3 or 5 days. For CM experiments, monocytes were seeded at a density of 1 × 106 cells per ml in CM obtained from NTT, RTT or RTT IRF3/7 cells with or without 20 µM COX1/2i (indomethacin) during media conditioning and subsequently supplemented with or without 10 µg ml–1 InVivoMab anti-mouse IFNAR1 anti-mouse (BioXCell) or isotype IgG1 control (BioXCell).
In vitro monocyte co-culture assay
Ly6C+Ly6A+ or Ly6C+Ly6A– monocytes were FACS-sorted from NTT tumours grown in Rag2–/– mice or BALB/c mice and co-cultured for 72 h with naive OT-1 T cells (1:3 ratio: 100,000 monocytes for 300,000 naive OT-1 cells) previously labelled with 0.25 µM CFSE for 30 min at 37 °C.
In vitro human monocyte assays
BLaER-1 cells were transdifferentiated into monocytes as previously described68. In brief, BlaER-1 transdifferentiation medium was freshly prepared by adding 10 ng ml–1 human recombinant (hr-)IL-3 (PeproTech), 10 ng ml–1 hr-M-CSF (PeproTech) and 100 nM β-oestradiol (Sigma-Aldrich) to complete RPMI medium. Cells were resuspended in transdifferentiation medium and plated in a 12-well plate at 0.7 × 106 cells per ml. Cells were incubated at 37 °C for 5–6 days until mature monocytes were differentiated. For CM experiments, BLaER-1 or MONO-MAC-1 human monocytes were seeded at a density of 0.7 × 106 cells per ml in CM obtained from NTT or RTT cells from the human melanoma cell lines A375, M249 and LOX or the human NSCLC cell line NCI-H358 with or without 20 µM COX1/2i (indomethacin) during media conditioning. Cells were cultured in CM for 24 h and collected for RNA extraction, as described above.
Evaluation of pMHCI cross-dressing on monocytes
For mismatched MHCI haplotype experiments, 1 × 106 YUMM1.7OVA NTT cells from C57BL/6 origin (H-2Kb) were injected in the flank of BALB/c (H2-Kd) mice. BALB/c mice were treated with anti-CD8 (50 µg in 100 µl, in-house produced), whereas control mice were treated with isotype control (rat IgG2b anti-keyhole limpet haemocyanin, clone LTF-2) starting the day before tumour engraftment and then every 3 days to avoid T cell-mediated mismatched MHCI rejection of YUMM1.7 cells. On day 10, tumours were collected and processed for flow cytometry staining of H2-Kb or FACS-sorted on the basis of Ly6A expression for in vitro assays.
Sample preparation for scRNA-seq
For scRNA-seq experiments involving TME characterization, tumours were isolated at day 10 after injection (72 h after ACT) and were processed as described above. The CD45+ live fraction was isolated by FACS, and approximately 1 × 105 cells were collected. For scRNA-seq of OT-1 T cells, tumours were isolated 5 days after i.t. injection of 4 × 106 T cells. Alive T cells were isolated from tumours by FACS for CD45+CD3+CD8+ markers. Dissociated cell concentrations were measured using NucleoCounter NC250 (Chemometec) following the manufacturer’s instructions. For scRNA-seq samples from experiments 3 and 4 (see below), a Chromium Next GEM Single Cell Fixed RNA Sample preparation kit was used according to the manufacturer’s protocol. In brief, 1 × 106 cells were fixed for 22 h at 4 °C, quenched and long-term stored at –80 °C according to 10x Genomics Fixation of Cells & Nuclei for Chromium Fixed RNA profiling (CG000478) using a Chromium Next GEM Single Cell Fixed RNA Sample preparation kit (PN-1000414, 10x Genomics). About 250,000 cells per sample were used for probe hybridization using a Chromium Fixed RNA Kit, Mouse Transcriptome, 4rxn × 4BC (PN-1000496, 10x Genomics), pooled equally and washed following the Pooled Wash Workflow as described in the Chromium Fixed RNA Profiling Reagent kit protocol (CG000527, 10x Genomics). For all the other scRNA-seq samples, a Chromium Next GEM Single cell 3′ kit with Dual Index was used according to the manufacturer’s instructions. GEMs were generated on Chromium X (10x Genomics) with a target of 10,000 cells recovered, and libraries prepared according to the manufacturer’s instructions (CG000527, 10x Genomics). Sequencing was performed on NovaSeq S4 lane PE150 (Illumina) with a target of 15,000 reads per cell.
scRNA-seq analysis of CD45+ TME
CD45+ immune cells were collected in four different 10x Genomics sequencing experiments. Experiment 1, Chromium Single Cell 3′ scRNA-seq samples were pre-processed using cellranger count (v.6.1.1) (YUMM3.3 samples: NTT/108155 and RTT/108157). Experiment 2, 3′ CellPlex multiplex experiment with 4 samples pre-processed using cellranger multi (v.6.1.1) (YUMM1.7OVA samples: NTT + ACT, RTT + ACT, RTT Ptgs1/2 KO + ACT, RTT CTRL ROSA26 + ACT). Experiments 3 and 4, Chromium Flex multiplex experiments with 4 samples each pre-processed using cellranger multi (v.7.1.0) and the built-in Probe Set (v.1.0.1 mm10-2020-A). Experiment 3, YUMM1.7OVA samples: RTT mCherry CTRL, RTT IRF3/7, RTT COX2i and RTT COX2i + 5-AZA, all ACT treated. Experiment 4, YUMM1.7OVA contained biological replicates of experiment 2 samples and untreated YUMM1.7OVA samples (noA): NTTnoA/271221, RTTnoA/271222, NTT/271223 ACT, RTT/271224 ACT. The prebuilt 10x Genomics mm10 reference refdata-gex-mm10-2020-A was used. Further processing was performed in R (v.4.2.2) with Seurat (v.4.3.0). For generating a CD45+ immune reference map, we integrated cells from the first three experiments as follows. The cellranger filtered feature–barcode matrices were used, retaining cells with more than 1,000 detected genes and less than 15% of mitochondrial and less than 40% of ribosomal RNA reads. An integrated feature–barcode matrix from the three experimental batches was generated accounting for the inclusion of a probe-based assay by keeping genes found in at least five cells in each experiment and excluding ribosomal and mitochondrial genes. Data were log-normalized, scaled (regressing out the difference between the G2M and S phase signature scores), dimensionality reduction was performed using principal component analysis on the top 3,000 most variable genes, and batch correction across batches was performed using Harmony71 (v.0.1.1). The 40 harmony embeddings were used for UMAP visualizations. The first 40 harmony dimensions were used to identify immune cell subclusters with a resolution of 0.5 that were further assigned to cell types using known markers and publicly available myeloid reference datasets21,72. Cells were scored for the expression of published signatures using the AddModuleScore function73. Wilcoxon rank-sum test implemented in Presto (v.1.0.0) was used to identify differentially expressed genes (DEGs). Seurat’s reference-based mapping was used to predict cell-type identity and map cells of the biological replicate experiment to our annotated reference set using the FindTransferAnchors and MapQuery functions after a quality control process retaining cells between 1,000 and 4,500 detected genes for 27,1222 and 27,1224 cells, respectively, and 1,300 and 8,000 detected genes for 27,1221 and 27,1223 cells, respectively, and limiting count tables to the gene universe of the reference. Depth-normalized counts for pseudobulk and GSEA functional analyses of this experiment were generated using cellranger aggr. Differences between ACT and untreated conditions (no ACT) from the replicate experiment (experiment 4) were explored on a pseudo-bulk level in an unsupervised clustering analysis with heatmap visualization. The fibroblast cluster was removed before further processing. Sum aggregation on the depth-normalized UMI counts was followed by variance stabilizing transformation, selection of the 300 most variable genes, standardization, k-means clustering (k = 3) and Enrichr analysis against the Reactome_2022 using Enrichr. The relative frequency bar plots depict the changes in the relative abundance of a cell type across different experimental conditions. For each condition, we calculated the normalized abundance of a specific cell type by comparing the absolute number of the cell type to the absolute number of all cells in the same condition. This normalization accounts for differences in total number of cells captured between conditions. We then calculated the relative cell abundance of the cell type in all conditions of the experiment. This was done by comparing the normalized abundance of the cell type to the sum of normalized abundances of the same cell type across conditions of the experiment. This step produces values between 0 and 1 for each condition for each cell type, with the sum of these values across all conditions of the experiment equalling 1 for each cell type.
scRNA-seq analysis intratumoral CD8+ OT-1Luc T cells
Single-cell gene expression of isolated NTT and RTT T cells was assayed in a Chromium Flex experiment, and read processing was performed using cellranger multi (v.7.1.0) using probeset (v.1.0.1 mm10-2020-A). Cellranger-filtered feature–barcode matrices were used and further filtered to retain cells with more than 800 detected genes, less than 10% of mitochondrial and less than 10% of ribosomal RNAs reads, and removal of cells of contaminant clusters was identified using SingleR and ImmGen reference (fibroblasts, MoMac populations). Data were log-normalized and scaled, and dimensionality reduction was performed using principal component analysis on the top 2,000 most variable genes. Harmony was used for the integration of cells from different samples, and 15 harmony embeddings were used for UMAP visualizations. Published tumour single-cell data were used for signature scoring29. Gene lists are provided in Supplementary Table 2.
RNA velocity analysis
To understand differentiation trajectories of myeloid cells within the TME, we performed RNA velocity analysis74 of the MoMac compartment. Loom files containing the splicing annotation were created for each sample using the velocyto run command from the package velocyto (0.17,17) with default parameters and with no masked intervals. The loom files were combined with the scRNA-seq object that had been filtered to keep the data for monocyte and macrophage populations (Monocyte_1, Monocyte_2, Infl_Mono, TAM_CCL6, TAM_Ctsk, TAM_C1q, TAM_H2-Ab1, TAM_Spp1 and TAM_cycling) and for each condition (NTT, RTT and RTT Ptgs1/2 KO). First-order and second-order moments were computed using scvelo (0.2.5) pp.moments (n_pcs = 30, n_neighbors = 30), and the dynamical model was run with default parameters. Python (v.3.8.12) was used.
SCENIC analysis
Gene regulatory networks for each cell population in each condition were calculated using SCENIC75. The motif database used was mm9-tss-centered-10kb-7species.mc9nr.feather. The co-expression network was calculated using GENIE3. The gene regulatory network was built using SCENIC wrapper functions.
Analysis of publicly available myeloid datasets and inflammatory signatures
For the melanoma and lung samples from a previously published23 dataset (Gene Expression Omnibus (GEO) identifier GSE154763), the raw counts were pre-processed as described in the publication, and clustering was calculated using a resolution of 0.8. The monocyte and inflammatory monocyte gene set was derived by using the wilcoxauc() function from presto and by selecting the genes with a log fold change > 0.6 (Supplementary Table 2). Then, the gene symbols were converted to human symbols. The human inflammatory monocyte gene set was used to calculate an enrichment score per cluster. In brief, the gene average expression was calculated for each cluster in the LUNG and MEL datasets on normalized data. Then, an enrichment score was calculated using GSVA with the following parameters: minSize = 5, maxSize = 500, kcdf = “Gaussian”. The projection of the signature on the UMAP embedding was done using the function AddModuleScore() and then plotting the resulting score using FeaturePlot() with min.cutoff = 0.3 for the inflammatory monocyte score and min.cutoff = 0.4 for the Monocyte_1 score. For another dataset43, the annotated seurat-object for myeloid populations corresponding to the original figure 4a was obtained, and gene sets were analysed as described above. For querying published inflammatory gene signatures, a previously published ISG+ DC signature37 was generated by taking the top DEGs in the cDC2 cluster37. The Bosteels Inf-cDC2 DC signature was previously generated37 and was obtained by re-analysing the scRNA-seq dataset (GEO identifier GSM4505993), in which the top 20 DEGs were taken in the identified inflammatory cDC2 cluster. All of them were subsequently scored in our dataset using the AddModuleScore73, and the resulting score was plotted using FeaturePlot(). Gene lists are provided in Supplementary Table 2.
Single-cell spatial transcriptomics of human melanoma samples
Single-cell spatial transcriptomics profiling was performed using the CosMx technology (Nanostring). Biopsy samples were obtained from patients with an age at diagnosis that ranged from 24 to 85 years with a median of 66 years; 34% were women and 66% were men. We obtained cell-segmented data for 74 FOVs (an area of 500 × 500 µm) from tissue microarray cores of 34 melanoma metastases, in total consisting of 980 genes × 171,536 cells. Tumour samples were obtained from 21 lymph nodes, 7 subcutaneous metastases, 1 lung metastasis and 1 brain metastasis and 4 not annotated, from 31 patients containing 72 FOVs. Two FOVs were from tonsils as control. Most tumour tissue were from patients who were treatment-naive at the time of surgery. Tissue collection was approved by the Regional Ethics committee at Lund University (numbers 191/2007 and 101/2013). Patients provided informed consent. The majority of tissue microarray cores contained tertiary lymphoid structures, and FOVs were preferentially directed to these regions. Low-quality FOVs, cells with <20 counts and potential multiplets of cells (area exceeding the sample geometric mean + 5 standard deviation) were discarded. Using Seurat, genes for which the mean expression was below 3× the median of the negative probe mean expression, and genes with the highest 99% quantile expression, MALAT1 and IGKC (due to potential spillover to neighbouring cells), were removed, which retained 641 genes. The data were normalized using SCTransform76, counts that were zero before SCTransform were restored, and counts were log-transformed as log2(counts+1). The top 30 principal components were used for UMAP reduction and clustering (k.param = 15, resolution = 0.5, Louvain algorithm). Resulting clusters were assigned to biological annotations using known marker genes, and annotations were mapped back to FOV coordinates. Expression of C1QC, CXCL9 or CXCL10 > 0 was considered as positive. Cell-type fractions were derived for each FOV. Pearson correlation values between cell-type fractions across FOVs were determined and displayed. In Fig. 2i, CXCL9+CXCL10+ macrophage/DCs (number 9 and number 10) were either CXCL9+ or CXCL10+. Macrophage/DCs (number 5) were negative for CXCL9, CXCL10 and C1qC.
Generation of the TME-COX and TME-IRF3/7 signature
For the TME-COX signature, the FindMarkers function was used in Seurat, with tresh.use = 0.25 and min.pct = 0.1, to compare RTT CTRL (ROSA26) and RTT Ptgs1/2 KO scRNA-seq samples. The top DEGs (log2 fold change ≤ 1.5, adjusted P value < 0.05) were used and converted to human orthologues using DIOPT77. For the TME-IRF3/7 signature, the FindMarkers function was used in Seurat, comparing RTT CTRL (mCherry) and RTT IRF3/7 and taking the top 40 DEGs.
TME signatures in immunotherapy-treated human samples
Gene expression data for patients receiving ICB were obtained from a previous study49 (NCBI BioProject accession number PRJEB23709). The TME-COX, TME-IRF3/7 and CD8+ T cell scores for each tumour sample were defined as the geometric mean of the expression values of each of the gene sets, respectively (Supplementary Table 4). The univariate Cox proportional hazards models, in which the TME-COX and TME-IRF3/7 scores were included as continuous variables, were used for testing the statistical association between gene signature expression and patient survival, separately for both signatures. The tumour samples were then divided into three groups on the basis of the signature score (bottom third, mid-third and top third) and Kaplan–Meier plots were generated for visualization. The association between signature expression and CD8+ T cell abundance was evaluated by calculating the Person’s correlation coefficient between the signature score and a CD8+ score for each signature separately. For this, all scores were normalized to a median of zero and standard deviation of one. The two overlapping genes were removed from the CD8+ signature before comparing it to TME-IRF3/7 signature expression. For evaluating the enrichment of TME-COX and TME-IRF37 gene signatures in responder and non-responder patients to TIL therapy (baseline) from a previous study43, mouse gene identifiers were first converted to human orthologues (with DIOPT v.9; best dcore = yes, best score reverse = yes, DIOPT score > 7) and single-cell level signature enrichment scores for the ‘humanized’ gene sets were calculated using AddModuleScore_UCell78.
Analysis of transcriptomics data
For plots shown in Fig. 3a, a cut-off of adjusted P value < 0.05 and log2 fold change > 2 and < –2 was used on DEGs expressed in YUMM1.7OVA NTT and RTT GFP+ cancer cells FACS-sorted out of tumours (Supplementary Table 3). Pathway enrichment analysis was performed using Enrichr79,80. For the plot in Extended Data Fig. 5i, upstream regulator analysis (Ingenuity)81 was used to identify upstream regulators using DEGs with an adjusted P value < 0.05.
Quantification of PGE2 and IFNβ by ELISA
For in vitro analysis of PGE2 production, 2 × 106 cells were seeded in 10 ml medium, and supernatants were collected after 48 h and kept at −70 °C until analysis. For IFNβ, 0.3 × 106 cells were seeded, and 1 ml of supernatant was collected from confluent cells in a 6-well plate after 48 h of culture and kept at –70 °C until analysis. For analysis of PGE2 and IFNβ from mouse tumours, whole tumours were isolated between days 4 and 10 after engraftment, accurately weighed and immediately snap-frozen in liquid nitrogen. They were stored at −70 °C until further processing. For PGE2 analysis, tumours were subsequently digested using a MACS dissociator according to the manufacturer’s protocol in PBS supplemented with 1 mM EDTA and 10 µM indomethacin. Lysate was further diluted in dissociation buffer depending on the tumour condition and weight (100 µl per mg of tumour) and further quantified using a PGE2 ELISA kit (Cayman) or a mouse IFNβ Quantikine ELISA kit (Biotechne) according to the manufacturer’s protocol. Values were normalized by taking into account dilution factors and tumour weight. For human IFNβ analysis from human cells, 1 × 106 A375, M249, LOX or NCI-H358 cells were injected into NSG mice and collected on day 21. Tumours were processed as described above and quantified using a Human IFNβ Quantikine ELISA kit (Biotechne) according to the manufacturer’s protocol.
Eicosanoid analysis from tumours by HPLC–MS
YUMM1.7OVA NTT and RTT tumours were isolated at day 10 after injection and weighed, and a solution of isopropanol and methanol (1:1, v/v) was added to the tissue for metabolite extraction. The material was subsequently homogenized and incubated for 1 h at −20 °C. The samples were then centrifuged at 14,000g for 3 min. A second extraction round was performed by adding 80% methanol and H2O (v/v) to the pellet and centrifuged, and both supernatants were combined. Finally, the samples were incubated for another 2 h at −20 °C, and after final centrifugation, the supernatants were stored at −70 °C until further analysis. Samples were subsequently measured on a ZIC-pHILIC column or a RP column. Metabolites were annotated using the compound discoverer 3.0 software (Thermo Fisher) using an internal database or the mzCloud database (at least 75% match on the basis of measured molecular weight and MS2 spectra). For filtering, a RSD of corrected quality control areas was used, being less than or equal to 25%. Group CV of at least 1 group is less than or equal to 40%.
Western blotting
Cells were lysed with RIPA buffer (Cell Signaling Technology) supplemented with complete Protease Inhibitor Cocktail (Sigma Aldrich) and HALT phosphatase inhibitor (Thermo Fisher Scientific). Lysates were sonicated and cleared by centrifugation at 14,000g for 10 min at 4 °C. Protein concentrations were quantified according to the manufacturer’s instructions using a BCA Protein Assay kit (Pierce, Thermo Fisher Scientific). Immunoblotting was conducted according to standard protocols. The primary antibodies used for immunoblotting were as follows: anti-vinculin (Sigma-Aldrich, 1:1,000), anti-COX2 (CST,1:1,000) and anti-H3 (acetyl K27) (Abcam, 1:5,000). The secondary antibodies used were as follows: anti-rabbit IgG HRP-linked (Cell Signaling Technology, 1:10,000) and anti-mouse IgG HRP-linked (Cell Signaling Technology, 1:10,000).
Volumetric IF microscopy and image analysis
Volumetric microscopy of mouse tumours was performed as previously described9. In brief, tumours were fixed in Antigenfix solution (Diapath) for 6–8 h, dehydrated in 30% sucrose overnight, embedded in TissueTek OCT freezing medium (Sakura Finetek) and stored at −80 °C. Using a Leica CM3050 S cryostat, consecutive sections of 50 µm thickness were generated, subsequently permeabilized, blocked and stained in 0.1 M Tris (Carl Roth) supplemented with 1% BSA, 0.3% Triton X-100 (Merck), normal mouse serum (Merck) and donkey serum (Merck). Stained sections were mounted in Mowiol (Merck) and imaged on an inverted TCS SP8 confocal microscope (Leica) using a HC PL APO CS2 ×20/0.75 NA objective. Images were acquired as tiled image stacks, covering whole tumour sections in the xy plane, with 2 µm z-spacing to provide 3D image volumes of at least 20 µm depth. For further analyses, images were adaptively deconvoluted using the Leica TCS SP8 LIGHTNING tool (v.3.5.7.23225) and analysed using Imaris 9.9 software (Oxford Instruments). The Imaris surface generation tool was used to reconstruct and visualize 3D objects for individual cells. Where indicated, signals outside rendered cells were masked to visualize intracellular proteins. For analysis of immune cell infiltration by histocytometry, statistics for object localizations were exported into Excel (v.16.88; Microsoft) and analysed using GraphPad Prism software (GraphPad). Quantification of the number of cells was performed relative to the volume of the imaged section. Interacting cells were described as being less than <5 µm apart from each other.
Antibodies for immunofluorescence microscopy
The following antibodies were used for staining of mouse tissues: anti-CD3 (BioLegend, clone 17A2), anti-CD103 (R&D Systems, goat polyclonal), anti-FSCN1 (Santa Cruz Biotechnology, clone 55-k2), anti-Ly6C (BioLegend, clone HK1.4) and anti-MHCII I-A/I-E (BioLegend, clone M5/114.15.2). All antibodies were either validated by the manufacturer or were previously reported for IF microscopy. The populations were defined as follows: T cells (CD3+), monocytes (Ly6C+CD103–MHCII+), cDC1s (FSCN1–CD103+MHCII+), CCR7+ cDC1 (FSCN1+CD103+MHCII+) and CCR7+ cDC2 (FSCN1+CD103–MHCII+). Nur77–GFP was directly assessed by transferring Nur77–GFP reporter OT-1 T cells.
Meta-analysis of NSAID immunotherapy cohorts
The meta-analysis was performed in accordance with the updated Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines82. The literature search was conducted using the PubMed (MEDLINE) database and last updated on 31 December 2023. The full search strategy is available in Supplementary Table 6. The literature review included studies of (1) adult patients with (2) melanoma or NSCLC (3) undergoing FDA-approved immunotherapy, including anti-PD1, anti-PD-L1 or anti-CTLA4, (4) co-medication with NSAIDs and (5) available sufficient patients’ outcome data to calculate odds ratios for overall response rates or hazard ratios for progression-free and overall survival. Patients were not excluded when receiving concomitant chemotherapy and/or radiotherapy. Included studies report time of overall survival, time of progressions-free survival and overall response rates (defined as complete responses and partial responses divided by patient population). All studies published since 1 January 2011 (FDA approval of first immunotherapy, for example, ipilimumab) were included. Survival data are reported as univariate or multivariate hazard ratios; if both were available, multivariate analysis was prioritized. Odds ratios and hazard ratios with 95% CIs for overall response rates, progression-free and overall survival from included studies were utilized to calculate the pooled odds and hazard ratios. The heterogeneity of the pooled results was evaluated using Q-tests to assess between-study heterogeneity and quantified by the Higgins I2 test. If P was <0.10 for the Q-test or I2 was >50%, significant heterogeneity was assumed, and the random-effects model was used to summarize the data. Statistical analysis was performed using R software (v.4.3.2) with meta (General Package for Meta-Analysis, v.7.0-0).
Statistical analysis and reproducibility
Statistical analyses were performed using GraphPad Prism (v.9.1.2 or newer) and Microsoft Excel (v.16.88). Normality of the data distribution was calculated using a D’Agostino and Pearson test or Shapiro–Wilk test. The number of samples (n) used per experiment and the statistical test used are indicated in the figure legends. All in vitro and in vivo experiments were repeated at least twice and always with multiple replicates, except for the following experiments that were performed only once: scRNA-seq involving pharmacological treatment of the YUMM1.7 RTT model, intratumorally injected T cells and the YUMM3.3 model. IF stainings for which representative images are shown were repeated at least twice, except for the NTT in Batf3–/– and Nur77 reporter experiment, which was performed once but with n = 3 tumours and was also confirmed with flow cytometry. Pharmacological combination treatments of the KPAR model were performed once. No statistical methods were used to determine sample size for in vivo experiments, and numbers were chosen on the basis of previous preliminary experiments. Scientists were not blinded to experimental groups, and experiments were repeated by different investigators. Mice were randomly assigned to treatment groups on the basis of tumour size at the day of treatment start or randomly allocated across separate cages when treatment had to be started at day 3. P values < 0.05 were considered significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Gene expression data from YUMM1.7 NTT and RTT cancer cells sorted from tumours have been previously deposited into the GEO with accession number GSE132443. Specifically, samples from NTT tumours (GSM3864154, GSM3864155 and GSM3864157) and RTT tumours (GSM3864170, GSM3864172 and GSM3864173) were used to generate plots in Fig. 3a and Extended Data Fig. 5i. Raw and processed files of the scRNA-seq generated in this study have been deposited into the GEO with accession number GSE241750. Expression data for patients receiving ICB are publicly available49 (accession number PRJEB23709). scRNA data from gene signatures in responder and non-responder patients to TIL therapy43, including the Seurat object for the myeloid compartment was obtained upon direct request. Expression data for the melanoma and lung samples are publicly available23 (GSE154763) and the raw count matrix was obtained upon request. All other formats of raw data are available upon request. Source data are provided with this paper.
References
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Spranger, S. & Gajewski, T. F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 18, 139–147 (2018).
Di Pilato, M. et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell 184, 4512–4530.e22 (2021).
Magen, A. et al. Intratumoral dendritic cell–CD4+ T helper cell niches enable CD8+ T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat. Med. https://doi.org/10.1038/s41591-023-02345-0 (2023).
Chen, J. H. et al. Human lung cancer harbors spatially organized stem-immunity hubs associated with response to immunotherapy. Nat. Immunol. 25, 644–658 (2024).
Duraiswamy, J. et al. Myeloid antigen-presenting cell niches sustain antitumor T cells and license PD-1 blockade via CD28 costimulation. Cancer Cell 39, 1623–1642.e20 (2021).
Jansen, C. S. et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019).
Meiser, P. et al. A distinct stimulatory cDC1 subpopulation amplifies CD8+ T cell responses in tumors for protective anti-cancer immunity. Cancer Cell 41, 1498–1515.e10 (2023).
Bayerl, F. et al. Tumor-derived prostaglandin E2 programs cDC1 dysfunction to impair intratumoral orchestration of anti-cancer T cell responses. Immunity 56, 1341–1358.e11 (2023).
Garris, C. S. et al. Successful anti-PD-1 cancer immunotherapy requires T cell–dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity 49, 1148–1161.e7 (2018).
Mellman, I., Chen, D. S., Powles, T. & Turley, S. J. The cancer-immunity cycle: indication, genotype, and immunotype. Immunity 56, 2188–2205 (2023).
Prokhnevska, N. et al. CD8+ T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor. Immunity 56, 107–124.e5 (2023).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 (2014).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020).
Haas, L. et al. Acquired resistance to anti-MAPK targeted therapy confers an immune-evasive tumor microenvironment and cross-resistance to immunotherapy in melanoma. Nat. Cancer 2, 693–708 (2021).
Seitter, S. J. et al. Impact of prior treatment on the efficacy of adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma. Clin. Cancer Res. 27, 5289–5298 (2021).
Ascierto, P. A. et al. Sequencing of ipilimumab plus nivolumab and encorafenib plus binimetinib for untreated BRAF-mutated metastatic melanoma (SECOMBIT): a randomized, three-arm, open-label phase II trial. J. Clin. Oncol. 41, 212–221 (2023).
Atkins, M. B. et al. Combination dabrafenib and trametinib versus combination nivolumab and ipilimumab for patients with advanced BRAF-mutant melanoma: the DREAMseq Trial-ECOG-ACRIN EA6134. J. Clin. Oncol. https://doi.org/10.1200/JCO.22.01763 (2022).
Zhang, Q. et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell 179, 829–845.e20 (2019).
Maier, B. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262 (2020).
Zilionis, R. et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 50, 1317–1334.e10 (2019).
Nirschl, C. J. et al. IFNγ-dependent tissue–immune homeostasis is co-opted in the tumor microenvironment. Cell 170, 127–141.e15 (2017).
Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809.e23 (2021).
Bill, R. et al. CXCL9:SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science 381, 515–524 (2023).
Siddiqui, I. et al. Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10 (2019).
Krishna, S. et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science 370, 1328–1334 (2020).
Kurtulus, S. et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1–CD8+ tumor-infiltrating T cells. Immunity 50, 181–194.e6 (2019).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Andreatta, M. et al. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat. Commun. 12, 2965 (2021).
Lacher, S. B. et al. PGE2 limits effector expansion of tumour-infiltrating stem-like CD8+ T cells. Nature https://doi.org/10.1038/s41586-024-07254-x (2024).
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Salmon, H. et al. Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 (2016).
Spranger, S., Dai, D., Horton, B. & Gajewski, T. F. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31, 711 (2017).
Böttcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037.e14 (2018).
Hammerich, L. et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 25, 814–824 (2019).
Gungabeesoon, J. et al. A neutrophil response linked to tumor control in immunotherapy. Cell 186, 1448–1464.e20 (2023).
Duong, E. et al. Type I interferon activates MHC class I-dressed CD11b+ conventional dendritic cells to promote protective anti-tumor CD8+ T cell immunity. Immunity 55, 308–323.e9 (2021).
Kwart, D. et al. Cancer cell-derived type I interferons instruct tumor monocyte polarization. Cell Rep. 41, 111769 (2022).
Tadepalli, S. et al. Rapid recruitment and IFN-I-mediated activation of monocytes dictate focal radiotherapy efficacy. Sci. Immunol. 8, eadd7446 (2023).
Bosteels, C. et al. Inflammatory type 2 cDCs acquire features of cDC1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity 52, 1039–1056.e9 (2020).
Wakim, L. M. & Bevan, M. J. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature 471, 629–632 (2011).
MacNabb, B. W. et al. Dendritic cells can prime anti-tumor CD8+ T cell responses through major histocompatibility complex cross-dressing. Immunity 55, 982–997.e8 (2022).
Barras, D. et al. Response to tumor-infiltrating lymphocyte adoptive therapy is associated with preexisting CD8+ T-myeloid cell networks in melanoma. Sci. Immunol. 9, eadg7995 (2024).
Zelenay, S. et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162, 1257–1270 (2015).
Caronni, N. et al. IL-1β+ macrophages fuel pathogenic inflammation in pancreatic cancer. Nature 623, 415–422 (2023).
Bonavita, E. et al. Antagonistic inflammatory phenotypes dictate tumor fate and response to immune checkpoint blockade. Immunity 53, 1215–1229.e8 (2020).
Muthalagu, N. et al. Repression of the type I interferon pathway underlies MYC- and KRAS-dependent evasion of NK and B cells in pancreatic ductal adenocarcinoma. Cancer Discov. 10, 872–887 (2020).
Obenauf, A. C. et al. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature 520, 368–372 (2015).
Gide, T. N. et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell 35, 238–255.e6 (2019).
Morotti, M. et al. PGE2 inhibits TIL expansion by disrupting IL-2 signalling and mitochondrial function. Nature https://doi.org/10.1038/s41586-024-07352-w (2024).
Wang, S.-J. et al. Effect of cyclo-oxygenase inhibitor use during checkpoint blockade immunotherapy in patients with metastatic melanoma and non-small cell lung cancer. J. Immunother. Cancer 8, e000889 (2020).
Wang, D. Y. et al. The impact of nonsteroidal anti-inflammatory drugs, beta blockers, and metformin on the efficacy of anti-PD-1 therapy in advanced melanoma. Oncologist 25, e602–e605 (2020).
Pelly, V. S. et al. Anti-inflammatory drugs remodel the tumor immune environment to enhance immune checkpoint blockade efficacy. Cancer Discov. 11, 2602–2619 (2021).
Chiappinelli, K. B. et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 162, 974–986 (2015).
Barry, K. C. et al. A natural killer–dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 24, 1178–1191 (2018).
van Elsas, M. J. et al. Immunotherapy-activated T cells recruit and skew late-stage activated M1-like macrophages that are critical for therapeutic efficacy. Cancer Cell 42, 1032–1050.e10 (2024).
Espinosa-Carrasco, G. et al. Intratumoral immune triads are required for immunotherapy-mediated elimination of solid tumors. Cancer Cell 42, 1202–1216.e8 (2024).
Kruse, B. et al. CD4+ T cell-induced inflammatory cell death controls immune-evasive tumours. Nature 618, 1033–1040 (2023).
Kersten, K. et al. Spatiotemporal co-dependency between macrophages and exhausted CD8+ T cells in cancer. Cancer Cell 40, 624–638.e9 (2022).
Jacquelot, N. et al. Sustained type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res. 29, 846–861 (2019).
Cilenti, F. et al. A PGE2–MEF2A axis enables context-dependent control of inflammatory gene expression. Immunity 54, 1665–1682.e14 (2021).
Meeth, K., Wang, J. X., Micevic, G., Damsky, W. & Bosenberg, M. W. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res. 29, 590–597 (2016).
Nazarian, R. et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468, 973–977 (2010).
Boumelha, J. et al. An immunogenic model of KRAS-mutant lung cancer enables evaluation of targeted therapy and immunotherapy combinations. Cancer Res. 82, 3435–3448 (2022).
Rathert, P. et al. Transcriptional plasticity promotes primary and acquired resistance to BET inhibition. Nature 525, 543–547 (2015).
Castle, J. C. et al. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genomics 15, 190 (2014).
Mayer, C. T. et al. Selective and efficient generation of functional Batf3-dependent CD103+ dendritic cells from mouse bone marrow. Blood 124, 3081–3091 (2014).
Gaidt, M. M., Rapino, F., Graf, T. & Hornung, V. Modeling primary human monocytes with the trans-differentiation cell line BLaER1. Methods Mol. Biol. 1714, 57–66 (2018).
Ochyl, L. J. & Moon, J. J. Whole-animal imaging and flow cytometric techniques for analysis of antigen-specific CD8+ T cell responses after nanoparticle vaccination. J. Vis. Exp. https://doi.org/10.3791/52771 (2015).
Michlits, G. et al. Multilayered VBC score predicts sgRNAs that efficiently generate loss-of-function alleles. Nat. Methods 17, 708–716 (2020).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Gerhard, G. M., Bill, R., Messemaker, M., Klein, A. M. & Pittet, M. J. Tumor-infiltrating dendritic cell states are conserved across solid human cancers. J. Exp. Med. 218, e20200264 (2021).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Hu, Y. et al. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinformatics 12, 357 (2011).
Andreatta, M. & Carmona, S. J. UCell: robust and scalable single-cell gene signature scoring. Comput. Struct. Biotechnol. J. 19, 3796–3798 (2021).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128 (2013).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Krämer, A., Green, J., Pollard, J. Jr & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530 (2014).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Acknowledgements
We thank the members of A.C.O.’s laboratory and J.Z.’s laboratory (Research Institute of Molecular Pathology) for discussions and proofreading the manuscript; P. S. Jung for the help with the summary scheme; S. Balay, E. Mancheño and J. van der Veeken for critical reading of the manuscript; G. Jonsson and J. Penninger for providing Itgax-cre mice; J. Moon for providing OT-1Luc Thy1.1 mice; V. Buchholz for providing Nur77–GFP OT-1 T cells; M. Bosenberg for providing the parental YUMM1.7 and YUMM3.3 cell lines; Z. Zhang and S. Cheng for sharing the raw count matrix for their scRNA-seq analysis; M. Gaidt for sharing the BLaER-1 cell line; staff at the BioOptics facility at the Vienna Biocenter for cell sorting; T. Grentzinger and staff at the Next Generation Sequencing Facility at the Vienna Biocenter for scRNA-seq; T. Köcher from the Metabolomics Facility at the Vienna Biocenter for the metabolomic analysis of eicosanoids; and M. Kostic for editing the manuscript. Research in the laboratory of A.C.O. is supported by Boehringer Ingelheim, the Austrian Research Promotion Agency (headquarter grant FFG-852936), the European Research Council (‘CombaTCancer’, grant number 759590 and ‘UnlockIT’ grant number 101125797), and the Vienna Science and Technology Fund (LS-16-063). M.V. is supported by the SNF postdoctoral mobility grant (grant number 214359), the Marie Skłodowska–Curie postdoctoral fellowship programme (grant number 101108907) and the VIP2 postdoctoral programme, which has received funding from the European Union’s Framework Programme for Research and Innovation Horizon 2020 (2014‐2020) (Marie Skłodowska–Curie grant agreement number 847548). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska–Curie grant agreement number 955951. S.V. is supported by the Sigrid Jusélius Foundation. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
A.E., G.E. and A.C.O. conceived the study, designed the experiments and interpreted the results. A.C.O. supervised the study. A.E. and G.E. developed experimental tools, performed in vitro experiments, in vivo treatment studies, flow cytometry analyses, gene expression profiling and parts of the computational analyses, and analysed the data. F.B. and A.-M.P. performed IF stainings. L.H.-H. established parental cell lines and phenotypes, experimental design and expression profiling. M.N. and L.C. analysed gene expression data, scRNA-seq results, including the SCENIC and RNA velocity analyses. E.P., F.A., T.V.N., M.M.i.O. and N.F. contributed to the generation of experimental tools and in vivo and in vitro studies. M.S. performed the meta-analysis of patient data. I.K. performed western blotting and helped with mouse colony maintenance and genotyping. F.B., M.V., S.M.C., S.R., F.H., J.Z. and T.W. contributed to experimental design, generation of experimental tools or in vivo experiments. G.C., D.D.L. and D.B. provided the TIL therapy of patients with melanoma scRNA-seq dataset. G.J. and M.L., provided spatial CosMx data and analysis of human melanoma samples. J.P.B. provided EP2/EP4 KO mice and supervised IF stainings and analyses. C.Q. and X.B. analysed patient data. S.V. analysed RNA-seq data and analysed signatures derived from the scRNA-seq in datasets of patients with melanoma. A.E., G.E. and A.C.O. wrote the manuscript, with input from S.V., J.P.B. and J.Z., and all authors read and approved it.
Corresponding author
Ethics declarations
Competing interests
The laboratories of A.C.O. and J.Z. received research support and funding from Boehringer Ingelheim. J.Z. is a founder and scientific advisor of Quantro Therapeutics. G.C. has received grants from Celgene, Boehringer-Ingelheim, Roche, BMS, Iovance Therapeutics, and Kite Pharma. The institution that G.C. is affiliated with has received fees for his participation on an advisory board or for presentation at a company-sponsored symposium from Genentech, Roche, BMS, AstraZeneca, NextCure, Geneos Tx, and Sanofi/Avensis. G.C. has patents in the ___domain of antibodies and vaccines targeting the tumour vasculature, as well as technologies related to T cell expansion and engineering for T cell therapy. G.C. has received royalties from the University of Pennsylvania regarding CAR T cell technology. G.C. is a named inventor on patent applications filed by the Ludwig Institute for Cancer Research pertaining to the subject matter disclosed herein. D.D.L. has received a grant from Hoffmann-La Roche. All other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Niroshana Anandasabapathy, Thomas Tueting and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Characterization of immune permissive versus suppressive TMEs in mouse melanoma models.
a, Representative images of bioluminescent live imaging (BLI) of T cell infiltration after adoptive CD8+ OT-1Luc T cell transfer (ACT) in YUMM1.7OVA NTT and RTT tumors. b, Scheme outlining the in vitro killing of NTT and RTT cells with activated CD8+ OT-1 T cells (1:1 ratio) (left), histograms (flow cytometry) displaying MHCI levels of NTT and RTT cells (middle) and normalized ratio of 50:50 NTT/RTT cells mixed cultures in an in vitro killing assay with CD8+ OT-1 T cells. Normalization was performed with NTT and RTT percentages from the same 50:50 mixed cultures without CD8+ OT-1 T cells to account for proliferation differences (n = 3 biological replicates) (right). Two-tailed unpaired Student’s t-test. c, UMAP of scRNA-seq of CD45+ cells of YUMM1.7OVA NTT and RTT tumors in Rag2−/− before ACT (n = 3 tumors pooled/group). d, Relative frequency of cell types across conditions in YUMM1.7OVA tumors post-ACT (n = 4 tumors pooled/group). e, Expression of myeloid cell markers in each cluster (scRNA-seq). f, Heatmap of scaled gene expression (scRNA-seq) across cell clusters between NTT and RTT YUMM1.7OVA tumors post-ACT. g, Response to immune checkpoint blockade (ICB, anti-PD1/CTLA-4) of YUMM3.3 NTT and RTT tumors (n = 6 NTT, n = 7 NTT + ICB, n = 8 RTT, n = 9 RTT + ICB). Two-way ANOVA with Tukey’s multiple comparison. h, UMAP of scRNA-seq of CD45+ cells of YUMM3.3 NTT and RTT (n = 1 tumor/group). i, Relative frequency of cell types across conditions. j, Heatmap of scaled gene expression (scRNA-seq) across cell clusters between YUMM3.3 NTT and RTT. Bar graphs and growth curves depict the mean ± s.e.m. ns = not significant.
Extended Data Fig. 2 Permissive TMEs act as local reservoirs for T cell expansion.
a, Representative immunofluorescence (IF) of YUMM1.7OVA NTT and RTT tumors 48 h post-ACT(n = 3 tumors/group). Scale bars depicted in image. b, Quantification of immune populations based on IF staining (n = 3 tumors/group). c, Quantification of relative T cell frequency and distance to the tumor periphery in NTT and RTT tumors. Percentages of T cells found in the tumor border or tumor center are depicted (n = 3 tumors per condition). d, Quantification of Nur77+ OT-1 cells in YUMM1.7OVA NTT and RTT tumors 48 h post-ACT (n = 6 tumors for NTT, n = 5 for RTT). e, Average distances between Nur77+ OT-1 cells and cDC1s or monocytes based on IF stainings (n = 6 tumors for NTT, n = 5 for RTT). f, Quantification of CD8+ OT-1Luc T cell infiltration by BLI post-ACT in YUMM1.7OVA NTT and RTT in Rag2−/− mice treated with FTY720 (n = 5 mice/group). g, Quantification of T cell proliferation by CFSE dilution via flow cytometry 72 h post intratumoral (i.tu.) T cell injection into NTT and RTT tumors in Rag2−/− (n = 5 mice/group). h, Relative frequencies of T cell phenotypes (flow cytometry) after activation in vitro prior to ACT (n = 1, experiment repeated 3 times). i, Gating strategy for the phenotypic characterization of T cells. j, Relative frequencies of CD8+ T cell populations based on defined T cell signatures29 of i.tu. injected T cells isolated and subjected to scRNA-seq 5 days post-transfer (n = 14 NTT, n = 18 RTT pooled tumors). k, Projection of a progenitor exhausted T cell signature score29 and Cxcr6 gene expression on scRNA-seq from intratumoral T cells. l, Scheme outlining the contralateral (CL) injection of tumors in opposite flanks of the same mouse followed by intravenous (i.v) or i.tu. ACT in one of the tumors. m, Growth curves depicting the response to i.v ACT of primary or CL tumor in the same mouse (n = 4 mice NTT/NTT, n = 3 mice RTT/RTT and n = 6 mice NTT/RTT). n, Quantification of T cell infiltration by BLI in mice harboring CL NTT/NTT tumors (n = 6 mice), RTT/RTT tumors (n = 3 mice) or an NTT and RTT tumor (n = 10 mice) after i.v ACT. o, Representative BLI images from Extended Data Fig. 2m, n. p, Flow cytometry characterization of the TME of tumors in the CL setting (n = 3 mice NTT/NTT, n = 3 mice RTT/RTT, n = 5 mice NTT/RTT tumors). q, Quantification of T cell infiltration by BLI post-ACT in NTT and RTT in Rag2−/− mice treated with FTY720 (n = 7 mice/group). r, Response to ACT of mice bearing CL tumors (NTT/RTT) and treated with FTY720 or untreated (CTRL), (n = 7 mice/group). s, Response to ACT of Rag2−/− mice bearing CL tumors (NTT/NTT) with one of the NTT tumors injected i.tu (n = 6 mice). t, Response to ACT of Rag2−/− mice bearing CL tumors (NTT/RTT) with RTT tumors injected i.tu (n = 6 mice). u, Representative BLI images of T cell infiltration in mice harboring NTT/NTT, RTT/ RTT or RTT/ NTT tumors, with one of the tumors receiving i.tu. T cell transfer (n = 6 mice). Bar graphs and growth curves depict the mean ± s.e.m. Error bars in s and t not visible due to synchronous tumor regression. Statistical analysis was performed with a two-tailed unpaired student’s t-test in b, c, d, and g. A one-way ANOVA with Tukey’s multiple comparisons test in f, n, p and q. Two-way ANOVA with Tukey’s multiple comparisons test in m and r on day 17 pi. ns = not significant. Arrow indicates day of ACT.
Extended Data Fig. 3 Inflammatory monocytes cross-dress with cancer-derived pMHCI complexes.
a, Scheme outlining the dendritic cell (DC) vaccination of YUMM1.7OVA RTT tumors with bone marrow-derived cDC1s (BM-DCs) injected i.tu. and treated with ACT. b, Left, representative BLI images and right, quantification of T cell infiltration by BLI (n = 6 mice CTRL and n = 7 mice for DC vaccination). c, Response to ACT of RTT tumors vaccinated with BM-DCs followed by ACT. Black arrow indicates the day of ACT and gray arrows the days of cDC1 vaccination (n = 6 mice CTRL and n = 7 mice for DC vaccination). d, Representative IF staining of YUMM1.7OVA NTT and RTT tumors in Batf3−/ − Rag2−/− mice, 48 h post ACT, scale bar in NTT = 500 µm, zoom-ins=20 µm and 10 µm, scale bar in RTT = 1000 µm, (n = 3 tumors/group). e, Quantification of relative T cell frequency and distance to the tumor periphery in NTT and RTT tumors injected in Rag2−/− Batf3−/− mice. Percentages of T cells found in the tumor border or tumor center are depicted (n = 3 tumors/group). f, Quantification of relative T cell frequency and distance to the next immune cell in NTT tumors in Rag2−/− Batf3−/− mice (n = 3 tumors). g, Scheme outlining the depletion of dendritic cells with diphtheria toxin (DT) in NTT tumors in Rag2−/− mice reconstituted with bone marrow from Rag2−/− Zbtb46-DTR mice. DT was administered every 3 days starting on the day of tumor injection for the duration of the experiment. h, Contour plots depicting the frequency of dendritic cells (left) and flow quantification (flow cytometry) (n = 3 tumors/group). i, Representative BLI images (left) and quantification of T cell infiltration (n = 5 mice for untreated, n = 6 DT treated) (right). j, Scoring of a monocyte derived dendritic cell (MonoDC) signature21 in the YUMM1.7OVA scRNA-seq. k, Scoring of the Bosteels et al.40 ISG signature (Supplementary Table 2) in the YUMM1.7OVA scRNA-seq. l, Quantification of Nur77+ or Nur77- OT-1 T cells interacting with Ly6c+ monocytes based on IF staining of NTT tumors 48 h post-ACT (n = 6 tumors). m, Median fluorescence intensity (MFI) quantification of SIINFEKL-H2Kb staining (n = 6 mice/group) (left) and histograms (right). n, MFI quantification of NTT-derived H2-Kb on BALB/c pMHC-I cross-dressed monocytes (n = 6 mice/group) in NTT and NTT B2m KO. o, Scheme of the process of cross-dressing and in vitro T cell activation (left) and quantification of OT-1 T cell proliferation after 72 h of co-culture with monocytes FACS-sorted from YUMM1.7OVA NTT tumors in BALB/c mice (n = 3 technical replicates). Bar graphs depict the mean ± s.e.m. Statistical analysis was performed with a two-tailed unpaired student’s t-test in b, e, h, i, l, m, n and o. ns = not significant.
Extended Data Fig. 4 Inflammatory monocytes correspond to ISG+ and CXCL9+ macrophages in human data-sets.
a, Inflammatory monocyte and Monocyte 1 gene signature projection on a UMAP, and enrichment scores (ES) calculated with Gene Set Variance Analysis (GSVA) for different myeloid populations in a human melanoma scRNA-seq myeloid data-set (MEL) (Cheng et al.23). b, Inflammatory monocyte and Monocyte 1 gene signature projection on a UMAP, and ES calculated with GSVA for different myeloid populations, in human non-small cell lung cancer (NSCLC) scRNA-seq myeloid data-set (Cheng et al.23). c, Inflammatory monocyte and Monocyte 1 gene signature projection on a UMAP, and ES calculated with GSVA for different myeloid populations, in human melanoma scRNA-seq myeloid data-set (Barras et al.43). See also Supplementary Table 2.
Extended Data Fig. 5 Oncogenic MAPK signaling counter-regulates the production of PGE2 and IFN-I.
a, Targeted metabolomic analysis of eicosanoids in YUMM1.7OVA NTT and RTT tumors isolated at day 10 post-injection from Rag2−/− mice (n = 3 tumors/group). b, Tumor growth of non-ACT RTT CTRL and RTT Ptgs1/2 KO (n = 7 mice/group) in Rag2−/− mice. c, Scheme outlining the injection of YUMM1.7OVA RTT Ptgs2 KO into Rag2−/− mice followed by ACT. d, Quantification of PGE2 by ELISA from YUMM1.7OVA RTT Ptgs2 KO tumors isolated at day 10 post-injection (n = 4 for RTT CTRL and n = 6 for RTT Ptgs2 KO, over 2 independent experiments). e, Survival of Rag2−/− mice harboringYUMM1.7OVA RTT CTRL or RTT Ptgs2 KO tumors upon ACT treatment (n = 5 mice/group). f, Quantification of PGE2 by ELISA from YUMM3.3 NTT and RTT tumors (n = 2 biological replicates). g, Tumor growth of YUMM3.3 RTT CTRL and RTT Ptgs2 KO (n = 5 mice/group) in Rag2−/− mice. h, Quantification of IFN-β by ELISA from YUMM1.7OVA NTT, RTT and RTT IRF3/7 tumors grown in Rag2−/− mice normalized to tumor weight (n = 4 tumors/group, pooled from 2 independent experiments). i, Upstream regulator analysis (Ingenuity) of differentially expressed genes in cancer cells sorted from RTT vs. NTT YUMM1.7OVA tumors grown in Rag2−/− mice. Benjamini-Hochberg correction for multiple testing. j, Tumor growth of non-ACT treated YUMM1.7OVA RTT CTRL (n = 5 mice) and RTT IRF3/7 (n = 4 mice) in Rag2−/− mice. k, Quantification of IFN-β by ELISA from YUMM3.3 NTT and RTT tumors grown in C57BL/6 mice normalized to tumor weight (n = 3 tumors/group). l, Heatmap of scaled ISG expression in YUMM1.7OVA RTT upon treatment with MEK inhibitor (MEKi) for 48 h. m, Western blot of RTT YUMM1.7OVA depicting COX2 protein levels upon treatment with RAF inhibitor (RAFi) or MEKi for 48 h. For gel source data see Supplementary Fig. 1. Experiment repeated 2 independent times. n, Heatmap of scaled gene expression in YUMM1.7OVA RTT cancer cells upon treatment with MEKi in vivo (n = 5 tumors per condition). o, ISG expression in YUMM1.7OVA RTT Ptgs2 KO cell line compared to NTT and RTT CTRL by RT-qPCR (n = 4 technical replicates). All expression values are depicted as the log2FC of the expression of each gene compared to NTT. p, Quantification of IFN-β by ELISA from YUMM1.7OVA RTT CTRL and RTT Ptgs2 KO tumors grown in Rag2−/− mice normalized to tumor weight (n = 6 tumors/group). q, Quantification of PGE2 by ELISA from YUMM1.7OVA RTT CTRL and RTT IRF3/7 tumors grown in Rag2−/− mice (n = 3 tumors/group). r, Quantification of IFN-β and PGE2 by ELISA from NTT and RTT variants of M249, LOX and H358 cell lines. For IFN-β analysis, tumors were grown in NSG mice and normalized to tumor weight (n = 3 tumors/group). For PGE2, supernatants from in vitro cell lines were analyzed (n = 2 biological replicates/group). s, Western blot of RTT, NTT and NTT NRAS variants of A375 human melanoma cell lines depicting COX2 protein levels upon treatment with RAFi for 48 h. For gel source data see Supplementary Fig. 1. Experiment performed once. Bar graphs depict the mean ± s.e.m. Statistical analysis was performed with a two-tailed student’s t-test in a, d, k, p, q and r and one-way ANOVA with Tukey’s multiple comparison in h. p-value in e was calculated using a Log-rank Mantel Cox test. ns = not significant. ND = Not detected.
Extended Data Fig. 6 High PGE2 and low IFN-I instruct an immune evasive TME.
a, Gating strategy for the identification of intratumoral myeloid populations. b, Flow cytometry myeloid characterization pre and 72 h post-ACT (n = 6 for RTT ± ACT, IRF37 RTT ± ACT, n = 5 for NTT ± ACT, RTT Ptgs2 KO + ACT, n = 4 RTT Ptgs2 KO). c, Flow cytometry characterization of dendritic cell populations pre and 72 h post-ACT, (n = 5 for NTT, RTT Ptgs2 KO ± ACT, IRF37 RTT + ACT, n = 6 for NTT + ACT, RTT ± ACT, IRF37 RTT). d, Relative frequency of cell types across conditions in YUMM1.7OVA (scRNA-seq), for RTT CTRL the RTT mCherry sample was used. e, Heatmap of scaled gene expression (scRNA-seq) between NTT, RTT CTRL, RTT Ptgs1/2 KO and RTT IRF3/7 for individual cell clusters. f, Flow cytometry characterization of SIINFEKL peptide on MHCI of dendritic cells isolated from tumors and pulsed ex vivo with SIINFEKL peptide (n = 6 tumors/group) and MFI quantification. g, Scheme outlining in vivo NK cell depletion in Rag2−/− mice harboring YUMM1.7OVA RTT Ptgs2 KO tumors. h, Representative plot depicting NK1.1+ cells in NK cell-depleted vs CTRL RTT Ptgs2 KO tumors measured by flow cytometry (left) and quantification of cDCs (n = 4 CTRL and n = 3 anti-NK1.1 treated tumors) (right). i, Representative BLI images (left) and quantification of CD8+ OT-1Luc T cell infiltration by BLI (right), (n = 3 mice for NTT, n = 5 mice for all other groups). j, Tumor response to ACT (n = 5 mice/group). Arrow indicates day of ACT. Bar graphs and growth curves depict the mean ± s.e.m. Data in i depicts the mean ± s.e.m. Statistical analysis was performed with a two-tailed unpaired student’s t-test in h. A one-way ANOVA with Tukey’s multiple comparison in f. Two-way ANOVA with Tukey’s multiple comparison in i. ns = not significant.
Extended Data Fig. 7 Type I and type II IFNs determine the inflammatory state.
a, b, Overall survival of melanoma patients stratified according to the TME-COX and TME-IRF3/7 signatures (Supplementary Table 4) (left) and correlation of intratumoral CD8+ T cell signature (right) in the Gide et al. RNA-seq data-set49. PCC=Pearson correlation coefficient. c, TME-COX and TME-IRF3/7 signatures projected on the myeloid UMAP from baseline tumors (split for responder and non-responders to TIL therapy) from the Barras et al.43 patient cohort (above) and individual score across different myeloid populations (below). d, Gene set enrichment analysis (GSEA) of NTT and RTT tumors pre and post-ACT based on scRNA-seq analysis. Normalized enrichment score (NES). Multiple correction testing with false discovery rate. e, Pathway enrichment analysis of the top variable genes in the immune tumor microenvironment (TME) from NTT and RTT tumors pre and post-ACT analyzed by scRNA-seq. 1, 2 and 3 depict each gene cluster identified. Benjamini-Hochberg correction for multiple testing. f, TME characterization of YUMM1.7OVA RTT Ptgs2 KO tumors pre and 72 h post-ACT upon αIFN-γ and αIFNAR1 treatment (n = 5 tumors/group, except n = 4 for isotype and αIFNAR + ACT). g, Trajectory analysis and pseudotime of the MoMac compartment of YUMM1.7OVA NTT and RTT tumors. h, Density plots of F4/80+ macrophages after exposure of BM-derived monocytes to PGE2 for 48 h (left) and quantification (right) (n = 3 replicates per condition). i, Scheme outlining the intratumoral (i.tu.) transfer of CD45.1+ BM-derived monocytes into NTT and RTT tumors injected into CD45.2+ Rag2−/− mice (left) and inflammatory monocytes frequencies 3 days after transfer (right) (n = 5 tumors/group). j, Scheme outlining the knockout of EP2 (Ptger2) and EP4 (Ptger4) in OT-1 T cells and i.v. injection into YUMM1.7OVA RTT tumor-bearing Rag2−/− mice (left) and tumor growth (n = 6 mice/group) (right). Arrow indicates the day of ACT. k, Expression of Ptger2 and Ptger4 across populations from the YUMM1.7OVA scRNA-seq. Bar graphs depict the mean ± s.e.m. Statistical analysis was performed with a two-tailed unpaired student’s t-test in h and i. p-value in a and b was calculated using a Cox’s proportional hazards model (survival) and with a two-sided Pearson’s correlation (correlations).
Extended Data Fig. 8 PGE2 suppresses myeloid responsiveness to IFN-I.
a, Tumor growth of YUMM1.7 (left) and YUMM3.3 (right) RTT in Itgax-Cre (CTRL) (n = 7 mice for YUMM3.3 and n = 8 for YUMM1.7) or CD11cCre(Itgax-Cre)Ptger2−/−/Ptger4fl/fl treated with anti-CD8 to deplete CD8+ T cells (n = 5 mice for YUMM3.3 and n = 6 for YUMM1.7) or isotype control (n = 7 mice for YUMM3.3 and n = 10 for YUMM1.7). b, TME characterization (flow cytometry) of RTT tumors in CD11cCre-Ptger2−/−Ptger4fl/fl mice (KOEP2/EP4) or CD11cCre control mice (CTRL) (n = 8 tumors/group). c, Quantification of Ly6A+ cells after exposing BM-derived Ly6C+ monocytes to conditioned media (CM) from cancer cell lines ± COX1/2 inhibitor (COX1/2i, indomethacin) during media conditioning ± αΙFNAR1 during 48 h of culture (n = 3 biological replicates per condition). d, MFI quantification of AXL, MHC-I and CD86 (flow cytometry) of BM-DCs treated with fresh media (C) or CM from NTT, RTT and RTT IRF3/7 cells for 24 h ± αΙFNAR1 or isotype control (n = 3 biological replicates per condition). e, Heatmap of scaled expression of ISGs in BM-DCs exposed to CM from RTT IRF3/7 treated with COX1/2i (indomethacin) or untreated, in the presence of αΙFNAR1 or isotype control measured by RT-qPCR (n = 4 technical replicates). f, Heatmap of scaled ISG expression in BM-DCs exposed to CM from NTT and RTT treated with MEKi or untreated, in the presence of αΙFNAR1 or isotype control (n = 4 technical replicates) measured by RT-qPCR. g, Scheme outlining the culture of human monocyte models to CM from cancer cell lines ± COX1/2i during media conditioning (left) and heatmaps of scaled ISG expression (right). h, Quantification of AXL+ BM-DCs treated with IFN-β and PGE2 (flow cytometry) (left) (n = 3 biological replicates) and heatmap of scaled ISG expression measured by RT-qPCR (right) (n = 4 technical replicates). Bar graphs and growth curves depict the mean ± s.e.m. Statistical analysis were performed with a two-way ANOVA with Tukey’s multiple comparisons test in a, a two-tailed unpaired student’s t-test (APCs, cDC1 and infl.monocytes) and two-tailed Mann Whitney U (total cDCs and infl.cDC2s) in b, one-way ANOVA with Tukey’s multiple comparison in c, d and h. ns = not significant.
Extended Data Fig. 9 Meta-analysis of retrospective clinical studies.
a-c, Forest plot of pooled odds ratios (ORs) and hazard ratios (HRs) with 95% confidence intervals (CI) across included studies of patients receiving ICB with and without concomitant non-steroidal anti-inflammatory drugs (NSAIDs) medication. Number of participants are included in each figure panel. a, Odds ratios comparing overall response rates. b, Hazard ratios comparing progression-free survival. c, Hazard ratios comparing overall survival. I2: I-squared; P: probability. d, PRISMA flow diagram of literature search and study selection process for meta-analysis. Statistical analysis was performed with a random effects model, data are presented as mean values ± 95% confidence interval. See also Supplementary Tables 5 and 6.
Extended Data Fig. 10 Pharmacological inhibition of PGE2 and type I IFN modulators resensitize cross-resistant tumors to immunotherapy.
a, Relative frequency of each cell type across conditions (scRNA-seq). b, Scoring of the Duong et al. inflammatory signature37 in RTT COX2i-treated tumors. c, Heatmap of scaled gene expression (scRNA-seq) in YUMM1.7 OVA RTT vehicle-treated tumors (CTRL) and RTT celecoxib (COX2i)-treated tumors (n = 3 tumors were pooled/group). d, Response of YUMM1.7OVA RTT tumors to vehicle + adoptive T cell transfer (ACT) (n = 7 mice), celecoxib (n = 4 mice), celecoxib + ACT (n = 6 mice) and etoricoxib ± ACT (n = 7 mice/group). Red arrow and box indicate COX2i treatment stop. e, UMAP of scRNA-seq of CD45+ cells from YUMM1.7OVA RTT mice treated with vehicle or COX2i + 5-AZA isolated 72 h post-ACT (n = 3 tumors pooled/group). f, Relative frequency of each cell type across conditions in e (scRNA-seq). g, Scoring of the Duong et al. inflammatory signature37 in COX2i + 5-AZA treated tumors. h, Response of YUMM1.7OVA RTT tumors to 5-azacytidine (5-AZA) (n = 4 mice), 5-AZA + ACT (n = 7 mice), vehicle+ACT (n = 8 mice), Flt3L+ACT (n = 7 mice), COX2i+ACT (n = 9 mice), COX2i + 5-AZA + ACT (n = 7 mice) and COX2i+Flt3L+ACT (n = 8 mice). i, Representative image of IF staining of vehicle-treated and COX2i+Flt3L RTT YUMM1.7OVA tumors 96 h post-ACT (n = 2 tumors per condition). Scale bar = 1000 µm, zoom-in = 50 µm. Black arrows indicates day of ACT.
Extended Data Fig. 11 Combination therapies in melanoma, lung, pancreatic and colorectal mouse cancer models.
a, Scheme outlining the re-injection of YUMM1.7OVA RTT cells into tumor-free responder mice from Fig. 5e (left) and tumor growth of re-challenged mice (n = 4 mice for COX2i + Ftl3L, n = 3 COX2i and n = 2 COX2i + 5-AZA) or naive control mice (n = 5 mice). b, Scheme outlining the injection of YUMM3.3 RTT tumors into C57BL/6 mice and the treatment regimen with COX2i, Flt3L, 5-AZA and anti-PD1/CTLA-4 (ICB) or isotype control. c, Response to control vehicle treatment or vehicle + ICB treatment of YUMM3.3 RTT tumors (n = 6 mice). d, Response to single treatments and ICB combination treatments of YUMM3.3 RTT tumors. COX2i, Flt3L, 5-AZA + ICB, COX2i+ICB and Flt3L+ICB (n = 8 mice), COX2i + 5-AZA + ICB and COX2i+Flt3L+ICB (n = 10 mice). e, Survival plot. f, Scheme outlining the injection of CT-26 RTT tumors into BALB/c mice and the treatment regimen with COX2i, Flt3L, 5-AZA and anti-PD1 (ICB) or isotype control. g, Response to treatment of CT26 RTT tumors. Vehicle and Vehicle+ICB (n = 8 mice), COX2i + 5-AZA + ICB and COX2i+Flt3L+ICB (n = 9 mice). h, Survival plot. i, Scheme outlining the intrapancreatic injection of EPP2Luc cells into C57BL/6 mice and the treatment regimen with COX2i, Flt3L and anti-PD1 (ICB) or isotype control. j, Left, representative BLI images of EPP2Luc tumor burden and right, quantification of control (n = 5 mice), anti-PD1 (n = 6 mice), anti-PD1 + COX2i + Ftl3L (n = 6 mice). k, Survival plot of j. l, Scheme outlining the injection of KPAR tumors into C57BL/6 mice and the treatment regimen with COX2i, Flt3L and anti-PD1 (ICB) or isotype control. m, Response to treatment of KPAR tumors, vehicle ± ICB (n = 6 mice) and ICB + COX2i + Ftl3L (n = 8 mice). n, Survival plot of m. o, Scheme summarizing the role of inflammatory monocytes in T cell restimulation and the functional convergence of PGE2 and IFN-I to determine an inflammatory TME, T cell restimulation and immunotherapy response. Bar graphs depict the mean ± s.e.m. Statistical analysis was performed with a one-way ANOVA with Tukey’s multiple comparisons test in j. Log-rank Mantel Cox test in h, k, n. ns = not significant.
Supplementary information
Supplementary Fig. 1
Uncropped western blot gel images.
Supplementary Tables
Supplementary Tables 1–8.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elewaut, A., Estivill, G., Bayerl, F. et al. Cancer cells impair monocyte-mediated T cell stimulation to evade immunity. Nature 637, 716–725 (2025). https://doi.org/10.1038/s41586-024-08257-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-08257-4
This article is cited by
-
Preoperative pan-immuno-inflammatory values and albumin-to-globulin ratio predict the prognosis of stage I–III colorectal cancer
Scientific Reports (2025)
-
Neoantigen enriched biomimetic nanovaccine for personalized cancer immunotherapy
Nature Communications (2025)
-
A lipid made by tumour cells reprograms immune cells
Nature (2025)
-
Advances in molecular pathology and therapy of non-small cell lung cancer
Signal Transduction and Targeted Therapy (2025)
-
Decoding STING’s roles in cancer: immunity, pain, dormancy, and autophagy
Molecular and Cellular Biochemistry (2025)