Abstract
Progenitors of intraepithelial T cells (IELps) migrate from the thymus to the intestines after birth where they develop into unconventional TCRγδ and TCRαβ lymphocytes in a process of extrathymic lymphopoiesis within cryptopatches. Mechanisms of IELp migration have remained unclear. Here we show that thymic IELps express the somatostatin receptor SSTR2, which contributes to their homing to the gut. IELp homing is Sstr2 dependent and correlates with neonatal induction of Sst encoding somatostatin in neuroendocrine and lamina propria stromal cells. The SSTR2 ligands somatostatin and cortistatin attract IELps in chemotaxis assays and somatostatin triggers IELp binding to the mucosal vascular addressin MAdCAM1. T cell transduction with Sstr2 confers homing to the neonatal colon. Human fetal thymic IELp-like cells express SSTR2 and intestinal stromal cells express SST at the time of initial T cell population, suggesting conserved mechanisms of progenitor seeding of the developing intestines. These results reveal an unexpected role for the SSTR2–somatostatin axis in early immune system development and describe a new role for a small peptide hormone G-protein-coupled receptor in developmental lymphocyte trafficking.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Supplementary Table 1 lists all published external scRNA-seq datasets used in this study, their nature and the specific figures they are part of in the paper. The mouse and human thymocytes (Teichmann, Haniffa and Maehr laboratories) imputed normalized cell × gene expression matrix used for analyses shown in Figs. 1 and 6 and in Extended Data Figs. 1 and 10, respectively, can be downloaded from the following link of the Stanford University Library: https://doi.org/10.25740/pq381cg6077. Source data are provided with this paper.
Code availability
Code for computational analyses is available upon request.
References
Ebrahimzadeh, P. R., Hogfors, C. & Braide, M. Neutrophil chemotaxis in moving gradients of fMLP. J. Leukoc. Biol. 67, 651–661 (2000).
Heagy, W., Laurance, M., Cohen, E. & Finberg, R. Neurohormones regulate T cell function. J. Exp. Med. 171, 1625–1633 (1990).
Del Rio, M. & De la Fuente, M. Chemoattractant capacity of bombesin, gastrin-releasing peptide and neuromedin C is mediated through PKC activation in murine peritoneal leukocytes. Regul. Pept. 49, 185–193 (1994).
Johnston, J. A. et al. Human T lymphocyte chemotaxis and adhesion induced by vasoactive intestinal peptide. J. Immunol. 153, 1762–1768 (1994).
Schratzberger, P. et al. Similar involvement of VIP receptor type I and type II in lymphocyte chemotaxis. J. Neuroimmunol. 87, 73–81 (1998).
McFadden, R. G. & Vickers, K. E. Bradykinin augments the in vitro migration of nonsensitized lymphocytes. Clin. Invest. Med. 12, 247–253 (1989).
Oomen, S. P. et al. Somatostatin is a selective chemoattractant for primitive (CD34+) hematopoietic progenitor cells. Exp. Hematol. 30, 116–125 (2002).
Naito, T., Shiohara, T., Hibi, T., Suematsu, M. & Ishikawa, H. RORγt is dispensable for the development of intestinal mucosal T cells. Mucosal Immunol. 1, 198–207 (2008).
Saito, H. et al. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science 280, 275–278 (1998).
Suzuki, K. et al. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity 13, 691–702 (2000).
Lambolez, F. et al. Characterization of T cell differentiation in the murine gut. J. Exp. Med. 195, 437–449 (2002).
Peaudecerf, L. et al. The role of the gut as a primary lymphoid organ: CD8αα intraepithelial T lymphocytes in euthymic mice derive from very immature CD44+ thymocyte precursors. Mucosal Immunol. 4, 93–101 (2011).
Wang, J. & Klein, J. R. Thymus–neuroendocrine interactions in extrathymic T cell development. Science 265, 1860–1862 (1994).
Sugahara, S. et al. Extrathymic derivation of gut lymphocytes in parabiotic mice. Immunology 96, 57–65 (1999).
Lin, T., Matsuzaki, G., Kenai, H., Nakamura, T. & Nomoto, K. Thymus influences the development of extrathymically derived intestinal intraepithelial lymphocytes. Eur. J. Immunol. 23, 1968–1974 (1993).
Lambolez, F. et al. The thymus exports long-lived fully committed T cell precursors that can colonize primary lymphoid organs. Nat. Immunol. 7, 76–82 (2006).
Laky, K. et al. Enterocyte expression of interleukin 7 induces development of γδ T cells and Peyer’s patches. J. Exp. Med. 191, 1569–1580 (2000).
Miller, A. F. & Falke, J. J. Chemotaxis receptors and signaling. Adv. Protein Chem. 68, 393–444 (2004).
Gu, Y. Z. & Schonbrunn, A. Coupling specificity between somatostatin receptor SST2A and G proteins: isolation of the receptor–G protein complex with a receptor antibody. Mol. Endocrinol. 11, 527–537 (1997).
Brazeau, P. et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179, 77–79 (1973).
Siler, T. M. et al. Inhibition of growth hormone release in humans by somatostatin. J. Clin. Endocrinol. Metab. 37, 632–634 (1973).
Sekiya, H. et al. The inhibitory effect of somatostatin on gastric motility in Suncus murinus. J. Smooth Muscle Res. 56, 69–81 (2020).
Shamsi, B. H., Chatoo, M., Xu, X. K., Xu, X. & Chen, X. Q. Versatile functions of somatostatin and somatostatin receptors in the gastrointestinal system. Front. Endocrinol. 12, 652363 (2021).
Tanaka, T., Miyadera, K. & Tani, S. Somatostatin inhibits pepsinogen secretion without influencing cytosolic free Ca2+ increase induced by carbachol and cholecystokinin octapeptide in rat chief cells. Biol. Pharm. Bull. 16, 767–770 (1993).
Zaki, M. et al. Somatostatin receptor subtype 2 mediates inhibition of gastrin and histamine secretion from human, dog, and rat antrum. Gastroenterology 111, 919–924 (1996).
de Lecea, L. et al. Cortistatin is expressed in a distinct subset of cortical interneurons. J. Neurosci. 17, 5868–5880 (1997).
Riedemann, T. Diversity and function of somatostatin-expressing interneurons in the cerebral cortex. Int. J. Mol. Sci. 20, 2952 (2019).
Samson, W. K. et al. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J. Biol. Chem. 283, 31949–31959 (2008).
Urban-Ciecko, J. & Barth, A. L. Somatostatin-expressing neurons in cortical networks. Nat. Rev. Neurosci. 17, 401–409 (2016).
Kernfeld, E. M. et al. A single-cell transcriptomic atlas of thymus organogenesis resolves cell types and developmental maturation. Immunity 48, 1258–1270 (2018).
Park, J. E. et al. A cell atlas of human thymic development defines T cell repertoire formation. Science 367, eaay3224 (2020).
The Immunological Genome Project. ImmGen at 15. Nat. Immunol. 21, 700–703 (2020).
Haber, A. L. et al. A single-cell survey of the small intestinal epithelium. Nature 551, 333–339 (2017).
Worthington, J. J., Reimann, F. & Gribble, F. M. Enteroendocrine cells—sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 11, 3–20 (2018).
Drokhlyansky, E. et al. The human and mouse enteric nervous system at single-cell resolution. Cell 182, 1606–1622.e23 (2020).
Gu, W. et al. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J. Biol. Chem. 293, 8089–8102 (2018).
Lavaert, M. et al. Integrated scRNA-seq identifies human postnatal thymus seeding progenitors and regulatory dynamics of differentiating immature thymocytes. Immunity 52, 1088–1104 (2020).
Zeng, Y. et al. Single-cell RNA sequencing resolves spatiotemporal development of pre-thymic lymphoid progenitors and thymus organogenesis in human embryos. Immunity 51, 930–948 (2019).
Stras, S. F. et al. Maturation of the human intestinal immune system occurs early in fetal development. Dev. Cell 51, 357–373.e5 (2019).
Elmentaite, R. et al. Single-cell sequencing of developing human gut reveals transcriptional links to childhood Crohn’s disease. Dev. Cell 55, 771–783.e5 (2020).
Beumer, J. et al. High-resolution mRNA and secretome atlas of human enteroendocrine cells. Cell 182, 1062–1064 (2020).
Ocampo Daza, D., Sundström, G., Bergqvist, C. A. & Larhammar, D. The evolution of vertebrate somatostatin receptors and their gene regions involves extensive chromosomal rearrangements. BMC Evol. Biol. 12, 231 (2012).
Patel, Y. C. Somatostatin and its receptor family. Front. Neuroendocrinol. 20, 157–198 (1999).
Krantic, S. Peptides as regulators of the immune system: emphasis on somatostatin. Peptides 21, 1941–1964 (2000).
Oomen, S. et al. Somatostatin induces migration of acute myeloid leukemia cells via activation of somatostatin receptor subtype 2. Leukemia 15, 621–627 (2001).
Solomou, K., Ritter, M. A. & Palmer, D. B. Somatostatin is expressed in the murine thymus and enhances thymocyte development. Eur. J. Immunol. 32, 1550–1559 (2002).
Mazo, I. B., Massberg, S. & von Andrian, U. H. Hematopoietic stem and progenitor cell trafficking. Trends Immunol. 32, 493–503 (2011).
Howie, D. et al. Extrathymic T cell differentiation in the human intestine early in life. J. Immunol. 161, 5862–5872 (1998).
Kennedy, K. M. et al. Over-celling fetal microbial exposure. Cell 184, 5839–5841 (2021).
Mishra, A. et al. Reply to Over-celling fetal microbial exposure. Cell 184, 5842–5844 (2021).
Mishra, A. et al. Microbial exposure during early human development primes fetal immune cells. Cell 184, 3394–3409.e20 (2021).
Eberl, G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat. Rev. Immunol. 5, 413–420 (2005).
Morbe, U. M. et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 14, 793–802 (2021).
Ruscher, R., Kummer, R. L., Lee, Y. J., Jameson, S. C. & Hogquist, K. A. CD8αα intraepithelial lymphocytes arise from two main thymic precursors. Nat. Immunol. 18, 771–779 (2017).
Weigmann, B. et al. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2, 2307–2311 (2007).
Campbell, J. J. et al. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 279, 381–384 (1998).
Ocon, B. et al. A mucosal and cutaneous chemokine ligand for the lymphocyte chemoattractant receptor GPR15. Front. Immunol. 8, 1111 (2017).
Sumida, H. et al. GPR55 regulates intraepithelial lymphocyte migration dynamics and susceptibility to intestinal damage. Sci. Immunol. 2, eaao1135 (2017).
Brulois, K. et al. A molecular map of murine lymph node blood vascular endothelium at single cell resolution. Nat. Commun. 11, 3798 (2020).
Lun, A. T., Bach, K. & Marioni, J. C. Pooling across cells to normalize single-cell RNA sequencing data with many zero counts. Genome Biol. 17, 75 (2016).
van Dijk, D. et al. Recovering gene interactions from single-cell data using data diffusion. Cell 174, 716–729.e27 (2018).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Acknowledgements
We thank L. de Lecea (Stanford University) for providing Cre-Sst and Cre-Cort mice. We also thank S. Ezine for technical advice regarding thymectomies on Il2rγ–/–Rag2–/– mice and critical discussion of IELp transfer results. We thank G. Ramos for mouse colony maintenance and L. Magalhaes for administrative support. This material is the result of work supported with resources and the use of facilities at the VAPAHCS. The contents of this publication do not represent the views of the Veterans Affairs or the US government. This work was funded by National Institutes of Health (NIH) grants R01 AI178113 and R01 AI047822, grant 1903-03787 from the Leona M. & Harry B. Helmsley Charitable Trust, and Regents of the University of California Tobacco-Related Disease Research Program (TRDRP) grants T31IP1880 and T33IR6609 to E.C.B. A.A. was supported by the California Institute for Regenerative Medicine (CIRM), award EDUC2-12677. M.X. was supported by TRDRP grant T31FT1867. Y.B. was supported by a research fellow award from the Crohn’s and Colitis Foundation of America (835171). M.G. was funded by the Department of Biology (Stanford University). B.O. was supported by a postdoctoral fellowship from the Ramon Areces Foundation (Madrid, Spain) and a research fellow award from the Crohn’s and Colitis Foundation of America (574148).
Author information
Authors and Affiliations
Contributions
B.O., K.F.B., M.G., H.H., A.A., Y.B., J.G. and M.K. performed the experiments. K.F.B., with assistance from M.X., performed scRNA-seq analysis. B.O. and E.C.B. wrote the paper, and K.F.B. and J.P. edited the paper. E.C.B. and B.O. conceived of the study, and E.C.B. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Daniel Mucida and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: S. Houston, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
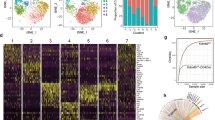
Extended Data Fig. 1 Mouse thymocyte scRNAseq profiling comprising the complete HSC to single positive trajectory. Mouse IELp GPCR and T cell markers expression profile.
(a) Top; relative positioning of the annotated cell subsets along the complete thymocyte developmental trajectory. Bottom; heatmap highlighting mRNA expression (relative to maximum per gene) of relevant genes. (b, c) Violin plots showing leukocyte trafficking receptors and T cell markers expression pattern among mouse thymocytes. Mouse IELp defined as previously indicated (see Methods). Data shown are imputed and shown as normalized log transformed expression values. The mouse thymocyte scRNAseq figure shown was generated mining a validated data set published by the Haniffa and Teichmann labs (Development Cell Atlas), as in Fig. 1a–c. The subset annotation shown in the figure was originally published by the authors. We annotated the early T cell progenitors as DN1-DN3/4 subsets based on the classic Cd44 vs Il2ra (CD25) relative expression.
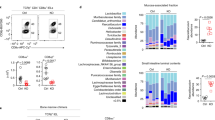
Extended Data Fig. 2 (A) Mouse IELp do not express GPR15 or CCR9 at the protein level.
Flow cytometric analysis of P1 neonatal mouse thymocytes confirms no classic gut homing GPCR expression by gut homing (α4β7+) mouse IELp. Neonatal TCRγδ cell subsets are used as positive internal controls for GPR15 and CCR9 staining. (B) Single and double positive mouse thymocytes do not migrate to SST or CORT. In vitro migration results of thymocyte subsets to CXCL12 (100 nM) as positive control, as well as SST-28 (1uM) and CORT-14 (100 nM). Data are shown as mean ± SEM (n = 6 to 8 per group) and are representative of 2 independent experiments. One-way ANOVA with Dunnett’s multiple comparisons test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Extended Data Fig. 3 Cortistatin is not expressed in the neonatal and adult mouse intestines.
Intestinal sections analyzed by immunofluorescence showing intestinal CORT expression using CORT td-Tomato reporter mice sacrificed shortly after birth and 8–10 weeks after birth. Phalloidin- AF488 in green for background (Scale bars = 100 μm).
Extended Data Fig. 4 β-tubulin or calponin-1 colocalization with SST td-Tomato positive cells in the adult mouse colon. Some neonatal and adult intestinal glia cells express somatostatin.
Colon sections analyzed by immunofluorescence showing β-tubulin (a), calponin-1 (b) or GFAP (glial marker) (c) staining (AF-488) and intestinal SST td-Tomato reporter signal in red. The Sst reporter mice were sacrificed early after birth or 8–10 weeks after birth. Phalloidin-AF488 in green for background (Scale bars = 50 μm). For GFAP, isolated areas of colocalization (yellow, indicated with arrows) were observed in the mucosal glia, within the lamina propria underneath the epithelium.
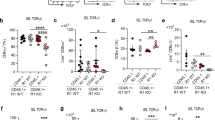
Extended Data Fig. 5 Effect of Sstr2 deficiency on gut IEL subset representation in neonatal (P10), 3 weeks (weaning) and 8–10 weeks old (adult) mice.
Flow cytometry analysis of colon and small intestine IEL subset relative representation, shown as % of total T cells, comparing Sstr2+/− (or WT for P10) vs Sstr2−/− 8–10 weeks old mice (a, b), 3 weeks old (weaning) mice (c, d) and neonatal (P10) suckling pups (e, f). Data are shown as mean ± SEM and are representative of at least 3 independent experiments. DN = double negative, indicating CD4- CD8β-. Unpaired two-tailed T-test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Extended Data Fig. 6 SSTR2 contributes to IEL repopulation in neonatally established competitive BM chimeras.
(a, b) Competitive bone marrow chimeras were set up 2 days after birth and analyzed 8 weeks later as young adults. Unconventional colon (A) and small intestine (B) IEL subsets are profiled and WT/WT as well as WT/Sstr2−/− ratios are shown. Results are internally normalized for each mouse to the ratio of the conventional TCRαβ + CD4 + and CD8+ IELs (exclusively of thymic origin where SSTR2 has no effect) from the same organ compartment. (c, d) BrDU incorporation by IEL subsets in 10–12 weeks old Sstr2−/− vs WT mice indicates heightened proliferation of CD8αα + TCRγδ+ (CD103 high) colon unconventional IEL in SSTR2-deficient mice. Unpaired two-tailed Student’s t test. *P ≤ 0.05, **P ≤ 0.01. DN = CD4-, CD8β-.
Extended Data Fig. 7 Reporters for SSTR2 chemotactic ligands are not detected in the lungs.
Immunofluorescence sections showing lung SST (left) and CORT (right) expression using the corresponding td-Tomato reporter mice sacrificed 8–10 weeks after birth. Phalloidin- AF488 in green for background. Scale bars = 100 μm.
Extended Data Fig. 9 The SSTR2-SST axis does not control DN T cell progenitor representation in the thymus.
(a) % of DN1; 1-2 transitional; 2 and 3 cells among DN1-3 T cell-committed progenitors at weaning. (b) Double positive, single positive and double negative thymocyte subsets cellularity in WT vs Sstr2−/− mice at weaning. (c) WT CD45.1 vs Sstr2−/− CD45.2 ratio in CD90 + CD44 + CD25 + SCA1+ cKIT+ α4β7 + thymocytes from competitive BM chimeras set up 2 days (P2) or 8 weeks after birth (8w). Results are normalized to the average of total TCRαβ + CD4+, TCRαβ + CD8β+ and B cell ratio in the spleen. Data are shown as mean ± SEM (n = 6 to 8 per group) and are representative of 2 independent experiments. Unpaired two-tailed T-test. *P ≤ 0.05. DN = double negative, indicating CD4- CD8β-.
Extended Data Fig. 10 Human thymocyte scRNAseq profiling comprising the complete double negative early progenitor to single positive trajectory. Human IELp-like cells features and relative abundance during fetal and post-birth stages in the thymus.
(a) Human thymocyte scRNA-seq profiling. Top; relative positioning of the annotated cell subsets along the complete thymocyte developmental trajectory. Bottom; heatmap highlighting mRNA scaled gene-wise (relative to maximum per gene) expression of indicated genes. (b, c) Violin plots showing leukocyte trafficking receptors and T cell markers expression pattern among human thymocytes. Data shown are imputed and normalized log transformed expression values. The human thymocyte scRNA-seq analysis shown was generated mining a validated and publicly available data set comprising first and second trimester, as well as post-birth thymus specimens published by Jong-Eun Park, et al.31. SMC = smooth muscle cells. The subset annotation shown in the figure was originally published by the authors. (d) % of gut homing human IELp-like among DN (early) thymocytes in sample comprising first and second trimester, as well as post-birth thymus specimens. No third trimester specimens were available in this data set. Human IELp-like cells are here defined as; CD34+, MPO+, CD7+, MMElow/−, and CD1A−; and those that are imprinted with a gut homing signature are triple positive for ITGA4, ITGB7 and SSTR2. ND = not detected.
Supplementary information
Supplementary Table 1
List and nature of the scRNA-seq datasets included in the study.
Source data
Source Data Figs. 2–5 and Extended Data Figs. 2, 5, 6 and 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ocón, B., Brulois, K.F., Hadeiba, H. et al. An SSTR2–somatostatin chemotactic axis drives T cell progenitor homing to the intestines. Nat Immunol 26, 607–618 (2025). https://doi.org/10.1038/s41590-025-02097-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-025-02097-8