Abstract
Nephrogenesis is completed before term birth, but preterm infants continue this process postnatally. It is unknown if preterm neonates are more susceptible to acute kidney injury (AKI) immediately after birth (during nephrogenesis) or later in postnatal development, and if this AKI timing impacts nephron number. Rabbits were exposed to gentamicin (100 mg/kg intraperitoneal) and indomethacin (5 mg/kg orally) on postnatal day (P) 0–3 (early-exposed) or P6-9 (late-exposed). Animals were euthanized three hours after last nephrotoxin dose or at 6 weeks of life. Histologic injury was assessed, and Kidney injury marker 1 (Kim1) expression was quantified. At 6 weeks, blood urea nitrogen (BUN) and serum creatinine (SCr) levels were measured. Ex vivo MRI imaging was performed with automated quantitation of nephron numbers. AKI was induced in early and late-exposed rabbits. At 6 weeks, we identified a mean of 170,972 glomeruli in the controls (n = 5), 159,655 in the early-exposed (n = 4), and 145,748 glomeruli in the late-exposed group (n = 3, a 14.8% reduction compared to control, p = 0.01). Late-exposed rabbits had elevated BUN and SCr relative to early-exposed. This study suggests that exposure to nephrotoxins during early postnatal nephrogenesis causes AKI but may have less impact on long-term nephron number than exposure during nephron maturation.
Similar content being viewed by others
Introduction
Preterm infants are vulnerable to acute kidney injury (AKI) due to frequent exposure to nephrotoxins in the neonatal intensive care unit (NICU)1,2,3. While nephrogenesis is typically completed before birth, preterm infants must continue this process postnatally4,5. Over 90% of all neonates under 28 weeks gestation are exposed to nephrotoxic medications6including known nephrotoxic agents such as gentamicin and indomethacin. However, the impact of these nephrotoxic exposures on the developing nephrogenic niche in the postnatal environment is poorly understood. AKI occurs in 38–50% of all preterm neonates1,7. Preterm birth is also associated with reduced nephron endowment and increased risk of chronic kidney disease (CKD)8,9,10,11,12,13,14,15,16,17,18. While previous literature suggested that postnatal nephrogenesis is truncated in the ex utero environment5more recent work suggests that preterm infants can continue nephrogenesis longer than previously reported, although nephrogenesis still ends 2 weeks earlier than age-matched in-utero controls with associated tubular and glomerular stress19. In adult mice studies, approximately 80% of proximal tubule (PT) recover after AKI through a process of dedifferentiation and regeneration within 2 weeks of AKI. The approximately 20% of PT that don’t recover exhibit the “failed repair” proinflammatory and profibrotic phenotype 2 weeks after injury20,21. The ability of developing neonatal PT to repair after injury, however, has not been well studied. Very little is understood regarding AKI in the neonatal setting, particularly with respect to development as a biological variable, the relative sensitivity to nephrotoxic injury, the capacity of immature proximal tubules (PT) to be injured and subsequently repair, and risk of progression to CKD. Given the increasing prevalence of preterm birth22 and the importance of early developmental programming on an individual’s long-term health11,14it is imperative to better define neonatal AKI mechanisms and potentially modifiable factors impacting lifetime risk for CKD.
In this study, we evaluated the impact of the timing of a nephrotoxic exposure during postnatal kidney development on the severity of both AKI and CKD. We utilized a rabbit model of postnatal nephrogenesis to simulate the period of postnatal nephrogenesis in the preterm infant, comparing postnatal kidney development in the human at ~ 24–32 weeks gestation(wg) to 34–40 wg. The rabbit was chosen both for its prolonged postnatal nephrogenesis relative to the mouse and its demonstration of arcading nephrogenesis further supporting its translatability to humans23. The nephron progenitor population in the rabbit is depleted by postnatal day 5 (P5), with ongoing glomerular and tubular maturation until P10 23. A recent publication by Charlton et al. found that rabbits exposed to gentamicin and indomethacin at 1 week of life resulted in a 63% reduction in nephron number at 6 weeks of life24. Based on our studies evaluating the timing of postnatal rabbit nephrogenesis, this period of insult occurred during nascent nephron maturation after depletion of the nephron progenitor pool. We hypothesized that rabbits exposed to nephrotoxic drugs during active nephrogenesis (P0–P3) would have lower nephron numbers at 6 weeks of age than those exposed during nephron maturation (P6–9). Surprisingly, our findings demonstrate that despite nephrotoxic injury being present during both ongoing nephrogenesis and at 1 week of life, those exposed at 1 week of life had worse fibrosis, biochemical evidence of CKD, a greater percentage of kidney injury marker 1 (Kim1) expression, and greater reduction in nephron number at 6 weeks of life compared to exposure during the first 4 days of life, suggesting the potential for discrete windows of susceptibility to nephron loss during nephron maturation.
Results
Nephrotoxic-induced acute kidney injury is more severe in the early-exposed group compared to late-exposed group (AKI analysis)
Thirty neonatal rabbits were analyzed in this study. Neonatal rabbits were exposed to daily gentamicin (100 mg/kg intraperitoneal (IP)) and indomethacin (5 mg/kg per oral (PO)) for four consecutive days. Experimental groups were either early-exposed in the first 4 days of life during ongoing nephrogenesis (P0-3, ~ 24–32 wg in the human) (n = 9) or late-exposed at 1 week of life during tubular maturation (P6–9, ~ 34–40 wg in the human) (n = 7). Intra-litter controls received normal saline (NS) (n = 8 and 6, respectively) (Tables 1, S1; Fig. S1). For acute kidney injury analyses, animals were euthanized three hours after the last nephrotoxic dose (P3 and P9). Euthanasia three hours after the last dosing was chosen to allow for peak levels of both indomethacin25 and gentamicin26 to be reached. No significant difference was seen in animal weights between exposed and controls at birth, before, or after last nephrotoxin exposure. PAS staining was performed to characterize histologic injury patterns within the kidneys. We identified histologic changes in both the early nephrotoxin-exposed group (n = 5) and late nephrotoxin-exposed group (n = 4) relative to controls (n = 5 and n = 4, respectively), including loss of brush border, intra-luminal vacuolization, and flattening of tubular epithelium (Fig. 1A). To better characterize histologic evidence of AKI in early vs. late-exposed groups, we standardized the cortical-cross-sectional areas between samples (see Methods) and quantified the number of tubules with flattened/lost brush border and luminal vacuolization. Through this approach, we confirmed that the early- and late-exposed groups both had histologic evidence of AKI (supporting that our model appropriately induces AKI). Additionally, injury was also seen in controls although to a lesser extent (Fig. 1B).
Periodic Acid-Schiff staining confirms acute kidney injury in both early and late-exposed rabbits. A Healthy tubules are defined by bright pink-staining brush border. Both early and late exposed rabbits demonstrate injury to the proximal tubule including dilatation of the proximal tubule/flattening of the tubular epithelium (yellow arrow) and vacuolization of the proximal tubular lumen (blue arrow). B Quantification of proximal tubular injury identified increased injury in both early and late exposed rabbits compared to controls.
We next evaluated for changes in RNA expression of kidney injury marker 1 (Kim1). We identified a 6.7x fold change in Kim1 in the early-exposed rabbits relative to controls, but only a 1.3x fold change in the late-exposed rabbit relative to controls (p = 0.002) (Fig. 2A). A statistically significant fold change in Kim1 in the late-exposed compared to early-exposed was maintained using two separate housekeeping genes Actb and Ppia (Fig. S2). After identifying increased Kim1, we next sought to determine where this injury occurred in the developing kidney. We evaluated for changes in RNA expression of HAVCR1 (Kim1) in the kidney using RNAScope™. Kim1 expression localized to the inner cortex in the early-exposed rabbits, sparing the nephrogenic zone and more superficial nephrons (Fig. 2C). The rabbits injured at 1 week of life had more diffuse expression of Kim1 throughout the cortex, but with a greater proportion of injury in the inner cortex (Fig. 2D). We next quantified the total area of Kim1 staining relative to the total cortical area (Fig. 2B) to evaluate if this would achieve results similar to the qPCR. The Kim1 percent expression was 36.2 ± 23.8% in early-exposed compared to early controls (5.0 ± 4.0%, p = 0.0079). The late-exposed percent injury was similar to that of the early-exposed (35.4 ± 24.9%), compared to 14.9 ± 14.3% in the late controls (p = 0.22). Although this did not identify a difference between injury in early- and late-exposed rabbits, it did demonstrate increased Kim1 in the animals exposed to nephrotoxins relative to controls, with a statistically significant difference in Kim1 expression in early-exposed compared to controls. In summary, acute kidney injury occurred in both early and late-exposed rabbits, but with increased Kim1 expression overall within the early-exposed rabbits, with the most severe injury occurring at the inner cortex in both.
Havcr1 (Kim1) is increased in the cortex of both early and late-exposed rabbits compared to saline controls, however injury is greater in those exposed early. A Kim1 qPCR fold change in nephrotoxin-exposed animals relative to age-matched control samples and normalized by changes in beta-actin levels. B Quantification of Kim1 in cortex of RNAScope demonstrates increased Kim1 in nephrotoxin-induced samples, with significantly increased Kim1 in early-exposed rabbits compared to controls. C Kim1 expression is localized to the cortico-medullary border/inner cortex in early-exposed rabbits while sparing the nephrogenic zone. D Late-exposed rabbits still favor injury in the inner cortex although Kim1 staining is more diffuse.
To evaluate the role of AKI on proliferation in the progenitor population, we performed proliferating cell nuclear antigen (PCNA) antibody staining, as PCNA is known to be expressed in the nephrogenic zone and downregulated during terminal differentiation of the nephron progenitor cells27. We identified strong proliferation signal within the nephrogenic zone in four of the five controls and three of the five experimental groups (Fig. S3A). No difference was seen in nephrogenic zone width between the controls and early-exposed rabbits based on this staining (Fig. S3B). Glomerular changes were next assessed using NPHS2 and WT1 antibodies. Similar size and appearance is seen in both experimental and control groups in both P3 and P9 for both markers (Fig. S4A–H).
Greater nephron loss, increased fibrosis and Kim1 expression, and higher CKD markers were found in late-exposed group compared to early-exposed group (CKD analysis)
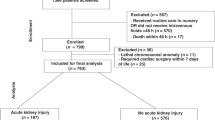
Prior to euthanasia at 6 weeks of life, rabbits were injected with cationic ferritin to label the glomerular basement membrane (see methods for further details). Ex vivo imaging of the rabbit kidneys was performed using 7T Biospec MRI system (Fig. S5). We identified a mean of 170,972 ± 12,205 glomeruli in the controls (n = 5), 159,655 ± 16,194 in the early-exposed animals (6.6% reduction compared to controls, p = 0.29), and 145,748 ± 7508 glomeruli in the late-exposed group (14.8% reduction compared to control, p = 0.01), (Tables 2, S2, Fig. 3A). Although no difference was seen between the weights of the animals, the kidney weight/body weight ratio of the late-exposed rabbits was significantly lower than controls (p = 0.034). No difference was seen in cortical volume or kidney weights between animals (Table 2).
Assessment of rabbits at 6 weeks of age demonstrates decreased nephron number, increased markers of CKD, and increased fibrosis in late-exposed rabbits compared to early-exposed rabbits. A A 15% reduction in nephron number was seen in rabbits exposed to nephrotoxins during tubular maturation (P6–9) compared to controls. B Although clinically small in difference, a statistically significant increase in serum creatinine and C blood urea nitrogen (BUN) was seen in the late-exposed rabbits compared to early-exposed rabbits. D–L Trichome staining was performed to assess for regions of fibrosis. Blue staining indicates fibrotic region of the kidney, with the most staining seen in late-exposed rabbits (J–L) compared to controls. Patches of fibrosis were also seen in early-exposed (G–I).
Serum creatinine and blood urea nitrogen (BUN) were measured on all rabbits at 6 weeks of age (Table 2). We identified a significant increase in serum creatinine in the late-exposed compared to both control and early exposed (0.7 ± 0.0 mg/dL vs. 0.5 ± 0.0 mg/dL, p = 0.028) (Fig. 3B-C). The BUN was also significantly increased in the late-exposed compared to early-exposed (12.00 ± 1.73 mg/dL vs. 7.75 ± 2.22 mg/dL, p = 0.036 (Fig. 3C). Urinalyses were performed on all rabbits, but no evidence of protein was detected in any sample.
Using trichrome stain, we evaluated fibrosis deposition in the controls, early-exposed, and late-exposed animals. We identified evidence of interstitial fibrosis in both the early and late-exposed cohorts relative to controls, with relatively increased fibrosis staining in the late-exposed group (Fig. 3D-L). Fibrosis was patchy and scattered, and deposition tended to be closer to the medullary border/mature portions of the kidney rather than the outer cortex.
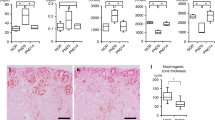
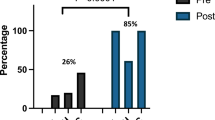
As sustained expression of protein KIM-1 is associated with kidney fibrosis28we evaluated for persistent Kim1 in the 6-week-old rabbit kidneys using RNAScope™. Unlike the AKI analysis where there was higher overall expression of Kim1, in this CKD analysis, there was overall less Kim1 expression within the cortex of any experimental group at 6 weeks of age. (Fig. 4A–D). However, the regions that were Kim1 + also co-stained for Krt8 (Fig. 4E–H), an epithelial marker upregulated after AKI and also identified as co-staining with Kim1 in LTL- PT failing to repair after AKI29. Additionally, one control had higher expression relative to the other controls (Figs. 4I, S5), and therefore the median values were used for further analyses. The Kim1 + signal identified in the control sample did not overlap with Krt8 (Fig. S6). The median area of Kim1 expression relative to the cortical area was 1.92% (0.81–2.5%) in the late-exposed samples, 1.15% (0.15–1.87%) in the early-exposed, and 0.77% in the controls (range 0.15–5.81%) (Fig. 4I). Although not statistically significant (p = 0.36) there is a trend towards increased Kim1 in the late-exposed compared to early-exposed rabbits, as well as a higher percent change in Kim1 expression relative to the median control (Fig. 4J), supporting the findings of lower nephron number and increased fibrosis in the later-exposed rabbits.
Late-exposed rabbits at 6 weeks of age express overall higher percentage of Kim1, with overlapping staining of injury marker Krt8. A RNAScope imaging of early exposed rabbit and B late-exposed rabbit at lower magnification and higher magnification (C–D). Co-staining of Kim1 and Krt8 on tissue can be seen in both early and late samples (E–H). I Despite one outlier, overall Kim1 expression increased in late-exposed compared to controls at 6 weeks of age, although overall expression decreased from acute injury phase. J Evaluating the percent fold change from the median control value, we identified a larger percent change in Kim1 expression in the late-exposed compared to early-exposed rabbits.
Discussion
As the resuscitation and survival of preterm neonates continue to improve30the windows of susceptibility during postnatal kidney development must be considered to reduce long-term morbidity in this growing population. This study sheds light on potential windows of vulnerability to nephrotoxic injury, suggesting that although acute kidney injury is more pronounced in those exposed immediately after birth (early-exposed), higher nephron loss and persistent injury are seen in those exposed at 1 week of life (late-exposed). In this current study, we used previously established dosing of gentamicin and indomethacin to induce AKI in the neonatal rabbit24. Although our injury was not as severe as the nephron loss reported by Charlton et al.24 who found a 63% reduction at 6 weeks of life, both studies support that exposure to nephrotoxins at 1 week of life is detrimental to long-term nephron numbers. Our current study supports that the timing of these exposures may play a critical role in how nephron loss occurs in the neonatal period.
While it would be preferred to avoid nephrotoxins in the neonatal period, their use remains critical for the survival of these infants. Aminoglycosides, including gentamicin, are considered first-line empiric therapy, as this combination is effective against group B strep, most strep and enterococcal species, and L monocytogenes31. Alternative therapies, including cefotaxime, are associated with an increased risk of death and therefore not recommended as first-line therapy32. Therefore, neonates will continue to be exposed to aminoglycosides despite their known nephrotoxicity6,33,34. Indomethacin use in the NICU can be prescribed for both intraventricular hemorrhage (IVH) prophylaxis and treatment of patent ductus arteriosus (PDA). The prostaglandin blockade associated with NSAID use, however, can reduce kidney blood flow, resulting in injury. AKI was found to occur in roughly one quarter of preterm infants who received NSAIDS35.
It remains unknown why the late-exposed rabbits are more susceptible to nephron loss at 6 weeks of age than the early-exposed group, nor can we determine from this study if one of the two nephrotoxins vs. their combinatory effect was the driver of either the short- or long-term outcomes. While we cannot exclude the possibility of increased cellular plasticity in a progenitor population, our data suggests that the kidney region of injury is not the undifferentiated and early nephron structures, but rather the newly functional tubules. Our in situ hybridization studies demonstrate that the more mature nephrons in the corticomedullary border are at higher risk of injury while the nephrogenic zone is relatively spared from injury based on lack of Kim1 expression. During kidney development, nephrons form in a centrifugal fashion, where the inner cortex nephrons are more mature than the outer/superficial cortex36. The increase in tubular maturation/transport also follows this pattern37and more immature tubules below the nephrogenic zone may be present immediately after birth than at 1 week of life (late-exposed), resulting in a greater proportion of injured nephrons. Megalin (LRP2) is the apical protein responsible for gentamicin endocytosis within the PT33,38,39. LRP2 is also one of the earliest tubular transport proteins expressed during development appearing before other more mature PT markers40. LRP2 expression more than doubles from 24 weeks gestation to term birth41. While this may explain long-term risk of the late-exposed animals, it does not explain why the acute kidney injury was worse in the early-exposed animals. Our data identified that exposure immediately after birth was associated with increased histologic injury on PAS and increased Kim1 expression using qPCR and RNAScope. The use of NSAIDs in this experiment is likely the reason that the early-exposed neonates were more susceptible to AKI. COX-2 inhibition in fetal and neonatal rodents impaired the development of the kidney cortex and reduced glomerular diameter in previous studies42. Importantly, this data does not support that nephrotoxins at earlier gestational ages are “safer”, and these results must be taken cautiously when translating findings in a full-term postnatal nephrogenesis model to a preterm infant.
Limitations of this study include a relatively small sample size. The sex of the rabbits could not be determined until after randomization/receiving experimental conditions. While we were not powered to identify differences between male and female rabbits, both sexes demonstrated a low nephron number after late exposure compared to controls. However, we cannot control for potential protective nature of female sex in AKI43,44. The sex of the rabbits could not be determined until after randomization/receiving exerpimental conditions. During the AKI analysis, our control groups contained higher percentages of female rabbits. In the CKD analysis, the control group at 6 weeks of age was mostly female, but so was our late-exposed cohort. Another important limitation of this study is defining injury within the NPC population. Lack of Kim1 expression may not indicate a lack of injury within the NPC. Although the reduction of PCNA staining in some of the samples at P3 may be artifactual due to the technical challenges of rabbit antibodies on rabbits, there is a trend for brighter staining in the controls, suggesting that the NPC may still be affected by this nephrotoxic exposure despite no difference in nephrogenic zone width. Additionally, although no obvious differences were noted in NPHS2 and WT1 glomerular staining, we did not perform quantitative analyses such as percentage of WT-1 positive cells/glomerular cells to detect more subtle differences between the two groups. Future work will also focus on alternative markers of injury, including cellular stress and apoptosis, within the progenitor population. Another limitation of this study is that the gestational period of the rabbit is 31–33 days. We based dosing on postnatal day for consistency rather than postconceptual age (PC) as we could not control P0 on E31 vs. E33. For example, the late-exposed rabbits were born at E31. The early-exposed rabbits were born on E31-33. Although we were able to collect fresh tissue to evaluate for RNA expression using qPCR for the AKI analysis, we could not obtain fresh tissue at 6 weeks of age due to the fixation protocol for MRI imaging. We also did not perform acid maceration studies or stereology to confirm our MRI quantification. However, our nephron numbers in controls (170,972 ± 12,205) are consistent with published data for nephron numbers in the rabbit, including both MRI (191,834 ± 49,150)24, and stereology (160,803 ± 11,838)45. Additionally, our data evaluating outcomes at 6 weeks are consistent with decreased nephron number, fibrosis, and increased Kim1 expression in the late-exposed rabbits. Another limitation of this study is that we cannot comment on changes in the duration of nephrogenesis of those exposed early, nor can we comment on any early changes nephron number to compensate for the original injury. Others have used unilateral nephrectomy during ongoing nephrogenesis to see if this impacts nephron number. In the sheep, nephrectomy at 100 days gestation (gestational period 150 days) resulted in a 45% increase in nephron number in the contralateral kidney without compensatory hypertrophy noted at 30 days of life46. In the rat, unilateral nephrectomy shortly after birth did not change nephron number but did lead to compensatory glomerular hypertrophy47. Although the differences identified may be species-dependent or due to additional postnatal stressors not encountered in utero, the biggest difference between these studies was the end point in which they were evaluated. The sheep was evaluated 30 days after birth, while the rat was observed 8 weeks after nephrectomy. Therefore, it cannot be concluded in the sheep if there was prolonged nephrogenesis, or just increased nephron formation within the same window of development. It is also unclear if compensatory hypertrophy and subsequent loss would have been evident in the sheep after a longer period of observation. Similarly, we did not evaluate for ongoing nephrogenesis past P3, nor nephron number on P9, but instead waited 6 weeks. We similarly can’t comment on increased or prolonged nephrogenesis in the P3 group, nor can we comment on compensatory hypertrophy that may be evident at 8 weeks. Further studies are indicated to see if nephron numbers were higher in those still undergoing nephrogenesis to compensate for any immediate loss. We did not see a difference in cortical volume, glomerular volume, or glomerular density, and glomeruli were smaller in both nephrotoxin groups compared to controls arguing against compensatory nephrogenesis in one group over the other. However, observing beyond 6 weeks may be needed for additional changes of compensatory hypertrophy. It remains unclear why one control rabbit had high levels of Kim1 at 6 weeks of age, as nephron number was not affected in this control. It is possible that the saline exposure itself caused damage due to the hyperosmolar load relative to milk48as we still see some injury in many of the controls. While this study evaluated AKI during two postnatal developmental windows, it did not evaluate the mechanisms of this injury. Future studies will be necessary to determine which nephrotoxin is responsible for this injury or if they are synergistic. Additional work will focus on these susceptibility windows, how various perinatal exposures may impact these windows, and on recovery after injury in the neonatal period. While many questions remain, this study emphasizes that we should remain vigilant regarding perinatal exposures during this vulnerable period of kidney development and that development should be considered a biological variable, as these early insults may have long-lasting detrimental effects on nephron endowment.
Materials and methods
Rabbits
This study was reviewed and approved by the Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee (IACUC). Timed-pregnant New Zealand rabbits were purchased from Charles River and ethically cared for per Cincinnati Children’s institutionally approved IACUC protocols 2021-0067 and 2022-0042, and methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines. Macroenvironmental conditions were maintained using the electronic building management system set to 68 F (+/- 1 F) and lighting on a 14:10 h light/dark cycle. Animals had ad libitum access to RO water and a pelleted rabbit diet (LabDiet 5326). Diet was supplemented daily with timothy hay and fresh produce. The study IDs and weights for all litters are listed in supplemental Table 1. Litter 1 included kits 1–6 (six total), litter two included kits 11–17 (seven total), litter 3 included kits 21–29 (nine total), and litter 4 included kits 51–58 (eight total). All kits from all litters were included (no exclusion) and were randomly numbered and divided between control and experiment groups. A Sharpie was used to number the kits and keep track of experimental groups. A total of 30 rabbit kits were analyzed for this study. Power analyses were performed for MRI quantification (see statistical methods), which were performed after analyzing 7 rabbits. Rabbits were euthanized after anesthetic overdose of pentobarbital (65 mg/ml concentration,0.08 ml-0.4 ml based on weight) followed by transcardiac perfusion as previously described23 using PBS and 10% normal buffered formalin (NBF). Rabbit kits were divided into experimental and control groups randomly. Rabbits were exposed to gentamicin (Fresenius Kabi USA, Cat# 6332301003, 40 mg/ml, diluted to 10 mg/ml in normal saline (NS), dose: 100 mg/kg intraperitoneal) and indomethacin (Sigma Aldrich; cat# 1341001, 5 mg/kg orally, diluted in NS to 0.67 mg/ml) on either P0-3 (early-exposed) or P6-9 (late-exposed). Intra-litter controls received oral and IP NS of equivalent volume. All rabbit kits were included from a litter regardless of sex. Kits were planned to be excluded a priori if they appeared ill; however, all kits remained healthy, and none were excluded from the study.
Acute kidney injury analyses
Animals were euthanized 3 h after their last nephrotoxic exposure on P3 or P9, respectively. After transcardiac perfusion with PBS, the right kidney was removed and fixed in 10% normal buffered formalin (NBF). The animal was then perfused with NBF and the left kidney was removed. Kidneys in NBF were fixed for 24 h at room temperature or 4 °C.
Periodic Acid-Schiff (PAS) staining and processing
PAS was performed with the assistance of the Cincinnati Children’s pathology core. Five-micron sections of paraffin embedded tissue were stained using the Ventana Symphony automated slide stainer and scanned using the Nikon Ti2 Upright Widefield Microscope at 20 × objective. Images were processed using NIS Elements software.
Quantification of acute kidney injury
Ten distinct regions per kidney (2000 × 2000 pixels) were chosen for quantifying injury. These regions included 5 outer cortex and 5 deep cortex/medullary border to standardize between all slides. Slides were reviewed by a clinical pathologist (KV) to train researchers on injury. Two researchers (SI and SY) were blinded to animal treatment. Injured tubules (based on loss of brush border, vacuolization, and flattened epithelium) were quantified relative to total number of proximal tubules.
Image processing and analysis
NIS-Elements 5.3.00 was used for image processing, measurement of nephrogenic zone width and conversion of ND2 files to TIF files. Statistical Analyses were performed using GraphPad Prism v9.
Quantitative PCR (qPCR)
qPCR was performed to assess for Kim1 expression. Kidney cortex was collected into RNA Later (cat#AM7020, Thermofisher Scientific) and stored at -20ºC. Rabbit kidneys were homogenized in RNASTAT 60 (cat#CS-110, AMSBio) and total RNA was extracted using GeneJET RNA Purification Kit (K0732, Thermofisher Scientific). cDNA was synthesized using SuperScript™ IV First-Strand Synthesis System (18091050, Thermofisher Scientific). qPCR was performed using Powerup SYBR green Mastermix (A25742, Thermofisher Scientific) in QuantStudio 5 Real time PCR system. Samples were normalized to Ppia and Actb and fold change was calculated relative to age-matched controls. Primers used are listed in Supplemental Methods.
PCR for sex determination of P3 and P9 rabbits
We used cDNA previously made from rabbit kidneys to detect SRY (242 bp PCR product). About 25 ng of cDNA was used for PCR (GoTaq Green Master Mix, Promega #M7122). PCR was done using following cycle conditions: first denaturation step 94 °C 2 min, 35 cycles of 94 °C 20 s, 64 °C 30 s, 72 °C 30 s and final extension step 72 °C 10 min using primers OcSRY21F: 5′-AGC GGC CAG GAA CGG GTC AAG-3′, and OcSRY23R: 5′-CCT TCC GGC GAG GTC TGT ACT TG-3′. PCR products obtained were analyzed by electrophoresis in 3% agarose gel containing sybr safe DNA Gel stain (Invitrogen #S33102) and visualized using BioRad ChemiDoc MP imaging system.
Single molecule fluorescence in situ hybridization using RNAScope
RNAScope was performed using the Multiplex Fluorescent V2 Assay (Advanced Cell Diagnostics, Inc.) according to the manufacturer’s protocol. Positive controls used were rabbit POL2RA (low abundance). The negative bacterial control (DapB) was used on all tissue samples. The probe of interest used was rabbit Havcr1. After completion of the manufacturer’s recommendation for RNAScope V2 kit, slides were incubated in biotinylated LTL (Vector Labs) and Guinea pig anti- Krt8/18 (Progen) overnight at 4 °C, followed by wash and secondary antibody incubation for 1 h with streptavidin conjugated to Alexa Fluor™ 647 (ThermoScientific) and Goat Anti-Guinea pig IgG H&L (Alexa Fluor® 750)(Abcam). Slides were washed and mounted with prolong gold antifade reagent. Images were acquired using the Nikon Ti-2 Spectra X Widefield Microscope using the 20× objective with all images of the same probe acquired with fixed optical/exposure configurations. The excitation wavelength for TRITC was narrowed to 523–572 nm to avoid any potential cross-excitation with Cy5.
Immunofluorescence
Five-micron sections of paraffin embedded tissue were used for Immunofluorescence. Immunofluorescence was performed as previously described23. Antibodies used included rabbit PCNA (Proteinech, 10205-2-AP; 1:200), Rabbit WT1-647 (Abcam, Ab202639; 1:250), Rabbit NPHS2 (Proteintech, 20384-1-AP; 1:200), Mouse Collagen IV (Sigma, SAB4200500; 1:200), mouse ecadherin (CDH1) (BD Biosciences, 610182; 1:500), and guinea pig Keratin 8 (Krt8) (Progen, GP-k8; 1:400). Briefly, to overcome the non-specific staining of a rabbit antibody on rabbit tissue, we performed a 5 min incubation using Scytek Superblock. After washing four times in PBS for 5–10 min at RT, we then performed an incubation in Scytek rabbit-to-rabbit blocking reagent for 1 h at RT followed by PBS wash × 4 for 10 min each before adding primary antibody. Images were acquired on the NikonNPHS2 and A1 Inverted Confocal Microscope and Nikon inverted AXR using the 20 × objective for PCNA, 20x and 60x for WT-1, and Nikon Ti-2 Spectra X Widefield Microscope at 10x for all other antibodies.
Nephrogenic zone width
Nephrogenic zone width was measured as previously described19 using two distinct regions from the PCNA-stained samples. The average width was calculated digitally using the Elements annotation tool measuring both area and length of total region.
Chronic kidney disease analyses
Cationic ferritin (CF) labeling and MRI imaging
Animals were euthanized 6 weeks after their last exposure. Ex vivo MRI imaging was performed after cationic ferritin labeling of the glomerular basement membrane as previously described24. Briefly, reach rabbit received an intramuscular injection of hydrocortisone sodium succinate (Pfizer) (10 mg/kg) to avoid allergic response prior to IV cationic ferritin (CF) as CF is a horse-based product. An IV was placed in the marginal ear vein. CF (Sigma Aldrich, St. Louis, MO; cat# F7879-2 ml, 1.92 mg/100 g body weight infused over 10–15 min) was infused into the rabbit. 90 min after completion of the CF infusion, the rabbits underwent anesthesia with isoflurane (3–5% via induction chamber), ketamine (35 mg/kg IV), and xylazine (5 mg/kg IV) followed by euthanasia via transcardial perfusion as discussed above.
MRI imaging and quantification
MRI imaging was performed using a 7T Biospec system (Bruker, Billerica, MA) utilizing a 38 mm inner diameter linear volume transmit coil (Bruker, Billerica, MA). Ex vivo MRI imaging was performed after cationic ferritin labeling of the glomerular basement membrane. Fixed kidneys were submerged in PBS and placed at isocenter. After acquiring localizer images, susceptibility weighted imaging was acquired using a 3D gradient recalled echo sequence (echo time 20 ms; repetition time 80 ms; flip angle 30°; field of view 28 mm x 28 mm x 36 mm; acquisition/reconstruction matrix 448 × 448 × 448; resolution 63 × 63 × 80 μm; 3 signal averages; read out bandwidth 30 kHz). DICOM files were converted to compressed nifti files using the MATLAB based dicm2nii (https://github.com/xiangruili/dicm2nii/). Images were then cropped tightly to the kidney surface to reduce memory demands. Cropped images were then manually segmented using Slicer 3D version 5.2.2. Further details on quantification can be found in Supplemental methods.
Trichrome staining
Trichome staining was performed with the assistance of the Cincinnati Children’s pathology core. Five-micron sections of paraffin embedded tissue were stained using the Ventana Symphony automated slide stainer and scanned using the Nikon Ti2 Upright Widefield Microscope at 20 × objective. Images were processed using NIS Elements software. Degree of staining was evaluated by clinical pathologist (KV).
CKD serum and urine studies
Blood was collected in Vacutainer® Plus Plastic EDTA Blood Collection Tubes (cat#BD Medical 367856, 3 ml) vial, spun at 1500g for 10 min at 4 °C. Supernatant was collected into a fresh 1.5 ml tube and stored at − 80 °C. Samples were sent to IDEXX Bioanalytics for BUN and SCr measurement. Urine samples were collected and spun at 1500g for 5 min at 4 °C. Supernatant was collected into a fresh 1.5 ml tube.
Statistical analyses
Statistical Analyses were performed using GraphPad Prism v9. Given a limited number of animals per litter, all animals were used, and then power analyses were performed to determine effect size of glomerular counts. This was performed as post-hoc analysis with all five control rabbits and supported an effect size of 4.11 and 99% power. Means and standard deviations were calculated for glomerular counts, glomerular volume, cortical volume, glomerular density, BUN and SCr. The normality of the data was assessed using Shapiro-Wilk test before using parametric vs. nonparametric comparisons. qPCR data were found to be normally distributed and comparisons were made using unpaired t-tests. All controls (early and late-exposed) were combined for 6-week analyses. Glomerular numbers were also normally distributed but with unequal standard deviations, so they were compared using an unpaired t test with Welch’s correction. Cortical volumes, glomerular volumes, glomerular densities, glomerular volume/cortical volume, and animal weights at 6 weeks of age were normally distributed and analyzed using one-way ANOVA. Differences between the groups were then compared using an unpaired t test. Kidney weights and kidney weights/body weights did not pass normality testing and were analyzed with Kruskal–Wallis testing.
Data availability
All data generated from this study is available within the manuscript. All MRI post-processing workflows will be documented in Github as described in supplemental methods.
References
Askenazi, D. J. et al. Prevalence of acute kidney injury (AKI) in extremely low gestational age neonates (ELGAN). Pediatr. Nephrol. 35, 1737–1748. https://doi.org/10.1007/s00467-020-04563-x (2020).
Hingorani, S. et al. Severe acute kidney injury and mortality in extremely low gestational age neonates. Clin. J. Am. Soc. Nephrol. 16, 862–869. https://doi.org/10.2215/cjn.18841220 (2021).
Sanderson, K. et al. Perinatal risk factors associated with acute kidney injury severity and duration among infants born extremely preterm. Pediatr. Res. https://doi.org/10.1038/s41390-024-03102-w (2024).
Sutherland, M. R. et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J. Am. Soc. Nephrol. 22, 1365–1374. https://doi.org/10.1681/ASN.2010121266 (2011).
Rodríguez, M. M. et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr. Dev. Pathol. 7, 17–25. https://doi.org/10.1007/s10024-003-3029-2 (2004).
Mohamed, T. H., Abdi, H. H., Magers, J., Prusakov, P. & Slaughter, J. L. Nephrotoxic medications and associated acute kidney injury in hospitalized neonates. J. Nephrol. 35, 1679–1687. https://doi.org/10.1007/s40620-022-01264-6 (2022).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child. Adolesc. Health. 1, 184–194. https://doi.org/10.1016/s2352-4642(17)30069-x (2017).
Barker, D. J. The intrauterine environment and adult cardiovascular disease. Ciba Found. Symp. 156, 3–10 (1991) (discussion 10–16).
Barker, D., Eriksson, J., Forsen, T. & Osmond, C. Fetal origins of adult disease: strength of effects and biological basis. Int. J. Epidemiol. 31, 1235–1239. https://doi.org/10.1093/ije/31.6.1235 (2002).
Barker, D. J. P. & Bagby, S. P. Developmental antecedents of cardiovascular disease: a historical perspective. J. Am. Soc. Nephrol. JASN. 16, 2537–2544. https://doi.org/10.1681/ASN.2005020160 (2005).
Barker, D. J. The fetal and infant origins of disease. Eur. J. Clin. Investig. 25, 457–463. https://doi.org/10.1136/bmj.301.6761.1111 (1995).
Barker, D. J. P., Bagby, S. P. & Hanson, M. A. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2, 700–707. https://doi.org/10.1038/ncpneph0344 (2006).
Barker, D. J., Bull, A. R., Osmond, C. & Simmonds, S. J. Fetal and placental size and risk of hypertension in adult life. BMJ (Clinical Res. ed.). 301, 259–262. https://doi.org/10.1136/bmj.301.6751.552 (1990).
Barker, D. J., Osmond, C., Golding, J., Kuh, D. & Wadsworth, M. E. BMJ 298, 564–567 (1989).
Lackland, D. T., Bendall, H. E., Osmond, C., Egan, B. M. & Barker, D. J. Low birth weights contribute to high rates of early-onset chronic renal failure in the southeastern United States. Arch. Intern. Med. 160, 1472–1476 (2000).
Sayer, A. A. et al. Are rates of ageing determined in utero? Age Ageing. 27, 579–583. https://doi.org/10.1093/ageing/27.5.579 (1998).
Hingorani, S. et al. Prevalence and risk factors for kidney disease and elevated BP in 2-year-old children born extremely premature. Clin. J. Am. Soc. Nephrol. 17, 1129–1138. https://doi.org/10.2215/cjn.15011121 (2022).
Crump, C., Sundquist, J., Winkleby, M. A. & Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: National cohort study. BMJ 365, l1346. https://doi.org/10.1136/bmj.l1346 (2019).
Carpenter, J., Yarlagadda, S., VandenHeuvel, K. A., Ding, L. & Schuh, M. P. Human nephrogenesis can persist beyond 40 postnatal days in preterm infants. Kidney Int. Rep. 9, 436–450. https://doi.org/10.1016/j.ekir.2023.10.032 (2024).
Kirita, Y., Wu, H., Uchimura, K., Wilson, P. C. & Humphreys, B. D. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc. Natl. Acad. Sci. USA. 117, 15874–15883. https://doi.org/10.1073/pnas.2005477117 (2020).
Ledru, N. et al. Predicting proximal tubule failed repair drivers through regularized regression analysis of single cell multiomic sequencing. Nat. Commun. 15, 1291. https://doi.org/10.1038/s41467-024-45706-0 (2024).
Walani, S. R. Global burden of preterm birth. Int. J. Gynaecol. Obstet. 150, 31–33. https://doi.org/10.1002/ijgo.13195 (2020).
Schuh, M. P., Yarlagadda, S., Alkhudairy, L., Preusse, K. & Kopan, R. Characterizing post-branching nephrogenesis in the neonatal rabbit. Sci. Rep. 13, 19234. https://doi.org/10.1038/s41598-023-46624-9 (2023).
Charlton, J. R. et al. Nephron loss detected by MRI following neonatal acute kidney injury in rabbits. Pediatr. Res. 87, 1185–1192. https://doi.org/10.1038/s41390-019-0684-1 (2020).
Helleberg, L. Clinical pharmacokinetics of indomethacin. Clin. Pharmacokinet. 6, 245–258. https://doi.org/10.2165/00003088-198106040-00001 (1981).
MacDougall, C. in Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13e (eds Laurence L. Brunton, Randa Hilal-Dandan, & Björn C. Knollmann) (McGraw-Hill Education, 2017).
Saifudeen, Z., Marks, J., Du, H. & El-Dahr, S. S. Spatial repression of PCNA by p53 during kidney development. Am. J. Physiol. Ren. Physiol. 283, F727–F733. https://doi.org/10.1152/ajprenal.00114.2002 (2002).
Humphreys, B. D. et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J. Clin. Investig. 123, 4023–4035. https://doi.org/10.1172/jci45361 (2013).
Liu, J. et al. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight. 2 https://doi.org/10.1172/jci.insight.94716 (2017).
Patel, R. M., Rysavy, M. A., Bell, E. F. & Tyson, J. E. Survival of infants born at periviable gestational ages. Clin. Perinatol. 44, 287–303. https://doi.org/10.1016/j.clp.2017.01.009 (2017).
Puopolo, K. M., Benitz, W. E. & Zaoutis, T. E. Management of neonates born at ≤ 34 6/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 142 https://doi.org/10.1542/peds.2018-2896 (2018).
Clark, R. H., Bloom, B. T., Spitzer, A. R. & Gerstmann, D. R. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics 117, 67–74. https://doi.org/10.1542/peds.2005-0179 (2006).
Dagil, R., O’Shea, C., Nykjær, A., Bonvin, A. M. & Kragelund, B. B. Gentamicin binds to the Megalin receptor as a competitive inhibitor using the common ligand binding motif of complement type repeats: insight from the Nmr structure of the 10th complement type repeat ___domain alone and in complex with gentamicin. J. Biol. Chem. 288, 4424–4435. https://doi.org/10.1074/jbc.M112.434159 (2013).
Randjelovic, P., Veljkovic, S., Stojiljkovic, N., Sokolovic, D. & Ilic, I. Gentamicin nephrotoxicity in animals: current knowledge and future perspectives. Excli J. 16, 388–399. https://doi.org/10.17179/excli2017-165 (2017).
Ting, J. Y., McDougal, K., De Mello, A., Kwan, E. & Mammen, C. Acute kidney injury among preterm infants receiving nonsteroidal anti-inflammatory drugs: a pilot study. Pediatr. Neonatol. 64, 313–318. https://doi.org/10.1016/j.pedneo.2022.06.018 (2023).
Evan, A. P., Gattone, V. H., Schwartz, G. J. & 2nd & Development of solute transport in rabbit proximal tubule. II. Morphologic segmentation. Am. J. Physiol. 245, F391–407. https://doi.org/10.1152/ajprenal.1983.245.3.F391 (1983).
Schwartz, G. J. & Evan, A. P. Development of solute transport in rabbit proximal tubule. I. HCO-3 and glucose absorption. Am. J. Physiol. 245, F382–390. https://doi.org/10.1152/ajprenal.1983.245.3.F382 (1983).
Nielsen, R., Christensen, E. I. & Birn, H. Megalin and Cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int. 89, 58–67. https://doi.org/10.1016/j.kint.2015.11.007 (2016).
Beenken, A. et al. Structures of LRP2 reveal a molecular machine for endocytosis. Cell 186, 821–836e813. https://doi.org/10.1016/j.cell.2023.01.016 (2023).
Marable, S. S., Chung, E., Adam, M., Potter, S. S. & Park, J. S. Hnf4a deletion in the mouse kidney phenocopies Fanconi renotubular syndrome. JCI Insight. 3 https://doi.org/10.1172/jci.insight.97497 (2018).
Charlton, J. R., Harer, M. W., Swan, C. & Nielsen, R. Immature Megalin expression in the preterm neonatal kidney is associated with urinary loss of vitamin carrier proteins. Pediatr. Res. 85, 405–411. https://doi.org/10.1038/s41390-018-0261-z (2019).
Kömhoff, M. et al. Cyclooxygenase-2-selective inhibitors impair glomerulogenesis and renal cortical development. Kidney Int. 57, 414–422. https://doi.org/10.1046/j.1523-1755.2000.00861.x (2000).
Kher, A. et al. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc. Res. 67, 594–603. https://doi.org/10.1016/j.cardiores.2005.05.005 (2005).
Boddu, R. et al. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am. J. Physiol. Ren. Physiol. 313, F740–F755. https://doi.org/10.1152/ajprenal.00049.2017 (2017).
Maduwegedera, D. et al. Sex differences in postnatal growth and renal development in offspring of rabbit mothers with chronic secondary hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R706–714. https://doi.org/10.1152/ajpregu.00458.2006 (2007).
Douglas-Denton, R., Moritz, K. M., Bertram, J. F. & Wintour, E. M. Compensatory renal growth after unilateral nephrectomy in the ovine fetus. J. Am. Soc. Nephrol. 13, 406–410. https://doi.org/10.1681/asn.V132406 (2002).
Woods, L. L., Weeks, D. A. & Rasch, R. Hypertension after neonatal uninephrectomy in rats precedes glomerular damage. Hypertension 38, 337–342. https://doi.org/10.1161/01.hyp.38.3.337 (2001).
Coates, M. E., Gregory, M. E. & Thompson, S. Y. The composition of rabbit’s milk. Br. J. Nutr. 18, 583–586. https://doi.org/10.1079/bjn19640051 (1964).
Acknowledgements
This project was made possible, in part, using the Cincinnati Children’s Bio-Imaging and Analysis Facility [RRID: SCR_022628], the Cincinnati Children’s Imaging Research Center [RRID: SCR_022631], and Veterinary Services Surgical Core.
Author information
Authors and Affiliations
Contributions
MPS conceived and designed research, SI, SY, LD, DL, EF, DW, VRM, and MPS performed experiments, SI, SY, SL, EF, KV, JF and MPS analyzed data, SI, SY, DL, JRC, JD, MPS interpreted results of the experiments, SI and MPS prepared figures, Si and MPS drafted manuscript, SI, DL, JRC, MPS edited and revised manuscript, all authors approved final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Indugula, S., Yarlagadda, S., Drumgool, L. et al. Windows of susceptibility to neonatal acute kidney injury and nephron loss in a rabbit model. Sci Rep 15, 21160 (2025). https://doi.org/10.1038/s41598-025-08685-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08685-w