Abstract
Anesthetics are widely used in electrophysiological tests to assess retinal and visual system functions to avoid experimental errors caused by movement and stress in experimental animals. To determine the most suitable anesthetic for visual electrophysiological tests, excluding ketamine and chloral hydrate due to regulatory and side effect concerns, this study investigated the effects of ethyl carbamate (EC), avertin (AR), and pentobarbital sodium (PS) on visual signal conduction in the retina and primary visual cortex. Assessments included flash electroretinogram (FERG), pattern electroretinogram (PERG), pattern visual evoked potentials (PVEP), and flash visual evoked potentials (FVEP), FERG and FVEP were used to evaluate the responses of the retina and visual cortex to flash stimuli, respectively, while PERG and PVEP assessed responses to pattern stimuli. The research showed that AR demonstrates the least disruption to the visual signal pathway, as evidenced by consistently high characteristic peaks in the AR group across various tests. In contrast, mice given EC exhibited the lowest peak values in both FERG and FVEP, while subjects anesthetized with PS showed suppressed oscillatory potentials and PERG responses. Notably, substantial PVEP characteristic peaks were observed only in mice anesthetized with AR. Consequently, among the three anesthetics tested, AR is the most suitable for visual electrophysiological studies.
Similar content being viewed by others
Introduction
In the retina, photoreceptor cells convert light signals into electrical signals, which are then transmitted to the inner nuclear layer of the retina through synapses. Here, a series of cells, including bipolar cells, amplify, modulate, and integrate these signals before passing them on to the retinal ganglion cells. These signals are subsequently transmitted through the optic nerve axons to the visual cortex, where they undergo further processing and integration, ultimately forming vision. The output of these processes constitutes one of the primary sensory inputs of the brain1,2,3,4,5. The degeneration of retinal neurons is one of the leading causes of vision loss, drawing substantial attention in both hereditary diseases such as retinitis pigmentosa, Stargardt disease, and Leber congenital amaurosis, and in complex age-related diseases like age-related macular degeneration. In recent years, there has been substantial progress in understanding the mechanisms of retinal neurodegenerative diseases, leading to considerable advancements in various biomedical therapies, including gene therapy and stem cell therapy6. These developments have brought hope for effective treatment of retinal neurodegenerative diseases7,8,9,10.
In preclinical proof-of-concept studies on the mechanisms and treatments of retinal degenerative diseases, we typically use a series of rigorous visual electrophysiological tests to assess whether the visual function of the corresponding animal models has been effectively improved. These tests include methods such as flash electroretinography (FERG), pattern electroretinography (PERG), pattern visual evoked potential (PVEP), and flash visual evoked potential (FVEP). These tests provide a comprehensive evaluation of the entire visual pathway, from retinal photoreceptor cells to the visual cortex of the brain11,12,13. FERG and FVEP are used to evaluate the responses of the retina and visual cortex to flash stimuli, respectively, while PERG and PVEP assess responses to pattern stimuli, focusing on signal transmission from ganglion cells to the primary visual cortex14,15,16,17. By conducting statistical analysis of these test results, we can gain a comprehensive understanding of the transmission and processing of visual signals at various levels. This, in turn, helps in studying the specific functions of different types of cells in visual signal conduction.
Since animals cannot actively cooperate with testing procedures like humans, using anesthetics to reduce experimental errors caused by movement and stress during electrophysiological recordings becomes crucial. Commonly used anesthetics include ketamine/xylazine and chloral hydrate. However, ketamine/xylazine is strictly regulated or even banned in many countries, including China, making its acquisition process extremely cumbersome, complex, and sometimes illegal. Chloral hydrate was once used for anesthesia and euthanasia in large animals. However, due to its pronounced side effects, such as vascular and respiratory depression, its use in veterinary practice has been greatly reduced18. In the research field, especially in studies involving laboratory animals like mice and rats, the use of chloral hydrate has been strictly limited, as many consider it to have sedative rather than anesthetic effects19. In humans, while chloral hydrate serves as a sedative and anxiolytic, concerns have been raised regarding potential adverse effects, including respiratory depression and gastrointestinal discomfort20,21,22. For these reasons, we did not use chloral hydrate in this study. Therefore, alternatives such as ethyl carbamate (EC), avertin (AR), and pentobarbital sodium (PS) have become substitutes for these animal anesthetics. It is important to note that these anesthetics have different effects on electroretinogram (ERG) results. For instance, wild-type rats under PS anesthesia do not exhibit oscillatory potentials (OPs) similar to those observed with ketamine or avertin23,24. Furthermore, even under identical stimulation conditions, the FERG amplitude in wild-type mice varies substantially depending on the anesthetic used25,26. To avoid inaccuracies in evaluating visual function, it is essential to conduct a comprehensive comparison of the effects of these three anesthetics on visual electrophysiological signals within a consistent experimental framework. Additionally, the visual neural pathway encompasses not only the photoreceptor cells in the outer nuclear layer (ONL) and the cells in the inner nuclear layer (INL) involved in FERG but also the retinal ganglion cell (RGC) layer and the visual cortex. In contrast to the extensive research on FERG, there is a notable lack of detailed comparisons regarding the impact of these anesthetics on PERG, FVEP, and PVEP signals, which reflect the status of these neurons.
This study investigated the effects of three commonly used anesthetics: EC, AR, and PS, on the complete visual signal transduction pathway under identical conditions. Compared to PS, which suppresses oscillatory potentials and PERG waveforms, mice anesthetized with AR consistently exhibited stable and high-amplitude characteristic peaks across all visual electrophysiological tests. Although the PERG results in the EC group were comparable to those in the AR group, their FERG and FVEP results were lower than those of the other two groups. Most notably, typical PVEP results were observed only in mice anesthetized with AR, while they were absent under the other two anesthetics. By conducting a series of visual electrophysiological tests, including FERG, PERG, PVEP, and FVEP, we determined that AR is the most suitable anesthetic among the three tested for visual electrophysiological research.
Results
Comparative analysis of anesthetic effects on induction and duration of anesthesia in mice
To assess the differential impact of various anesthetics on mice, we categorized eighteen two-month-old mice, characterized by stable physiological states and full maturity, into three cohorts: those receiving ethyl carbamate (EC), pentobarbital sodium (PS), and avertin (AR) for anesthesia.
In this study, we conducted four visual electrophysiological experiments, each requiring approximately 60 min of recording time. During these experiments, even minor movements by the mice could cause electrode displacement or detachment, leading to substantial experimental errors. To prevent this, all recordings were conducted under deep anesthesia to ensure the accuracy of the results. Based on previous research and experimental requirements, the anesthetic doses were set at 1.6 g/kg for ethyl carbamate27,28,29,30,31, 0.30 g/kg for avertin32,33,34,35,36, and 0.115 g/kg for pentobarbital sodium37,38,39,40,41,42. Under these doses, the deep anesthesia time in mice was approximately 70 min (EC group: 71 ± 14.05 min, AR group: 79.88 ± 3.93 min, PS group: 65.47 ± 3.05 min), thereby ensuring sufficient anesthesia for approximately 60 min of recording. After 70 min of deep anesthesia, the mice began to show gradual physiological changes, including subtle head movements during thoracic or thoracoabdominal breathing, corneal reflexes, and head lifting and neck turning movements. Stable electrical signals could no longer be obtained in mice exhibiting these movements. Therefore, all experimental recordings were performed during the deep anesthesia phase. The recording time (i.e., deep anesthesia duration) was kept consistent across the three groups (approximately 70 min), ensuring comparable physiological states and, consequently, data accuracy and reliability.
The deep anesthesia state was determined by the toe pinch reflex and further confirmed through stable EEG results. Once the mice exhibited no response to the toe pinch, the stability of the EEG was used to confirm the anesthesia state (Fig. S1). The moment EEG stabilization was achieved marked the beginning of the measurements. The induction time was defined as the interval from anesthetic administration to the loss of the toe-pinch reflex. The deep anesthesia time was as the physiological state in which the mice exhibit no response to external stimuli and complete loss of consciousness. The anesthesia time encompassed the entire period from the onset of induction until the restoration of consciousness and motor function. All assessment was based on both the toe-pinch reflex and EEG monitoring (Fig. S1). Statistical analysis revealed that the AR group exhibited the most rapid anesthesia induction, whereas the EC group demonstrated the longest induction and overall anesthesia duration, with substantial intra-group variability (Table 1).
Assessment of scotopic response and ERG parameters in mice administered with various anesthetics
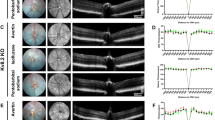
To objectively evaluate the scotopic responses of 18 two-month-old mice, we recorded their reactions under conditions of dark adaptation with incrementally intensified flash luminance (Fig. 1A). Considering that the frequency of oscillatory potentials (OPs) varies among species, and that in mice, as well as in humans, OPs typically exhibit frequencies between 75 and 80 Hz and 130–150 Hz43, we used a 30 Hz low-frequency filter in our methodology to isolate lower frequency components. This approach was intended to mitigate the effect of high-frequency filtering on the a-wave and b-wave, thus preserving their amplitudes for more precise fitting using established mathematical models (Fig. 1B). Subsequently, we harnessed the capabilities of the Matlab Statistical Toolbox to fit the scotopic ERG a-wave (Fig. 1C–E), extracting key parameters such as Rmax, Td, and S from the Lamb and Pugh models (Fig. 1F-H).
Scotopic ERG and a-wave Fitting. (A) Representative scotopic and photopic ERG responses in mice treated with different anesthetics. (B) The scotopic and photopic ERG waveforms from different groups were processed with a band-pass filter of 0.3–30 Hz to remove the influence of oscillatory potentials. (C–E) A-wave fitting waveforms for EC (C), AR (D), and PS (E). (F–H) Quantitative statistics of the a-wave related parameters, Rmax (F), S (G), and Td (H), derived from fitting with the Lamb and Pugh model. Representative data are presented as mean ± SEM; n = 6 independent biological replicates. Two-way ANOVA tests with Tukey’s multiple comparisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001. All experiments were independently performed at least three times to ensure repeatable results.
Dissecting the scotopic a-wave analysis, marked disparities were evident among the groups in terms of Rmax (F (2, 15) = 12.17, P < 0.001), S (F (2, 15) = 3.823, P = 0.05), and Td (F (2, 15) = 22.71, P < 0.001). Specifically, the a-wave Rmax of the EC group (2.581 ± 0.02244 log μV) was inferior to those of the AR and PS groups (2.750 ± 0.02365 and 2.773 ± 0.04052 log μV, respectively) (Fig. 1F), i suggesting that EC may suppress rod phototransduction signals.
Contrastingly, the S value, indicative of sensitivity to variations in light luminance, did not differ substantially across the EC, AR, and PS groups, with respective readings of 0.006748 ± 0.0002511, 0.006303 ± 0.0003810, and 0.009598 ± 0.001517. This uniformity underscores that the trio of anesthetics did not substantially influence the rod cells’ responsiveness to light stimuli (Fig. 1G).
Departing from the trends noted for Rmax and S, the Td parameter for the EC group (8.760 ± 0.1937 ms) substantially exceeded that of the AR (5.605 ± 0.3914 ms) and PS (7.586 ± 0.3813 ms) groups (Fig. 1H), indicating a markedly slower response to light in cells treated with EC. It is noteworthy that the PS group manifested the second-lengthiest Td value, hinting that PS may obstruct the signal transduction within the rod cells44.
Analysis of scotopic b-wave amplitude variations in ERG under different anesthetic treatments
In our investigation, we employed the Naka-Rushton equation to analyze the b-wave amplitude (R) in dark-adapted electroretinograms, revealing a dependency of R on the stimulus illumination. The b-wave fitting data for the dark-adapted ERG indicated significant intergroup variations in Rmax (F (2, 15) = 25.24, P < 0.001), N (F (2, 15) = 16.47, P < 0.001), and K (F (2, 15) = 94.78, P < 0.001) (Fig. 2).
Fitting of scotopic ERG b-waves. (A–C) Representative scotopic ERG b-waves of EC (A), AR (B) and PS (C). ERGs were recorded in dark-adapted mice with the light intensities increasing from − 3.6 to 2.15 log cd s m−2. (D–F) B-wave fitting waveforms of EC (D), AR (E) and PS (F). (G–I) Statistical analysis of the b-wave parameters, Rmax (G), N (H), and K (I), obtained by applying the Naka-Rushton equation. Representative data are presented as mean ± SEM; n = 6 independent biological replicates. Two-way ANOVA tests with Tukey’s multiple com-parisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001. All experiments were independently performed at least three times to ensure repeatable results.
Aligning with the a-wave findings, the Rmax for the dark-adapted b-wave in the EC group (3.012 ± 0.02970 log μV) was subpar compared to the AR (3.176 ± 0.02866 log μV) and PS (3.351 ± 0.04141 log μV) groups (Fig. 2G). The scotopic ERG b-wave mainly reflected the function of inner nuclear layer cells, which aggregates signals from photoreceptors before relaying them to amacrine and ganglion cells45. Given that the amplitude of the photoreceptor signals can influence those generated by bipolar cell transmission, the direct link between a-wave reduction and the subsequent b-wave diminishment remains to be elucidated.
Moreover, the N and K parameters for the dark-adapted b-wave in the AR group (N, 6.207 ± 0.3509; K, 5.152 ± 0.04133) were notably less than those in the EC and PS groups (N and K, 7.985 ± 0.2212 and 5.556 ± 0.01385 for EC group; 8.035 ± 0.1590 and 5.828 ± 0.04194 for PS group) (Fig. 2H, I), implying superior cell sensitivity in the AR group. It is important to observe that PS did not diminish the Rmax of the scotopic ERG a-wave (Fig. 1F) nor was the S value for the dark-adapted a-wave lowest in the PS group (Fig. 1G), indicating that PS did not exert marked inhibitory effects on rod-initiated signals. Nonetheless, the elevated K value of the dark-adapted b-wave in the PS group, the highest among the studied groups (Fig. 2I), suggests that PS might exert a modulatory influence on the sensitivity of bipolar cells46.
Characterization of oscillatory potential amplitudes and implicit times in scotopic ERG responses under anesthesia
To accurately delineate the peak amplitudes of OPs within the 75–80 Hz and 130–150 Hz frequency ranges, we processed the scotopic ERG response through a 65 Hz high-pass filter coupled with a 300 Hz low-pass filter. We defined the amplitude from the nadir of the initial negative wave to the zenith of the first positive wave as OP1, with subsequent peaks labeled consecutively as OP2 through OP5 (Fig. 3A). The cumulative OP amplitude (OPs) was calculated as the aggregate of OP1-OP5, while the implicit times (Ti) were gauged from the inception of each OP to the precise moment of stimulation.
Record of the oscillatory potentials. (A) Representative waveforms of oscillatory potentials (OPs) in three groups were recorded under dark adaptation, in response to a flash with a luminance of 1.4 log cd s m−2. (B) The frequency-___domain waveforms of oscillatory potentials (OPs) for three groups: EC (gray), AR (blue), and PS (green). (C, D) Quantitative statistics of the implicit time (C) and amplitude (D) of OPs across three groups. Representative data are presented as mean ± SEM; n = 6 independent biological replicates. Two-way ANOVA tests with Tukey’s multiple comparisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001. All experiments were independently performed at least three times to ensure repeatable results.
The frequency response spectra of the three anesthetic groups exhibit uniformity. Nonetheless, the peak responses of the EC and PS groups are markedly reduced compared to the AR group, suggesting that while EC and PS induce a certain suppression of frequencies, they still maintain the principal spectral components (Fig. 3B). In the within-group analysis of OP implict times, a significant interaction effect was observed (F(10, 90) = 98.35, P < 0.001), along with highly significant effects for both the row factor (F(5, 90) = 34,477, P < 0.001) and the column factor (F(2, 90) = 466.6, P < 0.001). Similarly, in the analysis of OP peak amplitudes, a significant interaction effect was detected (F(10, 90) = 41.79, P < 0.001), with both the row factor (F(5, 90) = 438.3, P < 0.001) and the column factor (F(2, 90) = 142.9, P < 0.001) also showing highly significant results. Our analysis revealed that the Ti values for the PS group (214.00 ± 1.84 ms) exhibited delays relative to the EC and AR groups (186.35 ± 1.98 and 185.42 ± 2.73 ms, respectively) (Fig. 3C), insinuating that PS impose inhibitory effects on OPs production. the OP peak amplitudes of the AR groups (2132.47 ± 116.86 μV) were substantially increased compared to the EC and PS group (907.03 ± 256.25 and 839.71 ± 176.86 μV, respectively) (Fig. 3D).
Analysis of cone function and photopic ERG response to anesthetics
To evaluate the effects of anesthetics on cone function, we performed photopic ERG against a white background illumination of 1.48 log cd s m−2. The PS group exhibited a singular peak profile, in contrast to the EC and AR groups, which each displayed a tri-phasic peak pattern (Fig. 4A–C).
Analysis of photopic ERG b-waves. (A–C) Exemplary photopic ERG b-waves were recorded from EC (A), AR (B), and PS (C) mice, with the light intensities progressively increasing from − 0.6 to 1.4 log cd s m−2 against a background illumination of 1.48 log cd s m−2. (D–F) Curves depicting the fitted b-wave responses for EC (D), AR (E), and PS (F). (G–I) Quantitative evaluation of b-wave parameters, such as R (G), N (H), and K (I), derived through the application of the Naka-Rushton equation. Representative data are presented as mean ± SEM; n = 6 independent biological replicates. Two-way ANOVA tests with Tukey’s multiple com-parisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001. All experiments were independently performed at least three times to ensure repeatable results.
To more comprehensively analyze the differences in b-wave of photopic ERG among different groups, we fitted the b-wave using the Naka-Rushton equation and analyzed the related parameters (Fig. 4D–F). The analytical fit utilizing the Naka-Rushton equation showed that Rmax (F (2, 15) = 7.759, P = 0.0049), K (F (2, 15) = 29.92, P < 0.001), and N (F (2, 15) = 5.717, P = 0.0143) differed substantially among the groups (Fig. 4G–I). Specifically, the Rmax for the b-wave during the AR group’s photopic ERG (2.977 ± 0.05866 log μV) exceeded those recorded for the EC (2.678 ± 0.08031 log μV) and PS (2.654 ± 0.05187 log μV) groups (Fig. 4G). Additionally, the AR group exhibited a notably lower N value (5.664 ± 0.2764) when contrasted with the PS group (8.978 ± 0.6912) (Fig. 4H). Relative to the EC and AR groups (4.844 ± 0.03474 and 4.756 ± 0.03853, respectively), the PS group presented the lowest K value in photopic ERG (4.455 ± 0.03853) (Fig. 4I).
Impact of anesthetic variation on mouse PERG through analysis of amplitude and latency
To evaluate the impact of anesthetics on ganglion cell signaling, we performed pattern electroretinograms (PERG) (Fig. 5A), which revealed distinct differences among the mice in various groups (Fig. 5B).
Characterization of PERG under varied anesthetic conditions in mice. (A) Schematic of the PERG experiment. (B) The waveforms of PERG results in mice under the influence of three different anesthetics: EC, AR, and PS, are illustrated. (C–D) The implicit times (C) and amplitude (D) for N1, P1, and N2 waves among the groups are quantified. (E–M) Statistical analysis of parameters following frequency-___domain analysis, including magnitude (E–G), phase (H–J), and power spectral density (PSD) (K–M). Compared to the other two groups, the PS group mice (G, J, M) demonstrated insensitivity to graphic stimuli when compared with the EC (E, H, K) and AR (F, I, L) groups. Representative data are presented as mean ± SEM; n = 6 independent biological replicates. Two-way ANOVA tests with Tukey’s multiple com-parisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001. All experiments were independently performed at least three times to ensure repeatable results.
The P1 and N2 waves exhibited significant intragroup variability in both implicit time and amplitude characteristics. For the P1 component, the analysis revealed F(2,15) = 40.58 with P < 0.001 for implicit time and F(2,15) = 45.98 with P < 0.001 for amplitude. Similarly, the N2 component showed F(2,15) = 5.708 with P = 0.01 for implicit time and F(2,15) = 230.1 with P < 0.001 for amplitude. In contrast, the N1 wave showed no significant intergroup differences in either implicit time (F(2,15) = 1.109, P = 0.36) or amplitude (F(2,15) = 2.658, P = 0.10). The N1 waves of the three groups of mice showed almost no differences, except that the implicit time of the EC group (20.61 ± 4.331 ms) was slightly higher than that of the AR group (13.13 ± 2.949 ms) (Fig. 5C).
Regarding the latency and amplitude of the P1 peak, the AR group exhibited the shortest implicit time (114.00 ± 3.824 ms) and the highest amplitude (17.28 ± 0.7070 μV), followed by the EC group with a latency of 110.2 ± 1.821 ms and an amplitude of 14.05 ± 0.9080 μV. The PS group demonstrated the longest latency (140.3 ± 1.397 ms) and the lowest amplitude (7.406 ± 0.5744 μV) (Fig. 5C, D).
The distinction among the three groups was most pronounced at the N2 peak, where the EC group’s performance was notably superior to that of the other two. The EC group exhibited the most robust N2 peak amplitude (23.44 ± 0.2596 μV) coupled with the shortest latency (229.7 ± 4.456 ms), a substantial enhancement compared to the AR group’s amplitude (23.34 ± 0.4336 μV) and latency (236.4 ± 3.264 ms), and the PS group’s amplitude (7.333 ± 0.4959 μV) and latency (245.1 ± 0.9631 ms) (Fig. 5C, D).
We conducted a frequency ___domain analysis of the results from three anesthetic groups (Fig. 5E–M). Although the phase results for all groups remained between − 4 and 4 rad, the magnitude (Fig. 5E–G) and Power Spectral Density (PSD) (Fig. 5K–M) for the PS group were substantially lower than those of the other two groups. This phenomenon corresponds with the time-___domain analysis results of the three groups’ PERG. In conclusion, the use of PS substantially prolonged the latency across all waves while also diminishing their amplitudes, suggesting a negative impact on the signal transmission process of ganglion cells.
Assessing the impact of anesthetics on visual signal transmission via FVEP
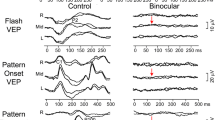
FERG and PERG are both visual electrophysiological experiments used to measure retinal signals. We conducted additional FVEP testing to evaluate the electrophysiological responses of the visual cortex to simple flash stimuli. The typical FVEP waveform consists of two positive peaks, P1 and P2, and one negative peak, N1, all of which were observed in the FVEP results across three groups (Fig. 6A).
The effects of different anesthetics on FVEP differ between anesthetics. (A) Representative FVEP waveforms from distinct groups: EC (bottom), AR (middle), and PS (top), elicited under a luminance of − 0.3 log cd s m−2. (B, C) The density plots show the density distribution of N1 (B) and P2 (C) amplitudes across various groups in their FVEP responses. (D–F) Latencies of P1 (D), N1 (E), and P2 (F) in different groups. (G–I) Peak amplitudes for P1 (G), N1 (H), and P2 (I) in different groups. Representative data are presented as mean ± SEM; n = 6 independent biological replicates. Two-way ANOVA tests with Tukey’s multiple com-parisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001. All experiments were independently performed at least three times to ensure repeatable results.
The temporal analysis indicates that the distribution of P1 implicit time shows no significant intergroup differences (F(2,15) = 0.8872, P = 0.43). In contrast, both the implicit time of N1 and P2 exhibit significant intergroup variations, with F(2,15) = 14.25, P < 0.001 for N1 and F(2,15) = 138.1, P < 0.001 for P2. Regarding amplitude analysis, the P1, N1, and P2 waves all demonstrate significant intergroup differences. Specifically, the P1 wave exhibits highly significant differences in amplitude (F(2,15) = 44.84, P < 0.001), while the N1 and P2 wave differences, though slightly less pronounced, remain statistically significant (N1: F(2,15) = 6.318, P = 0.01; P2: F(2,15) = 6.107, P = 0.01). These findings collectively suggest substantial intergroup variability in the amplitudes.
Compared to the other two groups, the AR group exhibited a more concentrated distribution of N1 and P2 peak amplitudes within a narrower range, indicative of stability in the results (Fig. 6B, C). There was no substantial difference in the latency of the P1 among the three FVEP groups (Fig. 6D), while the latency of the N1 characteristic peak in the PS group (36.22 ± 0.1776 ms) was higher than that of the EC group (34.31 ± 0.9270 ms) and the AR group (34.14 ± 0.8919 ms) (Fig. 6E).However, the peak of the PS group’s P2 was mostly concentrated around 60 ms and 75 ms, which differs from the distribution of the peak times for the P2 in the EC and AR groups (Fig. 6F).
The FVEP outcomes for the EC group were inferior compared to those of the other two groups, despite the P1 peak amplitude of the PS group (17.33 ± 1.926 μV) being lower than those recorded for the AR and EC group (60.48 ± 4.515 μV and 35.17 ± 2.710 μV respectively) (Fig. 6G). Specifically, the EC group exhibited substantially lower mean amplitudes for both N1 (274.6 ± 15.01 μV) and P2 (341.6 ± 17.94 μV) compared to the AR group, which demonstrated higher amplitudes of 340.1 ± 8.701 μV for N1 and 430.6 ± 15.28 μV for P2, and to the PS group, which had a mean N1 amplitude of 288.1 ± 16.36 μV and a P2 amplitude of 389.1 ± 20.45 μV (Fig. 6H, I).
Assessing the impact of anesthetics on visual signal transmission via PVEP
To further investigate the effects of anesthetics on the patterned visual signal pathway, we subsequently conducted PVEP assessments. This evaluation focused on analyzing the electrophysiological responses of the primary visual cortex to patterned stimuli, aiming to provide a more comprehensive analysis of the effects at the central nervous system level. Substantially, PVEP waveforms were exclusive to the AR group (Fig. 7A). In the PS group, no distinct waveform was observed, which may be related to the diminished signal conduction of RGCs. Conversely, the lack of substantial waveforms in the EC group stood in stark contrast to their definitive PERG outcomes. This suggests potential EC-mediated disruption in the signal transduction from RGCs to the visual cortex.
Evaluation of PVEP Outcomes. (A) Schematic representation of PVEP waveforms in EC (left), AR (center), and PS (right) mice. (B–C) Statistical analysis of the N1 (B) and P2 amplitudes (C) in the PVEP of AR mice. (D–I) Frequency ___domain analysis of the PVEP results for the AR group, including magnitude (D, E), phase (F, G), and power spectral density (PSD) (H, I). Frequency ___domain analysis results are statistically analyzed for recordings at a depth of 800 μm (D, F, H) and across all depths (E, G, I). Representative data are presented as mean ± SEM; n = 6 independent biological replicates. Two-way ANOVA tests with Tukey’s multiple com-parisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001. All experiments were independently performed at least three times to ensure repeatable results.
At a depth of 800 µm, PVEP N1 and P2 waves in the AR group reached their zenith, with amplitudes of 68.65 ± 4.89 µV and 69.57 ± 4.78 µV, respectively ((Fig. 7B, C). Fourier Transform analysis of the PVEP signals in AR mice elucidated the relationship between magnitude, phase, and PSD across frequencies (Fig. 7D–I). Similar to its PERG frequency ___domain analysis results, the AR group’s PVEP magnitude (Fig. 7D, E) and PSD (Fig. 7H, I) primarily respond within the 0–10 Hz range across virtually all depths. Similarly, the AR group’s phase is also concentrated between − 4 to 4 radians (Fig. 7F, G).
In summary, Fourier Transform analysis has unveiled primary PVEP signal frequencies at 0–10 Hz with notable amplitude and power, and a complex phase response.
Discussion
This study conducts a comprehensive comparison of the efficacy of three anesthetics—AR, EC, and PS—in recording electrophysiological signals from the retina and primary visual cortex in mice. In this research, 2–2.5 m pigmented C57BL/6J mice were selected as the experimental subjects because retinal development and light response vary greatly between pigmented and non-pigmented laboratory animals47,48. Compared to C57BL/6J mice, non-pigmented C57BL/6J-c2J mice exhibit substantial retinal degeneration. During development and early adulthood, these mice have abnormally high photoreceptor death rates, leading to substantially reduced ERG amplitudes, sometimes even less than half of those observed in C57BL/6J mice49. Additionally, albino mice show alterations in phototransduction gain and slower recovery after photobleaching50,51. Based on this, we selected C57BL/6J mice aged 2–2.5 months, as their retinas are fully developed at this stage52, and no substantial sex differences have been found in their visual electrophysiological signals53,54. Therefore, the results for male and female mice were not analyzed separately in this study. However, this method may not be applicable to older animals. As age increases, the protective effect of estrogen on vision becomes more evident, leading to less pronounced visual decline in older female animals compared to males55,56.
The research conducted a comprehensive comparative analysis of 35 parameters across four types of visual electrophysiological experiments (FERG, PERG, PVEP, and FVEP). The results showed no substantial differences among the three groups for five of these parameters (Table S1). However, in the remaining 30 parameters, the AR group outperformed the other two groups in 25 of them. Particularly in the PVEP-related parameters, only the AR-anesthetized mice exhibited typical responses, whereas no such responses were recorded in the EC and PS groups.
In addition to the AR group’s strong performance in 25 parameters and the five parameters showing no substantial difference among the three groups, the EC and PS group displayed slight advantages in the remaining five parameters. However, these advantages were not substantial enough to impact the overall conclusions of this study. For instance, the differences between the PS and AR groups did not result in qualitative changes. In the scotopic ERG b-wave Rmax, the PS group had the best performance (3.351), closely followed by the AR group (3.176), with a difference of only 0.175. This small variation was insufficient to alter the overall assessment. Similar trends were observed in other parameters, such as the N value of the scotopic ERG b-wave and the K value of the photopic ERG b-wave.
Previous studies have elucidated the substantial impact of anesthetics on systemic effects, highlighting the potential adverse effects of certain anesthetics57,58,59,60. Among the three anesthetics, avertin exhibited physiological effects most similar to those of ketamine/xylazine (KX), which has long been considered the gold standard for mouse experiments. Avertin had a milder impact on heart rate compared to ketamine/xylazine and sodium pentobarbital in experimental animals61,62,63. Although animals under EC anesthesia displayed physiological characteristics remarkably similar to the sleep state64, rats anesthetized with ethyl carbamate showed abnormal cardio-pulmonary patterns, including a marked decrease in heart rate during inspiration. Additionally, only mice anesthetized with pentobarbital returned to normal glucose levels within 60 min post-injection, while blood glucose remained elevated under the influence of the other anesthetics. The most pronounced increase was induced by ketamine/xylazine, followed by avertin, with EC causing a relatively lower rise65,66.
When comparing the effects of the three anesthetics on visual electrophysiological outcomes, AR’s performance was also the closest to KX. Based on previous studies67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106, we compiled amplitude for various waves from four visual electrophysiology experiments conducted using KX (Table S2). It is important to note that while some wave peaks, such as the scotopic ERG b-wave and PERG, were slightly lower in the KX group compared to the AR group, this may be attributed to differences in experimental conditions, electrode materials, and recording positions. Therefore, evaluating the efficacy of anesthetics solely through amplitude comparisons across studies is not comprehensive. Notably, unlike the EC and PS groups, mice anesthetized with KX did not exhibit any absence of typical responses in visual electrophysiological tests. Thus, compared to EC and PS, AR appears to be the most suitable alternative to KX as an anesthetic.
One of the particularly interesting phenomena observed in this study is the substantially reduced OPs amplitude in the scotopic ERG experiments for both the EC and PS groups. While the precise origin of OPs remains elusive, intravitreal injection of gamma-aminobutyric acid (GABA) specifically abolishes late OP components without substantially affecting early ones, a pattern closely mirroring the changes observed under PS and EC treatment107. PS affects the central nervous system through a series of complex mechanisms, including enhancing gamma-aminobutyric acid type A receptor function, inhibiting glutamate receptors, reducing voltage-gated sodium channel activity, activating potassium channels, and inhibiting acetylcholine receptors108,109,110,111,112. These combined actions contribute to its anesthetic effect. Studies on isolated rat retinas have shown that PS substantially reduces the activity of depolarizing bipolar cells without affecting photoreceptors. Moreover, secondary components of the ERG, including the scotopic threshold response and OPs, are strongly suppressed by PS109. These findings are consistent with our observations in PS-anesthetized mice, where the OPs in FERG were substantially suppressed and PERG, a key indicator of ganglion cell activity, was notably reduced. These results further confirm the profound impact of sodium pentobarbital on the electrical signals of specific retinal cells.
EC enhances the function of nicotinic acetylcholine, gamma-aminobutyric acid, and glycine receptors, while inhibiting N-methyl-D-aspartate receptor and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor receptors in a concentration-dependent manner113,114,115. Due to EC’s complex mechanism of action, accurately recording genuine electrical signals in mice under this anesthesia becomes exceptionally challenging. This difficulty arises from the extensive influence of the anesthetic on the mice’s nervous system, potentially leading to signal distortion or alteration, which makes it difficult to accurately reflect the true physiological state of the mice’s visual system.
AR indirectly activates gamma-aminobutyric acid type A and glycine receptors by modulating their agonists116. Compared to the direct mechanisms of EC and PS, this indirect regulation substantially reduces the potential impact on visual electrophysiological signal transmission. This likely explains why the FERG and PERG results in the AR group of mice were superior to those in the other two groups.
Another noteworthy observation is that, while FERG and PERG showed typical responses, the characteristic peaks of PVEP were absent in both the EC and PS groups, whereas FVEP performance remained normal. This may be related to the differences in the pathways involved in the processing of visual signals between pattern stimuli and flash stimuli. Pattern stimuli involve the cooperation of multiple brain regions, with the layers of the V1 region (L1 to L6) each playing distinct roles in this process, whereas the brain regions activated by flash stimuli are primarily concentrated in the V1 area. Specifically, pattern-reversal stimuli uniquely activate the cortex surrounding the intraparietal sulcus and the ventral occipital cortex, regions crucial for the analysis and integration of complex visual information 117,118. The intraparietal sulcus cortex is part of the dorsal visual pathway, responsible for processing spatial ___location, motion, and visually guided behaviors, with L5 of the V1 region interacting closely with this area. The ventral occipital cortex belongs to the ventral visual pathway, which handles object recognition, color, and shape analysis, with L2/3 and L6 of the V1 region interacting primarily with this area119. The PVEP results from the AR group, with peak values first increasing and then decreasing across depths, reflect this layer-specific functionality.
However, in the EC and PS groups, characteristic peaks were absent across all V1 depths, which may be related to the regulation of the brain’s GABAergic inhibitory system by PS and EC. This system plays a critical role in limiting the spread of layer-specific excitatory activity and maintaining topological projections120,121. When this system is disrupted, functional projections between layers may be affected, leading to weakened or absent VEP signals. Additionally, EC may suppress AMPA and NMDA receptors, thereby disrupting inter-layer functional projections and reducing neuronal population activity122. AR, on the other hand, indirectly activates GABA-A and glycine receptors by modulating their agonists116. This indirect regulation, as opposed to the direct mechanisms of EC and PS, likely explains why PVEP results in the AR group were unaffected.
Resting-state functional magnetic resonance imaging (rs-fMRI) further supports this conclusion, with AR and ketamine/xylazine showing favorable seed-based, global, and interhemispheric connectivity results, comparable to those of awake animals123. The connectivity results for ethyl carbamate were moderate117,118, and this suppression of brain activity was similarly observed in PS-anesthetized mice124.
The absence of characteristic peaks across all depths in the EC and PS groups suggests that these anesthetics may weaken VEP signals by disrupting the complex network connections between brain regions. Notably, the widespread nature of this effect—manifested by the lack of characteristic peaks across different depths—further strengthens the idea that EC and PS exert a nonspecific inhibitory effect on not only the visual system but also broader brain function.
Retinal dysfunction can extend to brain regions involved in visual information processing31,125,126,127,128,129, highlighting the importance of studying the visual system as an integrated whole. With advances in technology and deeper exploration, research in visual science has gradually expanded from focusing solely on the retina to encompassing the entire visual pathway, especially the brain’s visual processing areas. This multi-level research approach helps to uncover the complete mechanisms of visual information processing. However, the improper selection of anesthetics may suppress the function of brain regions responsible for visual processing, potentially leading to abnormal experimental outcomes. Therefore, the choice of anesthetics is particularly critical when conducting visual electrophysiological studies.
This study comprehensively evaluated the effects of three anesthetics on the visual signal pathway from the retina to the brain’s visual cortex under identical conditions. The responses to different stimulation patterns, including light and pattern stimuli, were analyzed in detail. The results indicate that EC and PS suppress visual signal transmission, particularly in the brain’s visual processing centers. AR proved to be the most suitable anesthetic among the three for studying visual electrophysiological signals, displaying distinct electrophysiological characteristics and yielding superior experimental results.
Materials and methods
Animals
In this study, 54 adult C57BL/6J mice, aged 2–2.5 months and weighing 20–26 g, were obtained from the Shanghai Model Organisms Center, Inc. The mice were housed under optimal laboratory conditions, with free access to water and timely provision of feed, in an environment maintained at a temperature range of 20–24 °C and relative humidity between 40 and 60%. A strict 12-h light/dark cycle was implemented to simulate natural conditions. Subjects were randomized into three study groups, in alignment with the 3R principles (Reduction, Replacement, and Refinement) that guide ethical animal research practices. The 54 mice were randomly divided into three experimental groups, each containing 18 mice. In each group, six mice were anesthetized using EC, AR, and PS, respectively, with no repeat anesthesia performed on the same mice. The study design for each group is as follows:
-
(1)
Group 1: Focused on a comprehensive assessment of retinal function, including scotopic and photopic ERG, as well as OPs detection. The sequence of experiments was scotopic ERG first, followed by photopic ERG, with OPs data collected synchronously during the scotopic ERG experiment. The data presented in Table 1 were derived from this group. The instrumentation employed for the experiments on mice in this group was the RetiMINER-C visual electrophysiology system (AiErXi Medical Equipment Co., Ltd., Chongqing, China);
-
(2)
Group 2: Examined the primary visual cortex’s response to simple flash stimuli, using FVEP detection. The equipment utilized for this group was the Celeris System (Diagnosys, Lowell, MA, USA);
-
(3)
Group 3: Focused on the retinal and visual cortex responses to patterned stimuli, with PERG and PVEP recorded simultaneously. The instrument used for this group’s investigations was also the Celeris System (Diagnosys, Lowell, MA, USA).
The experimental order followed a progression from light stimuli to patterned stimuli, with Group 1 conducted first, followed by Group 2, and then Group 3. Within each group, the anesthesia order followed the pattern: "EC, AR, PS, AR, PS, EC, PS, EC, AR." Specifically, the first mouse in each group was anesthetized with EC and the experiment was conducted; after completing the experiment, the second mouse in the group was anesthetized with AR for the next experiment; then, the third mouse was anesthetized with PS to complete its experiment, and so on, following the pre-determined order for anesthetizing and testing each mouse in the group. This approach maximized the simulation of randomization to minimize potential bias. Each recording session rigorously maintains the animal’s core body temperature between 37 °C and 38 °C. Ethical approval for the research protocol was granted by the Ethics Committee of Wuhan University of Science and Technology (wust202013). Throughout the study, all pertinent institutional and governmental standards concerning the ethical utilization of animals were rigorously observed. All experiments were conducted in accordance with the ARRIVE guidelines for the use of animals in ophthalmic and vision research.
Flash electroretinogram
Prior to electrophysiological recordings, mice were dark-adapted overnight and subsequently handled under subdued red illumination.We anesthetized the mice using intraperitoneal injections of ethyl carbamate (EC, 0.2 g/ml, Sigma-Aldrich, #U2500), avertin (AR, also known as 2,2,2-tribromoethanol, 0.02 g/ml, Sigma-Aldrich, #T48402), and pentobarbital sodium (PS, 0.01 mg/ml, Sigma-Aldrich, #P3761). The doses were 1.6 g/kg, 0.3 g/kg, and 0.115 g/kg, respectively. The total injection volume for each mouse ranged from 0.18 to 0.39 μL. Following the induction of anesthesia, the state of anesthesia was preliminarily assessed via a toe pinch reflex test. Needle electrodes were then strategically placed on the skin between the tail and both ears to capture and monitor the electrical signals (Fig. S1). In the formal experiment, the placement of the ground and reference electrodes will effectively shield the EEG and ECG signals, ensuring they do not interfere with the recorded visual electrophysiological signals. This electrophysiological feedback was utilized to confirm the attainment of a deep anesthetic state. Unless otherwise specified, all subsequent experiments will adhere to the standardized anesthesia confirmation protocol established in this study.
Upon verification of adequate anesthesia, mydriasis was induced using an ophthalmic solution containing tropicamide, phenylephrine hydrochloride, and atropine sulfate monohydrate. Hydroxypropyl methylcellulose was used to ensure that the mouse cornea remained hydrated throughout the experiment. A single application of hydroxypropyl methylcellulose can maintain corneal moisture for approximately 40 min. Since the experiment lasts about 60 min, hydroxypropyl methylcellulose was reapplied around the 35-min mark to ensure continuous corneal hydration. After application, the mice were given a 3-min adaptation period to allow sufficient absorption of the moisturizer by the eyes and to regain stability before the experiment continued. The flash electroretinogram was conducted by applying a silver wire loop electrode directly onto the corneal surface, a reference electrode on the forehead, and a ground clip on the tail. The impedance of the recording electrod was maintained within the range of 1.5 and 2 kΩ. Scotopic electroretinogram were elicited using a spectrum of white light flashes from − 3.6 to 2.15 log cd s/m2, with a flash duration of 1 ms and an average of three flashes. The interstimulus interval was adjusted based on light intensity, exceeding 10 s for lower intensities and 30 s for higher ones. A- and b-wave amplitudes were quantified from baseline to trough and trough to peak, respectively.
Following the scotopic electroretinogram trials, the mice underwent a 10-min adaptation under a 30 cd/m2 white light. Subsequently, their retinal responses to escalating light stimuli (− 0.6–1.4 log cd s/m2) were assessed under the same background illumination. The stimuli were applied at 5-s intervals, with each response quantified as the mean of three responses.
The photic stimuli were delivered using a bespoke LED apparatus, designed to uniformly project a homogenously distributed field of light onto the retina, with the white LED wavelength calibrated at 505 nm. The resultant bioelectrical signals were subjected to amplification through a precision direct current amplifier (DC Amplifier, GIMAmp, Chongqing IRC Medical Equipment Co., Ltd), followed by band-pass filtration within the frequency range of 0.3–300 Hz. The data were digitized at a sampling rate of 2000 Hz and processed using the RetiMINER-C visual electrophysiology system (AiErXi Medical Equipment Co., Ltd., Chongqing, China). Prior to each experimental session, calibration of the illumination intensity and amplifier adjustments were meticulously conducted using a photometric power meter (AQ2170, YOKOGAWA) and an electronic signal generator (FNIR-SISG-004A, FNIRSI), respectively.
Pattern electroretinogram and pattern visual evoked potential
Both PERG and PVEP experiments utilize the Celeris System (Diagnosys, Lowell, MA, USA) for consistent stimulation: a black and white horizontal striped stimulus with 2 Hz phase reversals, a spatial frequency of 0.06 cycles/degree, 100% contrast, and an intensity of 50 cd/m2. Each result is averaged over 200 repetitions. Following anesthesia, mydriasis in mice is induced with an ophthalmic solution of tropicamide, phenylephrine hydrochloride, and atropine sulfate monohydrate, while hydroxypropyl methylcellulose maintains corneal hydration. In PERG experiments, electrodes for recording, reference, and grounding are placed subcutaneously at the cornea, nasal bone, and tail, respectively. For PVEP, the recording electrode is placed in the occipital region, with reference and grounding electrodes matching the PERG setup.The impedance of the recording electrod was maintained within the range of 1.5 and 2 kΩ.
Flash visual evoked potential
The flash visual evoked potential (FVEP) recordings were conducted using the Celeris System (Diagnosys, Lowell, MA, USA), adhering strictly to the standardized procedures outlined in previous research130. Following the induction of anesthesia and confirmation of a deep anesthetic state, the cranial fur was meticulously shaved, and the skin was precisely incised and retracted to expose the lambdoidal suture. A microdrill with a 0.5 mm diameter was then used to carefully perforate the skull at the exact intersection of the sagittal and lambdoidal sutures.
The recording electrodes were inserted into the cerebral cortex at an optimized angle of 30–45 degrees through a hole until the impedance stabilized between 1.5 and 2 kΩ. At this point, the vertical depth (i.e., Z-axis depth) that the recording electrode penetrated into the cortex was approximately 1.5–1.6 mm. Ground and reference electrodes were positioned subcutaneously at the tail and snout, respectively. Throughout data acquisition, the impedance of the ground, reference, and recording electrodes was maintained within the 1.5–2 kΩ range to ensure stable and reliable signal quality.
A standardized flash intensity of 0.05 cd s/m2 was applied uniformly across all experimental sessions, with each eye independently exposed to 100 white light flashes at a frequency of 1 Hz via corneal stimulators. The elicited responses were efficiently captured within a 300 ms window, with a sampling rate of 2000 Hz to detect subtle potential changes. During signal processing, carefully selected filtering parameters were employed, including a low-frequency cutoff of 1.25 Hz and a high-frequency cutoff of 100 Hz, effectively eliminating noise and enhancing the signal-to-noise ratio and resolution.
Curve fitting
To more precisely and quantitatively characterize the electrophysiological differences, the electroretinogram (ERG) data were analyzed using the Lamb and Pugh model (1)131 and the Naka-Rushton Eq. (2)46. In the Lamb and Pugh model, the parameters R, I, S, T, and Td denote ERG amplitude, stimulus retinal illumination, response sensitivity, time since stimulus onset, and a minor delay before response onset, respectively; Rmax is the saturating amplitude of ERG, reflecting the maximal response capacity of retinal photoreceptors and indicating their light sensitivity. In the Naka-Rushton equation, K represents the half-saturation constant, indicating the light intensity required to elicit a half-maximal response, while N dictates the slope of the function, implying the retina’s dynamic range of response to varying light intensities132,133.
Statistical analyses
In MATLAB, perform precise fitting of scotopic and photopic ERG experimental data to extract key parameters of the Lamb-Pugh model and the Naka-Rushton equation, such as the maximum response (Rmax), time delay (Td), and the half-saturation constant (S). Data were presented as mean ± standard error of the mean (SEM), processed using GraphPad Prism and Origin software. Variance homogeneity was verified via an F-test to determine the independence of group datasets (DFn = 2, DFd = 15). An F-value exceeding 3.682 was interpreted as statistically significant, warranting subsequent t-test comparisons. Differences among groups were considered statistically significant at *p < 0.05, **p < 0.01, ***p < 0.001, as determined by two-way ANOVA tests with Tukey’s multiple comparisons.
Data availability
Authors declare that all relevant data supporting the findings of this study are available in this paper. Access to our raw data can be obtained from the corresponding authors upon reasonable request.
References
Baden, T., Euler, T. & Berens, P. Understanding the retinal basis of vision across species. Nat. Rev. Neurosci. 21, 5–20. https://doi.org/10.1038/s41583-019-0242-1 (2020).
Turner, P. L. & Mainster, M. A. Circadian photoreception: Ageing and the eye’s important role in systemic health. Br. J. Ophthalmol. 92, 1439–1444. https://doi.org/10.1136/bjo.2008.141747 (2008).
Diamond, J. S. Inhibitory interneurons in the retina: Types, circuitry, and function. Annu. Rev. Vis. Sci. 3, 1–24. https://doi.org/10.1146/annurev-vision-102016-061345 (2017).
Ernst, M. O. & Bulthoff, H. H. Merging the senses into a robust percept. Trends Cogn. Sci. 8, 162–169. https://doi.org/10.1016/j.tics.2004.02.002 (2004).
Zhang, W. H. Decentralized neural circuits of multisensory information integration in the brain. Adv. Exp. Med. Biol. 1437, 1–21. https://doi.org/10.1007/978-981-99-7611-9_1 (2024).
Xu, F. et al. Breaking genetic shackles: The advance of base editing in genetic disorder treatment. Front Pharmacol. 15, 1364135. https://doi.org/10.3389/fphar.2024.1364135 (2024).
Dalkara, D., Goureau, O., Marazova, K. & Sahel, J. A. Let there be light: Gene and cell therapy for blindness. Hum. Gene Ther. 27, 134–147. https://doi.org/10.1089/hum.2015.147 (2016).
Chen, X., Xu, N., Li, J., Zhao, M. & Huang, L. Stem cell therapy for inherited retinal diseases: A systematic review and meta-analysis. Stem Cell Res. Ther 14, 286. https://doi.org/10.1186/s13287-023-03526-x (2023).
Liang, Y., Sun, X., Duan, C., Tang, S. & Chen, J. Application of patient-derived induced pluripotent stem cells and organoids in inherited retinal diseases. Stem Cell Res. Ther. 14, 340. https://doi.org/10.1186/s13287-023-03564-5 (2023).
Qin, H. et al. Vision rescue via unconstrained in vivo prime editing in degenerating neural retinas. J. Exp. Med. https://doi.org/10.1084/jem.20220776 (2023).
Mobasserian, A. et al. Effect of pupil size on fixed-luminance flicker full-field electroretinogram magnitude. Clin. Ophthalmol. 16, 3733–3740. https://doi.org/10.2147/OPTH.S382207 (2022).
Jeon, S. J., Jung, K. I., Park, C. K. & Park, H. L. Macular blood flow and pattern electroretinogram in normal tension glaucoma. J. Clin. Med. https://doi.org/10.3390/jcm11071790 (2022).
Pojda-Wilczek, D., Maruszczyk, W. & Sirek, S. Flash visual evoked potentials (FVEP) in various stimulation conditions. Doc. Ophthalmol. 138, 35–42. https://doi.org/10.1007/s10633-018-9663-9 (2019).
Gotch, F. The time relations of the photo-electric changes in the eyeball of the frog. J. Physiol. 29, 388–410. https://doi.org/10.1113/jphysiol.1903.sp000965 (1903).
Granit, R. The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J. Physiol. 77, 207–239. https://doi.org/10.1113/jphysiol.1933.sp002964 (1933).
Popova, E., Kostov, M. & Kupenova, P. Effects of dopamine D(1) receptor blockade on the ERG b- and d-waves during blockade of ionotropic GABA receptors. Eye Vis. (Lond.) 3, 32. https://doi.org/10.1186/s40662-016-0064-4 (2016).
Liao, F., Liu, H., Milla-Navarro, S., Villa, P. & Germain, F. Origin of retinal oscillatory potentials in the mouse, a tool to specifically locate retinal damage. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24043126 (2023).
Cima, G. AVMA guidelines for the Euthanasia of animal: 2013 Edition. Javma-J. Am. Vet. Med. A 242, 715–716 (2013).
Flecknell, P. A. Laboratory animal anaesthesia and analgesia. Fifth edition. edn, (Academic Press, an imprint of Elsevier, 1996).
Merdink, J. L. et al. Kinetics of chloral hydrate and its metabolites in male human volunteers. Toxicology 245, 130–140. https://doi.org/10.1016/j.tox.2007.12.018 (2008).
Wandalsen, G. F., Lanza, F. C., Nogueira, M. C. & Sole, D. Efficacy and safety of chloral hydrate sedation in infants for pulmonary function tests. Rev. Paul Pediatr. 34, 408–411. https://doi.org/10.1016/j.rpped.2016.05.002 (2016).
Grissinger, M. Chloral hydrate: Is it still being used? Are there safer alternatives?. P T 44, 444–459 (2019).
Wu, Y. et al. AAV-mediated base-editing therapy ameliorates the disease phenotypes in a mouse model of retinitis pigmentosa. Nat. Commun. 14, 4923. https://doi.org/10.1038/s41467-023-40655-6 (2023).
Arsenijevic, Y. et al. Fine-tuning FAM161A gene augmentation therapy to restore retinal function. EMBO Mol. Med. 16, 805–822. https://doi.org/10.1038/s44321-024-00053-x (2024).
Meng, X. et al. In vivo genome editing via CRISPR/Cas9-mediated homology-independent targeted integration for Bietti crystalline corneoretinal dystrophy treatment. Nat. Commun. 15, 3773. https://doi.org/10.1038/s41467-024-48092-9 (2024).
Suh, S. et al. Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing. Nat. Biomed. Eng. 5, 169–178. https://doi.org/10.1038/s41551-020-00632-6 (2021).
Mariotti, L. et al. Interneuron-specific signaling evokes distinctive somatostatin-mediated responses in adult cortical astrocytes. Nat. Commun. 9, 82. https://doi.org/10.1038/s41467-017-02642-6 (2018).
Pinol, R. A. et al. Brs3 neurons in the mouse dorsomedial hypothalamus regulate body temperature, energy expenditure, and heart rate, but not food intake. Nat. Neurosci. 21, 1530–1540. https://doi.org/10.1038/s41593-018-0249-3 (2018).
Villette, V. et al. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice. Cell 179, 1590–1608. https://doi.org/10.1016/j.cell.2019.11.004 (2019).
Zhao, Q. et al. A multidimensional coding architecture of the vagal interoceptive system. Nature 603, 878–884. https://doi.org/10.1038/s41586-022-04515-5 (2022).
Liu, Y. et al. Whole brain functional connectivity in the early blind. Brain 130, 2085–2096. https://doi.org/10.1093/brain/awm121 (2007).
Zhao, Y. et al. SoNar, a highly responsive NAD+/NADH sensor, allows high-throughput metabolic screening of anti-tumor agents. Cell Metab. 21, 777–789. https://doi.org/10.1016/j.cmet.2015.04.009 (2015).
He, K., Jia, S., Lou, Y., Liu, P. & Xu, L. X. Cryo-thermal therapy induces macrophage polarization for durable anti-tumor immunity. Cell Death Dis. 10, 216. https://doi.org/10.1038/s41419-019-1459-7 (2019).
Larpthaveesarp, A. et al. Enhanced mesenchymal stromal cells or erythropoietin provide long-term functional benefit after neonatal stroke. Stroke 52, 284–293. https://doi.org/10.1161/STROKEAHA.120.031191 (2021).
Ma, C. et al. A novel inactivated whole-cell Pseudomonas aeruginosa vaccine that acts through the cGAS-STING pathway. Signal Transduct. Target Ther. 6, 353. https://doi.org/10.1038/s41392-021-00752-8 (2021).
Hu, J. et al. Melanopsin retinal ganglion cells mediate light-promoted brain development. Cell 185, 3124–3137. https://doi.org/10.1016/j.cell.2022.07.009 (2022).
Fujita, R., Ueda, M., Fujiwara, K. & Ueda, H. Prothymosin-alpha plays a defensive role in retinal ischemia through necrosis and apoptosis inhibition. Cell Death Differ. 16, 349–358. https://doi.org/10.1038/cdd.2008.159 (2009).
Liu, X. et al. Increased light exposure alleviates one form of photoreceptor degeneration marked by elevated calcium in the dark. PLoS One 4, e8438. https://doi.org/10.1371/journal.pone.0008438 (2009).
Bai, X. et al. Neuroprotection of SRT2104 in murine ischemia/reperfusion injury through the enhancement of Sirt1-mediated deacetylation. Invest Ophthalmol. Vis. Sci. 64, 31. https://doi.org/10.1167/iovs.64.4.31 (2023).
England, R. N., Preston, K. J., Scalia, R. & Autieri, M. V. Interleukin-19 decreases leukocyte-endothelial cell interactions by reduction in endothelial cell adhesion molecule mRNA stability. Am. J. Physiol. Cell Physiol. 305, C255-265. https://doi.org/10.1152/ajpcell.00069.2013 (2013).
Yamakawa, G. R. et al. The cholinergic forebrain arousal system acts directly on the circadian pacemaker. Proc. Natl. Acad. Sci. U. S. A. 113, 13498–13503. https://doi.org/10.1073/pnas.1610342113 (2016).
Yang, Y. et al. Bacteria eat nanoprobes for aggregation-enhanced imaging and killing diverse microorganisms. Nat. Commun. 13, 1255. https://doi.org/10.1038/s41467-022-28920-6 (2022).
Zhang, K., Yao, G., Gao, Y., Hofeldt, K. J. & Lei, B. Frequency spectrum and amplitude analysis of dark- and light-adapted oscillatory potentials in albino mouse, rat and rabbit. Doc. Ophthalmol. 115, 85–93. https://doi.org/10.1007/s10633-007-9061-1 (2007).
Shady, S., Hood, D. C. & Birch, D. G. Rod phototransduction in retinitis pigmentosa. Distinguishing alternative mechanisms of degeneration. Invest. Ophthalmol. Vis. Sci. 36, 1027–1037 (1995).
Gayet-Primo, J. & Puthussery, T. Alterations in kainate receptor and TRPM1 localization in bipolar cells after retinal photoreceptor degeneration. Front Cell Neurosci. 9, 486. https://doi.org/10.3389/fncel.2015.00486 (2015).
Evans, L. S., Peachey, N. S. & Marchese, A. L. Comparison of three methods of estimating the parameters of the Naka-Rushton equation. Doc. Ophthalmol. 84, 19–30. https://doi.org/10.1007/BF01203279 (1993).
Dorfman, A. L., Chemtob, S. & Lachapelle, P. Postnatal hyperoxia and the developing rat retina: Beyond the obvious vasculopathy. Doc. Ophthalmol. 120, 61–66. https://doi.org/10.1007/s10633-009-9208-3 (2010).
Polosa, A., Bessaklia, H. & Lachapelle, P. Strain differences in light-induced retinopathy. PLoS ONE 11, e0158082. https://doi.org/10.1371/journal.pone.0158082 (2016).
Bravo-Nuevo, A., Walsh, N. & Stone, J. Photoreceptor degeneration and loss of retinal function in the C57BL/6-C2J mouse. Invest. Ophthalmol. Vis. Sci. 45, 2005–2012. https://doi.org/10.1167/iovs.03-0842 (2004).
Nusinowitz, S. et al. Electroretinographic evidence for altered phototransduction gain and slowed recovery from photobleaches in albino mice with a MET450 variant in RPE65. Exp. Eye Res. 77, 627–638. https://doi.org/10.1016/s0014-4835(03)00217-3 (2003).
Gresh, J., Goletz, P. W., Crouch, R. K. & Rohrer, B. Structure-function analysis of rods and cones in juvenile, adult, and aged C57bl/6 and Balb/c mice. Vis. Neurosci. 20, 211–220. https://doi.org/10.1017/s0952523803202108 (2003).
Ferdous, S. et al. Age-related retinal changes in wild-type C57BL/6J mice between 2 and 32 months. Invest. Ophthalmol. Vis. Sci. 62, 9. https://doi.org/10.1167/iovs.62.7.9 (2021).
Chaychi, S., Polosa, A. & Lachapelle, P. Differences in retinal structure and function between aging male and female sprague-dawley rats are strongly influenced by the estrus cycle. PLoS ONE 10, e0136056. https://doi.org/10.1371/journal.pone.0136056 (2015).
Mazzoni, F., Tombo, T. & Finnemann, S. C. No difference between age-matched male and female C57BL/6J mice in photopic and scotopic electroretinogram a- and b-wave amplitudes or in peak diurnal outer segment phagocytosis by the retinal pigment epithelium. Adv. Exp. Med. Biol. 1185, 507–511. https://doi.org/10.1007/978-3-030-27378-1_83 (2019).
Hermenean, A. et al. Changes in retinal structure and ultrastructure in the aged mice correlate with differences in the expression of selected retinal miRNAs. Front Pharmacol 11, 593514. https://doi.org/10.3389/fphar.2020.593514 (2020).
Rodrigo, M. J. et al. Effect of age and sex on neurodevelopment and neurodegeneration in the healthy eye: Longitudinal functional and structural study in the Long-Evans rat. Exp. Eye Res. 200, 108208. https://doi.org/10.1016/j.exer.2020.108208 (2020).
Tian, W., Sasse-Middelhoff, H., Mosgaard, L. D. & Heimburg, T. Effect of anesthetics on action potential propagation. Biophys. J. 110, 150a–150a. https://doi.org/10.1016/j.bpj.2015.11.844 (2016).
de-Paula, E. & Schreier, S. Molecular and physicochemical aspects of local anesthetic-membrane interaction. Braz. J. Med. Biol. Res. 29, 877–894 (1996).
Gao, L. et al. Nicotinic acetylcholine receptors are associated with ketamine-induced neuronal apoptosis in the developing rat retina. Neuroscience 376, 1–12. https://doi.org/10.1016/j.neuroscience.2018.01.057 (2018).
Moult, E. M. et al. Evaluating anesthetic protocols for functional blood flow imaging in the rat eye. J. Biomed. Opt. 22, 16005. https://doi.org/10.1117/1.JBO.22.1.016005 (2017).
Hart, C. Y., Burnett, J. C. Jr. & Redfield, M. M. Effects of avertin versus xylazine-ketamine anesthesia on cardiac function in normal mice. Am. J. Physiol. Heart Circ. Physiol. 281, H1938-1945. https://doi.org/10.1152/ajpheart.2001.281.5.H1938 (2001).
Wang, L. The use of ultrasound for noninvasive monitoring of anesthetic effects in renal ischemia-reperfusion injury. J. Clin. Ultrasound https://doi.org/10.1002/jcu.23753 (2024).
Pachon, R. E., Scharf, B. A., Vatner, D. E. & Vatner, S. F. Best anesthetics for assessing left ventricular systolic function by echocardiography in mice. Am. J. Physiol. Heart Circ. Physiol. 308, H1525-1529. https://doi.org/10.1152/ajpheart.00890.2014 (2015).
Pagliardini, S., Greer, J. J., Funk, G. D. & Dickson, C. T. State-dependent modulation of breathing in urethane-anesthetized rats. J. Neurosci. 32, 11259–11270. https://doi.org/10.1523/JNEUROSCI.0948-12.2012 (2012).
Sanchez-Pozo, A., Alados, J. C. & Sanchez-Medina, F. Metabolic changes induced by urethane-anesthesia in rats. Gen. Pharmacol. 19, 281–284. https://doi.org/10.1016/0306-3623(88)90077-8 (1988).
Brown, E. T., Umino, Y., Loi, T., Solessio, E. & Barlow, R. Anesthesia can cause sustained hyperglycemia in C57/BL6J mice. Vis. Neurosci. 22, 615–618. https://doi.org/10.1017/S0952523805225105 (2005).
Storch, K. F. et al. Intrinsic circadian clock of the mammalian retina: Importance for retinal processing of visual information. Cell 130, 730–741. https://doi.org/10.1016/j.cell.2007.06.045 (2007).
Kim, J. Y. et al. Noncanonical autophagy promotes the visual cycle. Cell 154, 365–376. https://doi.org/10.1016/j.cell.2013.06.012 (2013).
Banskota, S. et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 185, 250–265. https://doi.org/10.1016/j.cell.2021.12.021 (2022).
Yang, M., Yao, J., Jia, L., Kocab, A. J. & Zacks, D. N. Preservation of retinal structure and function in two mouse models of inherited retinal degeneration by ONL1204, an inhibitor of the Fas receptor. Cell Death Dis. 15, 576. https://doi.org/10.1038/s41419-024-06970-6 (2024).
Yao, J. et al. Inhibiting autophagy reduces retinal degeneration caused by protein misfolding. Autophagy 14, 1226–1238. https://doi.org/10.1080/15548627.2018.1463121 (2018).
Chu-Tan, J. A. et al. Voluntary exercise modulates pathways associated with amelioration of retinal degenerative diseases. Front Physiol. 14, 1116898. https://doi.org/10.3389/fphys.2023.1116898 (2023).
Xing, Y. et al. Combination of Lactobacillus fermentum NS9 and aronia anthocyanidin extract alleviates sodium iodate-induced retina degeneration. Sci. Rep. 13, 8380. https://doi.org/10.1038/s41598-023-34219-3 (2023).
Riccitelli, S. et al. Glial Bmal1 role in mammalian retina daily changes. Sci. Rep. 12, 21561. https://doi.org/10.1038/s41598-022-25783-1 (2022).
Liu, H. et al. Reducing Akt2 in retinal pigment epithelial cells causes a compensatory increase in Akt1 and attenuates diabetic retinopathy. Nat. Commun. 13, 6045. https://doi.org/10.1038/s41467-022-33773-0 (2022).
Rutan Woods, C. T. et al. Downregulation of rhodopsin is an effective therapeutic strategy in ameliorating peripherin-2-associated inherited retinal disorders. Nat. Commun. 15, 4756. https://doi.org/10.1038/s41467-024-48846-5 (2024).
Strayve, D. et al. ROM1 contributes to phenotypic heterogeneity in PRPH2-associated retinal disease. Hum. Mol. Genet. 29, 2708–2722. https://doi.org/10.1093/hmg/ddaa160 (2020).
Rajala, A. et al. Pyruvate kinase M2 regulates photoreceptor structure, function, and viability. Cell Death Dis. 9, 240. https://doi.org/10.1038/s41419-018-0296-4 (2018).
Fu, C. et al. Mutant mice with rod-specific VPS35 deletion exhibit retinal alpha-synuclein pathology-associated degeneration. Nat. Commun. 15, 5970. https://doi.org/10.1038/s41467-024-50189-0 (2024).
Greenwald, S. H. et al. Gene therapy preserves retinal structure and function in a mouse model of NMNAT1-associated retinal degeneration. Mol. Ther. Methods Clin. Dev. 18, 582–594. https://doi.org/10.1016/j.omtm.2020.07.003 (2020).
Greenwald, S. H. et al. Mouse models of NMNAT1-leber congenital amaurosis (LCA9) recapitulate key features of the human disease. Am. J. Pathol. 186, 1925–1938. https://doi.org/10.1016/j.ajpath.2016.03.013 (2016).
Read, S. P., Cashman, S. M. & Kumar-Singh, R. POD nanoparticles expressing GDNF provide structural and functional rescue of light-induced retinal degeneration in an adult mouse. Mol. Ther. 18, 1917–1926. https://doi.org/10.1038/mt.2010.167 (2010).
Sanuki, R. et al. Protein-4.1G-mediated membrane trafficking is essential for correct rod synaptic ___location in the retina and for normal visual function. Cell Rep. 10, 796–808. https://doi.org/10.1016/j.celrep.2015.01.005 (2015).
Johnson, R. E. & Kerschensteiner, D. Retrograde plasticity and differential competition of bipolar cell dendrites and axons in the developing retina. Curr. Biol. 24, 2301–2306. https://doi.org/10.1016/j.cub.2014.08.018 (2014).
Soto, F., Watkins, K. L., Johnson, R. E., Schottler, F. & Kerschensteiner, D. NGL-2 regulates pathway-specific neurite growth and lamination, synapse formation, and signal transmission in the retina. J. Neurosci. 33, 11949–11959. https://doi.org/10.1523/JNEUROSCI.1521-13.2013 (2013).
Harun-Or-Rashid, M. et al. MCT2 overexpression rescues metabolic vulnerability and protects retinal ganglion cells in two models of glaucoma. Neurobiol. Dis. 141, 104944. https://doi.org/10.1016/j.nbd.2020.104944 (2020).
Schirmer, L. et al. Oligodendrocyte-encoded Kir4.1 function is required for axonal integrity. Elife https://doi.org/10.7554/eLife.36428 (2018).
Sultana, A. et al. Deletion of olfactomedin 2 induces changes in the AMPA receptor complex and impairs visual, olfactory, and motor functions in mice. Exp. Neurol. 261, 802–811. https://doi.org/10.1016/j.expneurol.2014.09.002 (2014).
Lu, Q. et al. Selective activation of ABCA1/ApoA1 signaling in the V1 by magnetoelectric stimulation ameliorates depression via regulation of synaptic plasticity. iScience 25, 104201. https://doi.org/10.1016/j.isci.2022.104201 (2022).
Jassim, A. H., Nsiah, N. Y. & Inman, D. M. Ocular hypertension results in hypoxia within glia and neurons throughout the visual projection. Antioxidants (Basel) https://doi.org/10.3390/antiox11050888 (2022).
Rocha, L. R. et al. Early removal of senescent cells protects retinal ganglion cells loss in experimental ocular hypertension. Aging Cell 19, e13089. https://doi.org/10.1111/acel.13089 (2020).
Yang, L. et al. Rescue of glaucomatous neurodegeneration by differentially modulating neuronal endoplasmic reticulum stress molecules. J. Neurosci. 36, 5891–5903. https://doi.org/10.1523/JNEUROSCI.3709-15.2016 (2016).
Cho, K. I., Yoon, D., Yu, M., Peachey, N. S. & Ferreira, P. A. Microglial activation in an amyotrophic lateral sclerosis-like model caused by Ranbp2 loss and nucleocytoplasmic transport impairment in retinal ganglion neurons. Cell Mol. Life Sci. 76, 3407–3432. https://doi.org/10.1007/s00018-019-03078-5 (2019).
Nishiguchi, K. M. et al. Retained plasticity and substantial recovery of rod-mediated visual acuity at the visual cortex in blind adult mice with retinal dystrophy. Mol. Ther. 26, 2397–2406. https://doi.org/10.1016/j.ymthe.2018.07.012 (2018).
Guo, X. et al. Preservation of vision after CaMKII-mediated protection of retinal ganglion cells. Cell 184, 4299–4314. https://doi.org/10.1016/j.cell.2021.06.031 (2021).
Chou, T. H. et al. Integrative properties of retinal ganglion cell electrical responsiveness depend on neurotrophic support and genotype in the mouse. Exp. Eye Res. 145, 68–74. https://doi.org/10.1016/j.exer.2015.11.008 (2016).
Xiong, S. et al. Stem cell transplantation rescued a primary open-angle glaucoma mouse model. Elife https://doi.org/10.7554/eLife.63677 (2021).
Guo, L. et al. Inducible Rbpms-CreER(T2) mouse line for studying gene function in retinal ganglion cell physiology and disease. Cells https://doi.org/10.3390/cells12151951 (2023).
Li, S. & Jakobs, T. C. Secreted phosphoprotein 1 slows neurodegeneration and rescues visual function in mouse models of aging and glaucoma. Cell Rep. 41, 111880. https://doi.org/10.1016/j.celrep.2022.111880 (2022).
Lagali, P. S. et al. Assessment of the uniform field electroretinogram for mouse retinal ganglion cell functional analysis. Doc. Ophthalmol. 147, 29–43. https://doi.org/10.1007/s10633-023-09933-y (2023).
Chou, T. H., Bohorquez, J., Toft-Nielsen, J., Ozdamar, O. & Porciatti, V. Robust mouse pattern electroretinograms derived simultaneously from each eye using a common snout electrode. Invest. Ophthalmol. Vis. Sci. 55, 2469–2475. https://doi.org/10.1167/iovs.14-13943 (2014).
Pangratz-Fuehrer, S., Kaur, K., Ousman, S. S., Steinman, L. & Liao, Y. J. Functional rescue of experimental ischemic optic neuropathy with alphaB-crystallin. Eye (Lond.) 25, 809–817. https://doi.org/10.1038/eye.2011.42 (2011).
Singh, S. K. et al. Astrocytes assemble thalamocortical synapses by bridging NRX1alpha and NL1 via hevin. Cell 164, 183–196. https://doi.org/10.1016/j.cell.2015.11.034 (2016).
McCurry, C. L. et al. Loss of Arc renders the visual cortex impervious to the effects of sensory experience or deprivation. Nat. Neurosci. 13, 450–457. https://doi.org/10.1038/nn.2508 (2010).
Ciucci, F. et al. Insulin-like growth factor 1 (IGF-1) mediates the effects of enriched environment (EE) on visual cortical development. PLoS ONE 2, e475. https://doi.org/10.1371/journal.pone.0000475 (2007).
Juricek, L. et al. AhR-deficiency as a cause of demyelinating disease and inflammation. Sci. Rep. 7, 9794. https://doi.org/10.1038/s41598-017-09621-3 (2017).
Dai, J. et al. Contribution of GABAa, GABAc and glycine receptors to rat dark-adapted oscillatory potentials in the time and frequency ___domain. Oncotarget 8, 77696–77709. https://doi.org/10.18632/oncotarget.20770 (2017).
Bradley, P. B. & Dray, A. Modification of the responses of brain stem neurones to transmitter substances by anaesthetic agents. Br. J. Pharmacol. 48, 212–224. https://doi.org/10.1111/j.1476-5381.1973.tb06907.x (1973).
Kapousta-Bruneau, N. V. Effects of sodium pentobarbital on the components of electroretinogram in the isolated rat retina. Vis. Res. 39, 3498–3512. https://doi.org/10.1016/s0042-6989(99)00079-6 (1999).
Mercado, J. & Czajkowski, C. Gamma-aminobutyric acid (GABA) and pentobarbital induce different conformational rearrangements in the GABA A receptor alpha1 and beta2 pre-M1 regions. J. Biol. Chem. 283, 15250–15257. https://doi.org/10.1074/jbc.M708638200 (2008).
Kuo, M. C. & Leung, L. S. Disruption of hippocampal multisynaptic networks by general anesthetics. Anesthesiology 127, 838–851. https://doi.org/10.1097/ALN.0000000000001861 (2017).
Shin, M. C. et al. Comparative effects of pentobarbital on spontaneous and evoked transmitter release from inhibitory and excitatory nerve terminals in rat CA3 neurons. Brain Res. Bull. 90, 10–18. https://doi.org/10.1016/j.brainresbull.2012.09.013 (2013).
Hara, K. & Harris, R. A. The anesthetic mechanism of urethane: The effects on neurotransmitter-gated ion channels. Anesth. Analg. 94, 313–318. https://doi.org/10.1097/00000539-200202000-00015 (2002).
Yoshiyama, M., Roppolo, J. R. & De Groat, W. C. Alteration by urethane of glutamatergic control of micturition. Eur. J. Pharmacol. 264, 417–425. https://doi.org/10.1016/0014-2999(94)00505-2 (1994).
Huh, Y. & Cho, J. Urethane anesthesia depresses activities of thalamocortical neurons and alters its response to nociception in terms of dual firing modes. Front Behav. Neurosci. 7, 141. https://doi.org/10.3389/fnbeh.2013.00141 (2013).
Krasowski, M. D. & Harrison, N. L. The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br. J. Pharmacol. 129, 731–743. https://doi.org/10.1038/sj.bjp.0703087 (2000).
Wu, T. et al. Altered regional connectivity reflecting effects of different anaesthesia protocols in the mouse brain. Neuroimage 149, 190–199. https://doi.org/10.1016/j.neuroimage.2017.01.074 (2017).
Wang, K. et al. Temporal scaling properties and spatial synchronization of spontaneous blood oxygenation level-dependent (BOLD) signal fluctuations in rat sensorimotor network at different levels of isoflurane anesthesia. NMR Biomed. 24, 61–67. https://doi.org/10.1002/nbm.1556 (2011).
Ding, S. L. Lamination, borders, and thalamic projections of the primary visual cortex in human, non-human primate, and rodent brains. Brain Sci. https://doi.org/10.3390/brainsci14040372 (2024).
Boly, M. et al. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J. Neurosci. 32, 7082–7090. https://doi.org/10.1523/JNEUROSCI.3769-11.2012 (2012).
Chen, Y., Li, S., Wu, F., Zou, L. & Zhang, J. Altered functional and directed connectivity in propofol-induced loss of consciousness: A source-space resting-state EEG study. Clin. Neurophysiol. 142, 209–219. https://doi.org/10.1016/j.clinph.2022.08.003 (2022).
Olivas, N. D., Quintanar-Zilinskas, V., Nenadic, Z. & Xu, X. Laminar circuit organization and response modulation in mouse visual cortex. Front Neural Circuits 6, 70. https://doi.org/10.3389/fncir.2012.00070 (2012).
Xie, H. et al. Differential effects of anesthetics on resting state functional connectivity in the mouse. J. Cereb. Blood Flow Metab. 40, 875–884. https://doi.org/10.1177/0271678X19847123 (2020).
Huang, W. et al. Magnetic resonance imaging (MRI) detection of the murine brain response to light: Temporal differentiation and negative functional MRI changes. Proc. Natl. Acad. Sci. U. S. A. 93, 6037–6042. https://doi.org/10.1073/pnas.93.12.6037 (1996).
Zhao, Y. et al. YAP in development and disease: Navigating the regulatory landscape from retina to brain. Biomed. Pharmacother 175, 116703. https://doi.org/10.1016/j.biopha.2024.116703 (2024).
Olavarria, J., Malach, R. & Van Sluyters, R. C. Development of visual callosal connections in neonatally enucleated rats. J. Comp. Neurol. 260, 321–348. https://doi.org/10.1002/cne.902600302 (1987).
Mogg, K., Garner, M. & Bradley, B. P. Anxiety and orienting of gaze to angry and fearful faces. Biol. Psychol. 76, 163–169. https://doi.org/10.1016/j.biopsycho.2007.07.005 (2007).
Butt, O. H., Benson, N. C., Datta, R. & Aguirre, G. K. The fine-scale functional correlation of striate cortex in sighted and blind people. J. Neurosci. 33, 16209–16219. https://doi.org/10.1523/JNEUROSCI.0363-13.2013 (2013).
Klugah-Brown, B. et al. The neurostructural consequences of glaucoma and their overlap with disorders exhibiting emotional dysregulations: A voxel-based meta-analysis and tripartite system model. J. Affect Disord. 358, 487–499. https://doi.org/10.1016/j.jad.2024.05.016 (2024).
Liu, S. et al. An optimized procedure to record visual evoked potential in mice. Exp. Eye Res. 218, 109011. https://doi.org/10.1016/j.exer.2022.109011 (2022).
Lamb, T. D. & Pugh, E. N. Jr. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol. 449, 719–758. https://doi.org/10.1113/jphysiol.1992.sp019111 (1992).
Gangadhar, D. V., Wolf, B. M. & Tanenbaum, H. L. Naka-Rushton equation parameters in electroretinogram analysis of daunomycin effects on retinal function. Doc. Ophthalmol. 72, 61–70. https://doi.org/10.1007/BF00155215 (1989).
Breton, M. E., Montzka, D. P., Brucker, A. J. & Quinn, G. E. Electroretinogram interpretation in central retinal vein occlusion. Ophthalmology 98, 1837–1844. https://doi.org/10.1016/s0161-6420(91)32041-4 (1991).
Acknowledgements
We thank members of Yao laboratory for their technical assistance.
Funding
This research was supported by National Natural Science Foundation of China (Nos. 82471107 and 31970930), Hubei Natural Science Foundation (Nos. 2020CFA069 and 2018CFB434), Neuroscience Team Development Project of Wuhan University of Science and Technology (Nos. 1180002 and 1180030), and Technology Development Projects of Wuhan University of Science and Technology (Nos. 2023H20134, 2024H10082 and 2024H20168).
Author information
Authors and Affiliations
Contributions
Conceptualization, K.Y.; Methodology, S.Z. and W.X.; Formal analysis, S.Z. and W.X.; Investigation, X.C., S.L., and F.X.; Resources, H.Q. and K.Y.; Data curation, S.Z. and W.X.; Writing—original draft preparation, H.Q. and S. Z.; Writing—review and editing, H.Q. and S. Z.; Supervision, K.Y.; Project administration, K.Y.; Funding acquisition, K.Y. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, S., Xu, W., Liu, S. et al. Anesthetic effects on electrophysiological responses across the visual pathway. Sci Rep 14, 27825 (2024). https://doi.org/10.1038/s41598-024-79240-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79240-2
This article is cited by
-
The Role of Non-coding RNAs in Diabetic Retinopathy: Mechanistic Insights and Therapeutic Potential
Molecular Neurobiology (2025)