Abstract
The deregulation of immune responses is what causes food allergy (FA) to occur. FA’s cause is still unknown. The goal of this study is to investigate the mechanism how the impaired production of IL-10 occurs in peripheral naive B cells of patients with FA. Samples from patients with FA and healthy controls (HC) were used to isolate CD19+ CD45R+ naive B cells from peripheral blood mononuclear cells (PBMC). Lipopolysaccharide (LPS) exposure was used to assess the expression of interleukin-10 (IL-10) in B cells. Although the FA and HC groups had similar total B cell counts, the FA patients had fewer IL-10+ B cell counts than the HC group. In peripheral B cells, the concentrations of IL-10 were inversely related to the concentrations of specific IgE, Th2 and Th1 cytokines in the serum. In patients with FA, peripheral B cells experienced impaired immune-suppressive functions. Galectin-9 could restore the defective induction of IL-10 expression in naive B cells of FA patients. In conclusion, FA patients with naive B cells experience impaired IL-10 induction. The induction of IL-10 in naive B cells of FA patients can be restored by galectin-9 treatment, which triggers B cells to differentiate into immune regulatory B cells.
Similar content being viewed by others
Introduction
Food allergy (FA) is an adverse response to food antigens by the immune system in the intestine. FA’s clinical symptoms are unique to each individual. It can be slight abdominal discomfort, diarrhea, severe abdominal pain, or even the life-threatening anaphylactic shock1. The current treatment for FA mainly focuses on controlling the clinical symptoms. The prevalence of FA in general populations is about 2–8% worldwide2. The social economy and human health have been greatly affected by FA3.
The consensus is that FA is a disease that affects the immune system4. FA has a pathological feature that involves Th2 cell polarization. Th2 cells that are polarized in FA aggregate in the intestinal tissues to produce too much Th2 cytokines, as indicated by their polarization. In general, dendritic cells present antigens to CD4+ T cells, which further induce plasma cells to produce IgE5. IgE is able to sensitize mast cells by binding to the high affinity receptor of IgE. The release of allergic mediators by sensitized mast cells after exposure to specific antigens leads to FA attacks2. In general, the immune regulatory system in the body is responsible for tightly controlling the activities of other immune cells. According to reports6, the immune regulatory system is dysfunctional due to the polarization status of Th2 cells in FA subjects.
In the body, the two major types of immune regulatory cells are regulatory T cells (Treg cells) and regulatory B cells (Breg cells), which have been well studied. It has been established that Treg cells or Breg cells in FA subjects are in a dysfunctional state6. In subjects with allergic diseases, it has been attempted to restore immune regulatory functions through the use of probiotics, curcumin, or adoptive transfer of Treg cells7. It is reported that galectin-9 (Gal9) is associated with the maintenance of immune homeostasis8 and limits immune inflammation9. Thus, we hypothesize that administration of Gal9 may restore the immune regulatory functions of Bregs. The results obtained from those approaches have been positive. Despite efforts to decrease the prevalence of FA and other allergic diseases in recent decades, they have continued to rise2. The maintenance of homeostasis in the intestine is a significant role played by Breg cells. A previous report10 has acknowledged that Breg cells in FA are deregulated. It is necessary to further explore the factors that lead to Breg cell dysfunction. This study demonstrated that FA patients have impaired the ability to incite IL10 expression in naive B cells. The immune-suppressive capacity of B cells in FA patients were defective. IL10 expression in FA Breg cells could be restored after being exposed to Gal9.
Results
Lower frequency of IL-10+ B cells was found in FA patients
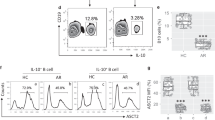
30 FA patients and 30 HC subjects provided the blood samples for PBMC preparation. The PBMCs were analyzed using flow cytometry (FCM). The frequency of B cells was the same in FA patients and HC subjects, but there was a significant difference in the number of IL-10+ B cells between the FA group and the HC group (Fig. 1A–F). In FA B cells (B cells obtained from FA patients), the median fluorescence intensity, MFI of IL-10 staining, was even lower than that in HC B cells (Fig. 1G). FA B cells released IL-10 at a significantly lower level than HC B cells (Fig. 1H). The supernatant showed the presence of IL-35 and TGF-β, but their concentrations did not differ significantly between the HC group and the FA group (Fig. 1I,J). Moreover, the annexin V staining (to detect apoptotic cells) was not detectable in IL-10+ B cells from both FA and HC groups (Fig. 1K,L). IL10 expression in peripheral B cells in FA patients is lower according to the results.
The frequency of IL-10+ B cells is less in FA patients. PBMCs were prepared with blood samples collected from FA patients (n = 30) and HC subjects (n = 30), and analyzed by FCM. (A) dead cells were gated out. (B) adherent cells were gated out. (C) B cells are gated. (D) counts of B cells. (E) IL-10+ B cells were gated. (F) counts of IL-10+ B cells. (G) amounts of IL-10 MFI in IL-10+ B cells. (H–J) B cells were isolated from PBMCs, and exposed to PMA/ionomycin in culture overnight (at 106 cells/ml). Bars show the amounts of indicated cytokines in culture supernatant. (K) apoptotic IL-10+ B cells were gated. (J) counts of apoptotic IL-10+ B cells. (K,L) quantities of IL-35 and TGF-β in the serum. The data of bars are presented as mean ± SD. Each dot in bars presents one sample. Statistics: Mann Whitney test. p values are presented in figures where appropriate. HC healthy control, FA food allergy, PBMC peripheral blood mononuclear cell, FCM flow cytometry, MFI median fluorescence intensity.
The amounts of IL-10 in B cells are associated with the FA associated cytokines and IgE in the serum
The serum was analyzed for the amounts of FA-associated cytokines and specific IgE (sIgE). The FA group showed a higher level of serum sIgE, eosinophil peroxidase (EPX), tryptase, IL-4, IL-5, and IL-13 than the HC group. The FA group had a lower level of IL-10. The HC group and FA group had similar amounts of IFN-γ (Fig. 2A). The amounts of IL-10 in peripheral B cells and serum were found to have a positive correlation. The levels of IL-10 MFI in B cells were negatively correlated with those of serum sIgE, IL-4, IL-5, and IL-13. The concentrations of serum IFN-β were not associated with the concentrations of IL-10 in peripheral B cells (Fig. 2B). The findings indicate that the levels of IL-10 in peripheral B cells could be linked to the pathogenesis of FA.
IL-10 amounts in peripheral B cells show relation with FA associated elements. The serum was isolated from blood samples collected from FA patients (n = 30) and HC subjects (n = 30), and analyzed by immunoCap (sIgE) and ELISA (the cytokines). (A) bars show means ± SD of the amounts of sIgE and indicated cytokines in the serum. (B) a heatmap shows correlation between IL-10 MFI (data are presented in Fig. 1E) and the FA associated elements. The coefficients (in white) are presented in each square. Statistics: Mann Whitney test (A) and Spearman correlation coefficient test (B). p values are presented in figures where appropriate. FA food allergy, HC healthy control, sIgE specific IgE, MFI median fluorescence intensity, IFNg IFN-γ.
The immune suppressive ability of FA B cells is impaired
The evaluation of peripheral pan B cells’ immune suppressive functions was done. B cells release IL-10 when exposed to non-specific cell activators (PMA/ionomycin). ELISA was used to determine the concentration of IL-10 in the culture supernatant. According to the results, the HC group had higher levels of IL-10 than the FA group. Effector immune cells (EICs) were prepared from the intestinal single cell suspension, from which B cells were removed by FCM cell sorting. Cytokines are released into the culture supernatant by EICs after exposure to PMA/ionomycin. In the FA group, there was a significant increase in the supernatant of cytokine sets, including EPX, ECP, MBP, IL-4, IL-5, and IL-13, compared to the HC group. The release of cytokines was effectively suppressed by adding HC B cells to EIC culture. The addition of FA B cells did not result in such an effect. JES5-2A5, a neutralizing anti-IL-10 antibody, was used to eliminate the suppressive functions of HC B cells (Fig. 3). FA B cells have impaired immune suppressive functions, as evidenced by the results (Fig. 3). It is likely that the impairment of IL-10 induction in B cells is the cause of it.
FA B cells are incompetent to suppress the cytokine release from EICs. B cells were isolated from FA PBMCs by FCM cell sorting; the remaining cells are used as EICs. EICs were treated with the conditions denoted on the X axis of bar graphs. The ratio of B cells and EICs was 1:4. The culture supernatant was collected 24 h later, and analyzed by ELISA. Bars show mean ± SD of the amounts of indicated molecules in supernatant. Each dot in bars presents one sample. Statistics: ANOVA + Bonferroni test. p values are presented in figures where appropriate. HC healthy control, FA food allergy, Activator: PMA (50 ng/ml)/ionomycin (100 ng/ml), HC.BC B cells isolated from PBMCs of HC subjects, FA.BC B cells isolated from PBMCs of FA patients, EICs effector immune cells (mainly including eosinophils and T cells), FCM flow cytometry, Ab A neutralizing anti-IL-10 Ab (100 ng/ml).
The inducibility of IL10 expression in FA B cells is defective
Our next step was to assess the induction of IL10 expression in naive B cells. IL10 mRNA was detected in HC B cells in a dose-dependent manner after two days of exposure to gradient concentrations of LPS in culture. The culture supernatant showed an increase in IL-10 levels. FA B cells only experienced a slight change in IL-10 expression, which was significantly less than that in HC B cells (Fig. 4A,B). Prompted by previous reports that MAF is critical for the IL10 gene transcription11, we examined the MAF levels in B cells. We found that LPS caused a significant increase in MAF mRNA, MAF protein, and Pol II in the IL10 promoter in HC B cells, but only a slight change in FA B cells (Fig. 4C–E). The findings indicate that the induction of IL10 expression in FA B cells is not functioning properly.
Induction of IL-10 in B cells of FA patients is impaired. (A,B) CD19+CD45R+ naïve B cells were isolated from PBMCs of HC subjects (n = 10) and FA patients (n = 10), and exposed to LPS at indicated concentrations for two days. Bars show mean ± SD of IL10 mRNA levels in B cells (A) and IL-10 in culture supernatant (B). (C−E) naïve B cells were stimulated by LPS (10 µg/ml) or PBS for 48 h and analyzed by indicated procedures. Bars show mean ± SD of MAF mRNA (C), MAF (D) or Pol II (E) in IL10 promoter locus of B cells. Each dot in bars presents one sample. Statistics: ANOVA + Bonferroni test (A,B) or Student’s t-test (C–E). p values are presented in figures where appropriate. HC healthy control, FA food allergy, FA B cell B cell of FA patients, HC.BC B cells of HC subjects, FA.BC B cells of FA patients.
Galectin-9 (Gal9) restores the inducibility of IL1it0 expression in FA B cells
Naive B cells were isolated from FA patients’ PBMCs and cultured overnight with Gal9 or both Gal9 and LPS. Gal9 exposure resulted in an increase in the expression of CMIP, MAF, and IL10 in FA B cells, as shown in the results. The expression of IL10 increased further when exposed to Gal9 and LPS concurrently, which was not observed in CMIP or MAF (Fig. 5A–C). CMIP was investigated as a factor in the increase in Gal9-increased expression of MAF and IL10 in FA B cells. CMIP is a critical factor in the induction of MAF as reported by previous studies11, we wondered if it also played a role in the Gal9-induced MAF production. To inhibit the expression of CMIP, the expression of CMIP was knocked down by treating a portion of FA B cells with CMIP RNA interference (RNAi; Fig. 5D). Gal9 did not have any effect on the CMIP¯ FA B cells (Fig. 5B,C). According to the results, Gal9 can restore the induction of IL10 expression in FA B cells.
Gal9 restores the IL10 expression in B cells of FA patients. Naïve B cells were isolated from PBMCs of FA patients, and treated with the conditions denoted on the X axis overnight. Bars show mean ± SD of the amounts of CMIP mRNA (A), MAF mRNA (B), and IL10 mRNA in B cells. Each dot in bars presents one sample. Statistics. ANOVA + Bonferroni test. p values are presented in figures where appropriate. (D) bars show CMIP mRNA in B cells treated with CMIP RNAi. LPS:10 µg/ml. Gal9:5 µg/ml. FA food allergy, PBMC peripheral blood mononuclear cell, RNAi RNA interference.
Gal9 confers on FA B cells the potential to acquire the immune suppressive capacity
From FA patients’ PBMCs, we made naive B cells and EICs (known as FA B cells and FA EICs). Gal9 incubation overnight was used to condition naive FA B cells, and they were then cocultured with FA EICs in the presence of PMA/ionomycin for two days. ELISA was employed to analyze the supernatant. EICs induced EPX, IL-4, IL-5, and IL-13 to be released into culture supernatant, as evidenced by the results (Fig. 6). FA EICs’ cytokines were effectively suppressed by FA B cells that were conditioned with Gal9 (Fig. 6). The results suggest that Gal9 has the potential to give naive FA B cells the ability to suppress the immune system.
Gal9 restores the immune regulatory capacity of B cells from FA patients. Naïve B cells and EICs were prepared with PBMCs of FA patients. The experimental setting is denoted on the X axis of bar graphs. Culture supernatant was collected 2 days later, and analyzed by ELISA. Bars present mean ± SD of indicated molecules. Each dot in bars presents one sample. Statistics: ANOVA + Bonferroni test. p values are presented in figures where appropriate. FA food allergy, FA B cell naïve B cell collected from FA patients. EIC effector immune cell, PBMC peripheral blood mononuclear cells. Gal9 FA B cells were incubated with Gal9 (5 µg/ml) overnight. Activator: PMA (50 ng/ml)/ionomycin (100 ng/ml).
Discussion
In the present study, it was discovered that IL-10+ B cells are present in peripheral blood of FA patients at a low frequency. The serum levels of FA-associated cytokines were correlated with the lower expression of IL10 in B cells. This indicates that the pathogenesis of FA is linked to the expression of IL-10 in peripheral B cells. FA B cells were unable to perform their immune-suppressive functions. Exposure to Gal9 could restore the defective induction of IL10 expression in FA B cells. The acquisition of the immune suppressive ability could be facilitated by Gal9 for FA B cells.
The EIC was used as an ex vivo experimental platform to test the immunosuppressive capacity of B cells. All immune cells in the blood are included in EICs, except for B cells. The release of immune cytokines by EICs was triggered by PMA/ionomycin, a non-specific cell activation agent. The culture supernatant was found to contain EPX, MBP, ECP, IL-4, IL-5, and IL-13 in the current case. The activators, PMA, and ionomycin can trigger the release of these cytokines by EICs. These cytokines have been documented as having significant roles in the pathogenesis of FA in previous studies12.
The current investigation is centered around the immune regulatory capability of peripheral B cells in individuals with FA. This role is generally agreed upon to be attributed to regulatory B cells (Breg cells)10. Breg cells are divided into multiple types according to10. The functional element of IL-10 is essential for the immune regulatory activities of these Breg cells, as shown in 10 13. The study of immune regulation has focused more on the expression of IL-10 in B cells13. The present evidence indicates that FA patients have a lower number of IL-10 B cells. The amount of IL-10 released by FA B cells is significantly less than that of HC B cells. FA B cells’ sources of IL-10 are defective, as suggested by the phenomenon.
Previous studies have indicated that patients with allergic and autoimmune diseases exhibit a lower prevalence of IL-10 B cells14. In animal model studies, similar phenomena were discovered15. Our findings are consistent with the initial research that demonstrates a reduction in the number of IL-10 B cells and a reduction in IL-10 expression in individuals with FA. Our findings are in accordance with those pioneering studies and demonstrate a lower prevalence of IL-10 B cells and low levels of IL-10 expression in patients with FA. This phenomenon has two possible explanations. IL-10+ B cells in FA patients are capable of apoptosis, which is one of the reasons. The comparison of apoptotic IL-10+ B cells between the HC group and the FA group makes it possible to exclude this possibility in the present study. Naive B cells are responsible for producing effector B cells. IL10 expression in naive B cells is crucial for the development of IL-10+ B cells. The current study revealed an impairment in the induction of IL-10 expression in naive B cells of FA patients. This phenomenon can be attributed to the abnormal decay of IL10 mRNA14,16. The current data indicates that FA B cells have lower levels of MAF mRNA. The IL10 gene is transcriptionally regulated by MAF17. According to the data, Gal9 exposure results in a rise in CMIP expression. According to previous reports17, CMIP is crucial for MAF’s expression. The expression of CMIP is first raised by Gal9. Inducing IL-10 production is a benefit to the restoration of immune regulatory functions of Bregs, which is facilitated by the latter’s promotion of MAF expression.
The importance of finding solutions to restore IL10 expression in FA B cells cannot be overstated. Gal9 has been shown to restore the ability to induce IL10 expression in FA B cells, according to the data. Previous reports indicate that regulation of B cell signaling can be done by Gal9 by binding to IgD/IgM-BCR18; current data suggest that IgE-BCR binding may be also involved. The suppression of immune diseases can be attributed to Gal9’s ability to regulate cells17. According to our data, Gal9 is capable of restoring the induction of IL10 in FA-naive B cells. Gal9 has been found to promote the expression of CMIP in B cells through a specific mechanism. This was not the case with B cells treated with LPS. The ability to induce IL-10 expression was acquired by FA naive B cells after combining LPS and Gal9. This is significant because it allows FA B cells to develop immune-suppressive abilities. Effective immune-suppressive functions were demonstrated in this study by Gal9 and LPS treatment of FA B cells.
The activities of Gal9 in immune regulation have been shown to have different results in previous studies, which is worth noting. In Hayen et al.‘s study19, they found that galectin-9 decreased anti-IgE-mediated basophil degranulation. The role of galectin-3 and galectin-9 in food allergy has been suggested by Steinert et al.20. According to our recent reports, the intestinal epithelium has low Gal9 expression21. These data suggest that there are multiple factors that impact Gal9. It can be connected to a variety of other events through its functions. According to current data, Gal9 and LPS can work together to restore the immune regulatory functions in Bregs from patients with FA. The role of Gal9 in immune regulation appears to be complex and requires further investigation.
Taken together, the immune-suppressive abilities of peripheral B cells are diminished in individuals with FA. Gal9 can restore the defective induction of IL10 expression in FA naive B cells.
Materials and methods
Reagents
Antibodies (Abs) of CD19 (Cat#: sc-390244, fluorochrome: AF488), IL-10 (sc-8438, AF594), CD45R (sc-19597, AF647), IgD (sc-53345, AF546), MAF (sc-293420), and Pol II (sc-21751) were purchased from Santa Cruz Biotech (Santa Cruz, CA). IgM Ab (743889, APC) was purchased from BD Bioscience (Franklin Lakes, NJ). CMIP Ab, galectin-9 protein, ELISA kits of EPX, MBP, ECP, tryptase, IL-4, IL-5, IL-13, IFN-γ, IL-35, TGF-β, and IL-10 were purchased from Dakewe BioMart (Shenzhen, China). Neutralizing anti-IL-10 Ab (JES5-2A5, ab189392) was purchased from abcam. Reagents and materials for RT-qPCR, Western blotting, and ChIP were purchased from Invitrogen (Carlsbad, CA). Annexin V kit was purchased from Sigma Aldrich (St. Louis., MO).
Ethics statement
The Human Ethics Committee at Zhengzhou University approved the study protocol with Approval Number H2022032. All experiments were performed in accordance with the Helsinki Declaration, local and national regulations. Each human subject signed a written informed consent.
Enrollment of human subjects
This study was conducted at our hospital and included FA patients. Our physicians used our routine procedures to diagnose FA. Patients who had FA for over two years and tested positive for serum specific IgE and skin allergens were diagnosed with FA. Patients who had severe organ diseases, autoimmune diseases, cancers, and those under treatment with immune suppressors for any reason were not included. Additionally, this study included healthy control (HC) subjects who were aged and gendered alike. In Table 1, the demographic data of human subjects is presented. At the remission stage, samples were collected from IgE-dependent FA patients.
Preparation of peripheral blood mononuclear cells (PBMCs)
Blood samples were obtained from each human subject by the ulnar vein puncture. PBMCs were isolated from the blood samples using the Percoll gradient density centrifugation.
Cell culture
RPMI1640 medium was used to culture cells. The medium was supplemented with 10% fetal calf serum, 0.1 mg/ml streptomycin, 100 U/ml penicillin, and 2 mM L-glutamine. Cell viability exceeded 99% as assessed using the Trypan blue exclusion assay. To analyze IL-10+ B cells, brefeldin A was added to the culture at 10 µg/ml for the last 4 h.
Flow cytometry (FCM)
The surface markers of cells were incubated with relevant Abs (labeled with fluorescence, diluted to 1 µg/ml) or isotype IgG for 30 min at 4 °C. After washing with FCM buffer [phosphate-buffered saline (PBS) containing 2% bovine serum albumin (BSA)] three times. A flow cytometer (BD FACSCanto II) was used to analyze the cells. To stain the intracellular molecules, cells were fixed with 1% paraformaldehyde (containing 0.05% Triton X-100) for 1 h. After washing with PBS, cells were processed using the same procedures for surface staining. The data were processed using a software package Flowjo (TreeStar Inc., Ashland, OR) using the data obtained from isotype IgG staining as the reference for gating. The ViD+ dead cells were gated out first. Then the adhesive cells were gated out under the FSC-A and FSC-H condition. The remainder cells were analyzed further.
Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR)
RNA was extracted from B cells harvested from relevant experiments. The RNA samples were converted to cDNA using a reverse transcription kit following the manufacturer’s instruction. The cDNA samples were amplified in a qPCR device (Bio Rad CFX96) with the SYBR Green Master Mix in the presence of specific primers, including IL10 (ccaagctgagaaccaagacc and aaggcattcttcacctgctc), MAF (aaggaggaggtgatccgact and tctcctgcttgaggtggtct), and CMIP (ccagtttgcttcaacccatt and gtaacaggagcccatgagga). The results were calculated with the 2−ΔΔCt method, and were presented as relative expression (RE) against the housekeeping gene ACTB.
Isolation of naïve B cells from PBMCs
PBMCs were stained with Abs of CD19-AF488, CD45R-AF647, IgD-AF546, and IgM-APC as described in FCM procedures. The CD19+CD45R+IgD+IgM+ naïve B cells were sorted with the FCM cell sorting procedures (BD Aria). The purity of isolated cells was exceeded 95% as checked by FCM.
Assessment of apoptotic IL-10+ B cells
In the procedures of assessing IL-10+ B cells by FCM, cells were co-stained with annexin v reagents following the manufacturer’s instruction. The annexin v+ cells were regarded as apoptotic cells.
Chromatin immunoprecipitation (ChIP)
The DNA and surrounding proteins were cross-linked by fixing B cells collected from relevant experiments with 1% formalin for 15 min. After lysing cells with a lysis buffer, the DNA was sheared into small pieces using an ultrasonic shear. Protein A/G agarose beads incubated for 2 h to preclear the pre-existing immune complexes in the lysates. The beads were eliminated. To anchor the IL10 promoter overnight, the IL10 promoter was incubated with an Ab of Maf, which is the transcription factor of the IL10 gene. Protein A/G agarose beads were used to precipitate immunocomplexes for 2 h. Centrifugation was used to collect the beads. An eluting buffer was used to elute the protein-DNA on the beads. DNA was recovered using a DNA extracting kit, and analyzed by qPCR in the presence of a pair of IL10 promoter primers (ggaaggtgaaggctcaatca and ttgtcttggatttgtgtgtgtg). The procedures were conducted at a temperature of 4 °C.
Assessment of the immune suppressive functions of B cells
The isolation of pan B cells or CD45R-positive B cells from PBMCs was done using FCM cell sorting procedures as described below. EICs (effector immune cells) were made up of the remaining cells of PBMCs. Non-cell activators, PMA (50 ng/ml) and ionomycin (100 ng/ml), were used in coculturing B cells with EICs at a ratio of 0.4 × 105:1.6 × 105/well in a 96-well plate. The culture lasted overnight. ELISA was employed to analyze the supernatant. In accordance with the manufacturer’s instructions, ELISA was used to determine the amounts of eosinophil peroxidase (EPX), major basic protein (MBP), eosinophil cationic protein (ECP), IL-4, IL-5, and IL-13 in the supernatant.
Induction of IL-10 in B cells
FCM cell sorting procedures were used to isolate naive B cells (CD19+CD45R+IgD+IgM+) from PBMCs. B cells were cultured in the presence of LPS at gradient concentrations or 10 µg/ml, or Gal9 (5 µg/ml) for two days. B cells had IL10 mRNA that was determined by RT-qPCR, while culture supernatant had IL-10 that was determined by enzyme-linked immunosorbent assay.
Statistics
Using the R packages (V4.4.1), Student’s t-test or Mann Whitney test were used to determine the difference between two groups. The ANOVA + Bonferroni test was used to conduct multiple comparisons for data from more than two groups. A significant criterion was determined by setting p < 0.05.
Data availability
Data are available upon request to Dr. Pingchang Yang.
References
Cosme-Blanco, W., Arroyo-Flores, E. & Ale, H. Food allergies. Pediatr. Rev. 41, 403–415 (2020).
Sicherer, S. H. & Sampson, H. A. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 141, 41–58 (2018).
Dierick, B. J. H. et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev. Pharmac. Outcomes Res. 20, 437–453 (2020).
Eiwegger, T., Hung, L., San Diego, K. E., O’Mahony, L. & Upton, J. Recent developments and highlights in food allergy. Allergy 74, 2355–2367 (2019).
Anvari, S., Miller, J., Yeh, C. Y. & Davis, C. M. IgE-mediated food allergy. Clin. Rev. Allergy Immunol. 57, 244–260 (2019).
Turner, P. J., Regent, L., Jones, C. & Fox, A. T. Keeping food-allergic children safe in our schools-time for urgent action. Clin. Exp. Allergy 50, 133–134 (2020).
Giganti, G. et al. Treg cell therapy: how cell heterogeneity can make the difference. Eur. J. Immunol. 51, 39–55 (2021).
Golden-Mason, L. & Rosen, H. R. Galectin-9: diverse roles in hepatic immune homeostasis and inflammation. Hepatology 66, 271–279 (2017).
Wang, W. et al. Galectin-9 targets NLRP3 for autophagic degradation to limit inflammation. J. Immunol. 206, 2692–2699 (2021).
Satitsuksanoa, P., Jansen, K., Głobińska, A., van de Veen, W. & Akdis, M. Regulatory Immune mechanisms in tolerance to food allergy. Front. Immunol. 9, 2939 (2018).
Liu, M. et al. Transcription factor c-Maf is essential for IL-10 gene expression in B cells. Scand. J. Immunol. 88, e12701 (2018).
Makiya, M. A. et al. Distinct CRTH2 + CD161+ (peTh2) memory CD4 + T-cell cytokine profiles in food allergy and eosinophilic gastrointestinal disorders. Clin. Exp. Allergy 53, 1031–1040 (2023).
Wang, L. et al. Piper nigrum extract attenuates food allergy by decreasing Th2 cell response and regulating the Th17/Treg balance. Phytother Res. 35, 3214–3225 (2021).
Tian, G. X. et al. CD38(+) B cells affect immunotherapy for allergic rhinitis. J. Allergy Clin. Immunol. 149 1691 – 701.e9 (2022).
Noh, J. et al. Tolerogenic effects of interferon-gamma with induction of allergen-specific interleukin-10-producing regulatory B cell (Br1) changes in non-IgE-mediated food allergy. Cell. Immunol. 273, 140–149 (2012).
Zeng, H. T. et al. Vasoactive intestinal peptide alleviates food allergy via restoring regulatory B cell functions. Immunobiology 224, 804–810 (2019).
Xu, X. et al. An association between elevated telomerase reverse transcriptase expression and the immune tolerance disruption of dendritic cells. Cell. Commun. Signal. 22, 284 (2024).
Cao, A. et al. Galectin-9 binds IgM-BCR to regulate B cell signaling. Nat. Commun. 9, 3288 (2018).
Hayen, S. M. et al. 2S protein Ara h 7.0201 has unique epitopes compared to other Ara h 7 isoforms and is comparable to 2S proteins Ara h 2 and 6 in basophil degranulation capacity. Clin. Exp. Allergy 48, 890–897 (2018).
Steinert, C. et al. Soluble IgE-binding factors in the serum of food-allergic patients: possible pathophysiological role of soluble FcεRI as protective factor. Clin. Transl Allergy 13, e12222 (2023).
Li, L. et al. Galectin-9 in synergy with NF-κB inhibition restores immune regulatory capability in dendritic cells of subjects with food allergy. Clin. Exp. Immunol. 213, 155–163 (2023).
Acknowledgements
This study was supported by research grants of the National Natural Science Foundation of China (32090052, 82405301), Shenzhen Key Medical Discipline Construction Fund (No.SZXK062), and Shenzhen science, technology, and innovation committee (SGDX20201103095609027); China Postdoctoral Science Foundation (Grant Number: 2023M740837, 2024M750659); Shenzhen Medical Research Fund (Grant Number: A2403058).
Author information
Authors and Affiliations
Contributions
Huang H, Li M, Song S, Feng S, Feng X, and Liu Y performed experiments, analyzed data, and reviewed manuscript. Yang P, Zheng P organized the study, supervised experiments. Yang P designed the project and prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, H., Li, M., Song, S. et al. Galectin 9 rescues the inducibility of IL-10 expression in regulatory B cells of patients with food allergy. Sci Rep 15, 196 (2025). https://doi.org/10.1038/s41598-024-84079-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84079-8