Abstract
Pulmonary fibrosis is a chronic interstitial lung disease with no curative therapeutic treatment, leading to significant mortality. The aims of this study were to investigate the regulatory mechanisms of mitophagy in the progression of pulmonary fibrosis. Through bioinformatics analysis, we identified the downregulation of long-chain fatty acyl-CoA synthetase 1 (ACSL1) as being associated with the severity of pulmonary fibrosis. A pulmonary fibrosis model was established through bleomycin (BLM) exposure both in vivo and in vitro. Mitoquinone (MitoQ) pretreatment significantly decreased redox damage, stabilized mitochondrial membrane potential (MMP), improved mitochondrial dynamics, and activated PINK1/Parkin-mediated mitophagy, thereby alleviating pulmonary fibrosis. In vitro, overexpression of ACSL1 mitigated mitochondrial damage and restored PINK1/Parkin-mediated mitophagy under BLM exposure. In contrast, ACSL1 inhibition exacerbated pulmonary fibrosis, and these adverse effects could not be reversed by MitoQ treatment. Taken together, our study reveals a novel mechanism underlying the pathogenesis of pulmonary fibrosis and suggests a potential therapeutic target for its treatment.
Similar content being viewed by others
Introduction
Pulmonary fibrosis is characterized as a chronic, progressive interstitial lung disease that leads to airway contraction and ultimately respiratory failure1,2. Clinically, it presents as progressive dyspnea and deterioration in lung function. Recent epidemiological studies indicate that the incidence of pulmonary fibrosis is on the rise, with a median survival of approximately 3–5 years after diagnosis1,3. Multiple risk factors, including smoking, environmental exposure, viral infections, and genetic predispositions, have been identified as high-risk contributors to pulmonary fibrosis4. Unfortunately, no current medications have been shown to effectively inhibit or reverse pulmonary fibrosis. Exploring the exact pathogenesis of pulmonary fibrosis should be contributed to provide a novel target for disease control.
Mitochondrial dysfunction, characterized by insufficient energy production by the mitochondria, is believed to play a significant role in the development and progression of various diseases, including pulmonary fibrosis5,6. Mitophagy, a form of selective autophagy, is crucial for maintaining mitochondrial quality control by removing damaged or dysfunctional mitochondria7,8,9. Insufficient mitophagy is associated with the pathological states of pulmonary fibrosis, particularly in aging lungs8,10,11. Impaired mitophagy leads to the accumulation of dysfunctional mitochondria, overproduction of reactive oxygen species (ROS), and alterations in mitochondrial dynamics, which contribute to oxidative stress and fibrotic processes7. There are various signaling mechanisms regulated mitophagy, with PTEN-induced putative kinase 1 (PINK1) and E3 ubiquitin ligase Parkin (Parkin) mediated mitophagy characterized as a key pathway in these mechanisms12. Deficiency in PINK1 can lead to mitochondrial degradation, promoting fibrosis13. Insufficient mitophagy in fibroblastic foci has been shown to involve a Parkin-mediated platelet-derived growth factor receptor signaling pathway in vivo14. Therefore, targeting mitophagy has emerged as a potential target for pulmonary fibrosis. Mitochondria-targeted antioxidants, such as Mitoquinone (MitoQ), have shown promise in reducing oxidative stress, improving mitochondrial function, and attenuating pulmonary fibrosis both in vivo and in vitro15. Also, tetrandrine has been found to suppress fibroblast activation and inhibit collagen deposition in vivo, possibly by rescuing impaired mitophagy through the inhibition of mitophagy-related protein expression16. However, the precise mechanisms by which mitophagy influences the process of pulmonary fibrosis have not been completely elucidated.
This study explores the underlying mechanism of mitophagy in pulmonary fibrosis through bioinformatics analysis, as well as in vitro and in vivo approaches. A pulmonary fibrosis rat model was established through bleomycin (BLM) exposure. MitoQ was utilized to assess the regulatory role of mitophagy in pulmonary fibrosis both in vivo and in vitro, focusing on the involvement of PINK1/Parkin-mediated mitophagy in this process. Subsequently, gain-of-function experiments were conducted in vivo to further elucidate the molecular mechanisms of mitophagy in pulmonary fibrosis. These findings may offer novel insights into the mechanisms driving the progression of pulmonary fibrosis.
Materials and methods
Bioinformatics analysis
The raw and clinical data for the GSE13469217 (n = 80) and GSE3253718 (n = 217) datasets were downloaded from the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/). The differentially expressed genes (DEGs) in idiopathic pulmonary fibrosis (IPF) patients compared to controls (|log2 Fold Change| >1 and adjusted P-value < 0.05) were identified, and intersected with autophagy-related genes19 (n= 803) and mitochondria-related genes20 (n= 1136) extracted from MitoCarta 3.0. Additionally, the St. George’s Respiratory Questionnaire (SGRQ) score and lung function parameters, including percent predicted forced vital capacity (FVC pre-bronchodilator % predicted) and percent predicted diffusion capacity of the lung for carbon monoxide (DLCO % predicted), were extracted from the GSE32537 dataset18. Correlation analysis between these factors and the expression levels of the DEGs was performed. To assess the robustness of the novel cell types identified in this study, the single-cell RNA sequencing of GSE13589321 and GSE12803322 datasets were obtained from IPF Cell Atlas (www.ipfcellatlas.com)23. The uMAP explorer plots of the DEGs expression within each specific cell were identified using cell type annotation.
Animal and treatments
A total of 24 male Wistar rats (6–8 w, 180–200 g) were housed under a 12/12-hour light/dark cycle at 25 °C with ad libitum access to food and water. The rats were randomly divided into four groups: the pulmonary fibrosis model was induced by BLM (APExBIO, USA) according to previous studies (Fibrosis group)24,25. On day 0, the rats in this group were exposed to BLM (3 mg/kg). In addition, the Fibrosis + MitoQ group received oral gavage of MitoQ (5 mg/kg/day, Glpbio, USA) for 28 consecutive days following BLM exposure. Rats treated with normal saline (Shuanghe Pharmacy, Anhui, China) or MitoQ (5 mg/kg/day) alone were assigned to the CON group and MitoQ group, respectively, as controls. After 28 days of exposure, the rats were anesthetized by intraperitoneal injection of 3% sodium pentobarbital (1 ml/kg) and sacrificed. Lung coefficients, calculated using the formula: Lung coefficient = (wet lung weight (g) / body weight (g)) × 100%, were used to assess lung damage in each group. Lung tissues were collected for further analysis. This study is performed in accordance with relevant guidelines and regulations. The animal experiments were conducted in accordance with ARRIVE 2.0 guidelines (Animal Research: Reporting of In Vivo Experiments, https://arriveguidelines.org)26, and these experiments were approved by the Ethics Committee of the Affiliated Hospital of Putian University (ID: 2024101).
Pathological staining and observation
First, right lung tissues were collected and fixed in 4% paraformaldehyde solution (Solarbio, China) overnight. After dehydration and embedding in paraffin, the tissues were cut into 4 μm slices for hematoxylin and eosin (H&E) and Masson staining to observe histomorphological changes. Second, immunohistochemistry (IHC) was conducted. The slides were permeabilized with 0.3% Triton X-100 (Solarbio, China) and blocked with bovine serum albumin (BSA, Beyotime, China) at room temperature. Next, the slides were incubated with primary antibodies overnight at 4 °C, followed by incubation with secondary antibody (HRP-labeled goat anti-rabbit IgG(H + L), Beyotime, China). The images were visualized under a microscope (Eclipse C1, Nikon, Japan).
Transmission electron microscope (TEM) examination
Fresh left lung samples were quickly cut into pieces approximately 1 mm3 in size and then fixed overnight with 2.5% glutaraldehyde solution. Next, the samples were treated with 1% osmium tetroxide for 2 h and dehydrated with a graded ethanol series (50–100%). Subsequently, the samples were embedded in Spurr’s epoxy resin and cut into ultra-thin sections. The sections were examined using a transmission electron microscope (TEM, HT7700, Hitachi, Japan). The dynamics of mitochondria were assessed and quantified by calculating the mitochondrial aspect ratio, defined as the ratio of the long diameter to the short diameter of the mitochondria, using Fiji (fiji.sc).
Cell culture and treatments
The A549 cell line (Procell, China), commonly used as a model for alveolar epithelial type 2 (AT2) cells, was cultured in RPMI 1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin (Hyclone, USA). Cells were maintained at 37 °C in a 5% CO₂ incubator (ThermoFisher, USA). To determine the optimal dosage, A549 cell lines were treated with varying concentrations of bleomycin (BLM, APExBIO, USA). Based on a previous study27, A549 cell lines were pretreated with MitoQ (4 µM, dissolved in DMSO, Glpbio, USA) for 2 h before BLM exposure, and subsequent experiments were performed 24 h post-BLM treatment.
For mechanistic studies, the role of long-chain fatty acyl-CoA synthetase 1 (ACSL1) was investigated. ACSL1 upregulation was achieved by transfection with the pCMV-ACSL1(human)-3×FLAG-Neo plasmid (Zoigene, Fuzhou, China), with the pCMV-T7-MCS-3×FLAG-WPRE-Neo plasmid serving as a negative control (plasmid sequencing details are shown in Figure S1). ACSL1 downregulation was induced using small interfering RNA (siRNA, GenePharma, Shanghai, China). A549 cell lines were seeded in 6-well plates and transfected with plasmids or siRNAs using Lipo8000™ Transfection Reagent (Beyotime, China) for 6 h. Following transfection, the cells were exposed to BLM for 24 h.
Cell viability assessment
Cell viability was assessed using a Cell Counting Kit-8 (CCK8) assay (Zomanbio, China) according to the manufacturer’s protocol. Briefly, A549 cell lines were cultured in 96-well plates and treated with the experimental drugs, while RPMI 1640 medium was used as the control. After exposure to BLM, 10% CCK8 solution was added to the culture medium and incubated for 2 h at 37 °C with 5% CO2 in an incubator. The absorbance (OD) at a wavelength of 450 nm was measured using a Thermo Multiskan FC enzyme reader (Thermo Fisher, USA). Cell viability was calculated using the following formula: Cell viability (%) = [(A(treatment) - A(control)) / (A(negative) - A(control))] × 100%.
Antioxidant system activity measurement
Lung tissues or treated A549 cell lines were collected and homogenized with ten times the volume of phosphate-buffered saline (PBS, pH 7.4) at 4 °C. The lung tissue homogenates were then centrifuged at 4 °C, and the supernatants were isolated and stored at -80 °C. To assess the concentrations of glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA) in rat lung tissue, commercially available GSH, SOD, and MDA kits (Solarbio, China) were used according to the manufacturer’s protocol. Measurements were taken using an ultraviolet-visible spectrophotometer (TU-1950, Puxi, China).
ROS production detection
ROS production was detected using the DCFH-DA kit (Zomanbio, China). A549 cell lines were treated with drugs in 6-well plates and then incubated with 1 µM DCFH-DA for 30 min at 37 °C in the dark. After incubation, the cells were observed under a fluorescence microscope (Nikon Eclipse Ti, Japan), and images were captured.
Mitochondrial quality determination
Mitochondrial quality control is crucial for cellular homeostasis28,29. First, mitochondrial membrane potential (MMP) levels were detected using a JC-1 kit (Solarbio, China). Treated A549 cell lines were incubated with 1 µM JC-1 working solution for 20 min at 37 °C in the dark and then observed under a fluorescence microscope (Nikon Eclipse Ti, Japan), where images were captured. Mitophagy selectively eliminates dysfunctional mitochondria to maintain mitochondrial quality control28,29. To assess changes in mitophagy in treated A549 cell lines, the cells were stained with MitoTracker Red CMXRos (Yeasen, China) and LysoTracker Green DND-26 (Yeasen, China) for 30 min. Images were captured using a laser scanning confocal microscope (Nikon C2, Japan).
Quantitative real-time RT-PCR (qRT-PCR)
Total RNA in A549 cell lines was isolated using TRIzol reagent (Invitrogen, US) and reverse-transcribed into complementary DNA (cDNA). cDNA was synthesized using HiScript II Q RT SuperMix for qPCR (Vazyme, China). qRT-PCR was performed using CFX Connect Real-Time PCR Detection System (BioRad, US) with an HQ SYBR qPCR Mix (without ROX) (Zomen, China). Relative mRNA expression was analyzed using the 2−ΔΔCT method, and GAPDH expression was used as an endogenous control (The primers used are shown in Table S1).
Western blotting assay
The protein extracts from lung tissues or treated A549 cell lines were lysed at 4 °C with RIPA buffer (Phygene, China), and the total protein concentrations were quantified using a BCA kit (Zomanbio, China). Protein samples (30 µg per well) were separated using 10% SDS-PAGE and transferred onto PVDF membranes. After blocking, the membranes were incubated overnight at 4 °C with primary antibodies against ACSL1 (Biodragon, China), PINK1 (Biodragon, China), Parkin (Proteintech, China), BECN1 (Solarbio, China), p62 (Biodragon, China), Drp1 (Solarbio, China), Mfn2 (Proteintech, China), α-SMA (Beyotime, China), FN1 (Immunoway, USA), and β-actin (Immunoway, USA). The membranes were then incubated with a secondary antibody (HRP-labeled goat anti-rabbit IgG(H + L), Beyotime, China) for 12 h at room temperature in the dark. Finally, protein levels were detected using BeyoECL Plus (Beyotime, China) with the JS-M9P system (P&Q Science and Technology, China) and quantified using Fiji (fiji.sc).
Statistical analysis
All experimental data were obtained from at least three independent experiments. Data analysis was conducted using R programming (version 4.3.1), and visualizations were created with the ggplot2 package. Results are presented as the mean ± SEM and were assessed for significant differences using one-way analysis of variance (ANOVA). Tukey’s HSD test was used for post-hoc comparisons between groups. P-values of less than 0.05 were considered statistically significant.
Results
Downregulation of ACSL1 contributes to mitophagy in IPF
Two raw datasets (GSE13469217 and GSE32537)18 were used to identify differentially expressed genes (DEGs) in IPF patients. As shown in Fig. 1A, the DESeq2 package30 was used to identify DEGs between IPF patients (n = 46) and controls (n = 26) in the GSE13469217 dataset, resulting in 3989 upregulated DEGs and 1298 downregulated DEGs. Using the limma package31with the GSE32537 dataset18, we identified 425 DEGs between IPF patients (n = 119) and controls (n = 50), comprising 311 upregulated and 114 downregulated genes (Fig. 1B).
Downregulation of ACSL1 Contributes to Mitophagy in IPF. (A) The volcano plot based on the GSE134692 dataset, with adjusted P value < 0.05 and |log2(fold change)| > 1. (B) The volcano plot based on the GSE32537 dataset, with adjusted P value < 0.05 and |log2(fold change)| > 1. (C) The Venn plot showing ACSL1 as the only gene intersection among the GSE134692 dataset, GSE32537 dataset, autophagy-related genes, and mitochondrial-related genes. (D) ACSL1 expression in IPF patients (n = 46) and controls (n = 26) based on the GSE134692 dataset. (E) ACSL1 expression in IPF patients (n = 119) and controls (n = 50) based on the GSE32537 dataset. (F) The scatter diagram shows a negative correlation between the expression of ACSL1 and St. George’s total score in IPF patients based on the GSE32537 dataset. (G) The scatter diagram shows a negative correlation between the expression of ACSL1 and FVC pre-bronchodilator % predicted in IPF patients based on the GSE32537 dataset. (H) The scatter diagram shows a negative correlation between the expression of ACSL1 and DLCO % predicted in IPF patients based on the GSE32537 dataset. Abbreviations: ACSL1, long-chain fatty acyl-CoA synthetase 1; DLCO % predicted, percent predicted diffusion capacity of the lung for carbon monoxide; GSE, Gene Expression Omnibus Series; FVC pre-bronchodilator % predicted, percent predicted forced vital capacity; IPF, idiopathic pulmonary fibrosis. Data are expressed as mean ± SEM. *** P < 0.001.
To assess the role of mitophagy-related genes in IPF, 803 autophagy-related genes19and 1136 mitochondria-related genes20 were obtained and intersected, identifying 57 genes as mitophagy-related. These mitophagy-related genes were then intersected with the DEGs from both the GSE13469217 and GSE3253718 datasets. Interestingly, ACSL1 was the only gene overlapping among them (Fig. 1C). Subsequently, downregulated levels of ACSL1 were observed in IPF patients compared to controls in both the GSE13469217 and GSE3253718 datasets (P < 0.05, Fig. 1D, E).
The GSE3253718 dataset also provided clinical characteristics, allowing for correlation analyses between the expression of ACSL1 and these characteristics. As shown in Fig. 1F-H, a negative relationship was indicated between ACSL1 levels and SGRQ scores, FVC pre-bronchodilator % predicted, and DLCO % predicted (**P* < 0.05). These findings suggest that decreased expression of ACSL1 might be associated with mitophagy in IPF.
MitoQ refrains pulmonary fibrosis in rat lungs following BLM exposure
MitoQ, a mitochondria-targeted antioxidant, plays a role in eliminating excessive mitochondrial ROS (mtROS) and improving mitophagy32,33. In this study, MitoQ treatment was used to assess mitophagy in pulmonary fibrosis rat models. The flowchart of these in vivo experiments is depicted in Fig. 2A. After 28 days of treatment, the body weight of the Fibrosis group decreased significantly compared to the CON group (Fig. 2B, P < 0.05), while a significant increase in body weight was observed in the MitoQ plus Fibrosis group compared to the Fibrosis group (Fig. 2B, P < 0.05).
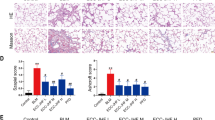
MitoQ Attenuates Pulmonary Fibrosis in Rat Lungs Following BLM Exposure. (A) The experimental flowchart. (B) Line plot showing the body weight of different groups of rats. (C) Comparisons of lung coefficients among the different groups. (D) Comparisons of GSH concentrations among the different groups. (E) Comparisons of SOD concentrations among the different groups. (F) Comparisons of MDA concentrations among the different groups. (G) H&E staining results for the different groups. (H) Masson staining results for the different groups. (I) Comparisons of fibrosis scoring among the different groups. (J) Levels of α-SMA using IHC staining. (K) Levels of FN1 using IHC staining. (L) Comparisons of the positive area for α-SMA. (M) Comparisons of the positive area for FN1. (N) Expression levels of α-SMA and FN1 measured by WB analysis, with β-actin used as a loading control. (O) Quantification of WB analysis. Abbreviations: BLM, bleomycin; GSH, glutathione; H&E, Hematoxylin and eosin; IHC, immunohistochemistry; MDA, malondialdehyde; SOD, superoxide dismutase; WB, western blotting. Data are expressed as mean ± SEM, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001.
After sacrifice, the lung tissues of the CON and MitoQ groups appeared healthy relatively, with a reddish color and good elasticity. In contrast, the lung tissues of the Fibrosis group exhibited a grayish color, noticeable edema, congestion, and reduced elasticity. Meanwhile, the MitoQ plus Fibrosis group showed milder symptoms compared to the BLM group, with only a few hemorrhagic spots present (Figure S2). The Fibrosis group showed a significantly increased lung coefficient (Fig. 2C, P < 0.05), and MitoQ treatment was able to decrease the abnormally elevated lung coefficient caused by BLM (Fig. 2C, P < 0.05).
Compared to the CON group, the levels of GSH and SOD were significantly decreased in the Fibrosis group (Fig. 2D-E, P < 0.05), while the levels of MDA were markedly increased (Fig. 2F, P < 0.05). MitoQ could alleviate these changes (Fig. 2D-F, P < 0.05). These data suggest that redox damage may play an important role in pulmonary fibrosis induced by BLM, and that MitoQ can relieve oxidative stress in the rat lung by inhibiting the production of ROS.
The results of H&E staining showed that aggregation of alveolar inflammatory cells in the Fibrosis group (Fig. 2G). Meanwhile, Masson staining illustrated a significant increase in fibrotic lesions in the Fibrosis group (Fig. 2H), with fibrosis scores higher than those in the CON group (Fig. 2I, P < 0.05). After MitoQ treatment, the severity of alveolar inflammation and pulmonary fibrosis was reduced compared to the Fibrosis group (Fig. 2G-I, P < 0.05).
The level of α-SMA is characterized as a crucial marker in the process of transforming lung fibroblasts into myofibroblasts during the development of pulmonary fibrosis34. IHC staining demonstrated an increase in the positive area of α-SMA in the Fibrosis group (Fig. 2J and L, and Figure S3A-B, P < 0.05), along with upregulated expression of α-SMA through WB assay (Fig. 2N and O, P< 0.05). FN1 is essential in driving myofibroblast differentiation in the development of pulmonary fibrosis35. The results of IHC staining and WB assays showed that the levels of FN1 were higher in the Fibrosis group compared to the CON group (Fig. 2K, M and N, and 2O, P < 0.05). Meanwhile, MitoQ pretreatment reduced the increased levels of α-SMA and FN1 observed in the Fibrosis group (Fig. 2J-O, P < 0.05). These findings indicate that MitoQ treatment can attenuate pulmonary fibrosis in rat lungs triggered by BLM.
MitoQ enhances ACSL1 and PINK1/Parkin-mediated mitophagy in rat lungs following BLM exposure
To assess the degree of mitophagy in vivo, TEM observation was conducted (Fig. 3A). In the CON group, mitochondria maintained their morphological integrity. In contrast, mitochondria in the Fibrosis group were significantly swollen, with intact membranes, sparse matrix granules, cavitation, and disrupted or absent cristae. The mitochondrial aspect ratio, defined as the ratio of the long diameter to the short diameter of the mitochondria, was used to evaluate mitochondrial function. Results indicated a reduced aspect ratio in the Fibrosis group compared to the CON group (Fig. 3B, P < 0.05). Additionally, a few autophagolysosomes were observed in the Fibrosis group. In comparison, the MitoQ treatment exhibited improved mitochondrial morphology, reflected in an increased mitochondrial aspect ratio (Fig. 3B, P < 0.05). These observations illustrate that BLM induced mitochondrial damage, while MitoQ treatment effectively ameliorated this damage.
MitoQ Enhances ACSL1 and PINK1/Parkin-Mediated Mitophagy in Rat Lungs Following BLM Exposure. (A) TEM images illustrating mitochondrial morphology. (B) The ratio of the long diameter to the short diameter of mitochondria. (C) Western blot (WB) analysis of Drp1, Mfn2, BECN1, and p62 expression, with β-actin used as a loading control. (D) Quantification of WB analysis. (E) Immunohistochemistry (IHC) staining for ACSL1. (F) Comparison of ACSL1 levels across different groups. (G) IHC staining for PINK1. (H) Comparison of PINK1 levels across different groups. (I) IHC staining for Parkin. (J) Comparison of Parkin levels across different groups. (K) WB analysis of ACSL1, PINK1, and Parkin expression, with β-actin used as a loading control. (L) Quantification of WB analysis. Abbreviations: ACSL1, long-chain fatty acyl-CoA synthetase 1; BECN1, Beclin-1; BLM, bleomycin; Drp1, dynamin-related protein 1; IHC, immunohistochemistry; Mfn2, mitofusin 2; PINK1, PTEN-induced putative kinase 1; Parkin, E3 ubiquitin ligase Parkin; TEM, transmission electron microscopy; WB, western blotting. Data are expressed as mean ± SEM, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001.
To evaluate mitochondrial fission in the Fibrosis group, the levels of Drp1 and Mfn2 were detected using a WB assay (Fig. 3C and D). The Fibrosis group showed upregulated levels of Drp1 and downregulated levels of Mfn2 (P< 0.05), indicating abnormal mitochondrial fission. MitoQ treatment improved mitochondrial fission. Dysfunctional mitochondrial fission can facilitate autophagy36. The decreased levels of BECN1 and increased levels of p62 in the Fibrosis group (Fig. 3C and D, P < 0.05) suggested insufficient autophagy was involved in BLM-induced pulmonary fibrosis in rats. MitoQ treatment mitigated the abnormal autophagy status.
As mentioned above, downregulated expression of ACSL1 might be related to mitophagy in IPF based on bioinformatics analysis. In our in vivo experiment, decreased levels of ACSL1 were observed in the Fibrosis group, along with insufficient PINK1/Parkin-mediated mitophagy, as shown by IHC and WB assays (Fig. 3E-L, and Figure S3C-D). Activation of mitophagy was involved in MitoQ treatment of pulmonary fibrosis induced by BLM. Collectively, MitoQ treatment might restore mitochondrial damage triggered by BLM through regulating the levels of ACSL1 and PINK1/Parkin-mediated mitophagy.
MitoQ relieves mitochondrial dysfunction and dysfunctional mitophagy in A549 cell lines exposed to BLM
The expression of ACSL1 in different cell types was investigated through single-cell analysis. Based on the GSE13589321 and GSE12803322 datasets, ACSL1 mainly enriches in alveolar type II epithelial cell (AT2), alveolar type I epithelial cell (AT1), monocytes, macrophages, and other cell types (Fig. 4 and Figure S4). Recently, damage and dysfunction of AT2 play a key role in the pathogenesis of IPF37,38. As previous studies27,39, the A549 cell lines, characterized as AT2 cell lines40, were used for further in vitro experiments.
ACSL1 expression in different cell types based on single-cell analysis. (A) uMAP plot of ACSL1 expression in the GSE135893 dataset. (B) uMAP plot comparing ILD patients and controls in the GSE135893 dataset. (C) uMAP plot showing ACSL1 expression among different cell types in the GSE135893 dataset. (D) uMAP plot of ACSL1 expression in the GSE128033 dataset. (E) uMAP plot comparing ILD patients and controls in the GSE128033 dataset. (F) uMAP plot showing ACSL1 expression among different cell types in the GSE128033 dataset. Abbreviations: ACSL1, long-chain fatty acyl-CoA synthetase 1; uMAP, uniform manifold approximation and projection; ILD, interstitial lung disease; GSE, Gene Expression Omnibus Series.
First, the suitable concentration of BLM exposure on the A549 cell lines was determined through CCK8 assay and WB detection. A concentration of 40 µM BLM was selected to establish a cell model of pulmonary fibrosis (Figure S5). Next, the role of MitoQ on treatment of A549 cell lines triggered by BLM was assessed, the results showed that the viability of A549 cell lines was reduced under BLM exposure (BLM group), but MitoQ pretreatment (MitoQ group) improved cell viability in A549 cell lines exposed to BLM (MitoQ + BLM group), as shown in Fig. 5A. Moreover, the levels of oxidant and antioxidant systems, including GSH, SOD, and MDA, were significantly altered in the BLM group (Fig. 5B-D, P < 0.05). MitoQ showed effectiveness in alleviating these oxidant and antioxidant changes (Fig. 5B-D, P < 0.05). Excessive ROS production in A549 cell lines was observed after BLM induction (Fig. 5E and G, P < 0.05), and MitoQ pretreatment could inhibit ROS generation, restoring these effects.
MitoQ improved redox damage, mitochondrial dysfunction, and PINK1/Parkin-mediated mitophagy in A549 cell lines exposed to BLM. (A) Comparison of cell viability among the different groups. (B) Comparison of GSH concentrations among the different groups. (C) Comparison of SOD concentrations among the different groups. (D) Comparison of MDA concentrations among the different groups. (E) Comparison of fluorescence intensity of ROS among the different groups. (F) Comparison of fluorescence intensity ratio of JC-1 monomers to JC-1 aggregates among the different groups. (G) Observation of DCFH-DA staining, scale bar: 50 μm, magnification: 20x. (H) Observation of JC-1 staining, scale bar: 20 μm, magnification: 40x. (I) Colocalization analysis of LysoTracker (Green) and MitoTracker (Red) staining. (J) Expression levels of α-SMA, FN1, Drp1, Mfn2, BECN1, p62, ACSL1, PINK1, and Parkin measured by WB assays, with β-actin used as a loading control. (K) Quantification of WB analysis. Abbreviations: BLM, bleomycin; Drp1, dynamin-related protein 1; GSH, glutathione; IHC, immunohistochemistry; MDA, malondialdehyde; Mfn2, mitofusin 2; PINK1, PTEN-induced putative kinase 1; Parkin, E3 ubiquitin ligase Parkin; SOD, superoxide dismutase; ROS, reactive oxygen species; WB, western blotting. Data are expressed as mean ± SEM, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001.
Mitochondrial dysfunction, characterized by excessive ROS, decreased MMP, and altered mitochondrial dynamics, contributes to the development of pulmonary fibrosis6. JC-1 staining showed that MitoQ could relatively increase the decreased MMP in A549 cell lines triggered by BLM (Fig. 5F and H, P < 0.05). Changes in Drp1 and Mfn2, indicative of altered mitochondrial dynamics, were observed in A549 cell lines stimulated by BLM (Fig. 5J and K, P< 0.05). Mitophagy is essential for regulating mitochondrial quality control11,41. Laser confocal observation demonstrated a decreased degree of mitophagy under BLM exposure, while MitoQ pretreatment improved mitophagy, reversing the insufficient mitophagy observed, as shown in Fig. 5I. The levels of ACSL1 and the PINK1/Parkin pathway were monitored. Following BLM exposure, decreased ACSL1 expression and inhibited PINK1/Parkin-mediated mitophagy were exhibited. These effects were alleviated by MitoQ pretreatment (Fig. 5J and K, P < 0.05). Additionally, BLM exposure led to the upregulation of α-SMA and FN1 (Fig. 5J and K, P < 0.05), these effects which were mitigated by MitoQ pretreatment. These findings suggest that excessive ROS production, decreased MMPs, and insufficient mitophagy were involved in the process of pulmonary fibrosis induced by BLM. MitoQ pretreatment appeared to restore mitochondrial function, particularly through the modulation of ACSL1 and PINK1/Parkin-mediated mitophagy.
Intervention of ACSL1 accelerates PINK1/Parkin mediated mitophagy in A549 cell lines exposed by BLM
To explore the regulatory mechanism of ACSL1 in mediating mitophagy in IPF, A549 cell lines were transfected with pCMV-ACSL1(human)-3×FLAG-Neo plasmid (oeACSL1 group) and pCMV-T7-MCS-3×FLAG-WPRE-Neo plasmid (ncACSL1 group), respectively. The expression of ACSL1 was significantly increased in the oeACSL1 group compared to the ncACSL1 and control (CON) groups (Figure S6A-B, P < 0.05). The oeACSL1 transfection promoted cell viability decreased by BLM exposure (Fig. 6A). The antioxidant role of oeACSL1 transfection was evidenced by increased levels of GSH and SOD (Fig. 6B and C, P < 0.05), and decreased levels of MDA (Fig. 6D, P < 0.05) and ROS generation (Fig. 6E and G, P < 0.05). The increased ratio of JC-1 monomers to JC-1 aggregates, indicating decreased MMPs, was stimulated by BLM (Fig. 6F and H, P< 0.05). This effect was ameliorated by oeACSL1 transfection. We measured the protein expression of Drp1 and Mfn2, which are involved in mitochondrial dynamic homeostasis42. BLM exposure increased Drp1 expression in A549 cell lines while downregulating Mfn2 expression. Notably, these changes were significantly attenuated by oeACSL1 transfection (Fig. 6J and K, P < 0.05). Similarly, overexpression of ACSL1 could rectify the insufficient mitophagy induced by BLM (Fig. 6I). These results indicate that ACSL1 may ameliorate mitochondrial dysfunction. Additionally, upregulation of ACSL1 activated mitophagy inhibition caused by BLM, as expected (Fig. 6I). BLM inhibited the expression of BECN1 while upregulating the expression of p62 and inhibiting PINK1/Parkin-mediated mitophagy. These phenomena were attenuated by oeACSL1 transfection (Fig. 6J and K). Together, ACSL1 may promote PINK1/Parkin-mediated mitophagy and contribute to the improvement of pulmonary fibrosis.
Overexperssion of ACSL1 alleviated redox damage, mitochondrial dysfunction, and PINK1/Parkin-mediated mitophagy in A549 cell lines exposed to BLM. (A) Comparison of cell viability among the different groups. (B) Comparison of GSH concentrations among the different groups. (C) Comparison of SOD concentrations among the different groups. (D) Comparison of MDA concentrations among the different groups. (E) Comparison of fluorescence intensity of ROS among the different groups. (F) Comparison of fluorescence intensity ratio of JC-1 monomers to JC-1 aggregates among the different groups. (G) Observation of DCFH-DA staining, scale bar: 50 μm, magnification: 20x. (H) Observation of JC-1 staining, scale bar: 20 μm, magnification: 40x. (I) Colocalization analysis of LysoTracker (Green) and MitoTracker (Red) staining. (J) Expression levels of α-SMA, FN1, Drp1, Mfn2, BECN1, p62, ACSL1, PINK1, and Parkin measured by WB assays, with β-actin used as a loading control. (K) Quantification of WB analysis. Abbreviations: BLM, bleomycin; Drp1, dynamin-related protein 1; GSH, glutathione; IHC, immunohistochemistry; MDA, malondialdehyde; Mfn2, mitofusin 2; PINK1, PTEN-induced putative kinase 1; Parkin, E3 ubiquitin ligase Parkin; SOD, superoxide dismutase; ROS, reactive oxygen species; WB, western blotting. Data are expressed as mean ± SEM, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001, NS. no significant.
Inhibition of ACSL1 in A549 cell lines was achieved using siRNA (siACSL1 group, ACSL1-homo-1324 selected, Figure S6C, P < 0.05), with a negative control (snACSL1 group) set for comparison. The results demonstrated that siACSL1 treatment reduced cell viability (Fig. 7A), increased ROS generation (Fig. 7B and H), and decreased MMP (Fig. 7C and I). It also exacerbated mitochondrial dysfunction and impaired mitophagy, as evidenced by abnormal levels of Drp1, Mfn2, PINK1, and Parkin (Fig. 7G and S7A). Compared to BLM treatment alone, siACSL1 pretreatment further increased cell death (Fig. 7A), accumulated ROS (Fig. 7B and H), reduced MMPs (Fig. 7C and I), and inhibited PINK1/Parkin-mediated mitophagy. Additionally, when A549 cells pretreated with siACSL1 and exposed to BLM were treated with MitoQ for 24 h, the adverse effects induced by siACSL1 and BLM were not alleviated by MitoQ (Fig. 7D, E, F, J, K and L, and Figure S7B). These findings suggest that ACSL1 is involved in PINK1/Parkin-mediated mitophagy in pulmonary fibrosis, and that MitoQ treatment is unable to reverse the mitophagy dysfunction caused by ACSL1 inhibition.
Inhibition of ACSL1 aggregated redox damage, mitochondrial dysfunction and PINK1/Parkin-mediated mitophagy. When BLM treated the A549 cell lines inhibited by siACSL1, (A) Comparison of cell viability among the different groups. (B) Comparison of fluorescence intensity of ROS among the different groups. (C) Comparison of fluorescence intensity ratio of JC-1 monomers to JC-1 aggregates among the different groups. (G). Expression levels of α-SMA, FN1, Drp1, Mfn2, BECN1, p62, ACSL1, PINK1, and Parkin measured by WB assays, with β-actin used as a loading control. (H). Observation of DCFH-DA staining, scale bar: 50 μm, magnification: 20x. (I) Observation of JC-1 staining, scale bar: 20 μm, magnification: 40x. When MitoQ treated the A549 cell lines inhibited by siACSL1 under BLM exposure, (D) Comparison of cell viability among the different groups. (E) Comparison of fluorescence intensity of ROS among the different groups. (F) Comparison of fluorescence intensity ratio of JC-1 monomers to JC-1 aggregates among the different groups. (J). Expression levels of α-SMA, FN1, Drp1, Mfn2, BECN1, p62, ACSL1, PINK1, and Parkin measured by WB assays, with β-actin used as a loading control. (K). Observation of DCFH-DA staining, scale bar: 50 μm, magnification: 20x. (L) Observation of JC-1 staining, scale bar: 20 μm, magnification: 40x. Abbreviations: BLM, bleomycin; Drp1, dynamin-related protein 1; GSH, glutathione; IHC, immunohistochemistry; MDA, malondialdehyde; Mfn2, mitofusin 2; PINK1, PTEN-induced putative kinase 1; Parkin, E3 ubiquitin ligase Parkin; SOD, superoxide dismutase; ROS, reactive oxygen species; WB, western blotting. Data are expressed as mean ± SEM, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001, NS. no significant.
Discussion
Globally, pulmonary fibrosis remains a chronic, progressive interstitial lung disease and is a major cause of mortality1,10. Although some drugs have been found to effectively attenuate the progression of pulmonary fibrosis43, the complete cure or preventive treatment has not yet been achieved. Therefore, novel approaches need to be explored. Currenttly, the role of mitochondrial dysfunction in the development of pulmonary fibrosis has garnered significant attention. Emerging evidence suggests that impaired mitochondrial function, including altered mitophagy, increased oxidative stress, and abnormal mitochondrial dynamics, plays a crucial role in the pathogenesis of pulmonary fibrosis6,41. Impaired mitophagy contributes to the accumulation of damaged mitochondria, thereby promoting cellular senescence8,44. Senescent cells secrete pro-fibrotic factors, exacerbating fibrosis in the progression of pulmonary fibrosis. Additionally, the activation of pro-fibrotic signaling pathways, such as the TGF-β signaling pathway, can induce fibroblast activation and differentiation into myofibroblasts. These myofibroblasts are responsible for the excessive deposition of extracellular matrix (ECM) components, including collagen45. Mitochondrial dysfunction in epithelial cells can trigger epithelial-mesenchymal transition (EMT) by activating stress signaling pathways, such as TGF-β146. EMT promotes the differentiation of epithelial cells into myofibroblasts, which express α-SMA and produce large amounts of ECM components, further advancing fibrosis46,47. AT2 cells adopt fibroblast-like behaviors, including enhanced migratory capacity and increased production of ECM proteins like FN1, contributing to excessive ECM deposition48. This leads to the thickening and stiffening of lung tissue, indicating that α-SMA and FN1 serve as important markers of pulmonary fibrosis progression47,48. In this study, the upregulation of α-SMA and FN1 was observed in the lung tissue of rats and A549 cell lines exposed to BLM, suggesting successful establishment of the pulmonary fibrosis model. Also, mitochondrial dysfunction was noted in rat lung tissue, and dysregulated mitochondrial dynamics were observed in vitro. These findings indicate that targeting dysfunctional mitochondria represents a promising therapeutic approach for pulmonary fibrosis.
Notably, strategies that enhance mitophagy have shown promise in preclinical models by reducing oxidative stress, improving mitochondrial function, and attenuating fibrosis6,15. MitoQ can accumulate in the mitochondria, where it specifically targets and neutralizes mtROS, helping to preserve mitochondrial function and integrity15,27. MitoQ treatment has been shown to alleviate cell apoptosis in A549 cell lines and barrier breakdown characteristic of acute lung injury (ALI) caused by lipopolysaccharide (LPS) by regulating Drp1-mediated mitochondrial fission. The inhibition of Nrf2 could block these protective effects of MitoQ49. Our previous study also demonstrated that MitoQ could reduce mtROS and inhibit mitochondrial-mediated apoptosis induced by PM2.527. PM2.5is a significant risk factor for mortality in patients with IPF15,50. PM2.5 can stimulate collagen I/III overproduction, EMT, and activation of the TGF-β1/Smad2 pathway in PM2.5-induced pulmonary fibrosis in vivo and in vitro, and these toxic effects might be alleviated by MitoQ pretreatment15. MitoQ combined with Yifei Decoction can improve lung injury in rats with pulmonary fibrosis by reducing the secretion of proinflammatory cytokines and inhibiting TGFβ1/NOX4 and PDGF/ROCK signaling pathways51, suggesting that MitoQ could inhibit excessive mitochondrial ROS production and promote EMT by activating some fibrogenic signaling pathways, thereby reducing fibroblast differentiation and ECM production. In this study, intervention with MitoQ reduced excessive ROS production, attenuated alterations in mitochondrial dynamics, and enhanced decreased mitophagy, leading to the treatment of pulmonary fibrosis both in vivo and in vitro. Moreover, MitoQ also prevented ferroptosis-characteristic mitochondrial changes, pulmonary epithelial cell death, and ALI, suggesting that MitoQ might mitigate ferroptosis induced by BLM52. Together, these findings indicate that MitoQ might play a variety of regulatory roles, improve pulmonary fibrosis, and serve as a potential therapeutic treatment.
Particularly, the PINK1/Parkin pathway is one of the best-characterized mechanisms of mitophagy12,53. MitoQ pretreatment has been shown to alleviate arsenic-induced acute liver injury and immune imbalance by activating the Nrf2/HO-1 and PINK1-mediated mitophagy pathways32. Additionally, MitoQ reduced the activation of hepatic stellate cells and blocked PINK1/Parkin-mediated mitophagy, thereby alleviating hepatic fibrosis33. Similarly, our findings revealed that MitoQ could attenuate pulmonary fibrosis by enhancing PINK1/Parkin-mediated mitophagy both in vivo and in vitro. Additionally, in PINK1/Parkin-mediated mitophagy, damaged mitochondria are marked for degradation and delivered to lysosomes for breakdown. Enhanced lysosomal acidification, as suggested by the Lysotracker staining results in our study, may indicate increased autophagic flux, potentially facilitating the clearance of dysfunctional mitochondria. This process plays a critical role in maintaining cellular homeostasis, particularly in the regulation of mitochondrial quality control54,55. Interestingly, our study demonstrated stronger Lysotracker staining in MitoQ-pretreated cells, even in the absence of BLM exposure, suggesting that MitoQ exerts a protective effect on cellular damage by enhancing antioxidant defenses and improving mitophagy. However, there are also researchers who propose an opposite view56. For instance, MitoQ has been reported to play a protective role in aortic fibrosis after PM2.5 exposure, but its regulatory mechanism showed that MitoQ was involved in reducing ROS/PINK1/Parkin-mediated mitophagy56. These data suggest that MitoQ might have a double-edged sword effect in the treatment of fibrotic diseases, and its reaction may be associated with mitochondrial quality control under different disease statuses.
Interestingly, ACSL1 is the only molecule identified by intersecting the raw data of IPF17,18and mitophagy-related genes. ACSL1 is an enzyme that plays a crucial role in lipid metabolism by converting free long-chain fatty acids into fatty acyl-CoA esters, which are essential intermediates in various metabolic pathways57,58. It has been suggested that ACSL1 is involved in facilitating cancer progression58,59. Compared to normal human bronchial epithelial cells, higher expression of ACSL1 is significantly correlated with lung adenocarcinoma60. Additionally, the expression of ACSL1 is significantly increased in the exfoliated urine cells and urine supernatant of diabetic nephropathy patients and is closely related to renal function61. In this study, downregulation of ACSL1 was observed in IPF patients through bioinformatics analysis. Similarly, the expression of ACSL1 decreased in the lung tissues of rats with pulmonary fibrosis and A549 cell lines triggered by BLM. The level of ACSL1 was negatively correlated with the SGRQ scores, which are used to measure the quality of life in patients with IPF62. Furthermore, there is an inverse trend between the level of ACSL1 and lung function parameters, including FVC pre-bronchodilator % predicted and DLCO % predicted. This study first proposes that the downregulation of ACSL1 is associated with the severity of IPF, providing a new target for the clinical treatment of IPF.
Recently, the regulatory mechanism of ACSL1 has been explored in further detail. Temporally induced knockout of ACSL1 in hearts results in the loss of fatty acid oxidation, which is associated with the activation of mTORC1, inhibition of autophagy, and an increase in the number of damaged mitochondria63. In the regulation of muscle function, ACSL1 deficiency leads to increased protein catabolism and defects in muscle fuel metabolism, resulting in exercise intolerance, muscle weakness, and myocyte apoptosis64. These findings suggest that ACSL1 might inhibit autophagy by activating mTORC1. Several studies have shown that ACSL1 is located in mitochondria and primarily directs fatty acids to the β-oxidation pathway in various cell types58,65. Overexpressed ACSL1 can increase fatty acid oxidation and ameliorate palmitate-induced insulin resistance in C2C12 myotubes65. The activity of ACSL1 can affect the composition of mitochondrial membranes by incorporating fatty acids into phospholipids. Changes in membrane composition can influence mitochondrial dynamics, including fission and fusion processes that are critical for mitophagy. Overexpression of ACSL1 in A549 cell lines transfected with a plasmid resulted in decreased ROS levels and improved MMPs, alongside an increase in PINK1 and Parkin expression. Conversely, ACSL1 inhibition had the opposite effect in A549 cells under BLM exposure, highlighting ACSL1 might play a regulatory role in PINK1/Parkin-mediated mitophagy. Additionally, MitoQ pretreatment was unable to reverse the inhibition of PINK1 and Parkin caused by ACSL1 downregulation via siACSL1, suggesting that ACSL1 may be crucial for the mitophagy-regulating effects of MitoQ.
Additionally, there are several limitations to this study. First, we focused exclusively on PINK1/Parkin-mediated mitophagy in the context of pulmonary fibrosis, without thoroughly investigating other signaling pathways or the phosphorylation status of PINK1, which may also play important roles in regulating mitophagy in pulmonary fibrosis. This limitation was due to the scope of our experimental capabilities. Second, the relationship between ACSL1 and the PINK1/Parkin pathway requires further exploration to fully understand its mechanistic role. Lastly, the clinical relevance of ACSL1 must be validated through clinical observational studies to assess its potential therapeutic value.
Conclusions
Collectively, this study is the first to investigate the biological function of ACSL1 in the process of pulmonary fibrosis. Bioinformatics analysis suggests that downregulation of ACSL1 might be associated with the severity of IPF in patients. Our in vivo and in vitro experiments demonstrated decreased levels of ACSL1 and PINK1/Parkin-mediated mitophagy in pulmonary fibrosis induced by BLM. MitoQ pretreatment was shown to alleviate these toxicological effects. urthermore, overexpression of ACSL1 alleviated the inhibition of PINK1/Parkin-mediated mitophagy caused by BLM exposure. Conversely, ACSL1 inhibition exacerbated the severity of pulmonary fibrosis, and these adverse effects could not be reversed by MitoQ treatment. Therefore, our data highlight the crucial role of ACSL1 in the process of pulmonary fibrosis through the regulation of PINK1/Parkin-mediated mitophagy and provide a potential therapeutic target for pulmonary fibrosis.
Data availability
The original data presented in the study are included in the manuscript/Supplementary Material. Further inquiries can be directed to the corresponding author.
References
Moss, B. J., Ryter, S. W. & Rosas, I. O. Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Annu. Rev. Pathol. Mech. Dis. 17, 515–546 (2022).
Hill, C. & Wang, Y. Autophagy in pulmonary fibrosis: friend or foe? Genes Dis. 9, 1594–1607 (2022).
Luppi, F., Kalluri, M., Faverio, P., Kreuter, M. & Ferrara, G. Idiopathic pulmonary fibrosis beyond the lung: understanding disease mechanisms to improve diagnosis and management. Respir Res. 22, 109 (2021).
Gandhi, S., Tonelli, R., Murray, M., Samarelli, A. V. & Spagnolo, P. Environmental causes of idiopathic pulmonary fibrosis. Int. J. Mol. Sci. 24, 16481 (2023).
Prakash, Y. S., Pabelick, C. M. & Sieck, G. C. Mitochondrial dysfunction in airway disease. Chest. 152, 618–626 (2017).
Rangarajan, S., Bernard, K. & Thannickal, V. J. Mitochondrial dysfunction in Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 14, S383–S388 (2017).
Ding, W. X., Yin, X. M. & Mitophagy Mechanisms, pathophysiological roles, and analysis. Biol. Chem. 393, 547–564 (2012).
Albano, G. D., Montalbano, A. M., Gagliardo, R. & Profita, M. Autophagy/Mitophagy in Airway diseases: impact of oxidative stress on epithelial cells. Biomolecules. 13, 1217 (2023).
Larson-Casey, J. L., He, C. & Carter, A. B. Mitochondrial quality control in pulmonary fibrosis. Redox Biol. 33, 101426 (2020).
Cala-Garcia, J. D., Medina-Rincon, G. J., Sierra-Salas, P. A., Rojano, J. & Romero, F. The role of mitochondrial dysfunction in idiopathic pulmonary fibrosis: New perspectives for a Challenging Disease. Biology. 12, 1237 (2023).
Sharma, A., Ahmad, S., Ahmad, T., Ali, S. & Syed, M. A. Mitochondrial dynamics and mitophagy in lung disorders. Life Sci. 284, 119876 (2021).
Tsubouchi, K., Araya, J. & Kuwano, K. PINK1-PARK2-mediated mitophagy in COPD and IPF pathogeneses. Inflamm. Regen. 38, 18 (2018).
Bueno, M. et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J. Clin. Invest. 125, 521–538 (2015).
Kobayashi, K. et al. Involvement of PARK2-mediated mitophagy in idiopathic pulmonary fibrosis pathogenesis. J. Immunol. 197, 504–516 (2016).
Li, Y. et al. MitoQ ameliorates PM2.5-induced pulmonary fibrosis through regulating the mitochondria DNA homeostasis. Chemosphere. 330, 138745 (2023).
Chu, L. et al. Tetrandrine alleviates pulmonary fibrosis by inhibiting alveolar epithelial cell senescence through PINK1/Parkin-mediated mitophagy. Eur. J. Pharmacol. 969, 176459 (2024).
Sivakumar, P. et al. RNA sequencing of transplant-stage idiopathic pulmonary fibrosis lung reveals unique pathway regulation. ERJ Open. Res. 5, 00117–2019 (2019).
Yang, I. V. et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax. 68, 1114–1121 (2013).
Deng, X. et al. Identification of 4 autophagy-related genes in heart failure by bioinformatics analysis and machine learning. Front. Cardiovasc. Med. 11, 1247079 (2024).
Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2021).
Habermann, A. C. et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv. 6, eaba1972 (2020).
Morse, C. et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir J. 54, 1802441 (2019).
Neumark, N., Cosme, C., Rose, K. A. & Kaminski, N. The idiopathic pulmonary fibrosis cell atlas. Am. J. Physiol. Lung Cell. Mol. Physiol. 319, L887–L893 (2020).
Della Latta, V., Cecchettini, A., Del Ry, S. & Morales, M. A. Bleomycin in the setting of lung fibrosis induction: from biological mechanisms to counteractions. Pharmacol. Res. 97, 122–130 (2015).
Moeller, A., Ask, K., Warburton, D., Gauldie, J. & Kolb, M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 40, 362 (2008).
Percie, D. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
Weng, A. B., Deng, M. M., Liu, X. D., Wang, H. B. & Lin, Q. MitoQ alleviated PM2.5 induced pulmonary epithelial cells injury by inhibiting mitochondrial-mediated apoptosis. Iran. J. Public. Health. 53, 614–624 (2024).
Roca-Portoles, A. & Tait, S. W. G. Mitochondrial quality control: from molecule to organelle. Cell. Mol. Life Sci. 78, 3853–3866 (2021).
Chen, W., Zhao, H. & Li, Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Sig Transduct. Target. Ther. 8, 333 (2023).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Ritchie, M. E. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Li, H. et al. The mitochondria-targeted antioxidant MitoQ ameliorates inorganic arsenic-induced DCs/Th1/Th2/Th17/Treg differentiation partially by activating PINK1-mediated mitophagy in murine liver. Ecotoxicol. Environ. Saf. 277, 116350 (2024).
Dou, S. Y. et al. MitoQ inhibits hepatic stellate cell activation and liver fibrosis by enhancing PINK1/parkin-mediated mitophagy. Open Med. 16, 1718–1727 (2021).
Frangogiannis, N. Transforming growth factor-\(\:\beta\:\) in tissue fibrosis. J. Exp. Med. 217, e20190103 (2020).
Kim, S. K., Jung, S. M., Park, K. S. & Kim, K. J. Integrative analysis of lung molecular signatures reveals key drivers of idiopathic pulmonary fibrosis. BMC Pulm Med. 21, 404 (2021).
Burman, J. L. et al. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J. Cell. Biol. 216, 3231–3247 (2017).
Parimon, T., Yao, C., Stripp, B. R., Noble, P. W. & Chen, P. Alveolar epithelial type II cells as drivers of lung fibrosis in idiopathic pulmonary fibrosis. Int. J. Mol. Sci. 21, 2269 (2020).
Wu, H. et al. Progressive Pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell. 180, 107–121e17 (2020).
Wang, Z. et al. Citrus alkaline extracts prevent endoplasmic reticulum stress in type II alveolar epithelial cells to ameliorate pulmonary fibrosis via the ATF3/PINK1 pathway. Phytomedicine. 89, 153599 (2021).
Khan, P. et al. Culture of human alveolar epithelial type II cells by sprouting. Respir Res. 19, 204 (2018).
Hara, H., Kuwano, K. & Araya, J. Mitochondrial quality control in COPD and IPF. Cells. 7, 86 (2018).
Youle, R. J. & van der Bliek, A. M. Mitochondrial fission, fusion, and stress. Science. 337, 1062–1065 (2012).
Thong, L., McElduff, E. J. & Henry, M. T. Trials and treatments: an update on Pharmacotherapy for Idiopathic Pulmonary Fibrosis. Life (Basel). 13, 486 (2023).
Pokharel, M. D. et al. Mitochondrial network dynamics in pulmonary disease: bridging the gap between inflammation, oxidative stress, and bioenergetics. Redox Biol. 70, 103049 (2024).
Yombo, D. J. K. et al. SEMA3B inhibits TGF\(\:\beta\:\)-induced extracellular matrix protein production and its reduced levels are associated with a decline in lung function in IPF. Am. J. Physiol. Cell. Physiol. 326, C1659–C1668 (2024).
Salton, F., Volpe, M. C. & Confalonieri, M. Epithelial–mesenchymal transition in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Med. (Kaunas). 55, 83 (2019).
Gd, M. et al. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 10, (2021).
Zhang, X. et al. Dissecting pulmonary fibroblasts heterogeneity in lung development, health and diseases. Heliyon. 9, e19428 (2023).
Hou, L. et al. MitoQ alleviates LPS-mediated acute lung injury through regulating Nrf2/Drp1 pathway. Free Radic Biol. Med. 165, 219–228 (2021).
Zhao, C. et al. Long-term exposure to PM2.5 aggravates pulmonary fibrosis and acute lung injury by disrupting Nrf2-mediated antioxidant function. Environ. Pollut. 313, 120017 (2022).
Chen, L. et al. Mechanism of Yifei Decoction Combined with MitoQ on Inhibition of TGF\(\:\beta\:\)1/NOX4 and PDGF/ROCK Signal Pathway in Idiopathic Pulmonary Fibrosis. Evid Based Complement Alternat Med 6615615 (2021). (2021).
Zhan, P. et al. Mitoquinone alleviates bleomycin-induced acute lung injury via inhibiting mitochondrial ROS-dependent pulmonary epithelial ferroptosis. Int. Immunopharmacol. 113, 109359 (2022).
Bingol, B. & Sheng, M. Mechanisms of mitophagy: PINK1, parkin, USP30 and beyond. Free Radic Biol. Med. 100, 210–222 (2016).
Weber, R. A. et al. Maintaining Iron Homeostasis is the key role of lysosomal acidity for cell proliferation. Mol. Cell. 77, 645–655e7 (2020).
Gómez-Virgilio, L. et al. Autophagy: a Key Regulator of Homeostasis and Disease: an overview of Molecular mechanisms and modulators. Cells. 11, 2262 (2022).
Ning, R. et al. The mitochondria-targeted antioxidant MitoQ attenuated PM2.5-induced vascular fibrosis via regulating mitophagy. Redox Biol. 46, 102113 (2021).
Zhou, Y. et al. ACSL1-Mediated Fatty Acid \(\:\beta\:\)-Oxidation Enhances Metastasis and Proliferation in Endometrial Cancer. Front. Biosci. (Landmark Ed). 29, 66 (2024).
Quan, J., Bode, A. M. & Luo, X. ACSL family: the regulatory mechanisms and therapeutic implications in cancer. Eur. J. Pharmacol. 909, 174397 (2021).
Ma, Y. et al. Long-chain fatty acyl-CoA synthetase 1 promotes prostate cancer progression by elevation of lipogenesis and fatty acid beta-oxidation. Oncogene. 40, 1806–1820 (2021).
Ma, Y. et al. The diagnostic value of ACSL1, ACSL4, and ACSL5 and the clinical potential of an ACSL inhibitor in Non-small-cell Lung Cancer. Cancers (Basel). 16, 1170 (2024).
Cao, Z. et al. ACSL1: a preliminary study that provides a new target for the treatment of renal fibrosis could bring new insights in diabetic kidney disease. Nefrologia (Engl Ed). 43 (Suppl 2), 38–46 (2023).
Swigris, J. J. et al. Psychometric properties of the St George’s respiratory questionnaire in patients with idiopathic pulmonary fibrosis. Eur. Respir J. 49, 1601788 (2017).
Grevengoed, T. J., Cooper, D. E., Young, P. A., Ellis, J. M. & Coleman, R. A. Loss of long-chain acyl-CoA synthetase isoform 1 impairs cardiac autophagy and mitochondrial structure through mechanistic target of rapamycin complex 1 activation. FASEB J. 29, 4641–4653 (2015).
Zhao, L. et al. Defective fatty acid oxidation in mice with muscle-specific acyl-CoA synthetase 1 deficiency increases amino acid use and impairs muscle function. J. Biol. Chem. 294, 8819–8833 (2019).
Nan, J. et al. An Essential Role of the N-Terminal Region of ACSL1 in Linking Free Fatty Acids to Mitochondrial \(\:\beta\:\)-Oxidation in C2C12 Myotubes. Mol. Cells. 44, 637–646 (2021).
Acknowledgements
The authors like to acknowledge the researchers at Key Laboratory of Translational Tumor Medicine in Fujian Province (Putian University, Putian, China) for their assistance with this study.
Funding
This study was supported by Fujian Provincial Natural Science Foundation of China (No. 2024J011468, No. 2020J011251); the Fujian Health Provincial Technology Project, China (No. 2020GGA078); Medical Research Foundation of Putian University(No. 2024107).
Author information
Authors and Affiliations
Contributions
Q.L. and Y.L. performed most of the experiments, Z.C. and M.D. analyzed data, Y.L, Z.Z, X.L, and Z.C contributed to experiments. Q.L. designed experiments, supervised the study, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study is performed in accordance with relevant guidelines and regulations. The animal experiments were conducted in accordance with ARRIVE 2.0 guidelines and were approved by the Ethics Committee of the Affiliated Hospital of Putian University (ID: 2024101).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
LIN, Q., LIN, Y., LIAO, X. et al. ACSL1 improves pulmonary fibrosis by reducing mitochondrial damage and activating PINK1/Parkin mediated mitophagy. Sci Rep 14, 26504 (2024). https://doi.org/10.1038/s41598-024-78136-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78136-5
Keywords
This article is cited by
-
The effective-compound compatibility of JHF inhibits fibroblast activation in pulmonary fibrosis by enhancing PINK1/PARK2-mediated mitophagy
Scientific Reports (2025)
-
Decreased Complex I Activity in Blood lymphocytes Correlates with Idiopathic Pulmonary Fibrosis Severity
Biochemical Genetics (2025)
-
The critical role of mitophagy in cell senescence-mediated pulmonary fibrosis and potential therapeutic strategies
Molecular Biology Reports (2025)
-
Transcriptomic analysis delineates potential regulatory network as therapeutic alternatives in chronic myeloid leukemia
Egyptian Journal of Medical Human Genetics (2024)