Abstract
Gastric cancer (GC) is globally recognized as the fifth most common cancer and the third leading cause of cancer-related mortality. Early metastasis in GC significantly contributes to its high mortality and unfavorable prognosis. However, the underlying mechanisms of this phenomenon remain largely unexplored. Among the various factors involved, AKR1C3 has emerged as a crucial component in the pathways of tumorigenesis and metastasis across multiple cancer types. Yet, the precise significance of AKR1C3 in GC patients’ prognosis and its role in GC progression remain elusive. This study illuminated the significant downregulation of AKR1C3 in GC tissues, linking it to an aggressive phenotype and poor prognosis. Interestingly, while AKR1C3 overexpression did not affect the proliferation of GC cells, it significantly inhibited their ability to invade and metastasize. The underlying mechanism appears to involve AKR1C3’s inhibition of the p-JNK pathway, which leads to reduced phosphorylation of IKKα/β and IKBα, lowering p-NF-κB levels and hindering its movement into the nucleus, thereby stifling the epithelial-mesenchymal transition (EMT) process in GC cells. These insights reveal AKR1C3’s tumor-suppressive effects in GC and suggest its potential as a diagnostic and prognostic biomarker, offering new avenues for targeted therapies in gastric cancer management.
Similar content being viewed by others
Introduction
Globally, gastric cancer (GC) ranks fifth in cancer incidence and is the third leading cause of cancer-related mortality, trailing only lung and colorectal cancer1. Despite therapeutic advancements, GC’s clinical outcomes remain disappointing due to late-stage diagnoses that preclude optimal surgery, and limited gains from chemotherapy. Advanced GC yields a median survival of less than 12 months2. Consequently, there is a pressing need to uncover novel biomolecules and signaling pathways driving gastric carcinogenesis and progression, in pursuit of improved insights and treatment avenues.
AKR1C3, a member of the aldo-keto reductase (AKR) superfamily consisting of over 190 members divided into 16 families, belongs to the C3 subtype of the aldosterone reductase family 1 (AKR1C3). Encoded by the AKR1C3 gene, it is a soluble monomeric NADP(H)-dependent oxidoreductase3. AKR1C3 plays a multifaceted role in cellular functions, including the regulation of estrogen, androgen, progesterone, and prostaglandin biosynthesis and metabolism4. Moreover, it has been strongly associated with the progression and prognosis of various types of tumors3. In several cancers, the expression of AKR1C3 has been found to be either upregulated or downregulated, irrespective of hormone dependency. Its overexpression has been linked to the conversion of prostaglandin D2 (PGD2) to prostaglandin F2 (PGF2), a process that promotes prostate cell proliferation through the activation of G-protein-coupled receptors for prostaglandin F2 (PGF2) and the PI3K/Akt signaling pathway5,6. AKR1C3 upregulation has also been shown to confer radiation resistance to prostate cancer cells by activating the MAPK pathway and reducing PPARγ expression7. While AKR1C3 is expressed in the superficial mucosal cells of the stomach and duodenum8, its mRNA and protein levels are lower in gastric cancer (GC) tissues compared to adjacent normal mucosal tissues, especially in diffuse and intestinal-type GC9. However, the precise role and molecular mechanisms of AKR1C3 in GC remain to be fully understood.
In this study, we investigated the expression patterns of AKR1C3 in primary gastric cancer (GC) tumors and evaluated its potential implications for the prognosis of GC patients. We conducted a comprehensive exploration of AKR1C3’s role in GC, particularly its impact on tumor growth, invasion, and migration, both in-vitro and in-vivo models. Our findings have revealed a frequent reduction in AKR1C3 expression in GC, which is strongly indicative of an unfavorable prognosis for patients. Upon closer examination of the mechanisms at play, we unraveled a pivotal role for AKR1C3 in preserving an epithelial phenotype in GC cells. Its absence, however, appeared to trigger a pronounced epithelial-mesenchymal transition (EMT), a phenomenon closely associated with heightened tumor aggressiveness. The regulatory impact of AKR1C3 on EMT is mediated through its modulation of critical signaling pathways, particularly the AKT and JNK/p-NF-κB pathways. Therefore, AKR1C3 might serve as a promising candidate for future interventions aimed at effective management of GC.
Results
AKR1C3 is downregulated in GC and its downregulation predicts poor prognosis in GC patients
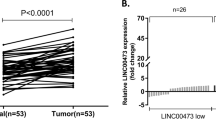
Using data sets from The Cancer Genome Atlas (TCGA), we initiated our investigation by analyzing AKR1C3 expression in GC. The findings from this initial analysis revealed that AKR1C3 expression was notably reduced in GC tissues when compared to adjacent noncancerous tissues (Wilcoxon rank sum test, P = 0.08) (Fig. 1a). To delve deeper into the clinical significance of AKR1C3 in GC, we conducted immunohistochemistry (IHC) using archived tumor samples collected from a cohort of 151 GC patients. The results demonstrated a significant decrease in AKR1C3 expression within primary GC tumors compared to adjacent normal tissues (paired t-test, P < 0.001) (Fig. 1b). Additionally, our qRT‒PCR and Western blot data (Fig. 1c and d) corroborated these findings, showing that both AKR1C3 mRNA and protein levels in GC tissues were significantly lower as compared to noncancerous tissues. Moreover, Pearson’s chi-square test was performed to investigate the links between AKR1C3 expression and various clinicopathologic features in GC patients. The results of these analyses highlighted associations between AKR1C3 expression and factors such as T classification, N classification, and clinical stage (Table 1). Lastly, we performed a Kaplan‒Meier analysis to assess the relationship between AKR1C3 protein expression, as determined through IHC, and patient outcomes. This analysis revealed that patients whose tumors exhibited low AKR1C3 expression had considerably shorter survival compared to those with high AKR1C3 expression as analyzed by Kaplan‒Meier analysis with log-rank test (P = 0.002) (Fig. 1e). These findings collectively suggest that AKR1C3 may function as a tumor suppressor in GC. The downregulation of AKR1C3 appears to be intricately linked to the development and progression of GC, underscoring its potential as a valuable therapeutic target in the management of this particular form of cancer.
AKR1C3 is downregulated in GC and predicts poor prognosis of patients. (a) The expression levels of AKR1C3 in GC samples and noncancerous tissue samples were examined from the TCGA database (P = 0.08, Wilcoxon rank sum test). (b) Representative images of IHC staining of 151 paired GC tissues and adjacent normal tissues using an anti-AKR1C3 antibody. The graph shows the scores of AKR1C3 in GC and NT ranging from 0 to 12. A paired t test was used to compute the P value (*** P < 0.001). (c,d) Expression of AKR1C3 in 8 pairs of GC tissues and their adjacent normal tissues was determined by real-time PCR (c) and Western blot (d) analysis. (e) Kaplan‒Meier survival analysis was performed to evaluate the correlation between AKR1C3 expression and overall survival in 138 GC patients (out of 151 total patients, 13 were lost to follow-up; maximum follow-up period was 79 months). Patients were stratified into high and low expression groups, revealing a significant difference in survival outcomes. The median survival time was 24 months for the low-expression group while it was not reached for the high-expression group (log-rank test, P = 0.002).
Effect of AKR1C3 on GC cell proliferation, migration and invasion in vitro
We conducted an extensive investigation into the functional impact of AKR1C3 on the malignant behaviors of GC, both in vitro and in vivo. Given the prognostic significance of AKR1C3 expression in GC, we aimed to determine how it influences the disease. To this end, we established GC cell lines with stable AKR1C3 knockdown or overexpression, as validated through Western blot analysis (Fig. 2a). Interestingly, altering AKR1C3 expression had no discernible effect on the proliferation rate of SNU-216 or AGS cells in vitro, as evidenced by a series of assays including the CCK-8 assay (Fig.S1a and b), and colony formation assay (Fig. S1c).
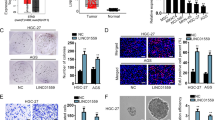
AKR1C3 affects the migration, invasion and metastasis of GC cells in vitro.(a) Western blot analysis confirmed stable overexpression or knockdown of AKR1C3 in AGS and SNU-216 cell lines, using GAPDH as a loading control.(b) The relative migration of cells was assessed using a Boyden chamber assay, where cells migrated through an uncoated filter toward a serum gradient. (c) Cell motility was evaluated using a wound-healing assay, monitoring the movement of GC cells into the cleared wound area. (d) Invasion capabilities were determined by the ability of GC cells to penetrate through a Matrigel-coated filter in a Boyden chamber. *P < 0.05, unpaired t test, pCDH versus pAKR1C3. *P < 0.05, one-way ANOVA with Dunnett’s post hoc test, pSuper versus psh1AKR1C3 and pSuper versus psh2AKR1C3. pCDH: empty vector transfected control cell line; pAKR1C3: stable AKR1C3 overexpressed cell line; pSuper: scramble shRNAi control transfected cell line; psh1/2AKR1C3: stable AKR1C3 shRNAi knockdown cell line.
Subsequently, we delved into the impact of AKR1C3 on cell migration, a critical factor in cancer metastasis. Using a Boyden two-chamber assay, where fetal bovine serum (FBS) in the opposite chamber stimulates cell migration, we observed that AKR1C3 overexpression inhibited migration (unpaired t-test, P < 0.05), while its knockdown had the opposite effect, enhancing cell migration (one-way ANOVA with Dunnett’s post hoc test, P < 0.05) (Fig. 2b). To further assess cell migratory capabilities, we conducted a wound-healing/scratch assay, a widely recognized measure of cell motility. As shown in Fig. 2c, cells overexpressing AKR1C3 exhibited slower migration compared to the control cells (unpaired t-test, P < 0.05). Conversely, AKR1C3-knockdown cells demonstrated accelerated wound closure compared to cells with scrambled shRNA controls (one-way ANOVA with Dunnett’s post hoc test, P < 0.05). To gauge invasive potential in the context of AKR1C3 expression, we conducted a modified Boyden chamber invasion assay, evaluating the number of cells invading through a Matrigel layer 24 h after plating on Matrigel-coated transwell inserts. As anticipated, AKR1C3 overexpression in GC cells significantly decreased their invasive capacity (unpaired t-test, P < 0.05). Conversely, AGS and SNU-216 cells with AKR1C3 knockdown displayed a marked increase in invasion ability (one-way ANOVA with Dunnett’s post hoc test, P < 0.05) (Fig. 2d). These results indicate that AKR1C3 plays a critical role in inhibiting the migration and invasion of GC cells.
Effect of AKR1C3 on GC cell metastasis potential in vivo
To further explore the role of AKR1C3 in GC metastasis in vivo, we injected NCG nude mice with AGS cells expressing either shRNA against AKR1C3 (AGS-psh1AKR1C3) or a control vector (AGS-pSuper). Eight weeks post-injection, we assessed lung metastases. The mice injected with AGS-psh1AKR1C3 cells exhibited a significantly higher number of nodules in their lungs upon gross examination compared to those injected with the control cells. Hematoxylin and eosin (H&E) staining further confirmed that the downregulation of AKR1C3 in GC cells notably enhanced metastasis to the lungs relative to control cells. The results confirmed that AKR1C3 inhibit metastasis of GC cells in vivo (unpaired t-test, P < 0.05) (Fig. 3).
AKR1C3 affects the migration, invasion and metastasis of GC cells in vivo. The extent of lung metastasis was evaluated in mice 8 weeks after tail vein injection with either AGS-pSuper or AGS-psh1AKR1C3 cells, showing significant differences in metastatic burden. *P < 0.05, unpaired t test, pSuper versus psh1AKR1C3.
AKR1C3 suppresses EMT in GC cells
Given the critical role of EMT initiation in cancer metastasis10, we sought to determine whether AKR1C3 could modulate EMT. Analysis of The Cancer Genome Atlas (TCGA) stomach cancer dataset through Gene Set Enrichment Analysis (GSEA) unveiled a negative correlation between AKR1C3 levels and EMT-activated cells (Fig. 4a). To delve deeper into this phenomenon, Western blot analysis was employed to assess the expression of EMT-related markers in AGS and SNU-216 cells with either AKR1C3 overexpression or knockdown. The results indicated that AKR1C3 overexpression led to an increase in the expression of epithelial markers (E-cadherin and ZO-1), accompanied by a decrease in mesenchymal markers (N-cadherin and vimentin), as well as EMT transcription factors (Slug and Snail) (unpaired t test, P < 0.05). Conversely, AKR1C3 knockdown had the opposite effect, resulting in a significant reduction in E-cadherin and ZO-1 expression and a substantial increase in N-cadherin, vimentin, Slug, and Snail expression (one-way ANOVA with Dunnett’s post hoc test, P < 0.05) (Fig. 4b). These findings collectively suggest that AKR1C3 hinders the migration and invasion of GC cells by suppressing the EMT process.
AKR1C3 suppresses EMT in GC cells. (a) GSEA plot demonstrating the inverse correlation between AKR1C3 expression and EMT-activated gene signatures in the TCGA stomach cancer dataset. (b) Western blot analysis showing the expression levels of EMT markers in cells with overexpressed or knocked-down AKR1C3. *P < 0.05, unpaired t test, pCDH versus pAKR1C3. *P < 0.05, one-way ANOVA with Dunnett’s post hoc test, pSuper versus psh1AKR1C3 and pSuper versus psh2AKR1C3.
AKR1C3 regulates the AKT, JNK and NF-κB signaling pathways
Numerous signaling pathways, including AKT, NF-κB, JNK, STAT3, MAPK, MEK, Notch1, GLI1, and HIF-1α, are recognized for their pivotal roles in regulating the process of EMT11. To pinpoint which of these signaling pathways might be influenced by AKR1C3 and contribute to EMT induction in GC cells, we conducted an analysis of a specific molecular signature in AGS and SNU-216 cells with either AKR1C3 overexpression or knockdown. As shown in Fig. 5 and Fig. S2, the upregulation of AKR1C3 in AGS and SNU-216 cells only resulted in a reduction of phosphorylation of AKT (Ser473), AKT (Thr308), JNK(Thr183/Tyr185), NF-κB (p-p65), and STAT3 (Tyr705) (unpaired t-test, P < 0.05). Conversely, AKR1C3 knockdown substantially increased the phosphorylation of AKT, JNK, NF-κB, and STAT3 (one-way ANOVA with Dunnett’s post hoc test, P < 0.05). To further investigate the influence of these pathways on cell migration, we conducted experiments using a panel of small-molecule inhibitors targeting NF-κB (EVP4593, 30 µM, 24 h), JNK (SP600125, 30 µM, 24 h), AKT (LY294002, 10 µM, 24 h), and STAT3 (S3I-201, 10 µM, 24 h) in AKR1C3 knockdown AGS cells. As depicted in Fig. 6a, the AKT inhibitor LY294002 significantly impaired cell migration in the shAKR1C3 cells compared to both treated and untreated control cells. Similar inhibitory effects on cell migration were observed with the JNK inhibitor SP600125 (Fig. 6b) and the NF-κB inhibitor EVP4593 (unpaired t-test, P < 0.05) (Fig. 6c). However, the STAT3 inhibitor S3I-201 did not significantly inhibit cell migration promoted by AKR1C3 knockdown (Fig. 6d). The results suggest that AKR1C3 promotes EMT in GC cells through the AKT, JNK, and NF-κB signaling pathways.
Analysis of the AKT, JNK, NF-κB, STAT3 signaling pathway mediating AKR1C3’s inhibition of EMT. Western blot analysis showing the expression of upstream proteins involved in EMT in both AGS and SNU216 cells with AKR1C3 overexpression or knockdown. *P < 0.05, unpaired t test, pCDH versus pAKR1C3. *P < 0.05, one-way ANOVA with Dunnett’s post hoc test, pSuper versus psh1AKR1C3 and pSuper versus psh2AKR1C3.
Effects of various inhibitors on cell migration. (a) The AKT inhibitor LY294002 significantly impaired cell migration in shAKR1C3 cells. (b) The JNK inhibitor SP600125 also reduced cell migration in shAKR1C3 cells. (c) The NF-κB inhibitor EVP4593 significantly inhibited cell migration in shAKR1C3 cells. (d) The STAT3 inhibitor S3I-201 did not show any significant effect on cell migration in shAKR1C3 cells. Representative images are displayed, quantification plots are provided for each inhibitor, with data presented as mean ± SD. *P < 0.05, unpaired t test.
AKR1C3 suppresses EMT via the AKT and JNK/NF-κB pathways
NF-κB is known to operate as a downstream signaling pathway for both JNK12 and AKT13,14. To ascertain whether JNK and AKT regulate EMT in GC cells through the NF-κB pathway or independently, we initiated experiments by introducing inhibitors targeting AKT (LY294002, 10 µM, 24 h) and JNK (SP600125, 30 µM, 24 h) into AKR1C3-knockdown AGS-psh1AKR1C3 and SNU-216-psh1AKR1C3 cells. When AGS-psh1AKR1C3 and SNU-216-psh1AKR1C3 cells were treated with the AKT-specific inhibitor LY294002, the phosphorylation level of NF-κB remained unaltered, while AKT phosphorylation decreased (unpaired t-test, P < 0.05). Conversely, treatment of AGS-psh1AKR1C3 and SNU-216-psh1AKR1C3 cells with SP600125 resulted in a reduction in phosphorylated JNK levels, a decrease in the phosphorylation level of NF-κB, and a reversal of the increasing trend of p-NF-κB caused by AKR1C3 silencing (Fig. 7a and b). These observations suggest that AKR1C3 exerts its inhibitory effects on GC cell motility and invasiveness through a mechanism involving both AKT and JNK/NF-κB signaling.
AKR1C3 modulation of EMT through AKT and JNK/NF-κB pathways. (a) NF-κB phosphorylation stimulated by AKR1C3 knockdown was not inhibited by LY294002 (an AKT inhibitor). (b) NF-κB phosphorylation increase due to AKR1C3 knockdown was counteracted by SP600125 (a JNK inhibitor). *P < 0.05, unpaired t test.
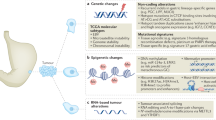
It is known that newly synthesized IκBα translocates to the nucleus, binds to NF-κB, leading to NF-κB dissociation from DNA, and subsequent export to the cytoplasm via a nuclear export sequence (NES) present on IκBα15. For the majority of NF-κB-activating stimuli, the multisubunit IκB kinase (IKK) that induces inducible IκB phosphorylation serves as the converging point. Both catalytic subunits IKK, IKKα and IKKβ, are capable of phosphorylating IκB correctly16. We evaluated the expression of IκBα, p-IκBα, IKKα, IKKβ, and p-IKKα/β in AKR1C3-overexpressing and AKR1C3-knockdown GC cells. Specifically, AKR1C3 overexpression led to a reduction in phosphorylated IκBα levels at serines 32 and 36 in GC cells (unpaired t-test, P < 0.05). Conversely, knocking down AKR1C3 activated IκBα phosphorylation (one-way ANOVA with Dunnett’s post hoc test, P < 0.05). Furthermore, our results demonstrated that AKR1C3 knockdown increased the levels of phosphorylated IKKα/β (Ser176/180) (one-way ANOVA with Dunnett’s post hoc test, P < 0.05) (Fig. 8a). When AGS-psh1AKR1C3 and SNU-216-psh1AKR1C3 cells were treated with the JNK-specific inhibitor SP600125 (30 µM, 24 h), the phosphorylation level of IKKα/β was reduced (unpaired t-test (P < 0.05) (Fig. 8b). To investigate whether JNK induces EMT by regulating the nuclear translocation of NF-κB in GC cells, we conducted a fractionation experiment to assess the levels of p-NF-κB in the cytoplasm and the nucleus. As shown in Fig. 8c, the nuclear and cytoplasmic levels of p-NF-κB decreased with AKR1C3 overexpression (unpaired t-test, P < 0.05) but increased with AKR1C3 knockdown (one-way ANOVA with Dunnett’s post hoc test, P < 0.05). As illustrated in Fig. 8d, these findings collectively suggest that AKR1C3 inhibits EMT by decreasing the phosphorylation levels of IKKα/β and IκBα, leading to a decrease in the distribution and quantity of p-NF-κB in the nucleus of GC cells.
Impact of AKR1C3 Regulation on NF-κB Localization and Function in GC Cells. (a) Overexpression of AKR1C3 decreased phosphorylation of IκBα and IKKα/β, whereas AKR1C3 knockdown led to increased phosphorylation of these proteins. (b) The increase in IKKα/β phosphorylation levels in response to shAKR1C3 can be inhibited by SP600125 (a JNK inhibitor). (c) Cell fractionation experiments showed that upregulation of AKR1C3 led to decreased expression of p-NF-kB in both the nucleus and cytosol of AGS and SNU-216 cells, while downregulation of AKR1C3 enhanced p-NF-kB expression. GAPDH was utilized as a loading control, with LaminB and GAPDH serving as markers for nuclear and cytosolic fractions, respectively. *P < 0.05, unpaired t test, pCDH versus pAKR1C3. *P < 0.05, one-way ANOVA with Dunnett’s post hoc test, pSuper versus psh1AKR1C3 and pSuper versus psh2AKR1C3. (d) Schematic model illustrating how AKR1C3 knockdown promotes malignant phenotypes in GC by activating p-JNK, which in turn activates phosphorylation of IKKα/β, leading to increased phosphorylation and activation of IκBα. This process facilitates the release and nuclear entry of p-NF-κB, which promotes EMT by upregulating transcription factors Snail and Slug, downregulating epithelial markers (E-cadherin and ZO-1), and upregulating mesenchymal markers (vimentin and N-cadherin).
Discussion
AKR1C3 plays a significant role in both hormone-dependent and hormone-independent cancers17 by influencing steroid hormone and prostaglandin-mediated signaling pathways18,19. Its expression in various endocrine organs such as the prostate20,21, breast22,23, adrenal grands24, and uterus25, facilitates the conversion of weak androgens and estrogens into their potent forms4, thereby promoting cancer progression through pathways like AKT and ERK6,17,26,27,28. AKR1C3 regulates epithelial-mesenchymal transition (EMT) by modulating the expression levels of vimentin, E-cadherin, and N-cadherin29. AKR1C3 also plays a pivotal role in the progression of hormone-independent tumors, including liver cancer30, gastrointestinal cancer9,31,32, lung cancer33, thyroid cancer34, acute myeloid leukemia35,36, HPV-negative oropharynx squamous cell carcinoma37, and nasal septum carcinoma38. It influences proliferation, invasion, and tumorigenesis of those hormone-independent cancers through the MAPK/ERK39, NF-κB30, and AKT38 pathways. While AKR1C3 is often recognized as an oncogenic marker, its downregulation has been noted in certain cancers, such as colon cancer32, Wilms’ tumor40 and neuroendocrine tumors of the aerodigestive tract8, depending on the specific cell type or context. In the present study, we observed a frequent downregulation of AKR1C3 in GC tissues compared to their adjacent normal gastric tissues. Low AKR1C3 expression correlated with advanced tumor stage, increased lymph node metastasis, and poorer clinical outcomes. Overexpression of AKR1C3 in GC cells suppressed migration and invasion in vitro, whereas AKR1C3 knockdown enhanced metastatic potential in vivo, suggesting its tumor-suppressive role. Mechanically, this effect was mediated through reduced phosphorylation of AKT and NF-κB through JNK signaling. Conversely, AKR1C3 knockdown had the opposite effect. These findings reinforce the critical role of AKR1C3 in suppressing GC progression and metastasis.
EMT is a critical process in cancer metastasis41,42, marked by the loss of cell adhesion and the acquisition of invasive traits43,44,45. In our in vitro experiments, AKR1C3 upregulation was correlated with increased epithelial markers (E-cadherin and ZO-1) and decreased mesenchymal markers (vimentin and N-cadherin). Conversely, AKR1C3 knockdown enhanced cell migration and invasion in vitro and metastatic potential in vivo. These findings suggest that AKR1C3 plays a role in mitigating cancer aggressiveness through suppression of EMT. EMT is driven by pathways, including AKT, JNK, NF-κB, which were significantly altered by AKR1C3 expression in our experiments. Inhibitors of these pathways reversed the migratory behavior of AKR1C3-knockdown cells, with JNK inhibition reducing NF-κB phosphorylation, highlighting a key role for the JNK/NF-κB axis. Further analysis showed that AKR1C3 knockdown increased IκBα and IKKα/β phosphorylation, activating the classical NF-κB pathway, which in turn promotes Snail and Slug expression to drive EMT.
Beyond EMT, AKR1C3 impacts cancer through other mechanisms. Overexpression of AKR1C3 mediates the conversion of PGD2, promoting prostate cell proliferation via FP and PI3K/AKT signaling pathways5,6. While AKR1C3 reduces PGD2 levels to limit the formation of anti-proliferative prostaglandins, its effects extend beyond prostaglandin metabolism. AKR1C3 appears to reduce intracellular reactive oxygen species (ROS) levels through the AKT/GSH signaling axis46 and promotes tumor growth by activating the NF-κB signaling independently of the androgen receptor and prostaglandin metabolic pathway. In gastric cancer, reduced levels of PGD2 and PTGDR2 have been linked to worse outcomes and higher mortality47 but our findings suggest that AKR1C3’s role in GC is largely prostaglandin-independent. It is widely acknowledged that the prevalence of gastric cancer is more pronounced in males than in females, a phenomenon substantiated by our clinical data. As shown in Table 1, the incidence of GC in this cohort demonstrated a male-to-female ratio of approximately 2.4:1, with 106 male and 45 female patients. Low AKR1C3 expression was predominant in both sexes, observed in 81.1% of males (86/106) and 86.7% of females (39/45). High expression was less frequent, found in 18.9% of males (20/106) and 13.3% of females (6/45). These findings highlight a predominant downregulation of AKR1C3 in GC across both sexes and suggest that sex-specific differences in AKR1C3 expression may contribute to the observed disparity in GC incidence and progression. Future studies should investigate how differential AKR1C3 expression affects prostaglandin metabolism in gastric cancer cells to better understand its role in the sex disparity in GC incidence. This could reveal how AKR1C3 influences hormone metabolism and contribute to the development of sex-specific therapies and personalized treatment strategies.
In summary, our findings suggest that AKR1C3 may function as a tumor suppressor in gastric cancer, consistent with previous reports such as Frycz et al.9. The downregulation of AKR1C3 in GC, which is strongly correlated with cancer progression and clinical outcomes, could potentially be harnessed as a valuable diagnostic and prognostic biomarker. While AKR1C3 inhibitors have shown promise in preclinical studies targeting several AKR1C3-proficient cancers, increasing the level or functional activity of AKR1C3 may represent another crucial treatment option for AKR1C3-deficient cancer types such as GC, where AKR1C3 functions as a tumor suppressor.
Materials and methods
TCGA sample information
The mRNA expression data and related clinical information for gastric cancer (GC) were downloaded from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov). Missing and uncertain clinical data were excluded, with the data extraction finalized in August 2022. Data extraction and merging were performed using Perl (version 5.3.0) ( https://www.perl.org/). Ultimately, we obtained 374 GC patient samples and 31 normal control samples from the TCGA database for our subsequent analyses. The TCGA database is publicly accessible, and all data are approved for analytical use, having obtained ethical approval. This study, based on open-source data, adheres strictly to the database’s release guidelines and access policies, thus not requiring additional ethical considerations.
AKR1C3 mRNA expression analysis
The analysis of AKR1C3 mRNA expression was based on the TCGA database using the package R ‘‘Limma’’, and the ‘‘ggplot2’’ package was used to draw a box plot for visualization using R software (https://cran.r-project.org/).
GSEA (gene set enrichment analysis)
The samples in TCGA were divided into high and low AKR1C3 expression groups based on the median level of AKR1C3 mRNA. Differential expression genes (DEGs) between these groups were identified using R package “DESeq2’’, with absolute logarithmic fold change (FC) > 0.58 and a false discovery rate (FDR) < 0.05 as the cut-off criteria48. Potential AKR1C3-regulated pathways were analyzed by GSEA. The “clusterProfiler” package in R software was utilized to investigate tumor hallmarks between the high and low AKR1C3 expression groups49, with visualizations generated using the ggplot2 package. A p-value < 0.05 and an FDR < 0.25 were considered statistically significant.
Clinical samples
A total of 151 human gastric cancer (GC) tissue specimens and their corresponding non-tumor gastric tissue counterparts were procured through surgical resections conducted at The Union Hospital of Fujian Medical University with IRB approval (2019KJCX052). None of the patients involved in this study had previously undergone chemotherapy or radiotherapy treatments prior to their surgical procedures. The tissue samples were promptly immersed in liquid nitrogen for rapid freezing and subsequently stored at a temperature of -80 °C until they were ready for further processing and analysis. All the patients participating in this study received signed written informed consent.
Cell culture
Two human GC cell lines, AGS and SNU-216, were obtained from the Chinese Academy of Sciences Committee Type Culture Collection Cell Bank (Shanghai, China) and Nanjing Cobioer Biosciences (Nanjing, China), respectively. The AGS cell line was derived from a gastric adenocarcinoma of a 54-year-old Caucasian female and is representative of an early-stage tumor50. The SNU-216 cell line was derived from the lymph node sample of a metastatic gastric carcinoma from a 46-year-old Korean female, representing an advanced stage of the disease51. AGS and SNU-216 cells were cultured in Dulbecco’s Modified Eagle’s Medium/F12 medium and RPMI-1640 medium (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum in a humidified incubator at 37 °C with 5% CO2. Cell lines were tested routinely for the absence of mycoplasma contamination by a MycoAlert detection kit (Lonza, Walkersville, MD, USA) and authenticated by short tandem repeat analysis every 6 months. The 293T cell line, derived from human embryonic kidney cells, was also used in this study. This cell line is widely used for its high transfection efficiency and was obtained from the Chinese Academy of Sciences Committee Type Culture Collection Cell Bank (Shanghai, China). 293T cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum in a humidified incubator at 37 °C with 5% CO2.
Tissue microarray and immunohistochemistry
A tissue microarray (TMA) was constructed with duplicate 1 mm cores using the aforementioned 151 GC and corresponding adjacent normal tissue samples. Immunohistochemistry (IHC) studies were performed by staining tissue sections using human anti-AKR1C3 antibody (1:200, Abcam, Cambridge, UK). The degree of AKR1C3 staining was quantified according to the following calculation: the score of stained tumor cells (0, ≤ 5% positive cells; 1, 5–25% positive cells; 2, 26–50% positive cells; 3, 51–75% positive cells; 4, ≥ 75% positive cells) multiplied with the score of staining intensity (0, no staining; 1, weak staining, light yellow; 2, moderate staining, yellow brown; 3, strong staining, brown) to obtain a final score ranging from 0 to 12. A final score of 3 or less was classified as low-expression group, while 4–12 as high-expression group.
RNA extraction and real-time quantitative PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNA was generated from 1 mg RNA using the PrimeScript™ RT reagent kit (Takara, Shiga, Japan) following the manufacturer’s instructions. Prime Script™ RT master mix was used for reverse transcription of RNA to produce first-strand cDNA, and then 10 pg cDNA and the SYBR Premix EX Taq kit (Takara, Shiga, Japan) were used for quantitative PCR (qPCR). β-actin was used as the internal control, and the 2−ΔΔCt method was used to calculate relative quantitation values.
Western blot analysis
Total cell lysates from various experiments were prepared by subjecting the tissues or cells to lysis using either RIPA buffer or Western & IP cell lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China. These lysis buffers were supplemented with the protease inhibitor PMSF (obtained from Amresco, Solon, Ohio, USA). The BCA Protein Assay kit (Beyotime Institute of Biotechnology, Shanghai, China) was used to determine the protein content. Proteins were separated via 10% SDS‒PAGE and transferred to a 0.45 μm PVDF membrane (AmershamHybond, GE Healthcare, München, Germany). The membranes were blocked with 3% BSA for 1 h at room temperature. Primary antibodies were incubated with the membrane at 4 °C overnight. Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. The protein bands were detected with enhanced chemiluminescence reagents (BeyoECL Star; Beyotime, Shanghai, China). Immunoblot imaging was performed, and ImageJ software was used to quantify Western blot band densities. The primary antibodies and dilution ratios were as follows: AKR1C3 (1:500, Abclonal, Wuhan, China), E-cadherin (1:1000, Santa Cruz Biotechnology), N-cadherin (1:1000, Cell Signaling), vimentin (1:1000, Cell Signaling), ZO-1 (1:1000, Cell Signaling), Snail (1:1000, Cell Signaling), Slug (1:1000, Cell Signaling), AKT (1:1000, Cell Signaling), p-AKT (Thr308) (1:1000,Cell Signaling), p-AKT (Ser473) (1:1000, Cell Signaling), JNK (1:1000, Cell Signaling), p-JNK(Thr183/Tyr185) (1:1000, Cell Signaling), NF-κB (p65) (1:1000, Cell Signaling), p-NF-κB (p-p65) (1:1000, Cell Signaling), STAT3 (1:1000, Cell Signaling), p-STAT3 (Tyr705) (1:1000, Cell Signaling), p38MAPK (1:1000, Cell Signaling), p42/44MAPK (1:1000, Cell Signaling), p-p42/44MAPK (1:1000, Cell Signaling), p-β-catenin(Ser33/37/Thr41) (1:1000, Cell Signaling), GSK3β (1:1000, Cell Signaling), p-GSK-3β (Ser9) (1:1000, Cell Signaling), MEK (1:1000, Cell Signaling), Notch1 (1:1000, Cell Signaling), GLI1 (1:1000, Cell Signaling), HIF-1α (1:1000, Cell Signaling), IKKα (1:1000, Cell Signaling), IKKβ(1:1000, Cell Signaling), IκBα(1:1000, Cell Signaling), p-IKKα/β (Ser176/180) (1:1000, Cell Signaling), p-IκBα (Ser32/36) (1:1000, Cell Signaling), GAPDH (1:2000, Cell Signaling), β-Tubulin (1:2000, Cell Signaling), and LaminB1(1:1000, Cell Signaling).
Plasmids and establishment of stably transduced GC cell lines
The human AKR1C3 (Gene ID: 8644) open reading frame was inserted into the eukaryotic lentiviral expression vector pCDH (Invitrogen Carlsbad, CA, USA). The recombinant lentiviral plasmids pCDH-AKR1C3 or the empty vector pCDH, along with the packaging plasmids (VSV-G, p-REV and pMDL), were co-transfected into 293 T cells to produce lentiviral particles. This transfection process was carried out using Lipofectamine-3000 (Invitrogen). The medium containing lentiviral particles was collected 48 h later and filtered through a 0.45 µm syringe filter. Subsequently, GC cells (AGS and SNU-216) were infected with the lentiviruses expressing AKR1C3 or control viruses. After another 48 h, puromycin at a concentration of 2 mg/ml was added to select stably transduced cells. This led to the establishment of stable AKR1C3-overexpressing clones (AGS-pAKR1C3 or SNU-216-pAKR1C3) and the control cells (AGS-pCDH or SNU-216-pCDH). For the generation of AKR1C3 knockdown clones, two shRNA sequences targeting different sites of AKR1C3 (sh1AKR1C3 and sh2AKR1C3) were synthesized and cloned into the pSuper-puro plasmid (Oligoengine, Seattle, USA). The recombinant plasmid pSuper-AKR1C3 or empty vector pSuper, which carries a non-targeting shRNA and the packaging plasmids pIK (Invitrogen) were cotransfected into 293T cells. The supernatants were collected and used to infect AGS or SNU-216 cells. After selection under puromycin, AKR1C3 knockdown cells (AGS-psh1AKR1C3, AGS-psh2AKR1C3, SNU-216-psh1AKR1C3, SNU-216-psh2AKR1C3) and control cells (AGS-pSuper or SNU-216-pSuper) were successfully established. The sequence of scramble shRNA was as follows: 5’-GCGAAAGATGATAAGCTAA. The shRNA-targeted sequences in AKR1C3 were as follows: sh1AKR1C3:5’-GATCCCCGGGCTTTGCCTGAT GTCTATTCAAGAGATAGACATCAGGCAAAGCCCTTTTTA-3’; sh2AKR1C3: 5’-GATCCCC GGACATATCACCTCTACTTTTCAAGAGAAAGTAGA G GTGATATGTCCTTTTTA-3’.
Cell proliferation assay
Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8, Dojindo, Kuma-moto, Japan). A total of 1 × 103 cells per well were plated in 96-well plates, and the CCK-8 assay was performed after 24, 48, 72, 96, 120–144 h. Ten microliters of CCK-8 solution was added and incubated at 37 °C for 2 h. The optical density (OD) values at 450 nm were recorded using a microplate reader (Bio-Tek, Winooski, VT, USA).
Colony formation assay
A total of 1,000 or 2,000 cells in complete medium were plated in 60-mm plates with DMEM/ F12 or RPMI-1640 containing 10% FBS and were then incubated at 37 °C and 5% CO2 for 2 or 3 weeks. After fixation with methanol for 10 min, the cells were dyed with crystal violet for 5 min. Then, the number of colonies that contained 50 or more cells was counted.
Cell migration and invasion assay
For the Transwell migration assays, 5 × 104 cells were plated in the upper chamber of a Transwell insert (8-mm pore size, BD Bioscience, USA). For the invasion assays, Transwell inserts were precoated with Matrigel (BD Bioscience) in the upper chamber, and 1 × 105 cells were seeded onto the upper chamber. The upper chamber was filled with 500 µl FBS-free medium, and the lower chamber was filled with 700 µl medium containing 10% FBS. Cells were incubated for 18 h for the migration assay and 24 h for the invasion assay and migration assay with the inhibitors at 37 °C with 5% CO2. Cells that had migrated or invaded to the lower surface of the filter were stained with 0.1% crystal violet in 20% methanol for 10 min, imaged, and counted using a Qimaging Micropublisher 5.0 RTV microscope camera (Olympus).
Wound-healing assay
Cells were seeded into 6-well plates and cultured to nearly 100% confluence. A 200 µl pipette tip was used to generate a linear wound of the same size. Cells were cultured at 37 °C in 5% CO2, and the wound widths were determined at 0, 24, 48, 72–96 h and photographed with a Qimaging Micropublisher 5.0 RTV microscope camera (Olympus).
Animal studies
All animal work performed was reviewed and approved by the Institutional Animal Care and Use Committee at Fujian Medical University (FJMU IACUC 2020-0073). All methods were carried out in accordance with relevant guidelines and regulations set forth by The Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International and are reported in accordance with ARRIVE guidelines. NCG mice were sourced from GemPharmatech Co., Ltd. (Nanjing, China) for an in vivo metastasis study. To simulate tumor metastasis, 1.5 × 106 cell suspensions of AGS-pSuper and AGS-psh1AKR1C3 (0.1 ml) were injected into the tail veins of six male NCG mice aged six weeks. The injections were administered on Day 0, Day 5, and Day 10, totaling three injections per mouse. Eight weeks post-injection, the mice were euthanized by exposure to gradually increasing concentrations of carbon dioxide, and the lungs were harvested to count the number of tumor nodules. This was accomplished through histopathologic analysis to evaluate the extent of metastasis.
Statistical analysis
Statistical analyzes were performed using R software v4.2.1 (https://cran.r-project.org/) and SPSS 22.0 (https://www.ibm.com/cn-zh/products/spss-statistics/). All data were from at least three independent experiments and presented as the mean ± standard deviation (SD). Student’s t test was used for pairwise comparisons between two groups while one-way analysis of variance (ANOVA) with Dunnett’s post hoc test was employed for comparisons among multiple groups to ensure appropriate statistical control for multiple comparisons. To evaluate differences in AKR1C3 expression levels between gastric cancer samples and noncancerous tissues from the TCGA database, the Wilcoxon rank sum test was applied, as it is suitable for non-normally distributed data. Pearson’s chi-square test was used to analyze the relationship between AKR1C3 expression and clinicopathological characteristics. Kaplan‒Meier analysis was performed to plot survival curves, with the log-rank test used to assess statistical significance between survival groups. P < 0.05 was considered statistically significant.
Data availability
All data generated or analyzed during this study are included in this manuscript.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Zhang, X. Y. & Zhang, P. Y. Gastric cancer: somatic genetics as a guide to therapy. J. Med. Genet. 54, 305–312. https://doi.org/10.1136/jmedgenet-2016-104171 (2017).
Xiao, X. Z., Lin, L. Y., Zhuang, M. K., Zhong, C. M. & Chen, F. L. Roles of AKR1C3 in malignancy. Chin. Med. J. (Engl). 134, 1052–1054. https://doi.org/10.1097/cm9.0000000000001379 (2021).
Penning, T. M. AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase/prostaglandin F synthase): Roles in malignancy and endocrine disorders. Mol. Cell. Endocrinol. 489, 82–91. https://doi.org/10.1016/j.mce.2018.07.002 (2019).
Downs, T. M., Burton, D. W., Araiza, F. L., Hastings, R. H. & Deftos, L. J. PTHrP stimulates prostate cancer cell growth and upregulates aldo-keto reductase 1C3. Cancer Lett. 306, 52–59. https://doi.org/10.1016/j.canlet.2011.02.027 (2011).
Wang, S., Yang, Q., Fung, K. M. & Lin, H. K. AKR1C2 and AKR1C3 mediated prostaglandin D2 metabolism augments the PI3K/Akt proliferative signaling pathway in human prostate cancer cells. Mol. Cell. Endocrinol. 289, 60–66. https://doi.org/10.1016/j.mce.2008.04.004 (2008).
Sun, S. Q. et al. Overexpression of AKR1C3 significantly enhances human prostate cancer cells resistance to radiation. Oncotarget 7, 48050–48058. https://doi.org/10.18632/oncotarget.10347 (2016).
Chang, T. S. et al. Expression of aldo-keto reductase family 1 member C3 (AKR1C3) in neuroendocrine tumors & adenocarcinomas of pancreas, gastrointestinal tract, and lung. Int. J. Clin. Exp. Pathol. 6, 2419–2429 (2013).
Frycz, B. A. et al. Transcript level of AKR1C3 is down-regulated in gastric cancer. Biochem. Cell. Biol. 94, 138–146. https://doi.org/10.1139/bcb-2015-0096 (2016).
Savagner, P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 21(Suppl 7), vii89-92. https://doi.org/10.1093/annonc/mdq292 (2010).
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196. https://doi.org/10.1038/nrm3758 (2014).
Li, W. et al. Melatonin induces cell apoptosis in AGS cells through the activation of JNK and P38 MAPK and the suppression of nuclear factor-kappa B: A novel therapeutic Implication for gastric Cancer. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 37, 2323–2338. https://doi.org/10.1159/000438587 (2015).
Liu, C. et al. RPS15A promotes gastric cancer progression via activation of the Akt/IKK-β/NF-κB signalling pathway. J. Cell. Mol. Med. 23, 2207–2218. https://doi.org/10.1111/jcmm.14141 (2019).
Efferth, T. & Oesch, F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med. Res. Rev. 41, 3023–3061. https://doi.org/10.1002/med.21842 (2021).
Arenzana-Seisdedos, F. et al. Nuclear localization of I kappa B alpha promotes active transport of NF-kappa B from the nucleus to the cytoplasm. J. Cell Sci. 110 (Pt 3), 369–378. https://doi.org/10.1242/jcs.110.3.369 (1997).
Karin, M. & Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu. Rev. Immunol. 18, 621–663. https://doi.org/10.1146/annurev.immunol.18.1.621 (2000).
Li, M. et al. AKR1C3 in carcinomas: From multifaceted roles to therapeutic strategies. Front. Pharmacol. 15, 1378292. https://doi.org/10.3389/fphar.2024.1378292 (2024).
Byrns, M. C., Steckelbroeck, S. & Penning, T. M. An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3alpha-HSD, type 5 17beta-HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem. Pharmacol. 75, 484–493. https://doi.org/10.1016/j.bcp.2007.09.008 (2008).
Yepuru, M. et al. Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 19, 5613–5625. https://doi.org/10.1158/1078-0432.Ccr-13-1151 (2013).
Cui, X. et al. Establishing a Proteomics-based signature of AKR1C3-related genes for predicting the prognosis of prostate cancer. Int. J. Mol. Sci. 24 https://doi.org/10.3390/ijms24054513 (2023).
Powell, K. et al. ERG/AKR1C3/AR constitutes a feed-forward loop for AR signaling in prostate cancer cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 21, 2569–2579. https://doi.org/10.1158/1078-0432.Ccr-14-2352 (2015).
Byrns, M. C. & Penning, T. M. Type 5 17beta-hydroxysteroid dehydrogenase/prostaglandin F synthase (AKR1C3): Role in breast cancer and inhibition by non-steroidal anti-inflammatory drug analogs. Chemico-Biol. Interact. 178, 221–227. https://doi.org/10.1016/j.cbi.2008.10.024 (2009).
Yin, Y. D. et al. The activity of SN33638, an inhibitor of AKR1C3, on testosterone and 17β-estradiol production and function in castration-resistant prostate cancer and ER-positive breast cancer. Front. Oncol. 4, 159. https://doi.org/10.3389/fonc.2014.00159 (2014).
Nakamura, Y. et al. Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J. Clin. Endocrinol. Metab. 94, 2192–2198. https://doi.org/10.1210/jc.2008-2374 (2009).
Rizner, T. L., Smuc, T., Rupreht, R., Sinkovec, J. & Penning, T. M. AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol. Cell. Endocrinol. 248, 126–135. https://doi.org/10.1016/j.mce.2005.10.009 (2006).
Wu, C. H. et al. Clinical implications of aldo-keto reductase family 1 member C3 and its relationship with lipocalin 2 in cancer of the uterine cervix. Gynecol. Oncol. 132, 474–482. https://doi.org/10.1016/j.ygyno.2013.11.032 (2014).
Park, S. et al. Inhibitory interplay of SULT2B1b sulfotransferase with AKR1C3 aldo-keto reductase in prostate cancer. Endocrinology 161 https://doi.org/10.1210/endocr/bqz042 (2020).
Yoda, T. et al. 11β-Prostaglandin F2α, a bioactive metabolite catalyzed by AKR1C3, stimulates prostaglandin F receptor and induces slug expression in breast cancer. Mol. Cell. Endocrinol. 413, 236–247. https://doi.org/10.1016/j.mce.2015.07.008 (2015).
Wang, B. et al. AKR1C3, a crucial androgenic enzyme in prostate cancer, promotes epithelial-mesenchymal transition and metastasis through activating ERK signaling. Urol. Oncol. 36, 472.e411-472.e420. https://doi.org/10.1016/j.urolonc.2018.07.005 (2018).
Zhou, Q. et al. A positive feedback loop of AKR1C3-Mediated activation of NF-κB and STAT3 facilitates proliferation and metastasis in hepatocellular carcinoma. Cancer Res. 81, 1361–1374. https://doi.org/10.1158/0008-5472.Can-20-2480 (2021).
Matsunaga, T. et al. Pathophysiological roles of autophagy and aldo-keto reductases in development of doxorubicin resistance in gastrointestinal cancer cells. Chemico-Biol. Interact. 314, 108839. https://doi.org/10.1016/j.cbi.2019.108839 (2019).
Li, Y. et al. ARID3A promotes the chemosensitivity of colon cancer by inhibiting AKR1C3. Cell. Biol. Int. 46, 965–975. https://doi.org/10.1002/cbin.11789 (2022).
Guise, C. P. et al. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res. 70, 1573–1584. https://doi.org/10.1158/0008-5472.Can-09-3237 (2010).
Wang, Y., Yang, J., Chen, S., Wang, W. & Teng, L. Identification and validation of a prognostic signature for thyroid cancer based on ferroptosis-related genes. Genes 13 https://doi.org/10.3390/genes13060997 (2022).
Morell, A. et al. Bruton’s tyrosine kinase inhibitors ibrutinib and acalabrutinib counteract anthracycline resistance in cancer cells expressing AKR1C3. Cancers 12 https://doi.org/10.3390/cancers12123731 (2020).
Wu, Z. et al. Upregulation of Tim-3 is associated with poor prognosis in acute myeloid leukemia. Cancer Med. 12, 8956–8969. https://doi.org/10.1002/cam4.5549 (2023).
Peraldo-Neia, C. et al. AKR1C3 is a biomarker and druggable target for oropharyngeal tumors. Cell. Oncol. (Dordr). 44, 357–372. https://doi.org/10.1007/s13402-020-00571-z (2021).
Lee, Y. J. et al. Cadmium-induced up-regulation of aldo-keto reductase 1C3 expression in human nasal septum carcinoma RPMI-2650 cells: Involvement of reactive oxygen species and phosphatidylinositol 3-kinase/Akt. Environ. Toxicol. Pharmacol. 31, 469–478. https://doi.org/10.1016/j.etap.2011.03.006 (2011).
Zhu, P. et al. Diagnostic and prognostic values of AKR1C3 and AKR1D1 in hepatocellular carcinoma. Aging 13, 4138–4156. https://doi.org/10.18632/aging.202380 (2021).
Azzarello, J. T. et al. Expression of AKR1C3 in renal cell carcinoma, papillary urothelial carcinoma, and Wilms’ tumor. Int. J. Clin. Exp. Pathol. 3, 147–155 (2009).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890. https://doi.org/10.1016/j.cell.2009.11.007 (2009).
Dongre, A. & Weinberg, R. A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 20, 69–84. https://doi.org/10.1038/s41580-018-0080-4 (2019).
Zhang, Y. & Weinberg, R. A. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front. Med. 12, 361–373. https://doi.org/10.1007/s11684-018-0656-6 (2018).
Mittal, V. Epithelial mesenchymal transition in tumor metastasis. Annu. Rev. Pathol. 13, 395–412. https://doi.org/10.1146/annurev-pathol-020117-043854 (2018).
Hashimoto, I. & Oshima, T. Claudins and gastric Cancer: An overview. Cancers 14, 290. https://doi.org/10.3390/cancers14020290 (2022).
Zhou, C. et al. Aldo-keto reductase 1C3 mediates chemotherapy resistance in esophageal adenocarcinoma via ROS detoxification. Cancers 13 https://doi.org/10.3390/cancers13102403 (2021).
Zhang, B. et al. PGD2/PTGDR2 signaling restricts the self-renewal and tumorigenesis of gastric cancer. Stem Cells (Dayton Ohio). 36, 990–1003. https://doi.org/10.1002/stem.2821 (2018).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics-a J. Integr. Biology. 16, 284–287 (2012).
Barranco, S. C. et al. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res. 43, 1703–1709 (1983).
Park, J. G. et al. Establishment and characterization of human gastric carcinoma cell lines. Int. J. Cancer. 70, 443–449. https://doi.org/10.1002/(sici)1097-0215(19970207)70:4<443::aid-ijc12>3.0.co;2-g (1997).
Acknowledgements
This study was supported by grants from Fujian Provincial Health Technology Project (2019-CX-14), Joint Funds for the Innovation of Science and Technology, Fujian Province (2019Y9083), Educational Research Project for Young and Middle-aged Teachers in Fujian Province (JAT200142) and and National Key Clinical Specialty Construction Projects of Fujian Province, China (grant number: 2023 − 1594).
Author information
Authors and Affiliations
Contributions
Liying Lin, Xinzhu Xiao and Xiaoxiong Guo prepared the material, performed experiments, analyzed data, as well as drafting and revision of the manuscript. Canmei Zhong, Mingkai Zhuang, Jie Xu, Yin Wang performed experiments. Fenglin Chen designed and supervised experiments as well as revised the paper. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, L., Xiao, X., Guo, X. et al. AKR1C3 mediates gastric cancer cell invasion and metastasis via the AKT and JNK/p-NF-κB signaling pathways. Sci Rep 14, 30263 (2024). https://doi.org/10.1038/s41598-024-82039-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82039-w