Abstract
Anaplastic thyroid carcinoma (ATC) is the most aggressive thyroid cancer, and it has a poor prognosis and high probability of metastatic recurrence. The long-term survival of cancer cells depends on their ability to settle in a favorable environment. Cancer cells interact with other cells in the tumor microenvironment to shape the “soil” and make it suitable for cell growth by forming an extremely complex tumor ecosystem. The extracellular matrix (ECM) is an essential component of the tumor ecosystem, and its biological and mechanical changes strongly affect tumor invasion, metastasis, immune escape and drug resistance. Compared to normal tissues, biological processes, such as collagen synthesis and ECM signaling, are significantly activated in ATC tissues. However, how ATC triggers changes in the properties of the ECM and its interaction with the ECM remain poorly characterized. Therefore, an in-depth study of the regulatory mechanism of the abnormal activation of ECM signaling in ATC is highly important for achieving the therapeutic goal of exerting antitumor effects by destroying the “soil” in which cancer cells depend for survival. In this research, we revealed the aberrant activation state of ECM signaling in ATC progression and attempted to uncover the potential mechanism of action of ECM components in ATC, with the aim of providing new drug targets for ATC therapy.
Similar content being viewed by others
Introduction
Thyroid carcinoma is one of the most common malignant endocrine cancers, and its incidence is increasing annually1. Anaplastic thyroid carcinoma (ATC) is an extremely malignant subtype of thyroid cancer that accounts for approximately 2% of cases, with a median survival of only 3–7 months2. Conventional therapies have limited efficacy in patients with ATC, and ATC patients often have a poor prognosis due to tumor expansion and distant metastasis3. Most ATC patients are diagnosed with advanced-stage cancer and miss the opportunity for surgical treatment, and there are currently no efficacious treatments that increase the overall survival of ATC patients4,5. Therefore, it is necessary to understand the cellular mechanisms of ATC to develop effective therapeutic targets and improve the survival rate of patients with this malignancy.
The tumor microenvironment (TME) refers to the internal environment in which tumor cells arise and live, and it is a complex integrated system that contains cellular components, such as cancer cells, fibroblasts and inflammatory/immune cells, and non-cellular components, such as the extracellular matrix (ECM) and cytokines6. A growing body of evidence suggests that the TME is not a ‘silent bystander’ but rather an ‘active promoter of oncogenesis’ in the process of cancer development7. Hypoxia, chronic inflammation and immunosuppression are prominent features of the TME, and these components form a highly complicated molecular network that makes the microenvironment a “blind zone” for the body’s anti-tumor immune response and immune intervention8. Recent immunotherapies, including immune checkpoint inhibitors, adoptive cellular immunotherapy, and cancer vaccines, restore and strengthen the ability of immune components in the TME to recognize and kill cancer cells, and these agents have shown superior therapeutic effects in the specific clearing of tumors compared to conventional therapies9. For patients with ATC, targeted blockade of programmed death-1/programmed death ligand-1 at immune checkpoints or the combination of molecularly targeted inhibitors and immunotherapy have shown promising therapeutic outcomes10. However, the high heterogeneity of individual tumors and the frequent occurrence of immune resistance limit the benefits of immunotherapy, and some patients do not benefit from it11. Understanding the mechanisms of immune regulation in the ATC microenvironment deepens our understanding of tumor cell behavior and aids in the development of new drugs to improve the antitumor efficacy of immunotherapy.
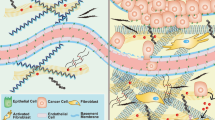
The ECM is a non-cellular component that provides biochemical components and fundamental structural support to tumor cells in the TME12. The main components of the ECM include collagen (approximately 90% of the ECM), fibronectin, elastin, laminin, hyaluronic acid, chondroitin sulfate, keratan sulfate, and heparan sulfate, and their quantity and cross-linking status are the main determinants of tissue stiffness13. The ECM is used as an intercellular filler, and it is an active substance and source of signaling molecules for intercellular communication, cell proliferation, and adhesion14. Active interactions between cancer cells and the TME often result in stiffening of the ECM, which leads to aberrant mechanotransduction and further malignant transformation during cancer progression15. The clinical application of ibrutinib, which is a small molecule compound that inhibits integrin (ECM receptor) signaling, for the treatment of lymphoid leukemias and lymphomas has demonstrated the feasibility of targeting aberrantly activated ECM in oncotherapy16. Notably, some studies have revealed the critical role of ECM remodeling in ATC cell metastasis17. Therefore, a comprehensive understanding of ECM derangement in the TME may help identify promising targets for ATC therapy.
In this study, we emphasize the significant role of ECM in ATC progression and its potential therapeutic targets for antitumor by summarizing the following knowledge: (1) the activation status of the ECM in ATC; (2) the potential function of the main components of the ECM in ATC; (3) the potential impact of the interaction between cancer cells and the ECM in the TME on ATC progression; and (4) potential therapeutic strategies targeting aberrant ECM signaling for ATC treatment.
Materials and methods
ATC data of public databases
The transcription data (GSE29265, GSE33630, GSE53072, and GSE65144) were downloaded from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/).
Gene ontology (GO) and kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analyses
Differentially expressed genes (DEGs) among the normal and ATC groups were displayed in the form of volcano plots by the “gplot” R package from Assistant for Clinical Bioinformatics (https://www.aclbi.com/static/index.html#/) platform. |Fold Change|> 2 and adjusted p < 0.05 were set as the statistical threshold value for differentially expressed genes. GO and KEGG pathway analyses were performed by the Assistant for Clinical Bioinformatics platform using ClusterProfiler package in R software, based on relevant cancer data in GEO database (GSE29265, GSE33630, GSE53072, and GSE65144). In the enrichment result, p less than 0.05 is considered to be a meaningful pathway.
Results
ECM signaling is significantly activated in ATC tissues
The GEO is a shared repository for high-throughput microarray and next-generation sequence functional genomic datasets. To determine the status of activated ECM signaling in ATC, we downloaded and extracted transcriptome data and corresponding clinical information from the GSE29265, GSE33630, GSE53072, and GSE65144 datasets. Differentially expressed genes (DEGs) between ATC and normal tissue were identified, and enrichment analysis was performed as described previously18. Notably, the downregulated DEGs in the four GEO datasets were closely related to extracellular structure organization and extracellular matrix organization (Figs. 1, 2, 3, 4), which suggested that ECM signaling played a crucial role in ATC progression. The most significantly enriched terms for GSE29265 were organelle fission, nuclear division, mitotic nuclear division, extracellular structure organization, extracellular matrix organization, and chromosome segregation, as shown in Fig. 1. Similarly, the most significantly enriched terms for GSE33630 were extracellular structure organization, extracellular matrix organization, and cytokine–cytokine receptor interaction; the most significantly enriched terms for GSE53072 were Herpes simplex virus 1 infection, cytokine–cytokine receptor interaction, extracellular structure organization, and extracellular matrix organization; the most significantly enriched terms for GSE65144 were Herpes simplex virus 1 infection, cytokine–cytokine receptor interaction, extracellular structure organization, and extracellular matrix organization, and neutrophil degranulation, neutrophil activation involved in immune response, as shown in Figs. 2, 3 and 4, respectively. A distinguishing feature of ATC is its ability to easily shape the tumor stromal microenvironment. We listed several genes that exhibit aberrant expression in ATC tissues and are involved in modifying the ATC immune microenvironment in Table 1. Because of the important regulatory role of the ECM in the malignant phenotype of ATC, there is an urgent need to elucidate the mechanism of ECM remodeling in ATC.
Differentially expressed genes and enrichment analysis between ATC and normal tissues based on the GSE29265 dataset. A-B Volcano map (A) and heatmap (B) of differentially expressed genes was constructed on normal and ATC group. (C) The most significantly enriched terms for GSE29265 were organelle fission, nuclear division, mitotic nuclear division, extracellular structure organization, extracellular matrix organization, and chromosome segregation.
Differentially expressed genes and enrichment analysis between ATC and normal tissues based on the GSE33630 dataset. A-B: Volcano map (A) and heatmap (B) of differentially expressed genes was constructed on normal and ATC group. (C) The most significantly enriched terms for GSE33630 were extracellular structure organization, extracellular matrix organization, and cytokine–cytokine receptor interaction.
Differentially expressed genes and enrichment analysis between ATC and normal tissues based on the GSE53072 dataset. A-B: Volcano map (A) and heatmap (B) of differentially expressed genes was constructed on normal and ATC group. (C) The most significantly enriched terms for GSE53072 were Herpes simplex virus 1 infection, cytokine–cytokine receptor interaction, extracellular structure organization, and extracellular matrix organization.
Differentially expressed genes and enrichment analysis between ATC and normal tissues based on the GSE65144 dataset. A-B: Volcano map (A) and heatmap (B) of differentially expressed genes was constructed on normal and ATC group. (C) The most significantly enriched terms for GSE65144 were Herpes simplex virus 1 infection, cytokine–cytokine receptor interaction, extracellular structure organization, and extracellular matrix organization, and neutrophil degranulation, neutrophil activation involved in immune response.
The function of the main components of the ECM in TME
Cancer cells remodel the ECM by interacting with stromal cells to promote covalent intermolecular cross-linking and the massive deposition of supramolecular aggregates, such as fibrillar collagens, and this ECM remodeling promotes tumor progression and influences cancer cell invasion25. Most proteins in the ECM are categorized into two groups, fibrous proteins (including collagen, fibronectin, elastin, and laminin) and glycosaminoglycan proteins (consisting of hyaluronic acid, chondroitin sulfate, keratan sulfate, and heparan sulfate)26.
Collagen
Fibroblasts primarily produce collagen, which is located in bones, tendons and skin27. Collagen accumulate in and around tumors during tumorigenesis progression and contribute to the promotion of tumor cell growth, metastasis, and drug resistance28,29. Notably, whether collagen deposition is a promoter or inhibitor in ATC progression remains controversial. Some collagens restrain cancer progression via multiple mechanisms, including inhibition of tumor proliferation by promoting cancer cells to enter and remain in a dormant state. Cancer cells in collagen-deficient tumors produce increased levels of chemokines to attract myeloid-derived suppressor cells (MDSCs), which induce an immunosuppressive microenvironment to limit the effectiveness of immunotherapy30,31. As a major component of the ECM, the quantity and post-translational modifications of collagen often change dramatically during cancer progression, which enable the construction of a complex collagenous mesh that has a fundamental impact on the behavior of cancer cells and other cells in the TME32,33. Collagen degradation is primarily governed by matrix metalloproteinases (MMPs). MMP-1, MMP-8, MMP-13, and MMP-14 cleave fibril-forming collagens I, II, and III, and MMP-2 and MMP-9 cleave denatured collagens and collagen IV34. Several collagen subtypes and MMPs are strongly associated with the development and progression of thyroid cancer, which suggests that remodeling of the ECM is a key feature of the TME that promotes the malignant processes of thyroid cancer and collagen subtypes and MMPs are candidate therapeutic targets and biomarkers for advanced stages of this malignancy35,36,37.
A recent single-cell transcriptomics study revealed that excessive activation of collagen and collagen-interacting receptors were critical processes in the malignant transformation of ATC38. Current studies elucidated the role of collagen in the ATC process from two main perspectives. One perspective is collagen synthesis and degradation. Many genes involved in collagen degradation affect the progression of ATC by altering collagen deposition39,40. The second perspective is the activation of cancer-associated fibroblasts (CAFs). Knockdown of CREB3L1 remodeled the tumor stromal microenvironment, and the presence of CAFs inhibited the growth of ATC spheroids and the metastasis of ATC cells41. Collagen is also produced from a subset of tumor cells42. We hypothesized that the state of collagen in ATC was a dynamic process, with alterations in components and content as cancer cells proliferate, expand and migrate. However, TAM-modified collagen also triggers signals that affect the interaction between cancer cells and surrounding mesenchymal cells or immune cells. There is also a great deal of variation in the collagen that borders or encapsulates the interior and exterior of the tumor tissue. The dynamic changes and differences in collagen, and the different sources of production, obscure its role in cancer progression. Therefore, constructing an intelligent model to reveal the progression status of ATC based on dynamic changes in collagen may be an effective approach.
Other components of the ECM
The content of fibronectin in the ECM is low. However, its role in tumor transformation should not be ignored, primarily because fibronectin connects various structural proteins to form an integrated matrix. Fibronectin also recognizes and binds integrins on the cell membrane and induces integrin attachment, which profoundly impacts intracellular signaling. Fibronectin binds directly to growth factors, such as insulin-like growth factor (IGF), fibroblast growth factor (FGF), transforming growth factor-β (TGF-β), hepatocyte growth factor (HGF), and platelet-derived growth factor (PDGF)43,44,45,46. Elastin is the main component of elastic fibers that maintains the toughness and strength of tissues by resisting tissue deformation or rupture together with collagen47,48. Laminin and collagen form the components of the basement membrane, which is involved in the process of vascular maturation49,50. Hyaluronic acid (HA) is a high molecular weight glycosaminoglycan that mechanically increases the elastic viscosity of the ECM51. HA also acts as a sieve to impede and immobilize large molecular particles that may be recognized by many types of cells during cell movement, invasion, proliferation and inflammation52,53. Despite the current lack of evidence confirming the role of these components of the ECM in the ATC process, their roles cannot be ignored. A recent study revealed that thyroid cancer cells cultured in different dimensional states differed significantly in hypoxia, ECM, cytoskeleton, thyroid-specific proteins, and thyroid transcription factors54. Three-dimensional spheroids from patients with thyroid cancer showed a decrease in the expression of thyroid differentiation markers54. Based on the essential role of ECM components in maintaining the spherical morphology of cancer cells, we hypothesized that these components played a crucial role in the malignant progression of ATC.
Fibroblasts
Fibroblasts directly produce structural macromolecules, such as collagen, fibronectin, and laminin, and secrete many enzymes involved in the modification and degradation of these structural macromolecules, such as lysyl hydroxylases and MMPs, to play a central role in the formation and renewal of the ECM55,56. Fibroblasts are highly flexible cells that convert signals from multiple sources, including TGF-β, tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1, repeated mechanical stretching, and repeated mild heat shocks, into alterations in ECM components57,58. Cancer-associated fibroblasts (CAFs), which originate primarily from mesenchymal cells and resident fibroblasts, are among the most important cell components in the TME of most solid tumors and are activated and reprogrammed in response to paracrine factors and cytokines produced and released by tumor cells59. Many studies suggest that fibroblast-mediated collagen remodeling within the TME facilitates the progression of thyroid cancers, and increased expression of CAFs is a risk factor for worse thyroid cancer clinicopathological features60,61,62. CAFs and senescent thyroid cancer cells co-occur in various histotypes of BRAF-driven thyroid tumors and localize at the tumor invasive front, which suggests that crosstalk between CAFs and thyroid cancer cells plays an essential role in the invasive process of thyroid tumors63. There is some evidence that the inhibition of the CAF-like properties of ATC cells via the blockade of ECM signaling, which remodels the tumor stromal microenvironment and drives malignancy in ATCs, may be a novel strategy for suppression of the malignant transformation of ATCs41. Fibroblasts are one of the major cell types in the TME, and targeting fibroblasts to block cell‒cell communication in ATC cells is an immunotherapeutic opportunity64.
Interaction of the ECM with TME components
Most cancers exhibit greater matrix stiffness (depending on the ECM components and proportions) than their corresponding normal tissues. Therefore, ECM stiffness is a unique feature and promising therapeutic target65,66. However, we hypothesize that the interaction of the ECM with cancer cells, stromal cells, and immune cells in the complex molecular regulatory network of the TME is the key to unraveling the malignant process of ATC. Chemokine-chemokine receptor interactions, such as the TGF-β/Smad2/3 and CXCL12/CXCR4 signaling pathways, are the most significant drivers of the transformation of normal fibroblasts to CAFs and macrophages to the M2 type of macrophages, which further facilitate ECM deposition and cancer malignancy67. This interaction also directly or indirectly affects ECM stiffness. Overall, the production of ECM components and enzymes is further accelerated when tumor cells receive external signals from growth factors. In contrast, some enzymes that catalyze ECM degradation are inhibited in the TME67. Notably, the upregulation of MMP activity in a stiffened TME also increases vascular proliferation, invasion, and neovascular branching68. The ECM acts as a reservoir for growth factors, and ECM degradation contributes to the release of growth factors and cytokines that facilitate cancer progression. However, the motility of cancer cells is activated when the local matrix is degraded, which further confirms the complexity and dynamics of the ECM regulatory network.
The ECM in the TME promotes the proliferation, migration and vascularization of cancer cells and the transformation of tumor cells to cancer stem cells (CSCs). Cancer cells hijack surrounding stromal cells, immune cells, inflammatory cells, and the ECM is a vehicle for inducing cancer cell expansion. The specific pathways and mechanisms involved have been detailed previously67. The ECM acts as a physical barrier and compresses micro blood vessels or causes them to leak, which partially reduces the efficacy of chemotherapies and immune therapies66,69. The negative effect of the ECM on the infiltration of tumor-killing immune cells in the TME is also well understood. The tumor-killing immune cells are more inclined to migrate during the ECM-rich encapsulation of tumors. ECM-rich tumors create a hypoxic environment that allows enhanced glycolytic metabolism and acidification, which further induces an immunosuppressive microenvironment70. ECM proteins also directly enhance the activity of Tregs to induce cancer cells to express more PD-L1, or drive M2 polarization, which inhibits CD8+ T-cell-mediated anti-tumor immune responses71,72. Altogether, these results suggest that the cross-talk between the ECM and microenvironmental cells largely controls cancer progression. Therefore, we hypothesize that aberrantly activated ECM signaling appears to be critical for the rapid expansion of ATC cells.
Receptors for the ECM in TME
Receptor proteins are important factors for ECM communication with the surrounding environment73. Many ECM-associated receptor proteins, including integrin, discoidin ___domain receptors (DDRs), CD44, and receptor for hyaluronan-mediated motility (RHAMM), are highly upregulated in many solid tumors and correlate with malignancy and poor prognosis74,75. Notably, inhibitors or antibodies against these receptors have been widely used in preclinical and clinical studies76. Cancer cells respond to their surroundings by sensing ECM-mediated mechanical stress or molecular stimuli via unidirectional (receptors sense ECM stiffness then transmit this signal to cells) or bidirectional (cells sense signals received by receptors and transmit signals through receptors) signaling driven by receptor proteins77. Notably, these receptors have a distinct division of labor, and different subtypes receive the corresponding ECM signals. For example, DDR1 binds to collagen types I and IV, and DDR2 interacts with collagen types I, II, and X. HA is the major ligand for membrane-bound RHAMM67. The binding of lymphocytes to fibronectin is also facilitated by CD44, which is critical for lymphocyte infiltration into the TME78,79. A preclinical trial demonstrated that recombinant fibronectin CH296 stimulated T cells to achieve robust tumor suppression in patients with advanced cancer80, which suggesting that blockade of ECM-cancer cell crosstalk is a potential anti-cancer strategy. This meticulous cross-linking constitutes a complex network of ECM-cancer cell interactions. We hypothesize that cancer cells hijack the ECM in the TME to sense changes in their surroundings and use it as a territory to send aggressive signals to surrounding or distant cells or organs. Cancer cells wrap surrounding normal cells via the ECM to form a tumor environment and construct an immunosuppressive microenvironment that is conducive to cancer cell expansion. We hypothesize that the rapid expansion of ATC is closely related to the dysregulation of ECM receptor signaling.
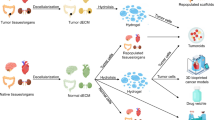
Matrix components as potential therapeutic targets for ATC
Drugs that inhibit collagen synthesis (halofuginone, Fresolimumab) or induce collagen degradation (collagenases) have been investigated extensively in animal and preclinical studies81,82. Mechanistically, these drugs weaken the stiffness of the ECM and contribute to more efficient drug delivery to solid tumors83,84,85. Several strategies aimed at modulating the number or activity of MMPs or approaches that indirectly increase the synthesis of collagenases also contribute to cancer therapy86. A number of drugs that use ECM components as therapeutic targets (e.g., 4-methylumbelliferone, which was designed to facilitate greater penetration of anticancer drugs into the interior of solid tumors) or that use ECM components as targets for precise drug delivery (BC-1, L19) also offer new opportunities for solid tumor therapy67. However, these methods have some drawbacks. The degradation of collagen releases many growth factors, and whether this release exacerbates the inflammatory response of tumors is not known, Whether ECM degradation contributes to cancer cell invasion must be further investigated. We postulated that the inhibition of collagen cross-linking is a novel strategy for inhibiting the progression of ATC. Lysyl oxidase (LOX, an ECM-remodeling enzyme) catalyzes collagen cross-linking and is frequently upregulated in thyroid cancer87. Several clinical trials confirmed the use of an anti-LOXL2 monoclonal antibody (simtuzumab) with no clinically significant effect in patients with pulmonary or hepatic fibrosis88,89. However, this unexpected negative result may be related to an ineffective antibody, and other members of the LOX family may be more appealing candidates for cancer treatment.
Significant breakthroughs in the past decade have been made in controlling cancer progression via targeted cell–ECM interactions. Collagen, proteoglycans, and glycoproteins also form ECM in the bone marrow and lymph nodes. However, unlike solid tumors, leukemia (so-called “liquid carcinoma”) cells form only temporary connections to the ECM in structured niches within the bone marrow and lymphoid organs90. Ibrutinib (an inhibitor of bruton tyrosine kinase) controls integrin α4β1-mediated adhesion to fibronectin, VCAM-1, and chemotaxis signals mediated by CXCL12-, CXCL13-, and CCL19-induced signaling, and it has been approved for patients with chronic lymphocytic leukemia (CLL)91. The RHAMM-HA interaction facilitates cell motility, but the RHAMM-R3 peptide vaccine triggered anti-cancer immune responses in CLL patients92,93. These results reveal that targeting cell-ECM interactions is a highly viable approach against malignant tumors, including ATCs.
Conclusion
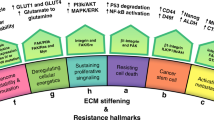
ATC is a rare subtype of thyroid carcinoma and is extremely aggressive. Treating ATC in the traditional manner of treating differentiated thyroid tumors is ineffective in most cases, and due to the highly aggressive and recurrent properties of ATC, its therapeutic options are mainly palliative. Therefore, there is an urgent need to find new therapeutic approaches, and targeted therapy in the ATC microenvironment may be a new way94. Polymorphonuclear neutrophils (PMNs) are critical effector cells that orchestrate the inflammatory response in TME. PMNs contain and release an excess of mediators, including granular enzymes [e.g., myeloperoxidase (MPO), pentraxin-3 (PTX3) and matrix metalloproteinase-9 (MMP-9)], and neutrophil extracellular traps (NETs), and those seem to correlate with malignancy and severity of progressive thyroid tumor95. Cristinziano et al.96 discovered that conditioned media with ATC cell lines induced the release of NETs, and thus targeting neutrophil activation may contribute to inhibiting ATC proliferation. In addition, the combination of BRAF inhibitors and immunotherapy in ATC is a promising therapeutic approach to maximize treatment efficacy. For example, combination therapy with the BRAFV600E inhibitor PLX4720 and an anti-PD-L1 or anti-PD-1 antibody significantly improved mouse survival, and the reduction in tumor volume was associated with an increase in the number and activity of effector immune cells97. Moreover, the interaction of ECM with cancer cells not only facilitates tumor development and progression, but also largely controls most of the characteristic hallmarks of tumorigenesis, providing new opportunities for the treatment of ATC.
Cancer progression is driven by continuous interactions between cancer cells, the ECM, and other cell types present in the TME. ECM components contribute significantly to the microenvironment of most individual cells in the body, and their dysregulation is closely linked to the development of many diseases, including cancer. However, the therapeutic role of EMT in thyroid cancer is often underestimated. The main challenge lies in the current lack of materials that accurately mimic the ECM in vitro due to its complex physical and biological properties, and the delicate interactions between different components of the ECM. There is a lack of systematic comparisons of differences in specific ECM components in different cancers, which is an essential step for the development of specific therapeutic and sensitive detection strategies. Therefore, current applications of the ECM in cancer primarily include adjuvant therapy for immunotherapy or are considered an imaging tool for tumors, and its central role in tumor therapy remains unexplored.
ATC is a subtype of thyroid cancer that occurs in a small percentage of patients but has a high degree of malignancy and poor patient prognosis. Clinically, it generally manifests as a fast-growing goiter or nodule that is firm to the touch and adheres resolutely to the underlying structures. We revealed the important role of ECM components in the malignant transformation of ATC, which suggested that ECM components were promising therapeutic targets. We hypothesized that cancer cells wrapped and hijacked the ECM to become part of the tumor microenvironment, which promoted the sending of signals of their own aggression to surrounding or distant tissues or organs and altered the state of the surrounding environment to form an immunosuppressive microenvironment that was conducive to the growth of cancer cells. Therefore, ATC undergoes significant infiltration and metastasis at an early stage.
During the malignant transformation of ATC, a variety of immune cells that were previously capable of tumor killing are transformed into promoters that advance the malignant progression of the tumor, including but not limited to the transformation of CD8+ T cells to depleted T cells and the polarization of macrophages to M2-type macrophages. These results suggest that cancer cells must release some signal or pressure to promote a decrease in immune cell activity in the TME. We hypothesize that the continuous interactions between the ECM and cancer cells play a crucial role in this process. Therefore, sufficient attention should be given to ECM-cancer cell interactions in future studies to reveal their key mechanisms in the process of cancer immunosuppression, which will provide new opportunities for the treatment of ATC (Fig. 5).
Data Availability
The original data contributing to the findings presented in the study were obtained from Assistant for Clinical Bioinformatics (https://www.aclbi.com/static/index.html#/). Further inquiries may be addressed to the corresponding author.
References
Tao, Y. et al. New insights into immune cells and immunotherapy for thyroid cancer. Immunol. Invest. 52(8), 1039–1064 (2023).
Wang, J. et al. Customizing cancer treatment at the nanoscale: A focus on anaplastic thyroid cancer therapy. J. Nanobiotechnol. 21(1), 374 (2023).
Bhattacharya, S. et al. Advances and challenges in thyroid cancer: The interplay of genetic modulators, targeted therapies, and AI-driven approaches. Life Sci. 332, 122110 (2023).
Abe, I. & Lam, A. K. Anaplastic thyroid carcinoma: Updates on WHO classification, clinicopathological features and staging. Histol. Histopathol. 36(3), 239–248 (2021).
Leandro-Garcia, L. J. & Landa, I. Mechanistic insights of thyroid cancer progression. Endocrinology 164(9), bqad118 (2023).
Luo, H. et al. The nexus of dynamic T cell states and immune checkpoint blockade therapy in the periphery and tumor microenvironment. Front. Immunol. 14, 1267918 (2023).
Asadi M. et al. Immune features of tumor microenvironment: A genetic spotlight. Cell Biochem. Biophys. 82(1), 107–118. https://doi.org/10.1007/s12013-023-01192-7 (2023).
Xiao, Y. & Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 221, 107753 (2021).
Hinshaw, D. C. & Shevde, L. A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 79(18), 4557–4566 (2019).
Lu, X. et al. Immunotherapy for anaplastic thyroid carcinoma: The present and future. Zhejiang da Xue Xue Bao Yi Xue Ban 50(6), 675–684 (2021).
Pitt, J. M. et al. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 27(8), 1482–1492 (2016).
Mao, X. et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 20(1), 131 (2021).
Najafi, M., Farhood, B. & Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell Biochem. 120(3), 2782–2790 (2019).
Mohan, V., Das, A. & Sagi, I. Emerging roles of ECM remodeling processes in cancer. Semin. Cancer Biol. 62, 192–200 (2020).
Belli, C. et al. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 65, 22–32 (2018).
Yang, H. et al. Tumor-infiltrating lymphocytes are functionally inactivated by CD90+ stromal cells and reactivated by combined Ibrutinib and Rapamycin in human pleural mesothelioma. Theranostics 12(1), 167–185 (2022).
Zhao, G. et al. Tunicamycin promotes metastasis through upregulating endoplasmic reticulum stress induced GRP78 expression in thyroid carcinoma. Cell Biosci. 10, 115 (2020).
Xia, J. K. et al. Deregulated bile acids may drive hepatocellular carcinoma metastasis by inducing an immunosuppressive microenvironment. Front. Oncol. 12, 1033145 (2022).
Ning, J. et al. METTL3 inhibition induced by M2 macrophage-derived extracellular vesicles drives anti-PD-1 therapy resistance via M6A-CD70-mediated immune suppression in thyroid cancer. Cell Death Differ. 30(10), 2265–2279 (2023).
Bao, L. et al. Targeting SIGLEC15 as an emerging immunotherapy for anaplastic thyroid cancer. Int. Immunopharmacol. 133, 112102 (2024).
Weber, F. et al. Increased cytoplasmatic expression of cancer immune surveillance receptor CD1d in anaplastic thyroid carcinomas. Cancer Med. 8(16), 7065–7073 (2019).
Huang, Y. et al. Identification of an immune-related key gene, PPARGC1A, in the development of anaplastic thyroid carcinoma: In-silico study and in-vitro evaluation. Minerva Endocrinol. (Torino) 47(2), 150–159 (2022).
Xu, T. et al. Immunological characteristics of immunogenic cell death genes and malignant progression driving roles of TLR4 in anaplastic thyroid carcinoma. BMC Cancer 23(1), 1131 (2023).
Das, S. et al. Tumor cell-derived IL1beta promotes desmoplasia and immune suppression in pancreatic cancer. Cancer Res. 80(5), 1088–1101 (2020).
Gupta, R. Epigenetic regulation and targeting of ECM for cancer therapy. Am. J. Physiol. Cell Physiol. 322(4), C762–C768 (2022).
Yuzhalin, A. E. et al. Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochim. Biophys. Acta Rev. Cancer 1870(2), 207–228 (2018).
De Martino, D. & Bravo-Cordero, J. J. Collagens in cancer: Structural regulators and guardians of cancer progression. Cancer Res. 83(9), 1386–1392 (2023).
Chen, Y. et al. Oncogenic collagen I homotrimers from cancer cells bind to alpha3beta1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell 40(8), 818-834.E9 (2022).
Niu, J. et al. Identification of the collagen family as prognostic biomarkers in papillary thyroid carcinoma. Endocrine 78(3), 491–506 (2022).
Di Martino, J. S. et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat. Cancer 3(1), 90–107 (2022).
Chen, Y. et al. Type I collagen deletion in alphaSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 39(4), 548-565.E6 (2021).
Xu, S. et al. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 17(1), 309 (2019).
Romer, A. M. A., Thorseth, M. L. & Madsen, D. H. Immune modulatory properties of collagen in cancer. Front. Immunol. 12, 791453 (2021).
Jablonska-Trypuc, A., Matejczyk, M. & Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 31(sup1), 177–183 (2016).
Huang, C. et al. The prognostic potential of alpha-1 type I collagen expression in papillary thyroid cancer. Biochem. Biophys. Res. Commun. 515(1), 125–132 (2019).
Ivkovic, I. et al. Role of matrix metalloproteinases and their inhibitors in locally invasive papillary thyroid cancer. Biomedicines 10(12), 3178 (2022).
Kameyama, K. Expression of MMP-1 in the capsule of thyroid cancer–relationship with invasiveness. Pathol. Res. Pract. 192(1), 20–26 (1996).
Lu, L. et al. Anaplastic transformation in thyroid cancer revealed by single-cell transcriptomics. J. Clin. Invest. 133(11), e169653 (2023).
Chen, Y. et al. CTHRC1 promotes anaplastic thyroid cancer progression by upregulating the proliferation, migration, and invasion of tumor cells. PeerJ 11, e15458 (2023).
Yoshida, T. et al. Membrane type 1 matrix metalloproteinase regulates anaplastic thyroid carcinoma cell growth and invasion into the collagen matrix. Biochem. Biophys. Res. Commun. 529(4), 1195–1200 (2020).
Pan, Z. et al. CREB3L1 promotes tumor growth and metastasis of anaplastic thyroid carcinoma by remodeling the tumor microenvironment. Mol. Cancer 21(1), 190 (2022).
Angre, T. et al. Role of collagen regulators in cancer treatment: A comprehensive review. Anticancer Agents Med. Chem. 22(17), 2956–2984 (2022).
Lin, T. C. et al. Fibronectin in cancer: Friend or foe. Cells 9(1), 27 (2019).
Rick, J. W. et al. Fibronectin in malignancy: Cancer-specific alterations, protumoral effects, and therapeutic implications. Semin. Oncol. 46(3), 284–290 (2019).
Kumra, H. & Reinhardt, D. P. Fibronectin-targeted drug delivery in cancer. Adv. Drug Deliv. Rev. 97, 101–110 (2016).
Farooq, F. et al. Shielding and nurturing: Fibronectin as a modulator of cancer drug resistance. J. Cell Physiol. 238(8), 1651–1669 (2023).
Cox, T. R. The matrix in cancer. Nat. Rev. Cancer 21(4), 217–238 (2021).
Li, J. et al. Elastin is a key factor of tumor development in colorectal cancer. BMC Cancer 20(1), 217 (2020).
Ruan, J. et al. Exogenous laminin exhibits a unique vascular pattern in the brain via binding to dystroglycan and integrins. Fluids Barriers CNS 19(1), 97 (2022).
Mori, T. et al. Downregulation of a newly identified laminin, laminin-3B11, in vascular basement membranes of invasive human breast cancers. Cancer Sci. 102(5), 1095–1100 (2011).
Vasvani, S., Kulkarni, P. & Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 151, 1012–1029 (2020).
Abatangelo, G. et al. Hyaluronic acid: Redefining its role. Cells 9(7), 1743 (2020).
Della Sala, F. et al. Advances in hyaluronic-acid-based (Nano) devices for cancer therapy. Macromol. Biosci. 22(1), e2100304 (2022).
Oh, J. M. et al. Different expression of thyroid-specific proteins in thyroid cancer cells between 2-dimensional (2D) and 3-dimensional (3D) culture environment. Cells 11(22), 3559 (2022).
Moretti, L. et al. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 298(2), 101530 (2022).
Haydont, V. et al. Fibroblasts from the human skin dermo-hypodermal junction are distinct from dermal papillary and reticular fibroblasts and from mesenchymal stem cells and exhibit a specific molecular profile related to extracellular matrix organization and modeling. Cells 9(2), 368 (2020).
Peng, D. et al. Targeting TGF-beta signal transduction for fibrosis and cancer therapy. Mol. Cancer 21(1), 104 (2022).
Chakravarthy, A. et al. TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 9(1), 4692 (2018).
Fozzatti, L. & Cheng, S. Y. Tumor cells and cancer-associated fibroblasts: A synergistic crosstalk to promote thyroid cancer. Endocrinol. Metab. (Seoul) 35(4), 673–680 (2020).
Jolly, L. A. et al. Fibroblast-mediated collagen remodeling within the tumor microenvironment facilitates progression of thyroid cancers driven by BrafV600E and Pten loss. Cancer Res. 76(7), 1804–1813 (2016).
Zhu, L. et al. Cancer-associated fibroblasts in papillary thyroid carcinoma. Clin. Exp. Med. 23(6), 2209–2220 (2023).
Hu, H. et al. Transcriptome analysis revealed potential neuro-immune interaction in papillary thyroid carcinoma tissues. Diseases 11(1), 9 (2023).
Minna, E. et al. Cancer associated fibroblasts and senescent thyroid cells in the invasive front of thyroid carcinoma. Cancers (Basel) 12(1), 112 (2020).
Chakraborty, S. et al. Disruption of cell-cell communication in anaplastic thyroid cancer as an immunotherapeutic opportunity. Adv. Exp. Med. Biol. 1350, 33–66 (2021).
Patwardhan, S. et al. ECM stiffness-tuned exosomes drive breast cancer motility through thrombospondin-1. Biomaterials 279, 121185 (2021).
Jiang, Y. et al. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 15(1), 34 (2022).
Huang, J. et al. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 6(1), 153 (2021).
Pittayapruek, P. et al. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 17(6), 868 (2016).
Thenappan, T., Chan, S. Y. & Weir, E. K. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 315(5), H1322–H1331 (2018).
Mhaidly, R. & Mechta-Grigoriou, F. Fibroblast heterogeneity in tumor micro-environment: Role in immunosuppression and new therapies. Semin. Immunol. 48, 101417 (2020).
Freeman, P. & Mielgo, A. Cancer-associated fibroblast mediated inhibition of CD8+ cytotoxic T cell accumulation in tumours: Mechanisms and therapeutic opportunities. Cancers (Basel) 12(9), 2687 (2020).
Horn, L. A. et al. Remodeling the tumor microenvironment via blockade of LAIR-1 and TGF-beta signaling enables PD-L1-mediated tumor eradication. J. Clin. Invest. 132(8), e155148 (2022).
Alldinger, S. et al. Roles of an extracellular matrix (ECM) receptor and ECM processing enzymes in demyelinating canine distemper encephalitis. Dtsch. Tierarztl. Wochenschr. 113(4), 151-2–151-6 (2006).
Niland, S., Riscanevo, A. X. & Eble, J. A. Matrix metalloproteinases shape the tumor microenvironment in cancer progression. Int. J. Mol. Sci. 23(1), 146 (2021).
Yuan, Z. et al. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 22(1), 48 (2023).
Mosquera, M. J. et al. Extracellular matrix in synthetic hydrogel-based prostate cancer organoids regulate therapeutic response to EZH2 and DRD2 inhibitors. Adv. Mater. 34(2), e2100096 (2022).
Zhang, W. et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br. J. Cancer 121(10), 837–845 (2019).
Nakajima, Y. et al. Critical role of the CD44lowCD62Llow CD8+ T cell subset in restoring antitumor immunity in aged mice. Proc. Natl. Acad. Sci. U. S. A. 118(23), e2103730118 (2021).
Kong, T. et al. CD44 promotes PD-L1 expression and its tumor-intrinsic function in breast and lung cancers. Cancer Res. 80(3), 444–457 (2020).
Ishikawa, T. et al. Phase I clinical trial of fibronectin CH296-stimulated T cell therapy in patients with advanced cancer. PLoS One 9(1), e83786 (2014).
Su, H. & Karin, M. Collagen architecture and signaling orchestrate cancer development. Trends Cancer 9(9), 764–773 (2023).
Martins Cavaco, A. C. et al. Collagen biology making inroads into prognosis and treatment of cancer progression and metastasis. Cancer Metastasis Rev. 39(3), 603–623 (2020).
Chen, P., Cescon, M. & Bonaldo, P. Collagen VI in cancer and its biological mechanisms. Trends Mol. Med. 19(7), 410–417 (2013).
Sarwar, M. et al. Collagen I dysregulation is pivotal for ovarian cancer progression. Tissue Cell 74, 101704 (2022).
Nissen, N. I., Karsdal, M. & Willumsen, N. Collagens and cancer associated fibroblasts in the reactive stroma and its relation to cancer biology. J. Exp. Clin. Cancer Res. 38(1), 115 (2019).
Zinger, A. et al. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano 13(10), 11008–11021 (2019).
Boufraqech, M. et al. miR30a inhibits LOX expression and anaplastic thyroid cancer progression. Cancer Res. 75(2), 367–377 (2015).
Muir, A. J. et al. Simtuzumab for primary sclerosing cholangitis: Phase 2 study results with insights on the natural history of the disease. Hepatology 69(2), 684–698 (2019).
Raghu, G. et al. Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: A randomised, double-blind, controlled, phase 2 trial. Lancet Respir. Med. 5(1), 22–32 (2017).
Hatfield, K. J., Reikvam, H. & Bruserud, O. The crosstalk between the matrix metalloprotease system and the chemokine network in acute myeloid leukemia. Curr. Med. Chem. 17(36), 4448–4461 (2010).
de Rooij, M. F. et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 119(11), 2590–2594 (2012).
Pilarski, L. M. et al. Potential role for hyaluronan and the hyaluronan receptor RHAMM in mobilization and trafficking of hematopoietic progenitor cells. Blood 93(9), 2918–2927 (1999).
Schmitt, M. et al. RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood 111(3), 1357–1365 (2008).
Ferrari, S. M. et al. Immune and inflammatory cells in thyroid cancer microenvironment. Int. J. Mol. Sci. 20(18), 4413 (2019).
Modestino, L. et al. Neutrophil extracellular traps and neutrophil-related mediators in human thyroid cancer. Front. Immunol. 14, 1167404 (2023).
Cristinziano, L. et al. Anaplastic thyroid cancer cells Induce the release of mitochondrial extracellular DNA traps by viable neutrophils. J. Immunol. 204(5), 1362–1372 (2020).
Gunda, V. et al. Combinations of BRAF inhibitor and anti-PD-1/PD-L1 antibody improve survival and tumour immunity in an immunocompetent model of orthotopic murine anaplastic thyroid cancer. Br. J. Cancer 119(10), 1223–1232 (2018).
Funding
This work was supported by the Guizhou Provincial People’s Hospital Talent Fund (grant number 2023-41).
Author information
Authors and Affiliations
Contributions
X.J.K. conceived and designed the study, performed the data analysis and interpretation. X.J.K., S.Y.Y., and C.X.X. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
The manuscript has never been submitted to more than one journal for simultaneous consideration. The submitted work is original and has not been published elsewhere in any form or language.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xia, J., Shi, Y. & Chen, X. New insights into the mechanisms of the extracellular matrix and its therapeutic potential in anaplastic thyroid carcinoma. Sci Rep 14, 20977 (2024). https://doi.org/10.1038/s41598-024-72020-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72020-y