Abstract
The zebrafish (Danio rerio) is one of the most widely used research model organisms funded by the United States’ National Institutes of Health, second only to the mouse. Here, we discuss the advantages and unique qualities of this model organism. Additionally, we discuss key aspects of experimental design and statistical approaches that apply to studies using the zebrafish model organism. Finally, we list critical details that should be considered in the design of zebrafish experiments to enhance rigor and data reproducibility. These guidelines are designed to aid new researchers, journal editors, and manuscript reviewers in supporting the publication of the highest-quality zebrafish research.
Similar content being viewed by others
Introduction
Science is an ever-evolving process, and our ability to formulate and study scientific questions continuously improves in both experimental design and technological implementation. In the biological sciences, this evolution is associated with increased interdisciplinary and collaborative approaches, encouraging scientists to cross into new fields to take advantage of novel tools. During this transition, scientists may bring assumptions and practices accepted in their previous field and apply them to the new one. However, many disciplines have independent assumptions and experimental design expectations that are taught and passed down through training, mentorship, and expertise. These rules or design guidelines are often unwritten, as is the case within the zebrafish community. Therefore, this review aims to outline critical resources available to the zebrafish researcher, describe major biological characteristics to consider when using the zebrafish as compared to other vertebrates, and propose guidelines for taking advantage of these attributes. We will review statistical perspectives that assist experimental designs and data analysis when using zebrafish. Finally, we will detail the critical reporting elements expected within a manuscript to judge methodological rigor and potential for data reproducibility.
Guidelines for rigorous and reproducible experimental results in the zebrafish
Overview of the zebrafish model and resources available
As a relative newcomer in the biological model field, the use of the zebrafish has expanded dramatically, with the number of publications rising steeply in the 2000s (Supplemental Fig. 1). This rapid expansion of zebrafish studies is due to the many advantages and relative accessibility of this model. Briefly, the genome of this vertebrate organism is fully sequenced and largely annotated1. Within the Online Mendelian Inheritance in Man website, 82% of disease-relevant genes contain a zebrafish ortholog1. Further, the zebrafish shares most organ systems2,3,4 with other vertebrates. Like the invertebrate research organisms5,6,7,8, the zebrafish genome is highly amenable to mutagenesis and transgenesis9,10. Zebrafish embryogenesis is rapid, with its developmental timeline more comparable to Drosophila than that of mammals. Notably, the embryos are optically translucent during development, facilitating imaging studies. As the zebrafish develops past embryogenesis, which is completed by “hatching” at 2–3 days post fertilization (dpf), the animal continues to grow and form pigment. However, it is possible to prevent pigment formation using phenyl-thio-urea (PTU) (Fig. 1c) until around 7 dpf11,12,13. Past 7 dpf, the translucency decreases, though there are genetic mutants, such as the casper (Fig. 1b), absolute, and crystal lines14,15, that allow for imaging of both larval and adult tissues. Depending upon fish husbandry practices, it requires 2–4 months for the zebrafish to mature enough to breed13. Another advantage is the large sample sizes available for experimentation due to the ease of husbandry and number of embryos per mating pair, which is not easily accomplished in other vertebrate organisms16,17. These features make the zebrafish one of the only vertebrates wherein large-scale forward genetic studies are relatively inexpensive and can be performed by a single lab9,18,19,20,21,22. All these factors make the zebrafish an ideal model for bridging the gap between invertebrates and mammals (Table 1).
Zebrafish are, by convention, oriented with their head to the left and tail to the right. The two common orientations are a lateral/side view (a, c, e) or a top/dorsal view (b, d, f). Labeled in the WT 5 pdf larvae (a) is the eye and swim bladder. a A side view of WT pigmentation pattern of the TLF line. b From the top view, the WT pigment obscures the zebrafish brain and upper digestive tract. c–d The zebrafish casper mutant line prevents most pigmentation from forming except for the eyes and around the swim bladder. E-F) WT embryos were exposed to PTU after gastrulation (at the start of somitogenesis) but before pigment started forming at 24 hpf. PTU can be added after the start of pigment formation, but this will cause an incomplete loss of pigment. Adding PTU prior to somitogenesis will inhibit gastrulation, and the embryos will not develop correctly. When comparing the WT and the WT + PTU zebrafish, despite these larvae coming from the same clutch, the PTU has slowed down development (compare swim bladder size and yolk). This is one example of when careful timing is important for direct comparison work.
The early, large (1 mm) single-cell zebrafish embryo is accessible to rapid and scaled experimental manipulation, which has led to a diverse array of creative technologies in the field. Microinjection is perhaps the most notable, with gain of function from synthetic mRNA23,24, loss of function using morpholinos25,26,27, rapid transgenics via artificial transposons28, and a rich array of gene editing tools29 that can be directly delivered via this method. Each of these has important scientific constraints to consider for implementation and are discussed here (see below).
Additionally, the zebrafish community has embraced the ever-growing capability to collect and store large amounts of data by creating many online databases summarizing the knowledge and making these tools freely available. One critical resource is The Zebrafish Information Network (ZFIN, https://zfin.org/)1,30. This curated database has information or links to genetic sequences, mutations, targeted antisense reagents (morpholinos26,27), antibodies, publications, and more. Additionally, this website grants access to The Zebrafish Book31, which contains practical standard operating procedures for general zebrafish husbandry and other experimental protocols. This text is an excellent resource when starting to work with the zebrafish. Directly linked to the ZFIN website is the Zebrafish International Resource Center (ZIRC, http://zebrafish.org/), which maintains many zebrafish lines available for purchase. This includes wild type (WT), transgenic and mutant lines. Laboratories worldwide send their mutant and transgenic lines to this facility for disbursement to other community members. In addition to these core resources, other free databases, both internationally and locally, are available32. Finally, the zebrafish community is highly collaborative and has many national and international societies that foster strong connections (Table 2).
Genetic variability within the zebrafish
Next, we will discuss key characteristics that will help leverage the advantages of the zebrafish. When exploring differences between research organisms, one critical distinction between zebrafish and other vertebrates is its extensive genetic variability. While it is commonplace to use isogenic and highly inbred mammalian models to reduce variability, the laboratory-based “WT” strains of zebrafish show significant genetic heterogeneity. For example, one study looking at single nucleotide polymorphisms (SNPs) between different zebrafish lines demonstrated a 7% interstrain genetic variation in inbred research animals, with WT lines having as high as 37% genetic variation33,34. Despite multiple attempts, isogenic lines with stable fecundity are rare35.
Due to this consideration, there are multiple laboratory-based “WT” lines, which include Tubingen (TU; strain used to sequence the zebrafish genome), AB, Tupfel long fin (TL), Sanger AB Tubingen (SAT), etc. Each of these lines behaves somewhat differently and has unique genetic and physical traits36,37. Additionally, when maintaining the WT lines within a facility, it is critical to maintain genetic diversity and prevent bottlenecks where the next generation offspring comes from a limited number of breeding pairs. The best solution is to obtain each new generation from a stock center or create new lines by combining clutches of at least 15–25 crosses.
In addition to genetic variability, around 340 million years ago, the zebrafish ancestor underwent a genome duplication event38,39. It has been found that, of the 70% of zebrafish orthologs to human genes, 47% have a single ortholog, whereas the remainder have more than 1 orthologue1. This has both advantages and disadvantages. With more than one gene performing the same function, it is possible to create a hypomorph by mutating one of the orthologs. Furthermore, many duplicate genes have subfunctionalized, leading to the actions of a given gene being split into two or more paralogs performing a distinct subset of the original gene’s functions40. This can be advantageous, as a subset of the original gene functions can be more easily studied when subfunctionalized to one paralog. However, to create a null mutant comparable to a human genotype, multiple genes may need to be targeted.
This heterogeneity and genome duplication could hinder the adoption of an organism for experimentation if there is a limited number of offspring. For example, the average mouse litter size varies from 2–12 pups, limiting the sample number41. However, a single zebrafish mating pair will lay clutches of 70–300 eggs17, allowing for a higher sample size and helping overcome genetic variability. In fact, when genetic variability is combined with an increased sample number, this becomes a distinct advantage of the zebrafish model, particularly when modeling human disease, where genetic heterogeneity is an important variable.
How genetic variability impacts experimental outcomes depends upon the hypothesis being tested, the experimental design, and the methods used. The power of the model is the ability to collect data from a single cell, an individual animal, or a subpopulation of fish, all of which can be defined as a single sample. Background variation provides noise that can make it challenging to link a genetic perturbation or a treatment condition to observed phenotypes. However, the background genetic diversity makes the zebrafish an excellent model for human disease, as humans are similarly diverse. Increasingly within mouse literature, it has been noted that isogenic lines limit the reproducibility and application of the data collected42. Combined with the large number of offspring available, the genetic diversity grants the zebrafish model further insight into the genotype-phenotype relationship. This is particularly critical when studying drugs. The zebrafish is an excellent model to study the variability of drug activity within a genetically heterogeneous population. Thus, using this model for drug testing will more truly emulate a human population as compared to an inbred mouse model. However, it is critical that the increase in variability, and thus larger error bars, be considered in the experimental design and carefully balanced with the number of animals needed to achieve statistical significance.
Maternal contribution to early development in zebrafish
Another benefit of the zebrafish is the ability to study maternal gene contribution to early development within a vertebrate animal. The onset of zygotic genome activation is preceded by the embryo’s exclusive reliance on maternal gene products for development. These maternal RNAs and proteins in zebrafish progressively decrease as the zygotic genome takes over at ~3 h post fertilization (hpf)43. Many zygotically expressed genes are also expressed maternally. Embryos containing homozygous mutations in proteins required for development may develop for several days due to their heterozygous female parent providing the normal transcript44,45. Hence, even a complete loss-of-function phenotype may not be reflected as embryonic lethality. Maternal gene function must also be perturbed to examine the complete loss of both maternal and zygotic function.
Gene editing within the zebrafish
The zebrafish has a rich array of tools available for genetic manipulation. There are two broad categories of technology to decrease gene function. First, knockdown technologies decrease gene function without altering the genome. Morpholinos (MOs) were classically used and are still deployed today to knockdown genes25. There are two targets for MOs. First, MOs can target the start codon, which prevents translation. Second, the MO targets a splice site which prevents correct splicing and, in turn, likely causes protein truncation46. Using MOs enables the rapid screening for loss of function phenotypes and can be an excellent way to study a gene’s function. This technology is most commonly used to study function during the first 2–3 days post fertilization (dpf). MOs have been shown to increase p53 signaling in the zebrafish embryo and larvae, with neurons the most sensitive cell type27,47. Therefore, conclusions on the effect of a MO on any developmental process, particularly within the neural tissue, should be carefully assessed for p53 signaling. In addition to MOs, CRISPR-based RNA targeting technology has recently become available. This enables a different mechanism than MOs for RNA targeting to knockdown gene expression or alter splice sites. As both these technologies improve, they offer options for development-specific timing of gene knockdown48,49. This is particularly important for genes critical for early development but whose function in later development or adulthood is still unknown due to early lethality50.
The second category is gene knockout through DNA manipulation. A variety of techniques have been used to mutate the zebrafish genome, each with advantages and disadvantages. Classically, N-ethyl-N-nitrosourea (ENU) was used for chemical mutagenesis for large-scale genetic screens20,51. While this method provides many single base-pair genomic changes, phenotype screening and subsequent gene cloning can be laborious51. An alternative approach is insertional mutagenesis, which utilizes retroviruses or transposons to insert DNA, although the mutation frequency is considerably lower. Due to the size of the inserted sequences, these technologies allow for faster and more precise localization of the mutated target gene52.
Lastly, when the gene of interest is known, using zinc fingers53, transcription activator-like effector nucleases (TALENS)54 or clustered regularly interspaced short palindromic repeats together with CRISPR-associated protein (CRISPR/Cas9)55 to create double-strand breaks, which in turn is repaired either by non-homologous end joining or homology-directed repair56,57, can be used to create mutant lines58. While the zinc finger technology53 is mostly obsolete within the zebrafish, TALEN56 and CRISPR55 are still cost-effective and practical options. The CRISPR system is unique due to its effectiveness in multiplex genome editing as well as its versatility for both single gene of interest mutagenesis and large-scale targeted mutagenesis screens59,60,61. A shortcoming of using CRISPR technology is off-target effects, with a recent estimate of approximately 26% of founder offspring carrying an off-target mutation62. Therefore, careful design of CRISPR targets, Cas9 protein choice63,64,65, and screening of multiple first-generation offspring are needed to decrease unwanted mutations. More recently, F0 crispants have emerged as a rapid screening tool. This technology allows for mutant phenotype screening in injected embryos at a similar efficiency to siRNA and MO21,66. As opposed to MO, the F0 crispant’s DNA is mutated, and thus, its effect does not fade. Another benefit of the technology is a decrease in screening time because the mutated animals do not need to be raised to generate a stable line prior to phenotypic analysis67,68.
Despite these technologies making genome manipulation straightforward, there are a few caveats to consider when creating a mutant animal. As stated previously, the zebrafish has high sequence diversity. Therefore, when using these specific double-strand DNA break technologies, it may be necessary to assess your gene of interest either through a SNP database or sequencing your zebrafish before TALEN/CRISPR design69,70. This is particularly true for fish that are not known laboratory lines, as these fish tend to have more sequence variability when compared to previously described zebrafish lines.
Next, as stated above, the zebrafish underwent a partial genome duplication71. Therefore, it is crucial to consider the paralogs and orthologs within the genome that can partially or completely compensate for the gene loss39. It may be that a single mutant within the zebrafish can act as a hypomorph, and observing a phenotype may require more than a single gene mutation. Alternatively, many duplicate genes have subfunctionalized thus displaying only a subset of phenotypes seen in other vertebrates40, so again, multiple mutations may be required to completely phenocopy a human disease.
Additionally, maternal contribution to early development is critical. There are two strategies to mutate the maternal contribution to the embryo. First, germ-line replacement, where germ cells from homozygous zygotic mutant embryos are transplanted into WT embryos depleted of their germ cells and raised to adults72, is used to create embryos with both maternal and zygotic homozygous mutations. Second, gene editing provides a feasible approach to investigating the maternal contribution during early development in zebrafish. Researchers can employ the CRISPR/Cas9 system directed to the germ line to study such maternal-effect or maternal-zygotic phenotypes73,74. Alternatively, rescue of the zygotic lethal mutation can be attempted by injecting WT mRNA of the gene into mutant embryos and raising them to adulthood to generate homozygous mutant mothers. The offspring could then be analyzed to determine maternal function75,76.
The zebrafish is a strong developmental model
While there is a growing body of work in adult fish, the primary historical strength of the model has been within developmental biology19. Zebrafish embryos develop outside the body. Therefore, development is visible from the one-cell stage77. This enables a non-invasive, in-depth analysis of development in a vertebrate model. The zebrafish embryo also develops quickly. Within 24 h of fertilization (hpf), a complete body plan can be observed, and within 7 days, the embryo grows into a fully functional larva11. During this time, the zebrafish is largely translucent, especially in the first 24 h before pigment cells appear, making imaging straightforward. Additionally, during these first 7 days, the animals are simple to care for. Reliant on a yolk sac, zebrafish embryos, and larvae do not need to be fed and can live in simple aqueous media in a 28 °C incubator31. This allows zebrafish to be collected in large numbers at very specific developmental time points. A summary of many key zebrafish life cycle milestones can be seen in Fig. 278,79,80,81,82,83.
Shown are critical milestones in zebrafish development (note images are not shown to scale). However, the embryos and larvae are mm in size, with the single cell at fertilization being 1 mm, whereas the juveniles and adults are cm in size. The mid-blastula transition (MBT) is when the time between cell cycles increases, and cell synchrony is lost77. Zygotic genome activation quickly follows, and maternal transcripts begin to be depleted. Gastrulation begins by just over 5 hpf, and somitogenesis begins 5 h later. The neural tube is formed by ~17 h, and primary neurogenesis begins at around 2 dpf, with secondary neurogenesis beginning at 3 dpf. The zebrafish have a mature nervous system by 4 dpf78. The heart starts beating by 24 h, and the larval kidney (the pronephros) begins to function at around 2 dpf79. The yolk sac is depleted by ~5–6 dpf. The mature digestive system is fully functional by 7 dpf, but the larvae can begin free feeding as early as 5 dpf80,81. The gonads begin to differentiate in the juvenile fish by around 20–25 dpf82. At ~3 months, the fish is fully mature and capable of mating. All times are developmental stages when the fish is developing at the standard 28.5 °C.
Such rapid development and ease of observation come with some precautions. Because the zebrafish develop so quickly, it is critical to accurately stage zebrafish embryos and larvae. For example, mRNA expression within various organ systems can wax and wane within a relatively short period of time, as seen in the short stature homeobox 1 (shox 1) gene expression (Fig. 3a). To address the challenge, zebrafish development has been extensively studied and the stereotypical developmental milestones are carefully delineated. These developmental stages are defined by both morphological features and chronological time passed since fertilization ((hpf or dpf); https://zfin.org/zf_info/zfbook/stages/index.html)11,12. It is through carefully defined and standardized stages that rapidly developing organ systems such as the vasculature (Fig. 3b) have been contextualized in development. This enables data from different labs as well as different genetic strains and mutants to be compared within that developmental context.
a Whole mount in situ hybridization mRNA stain of Short stature homeobox 1 (shox1) throughout the first 5 days of development exemplifies how a gene can change intensity (black arrows point to the brain) and ___location of expression (red arrows point to the fin bud, which is only expressed in 1.5–3 dpf; black arrowhead points to the pharyngeal arches which begin expression at 2 dpf) in a relatively short period of time. b The VEGF receptor (kdr-like) florescent line (Tg(kdrl:Hsa.HRAS-mCherry)s916) demonstrates how quickly an organ system, the vasculature, changes and develops in the first 4 days of zebrafish development. ISV—intersegmental vessels (a—arterial, v—venous); DA—dorsal aorta; PCV—posterior cardinal vein.
When staging adult zebrafish, age, body length and morphology must be considered. Zebrafish are adults at ~90 dpf at 28.5 °C13. Because various factors can influence growth, age is not the most reliable measure of maturation. It is recommended to use standard length, a measure of the length from the snout to the tail (not including the tail fin), to assess maturation12. Fin morphology, specifically pattern, has also been used to determine growth12,84. Body weight is not typically used to stage zebrafish, but it can be used to evaluate the health status of the zebrafish85,86.
Using both morphological markers and time post fertilization to stage development is critical because of the rapid development and the many exogenous factors that affect embryonic and larval development. Many factors can change the speed of zebrafish development31. For example, raising zebrafish at a warmer temperature accelerates development. At the standard 28 °C, zebrafish embryos reach the 20-somite stage at 19 hpf. At 33 °C, they reach the same stage by 14.5 hpf, and at 25 °C in 23.5 h—an almost 10-h difference (https://zfin.org/zf_info/zfbook/stages/figs/fig2.html)11.
Another key example is that the density of zebrafish within a Petri plate can influence the embryo’s development. Generally, it is considered best practice to raise around 50 embryos per 100 mm Petri plate. As the density increases, there is an increased likelihood of asynchronous growth rates, causing a large variation in the stage of the embryos87. This density effect continues into adulthood88. Additionally, the increased density causes an increased likelihood of water contamination with amoeba, bacteria, or mold, which also affects the health of the embryos. When considering juvenile and adult fish, the feeding regimen is another important example that can alter animal growth89,90.
Finally, it is particularly critical to be aware of the developmental stage when comparing WT to an animal that has been manipulated. For example, when using knockdown technology such as morpholinos, their injection generally alters the embryo’s development causing them to develop slower than their uninjected counterparts. It is critical to look at the embryo’s morphology to determine its stage and/or inject a control morpholino at the same concentration, which often causes a similar developmental delay. Within mutant lines, inbreeding of heterozygous mutants produces age-matched WT, heterozygous, and homozygous mutant animals within the same clutch. For experiments requiring exact timing, individual clutches should be collected soon after birth and kept separate because fish can produce embryos throughout the morning and, therefore, the embryos can be laid multiple hours apart. Even in homozygous mutants and maternal-effect mutants, stage matching should be done. Therefore, when comparing experimental and control groups, stage matching using both time and morphology should be considered while designing experiments and analyzing data12.
A summary of common environmental factors that can alter the development and health of both the larval91,92 and adult84,93,94 zebrafish is summarized in Table 3. Many of these are listed above and have been formally studied. However, some examples in the table are from observations and have not been well quantified. For example, most of those who work with zebrafish embryos have observed that, if the density of embryos is too high, the embryos develop at a different rate, resulting in a plate with fish ranging from the 3-somite to 25-somite stages within that same dish. Additionally, most have observed that, if the water is not consistently changed, fungus or amoebas will grow and overrun the embryos, causing a slowing of development and generally unhealthy animals. With adult animals, we have observed changes in mating rates, particularly during daylight savings time, when the light/dark cycle is altered. While these are common observations, they have not been formally quantified.
There are many considerations when deciding the developmental windows suitable for particular experiments. For example, cell fate within organ morphogenesis, cell movement, and migration patterns tend to be studied within the first 24 h; however, they can be studied later in development using a subset of cells95,96,97. One specific example of this is the study of the connection between vascular and neural development, which occurs within the first 24–48 h. Choosing the correct developmental stage is critical, especially in neurological and behavioral experiments, because the behavioral complexity and neural connections may not be as developed in certain larval stages when compared to adult zebrafish or other mammalian models.
In addition, working with extremely young embryos, such as in the first hour of development, can be challenging. During this critical time of development, the embryo changes radically over a very short time span due to cell divisions occurring approximately every 15 min for the first 10 rounds, so even relatively subtle differences within a single clutch of embryos can have substantive consequences in experimental outcomes. For example, those embryos spawned first may be at a different stage than those laid last11. Therefore, it is critical to closely time the embryos using previously defined developmental markers11. This not only ensures consistency within your replications but also improves reproducibility. Moreover, for the first few divisions, the cells are very fragile, and manipulating these embryos is technically challenging. Even after fixation, the embryos are easily damaged during the RNA or protein staining protocols. Therefore, when first beginning to work with these young embryos, plan on additional time and embryos to replace the ones that are ruined during the process.
In contrast, when using older zebrafish larvae, other considerations arise. For this article, we focus on zebrafish dpf and beyond due to key developmental milestones. We also recognize that international regulatory bodies may use different criteria for specifying regulatory practices. Thus, local animal welfare oversight rules may be an important consideration in the study design for any zebrafish experimentation. First, the yolk sac, which supplies nutrients to the growing animal, is exhausted by 6–dpf, and the growing larva needs to start eating98. Experiments performed on larvae that have exhausted their yolk should have a feeding regimen. Some of the larvae may be starving and sick without feeding, making data more challenging to interpret. In addition, humanely handling and caring for experimental fish are an important issue after dpf, at which point neuroendocrine response to exogenous stress has developed99,100,101. While established protocols (IACUC or institutional animal care and use committee) differ by individual institutions, the beginning of 4 dpf usually marks when the experimental animal should be cared for with a full measure (e.g. analgesics, anesthesia) in the zebrafish field. Second, later-stage larvae are larger, and their epithelial tissue is no longer as permeable. Therefore, many imaging and staining techniques are more difficult at this stage. After 6 to 7 dpf, most RNA or protein staining protocols may need to be conducted on sectioned tissue or after manual skin removal due to increased tissue density depending on the target mRNA/protein depth within the animal.
Organ system size and scale
Another difference in the zebrafish model lies within the scale of the cell types and tissues seen within the animal. This is exemplified by comparing the zebrafish brain to the mouse or human brain. The human brain is large, on average 8.6 × 109 neurons102, anatomically difficult to access through the skull, ethically complex to justify invasive studies, and biophysically challenging to investigate with non-invasive methods. The mouse brain, at approximately 7 × 107 neurons, is still too large to visualize in its entirety without removing the brain and examining sections or using disruptive clarity approaches103. The brain is encased within the skull, and looking at small live tissue sections is still difficult. On the other hand, the larval zebrafish brain contains approximately 100,000 neurons with the forebrain, midbrain, and hindbrain aligned along the anterior-posterior axis, which improves imaging capabilities. Still, the 100,000 neurons within the zebrafish larval brain provide analogous structures to the human and mouse brain104,105. The small size, translucency, and structural analogs enable the visualization of the entire brain and comparative studies. Other examples can be seen in the kidney, which contains a single glomerulus in the embryo, and the gastrointestinal system, which is a single straight lumen within the embryo. The zebrafish may be the only vertebrate model system where the cell biology of live intestinal cells can be assayed in the presence of bile mucus and symbiotic organisms106,107. These “scaled-down” analogs of human tissues allow for the rapid and single-cell-level resolution of cell-cell interactions and developmental processes within a live animal.
Experimental design and the zebrafish model
Sound experimental design and statistical analysis are foundational to collecting unbiased data and accurately interpreting experimental outcomes. To ensure scientific rigor, many scientific journals are starting to expect manuscripts to justify the statistical analysis108,109. This is also become an expectation within the zebrafish community. Here, we review unique considerations when creating sound experimental designs when using zebrafish.
Definition of replicates when using the zebrafish
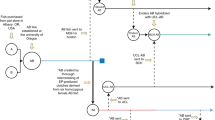
In statistics, the “n” typically refers to the number of sampled organisms versus the population size “N”. Within many areas of experimental science, the number of experimental replicates equals the “n”. However, when using an in vivo research organism, there are additional requirements that must be accounted for to ensure the correct sampling of the population. According to the United States’ National Institutes of Health (NIH) there are two distinct subsets of variation that must be accounted for when considering biological sampling (https://www.nigms.nih.gov/training/documents/module3-biological-and-technical-replicates.pdf). First, the samples must have independent technical replicates to account for the random noise of the methodology and equipment (reviewed in ref. 110). Second, biologically distinct samples that capture the variation of the animal or biological model system must be used to account for genetic and environmental noise (Fig. 4a, b).
a Using a single mating pair’s progeny, technical replicates can be conducted (the Eppendorf tube stands in for any data output with a–c representing three technical replicates). This data will only give information on the variation due to the experimental method. b Biological replicates are also necessary to sample the population appropriately. Using the progeny from different mating pairs ensures genetic diversity, which is the basis of biological variability. c A single experiment typically uses many different mating pairs to create biological diversity within the pool of progeny tested. The experiment shown here has a single technical replicate with multiple conditions (control, con; treatment 1, Tx 1; treatment 2, Tx 2). However, technical replicates are achieved using these progenies, but these are not adequate biological replicates. d Multiple experiments using different tanks of outcrossed adult fish allow for both technical and biological replicates.
It is within the second type, the biological replicates, where field-specific expectations and standards come into play. As stated above, each zebrafish line is a genetically diverse population. Therefore, it is critical to account for both technical and biological variability within the experimental protocol. Additionally, it is customary that a minimum of three experiments be performed to obtain a confidence interval and account for both biological and technical replicates.
For example, imagine an experiment assessing the efficacy of two drugs at a single dose. There will be three treatment conditions (e.g. “Control”, “Tx 1”, and “Tx 2”). Now, depending upon the methodology downstream, this single experiment can have either one or multiple zebrafish within each sample. When using multiple zebrafish, if they are from a mix of multiple mating pairs, this helps account for biological/genetic diversity. Typically, with multiple animals within an experiment, the data are averaged to produce a single output toward the ‘n’. Within this scenario, the number of zebrafish does not equal the number of experiments performed (Fig. 4). Next, this single experimental set should be repeated multiple times, ideally on different days. This way, the “Control”, “Tx 1”, and “Tx 2” groups will have multiple data points collected over the different experimental times (Fig. 4d). This helps avoid unforeseen and unnoticed factors that might affect the experimental outcome. For example, a malfunction within an incubator’s thermostat, pH level in the aquarium widely fluctuates, room temperature changes due to weather, microbiomes in Petri dishes where larvae are kept vary, and many others. Performing experiments on different days ensures a data point’s validity and the measurements’ precision over a temporal scale. Additionally, performing experiments on separate days requires clutches derived from multiple different mating pairs, which is critical for genetic and biological variation. If a single animal is used for each experiment, it is important to ensure genetic diversity in the samples chosen (Fig. 4b). In the end, the expectation is that the experiment was performed, at a minimum, in triplicate to ensure adequate replicates regardless of the actual number of zebrafish tested.
Determine the number of zebrafish required per replicate
The number of zebrafish required per experiment depends largely upon two key elements, the type of data being collected and the variability of the data. Two distinct types of data that can be collected within the zebrafish, observational (or non-quantifiable) and quantifiable data. Observational data is considered data that can be described but not necessarily changed into a quantifiable number. For example, gene and protein expression patterns during development and how they are altered when a manipulation is performed are considered observational data. Which organ systems the gene is expressed in and how that changes over time cannot be readily converted into a number. It is critical, however, that this data be replicated. For example, when performing an in-situ hybridization experiment, approximately 10 or more embryos or larvae from at least three separate clutches at a given stage should be assessed to ensure that the pattern seen is consistent at that time point. Quantifiable data, alternatively, can be expressed as a number or percentage. So, while the examples above are observational, some aspects can also be quantitative by measuring the number of mRNA transcripts with quantitative PCR or the immunofluorescence intensity levels. Therefore, when considering the number of zebrafish per replicate, it is critical to decide the type of data output first.
Prior to experimentation, it is critical to define what you will consider a single replicate. Due to the large number of progeny available, the number of individual zebrafish within one biological replicate can range from 3–15 animals. In general, the more animals included in a single replicate, the more accurate the output tends to be. When deciding on the actual number of animals, it is important to consider how much variability is expected. Some data tend to have greater variability and require a larger number of both biological and technical replicates. Examples of such data are behavioral outcomes. Behavior fluctuates significantly between animals, and identifying the true mean takes many replicates. In such cases, the number of fish is often several tens (adults) to hundreds (larvae) of individual animals to produce a single data point. In comparison, some data sets have less variability or contain categorical data. Within the zebrafish community, such data can be those defining cell types or developmental processes.
Although a large number of zebrafish per replicate is desirable, it is understood that cost and technical limitations significantly influence the number of animals used. “The Guide for Care and Use of Laboratory Animals”111 requests minimizing the number of vertebrate animals used. Therefore, to be good stewards of the animals within our care and the monetary resources awarded, it is important to consider using the minimum number of animals required while performing valid and reproducible experiments. Nevertheless, we cannot sacrifice experimental rigor, as a wrong or incomplete answer to a question wastes all of the animals.
As long as these ethical considerations are accounted for, measuring multiple data points for an experimental group and taking the average value from the collective data points as a single data point is one of the unique strengths the zebrafish model has, whether the data type is qualitative or quantitative. Such a single data point represents a sample mean. By the central limit theorem, these data points are likely to be normally distributed regardless of the distribution of the population from which the samples are drawn112,113. Whereas making a composite data point from multiple animals or by averaging multiple measurements and combining multiple such experiments are a common practice in invertebrate model systems, zebrafish are among the few vertebrate models that permit this type of rigorous experimental design.
Randomizing samples
Due to the genetic heterogeneity in the zebrafish, randomization starts with obtaining embryos from multiple parents. Whether an experiment uses larval or adult fish, the subject fish of an experiment should be obtained from multiple mating pairs of different lineages, which ideally means different fish tanks, to avoid lineage-specific biases. The embryos obtained from multiple parental pairs should be combined and then placed into Petri dishes, ensuring that each dish will contain embryos from all lineages. Otherwise, some dishes will contain embryos from a mating of one pair of fish while other dishes contain embryos from a mating of another pair. This, in turn, produces data that may be confounded by family-specific responses. When embryos can be collected from only one mating pair, the experiment can still be performed. However, ideally repeat experiments should be done with a different set of embryos from different parental pairs and on different days to ensure both technical and biological replicates are performed.
How the collected fish are distributed into experimental groups is also important. This is particularly true after the animals are out of the chorion and freely swimming. At this point, collecting poorly swimming larvae first is a common error that inadvertently occurs because it is easier to catch such larvae. This type of error, in turn, introduces variations in the collected groups that are not factored in the original experimental design. Such variabilities may represent differing baseline activity levels, nervousness, hormonal levels, and developmental delay. Alternatively, if there is no obvious visual phenotype to differentiate mutant from WT siblings, mutant animals may be collected into a particular experimental condition, either earlier or later, depending upon whether the mutation leads to hyper- or hypo-activity. Similarly, where the larvae are located in the plate matter, the larvae collected from the central area of a dish and those collected by the wall of a dish may display different behaviors or biochemical profiles. It is essential to randomize those collections into experimental groups. The same criteria apply to adult fish. When collecting adult fish from a housing tank, earlier and later caught fish should be distributed randomly to diverse experimental groups.
Improving reproducibility
As the scientific community strives to achieve experimental reproducibility, this review is intended to create guidelines for rigorous and reproducible experiments using the zebrafish model. The key to reproducibility has two primary facets. First, as discussed above, the experiment should be designed with key details in place from the beginning, including technical and biological replicates and analytical methods determined based on the best understanding of the specific scientific context. Second, all pertinent information for the experiment replicates should be provided to the scientific community. Here, we review key topics to increase reproducibility in zebrafish research and list critical factors that should be included in a manuscript (Table 4).
Information about the zebrafish used during experimentation
As with other animal model systems, following the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines is now strongly recommended114,115. When considering zebrafish specifically, the methods section of a manuscript should provide details on animal husbandry. These practices for zebrafish care are well defined and part of basic operating procedures found in sources such as Westerfield’s Zebrafish book31, Zebrafish: a practical approach17, or Zebrafish in Biomedical Research116. If practices deviate from the standards found within those sources, those should be detailed within the manuscript. This includes but is not limited to the light/dark cycle, feeding regimen, and water conditions.
The zebrafish are genetically diverse, and it is important to maintain that diversity. Therefore, the outbreeding frequency and the lines used should be included within a manuscript. For those who do not have access to the laboratory lines or decide to use local fish from a pet store, fish farm, or the wild, it is critical to note this in any publications. Should a mutant be created within that background and the sequence varies from the published genome, this should also be noted in the publication. Additionally, it is ideal that any new laboratory WT line established be sent to the local zebrafish repository.
How the zebrafish embryos and larvae are raised is another critical piece of information. In most institutions, young fish (until 6-7 dpf) are raised in a Petri dish in an incubator at approximately 28.5 °C. The light/dark cycle of the embryos is also standardized in The Zebrafish Book. However, when the lights are turned on and off can be adjusted for optimum experimental timing depending upon the data being collected. For instance, if experiments require the embryos to be raised without any light in an incubator or in a light/dark cycle, the protocol details should be reported. For many studies, reporting the times for in vivo experimental steps during the light/dark cycle may be important, as results may depend upon hormonal levels (e.g. the circadian rhythm) or time since feeding (for those animals 7 dpf and older). Experimentation on adult animals requires specific details along with the ones described for larvae. This includes any changes to the standard husbandry, such as changes to diet, temperature, pH of the water, the number of fish per tank above or below the standard practice, and the light/dark cycle. The adult zebrafish require the sex of the fish used in the experiment to be stated. If only a single sex is used, a reason should be given. If a mix of the sexes is used, a percentage of male to female should be stated.
Since the zebrafish has many strains of “WT” that are generally available through zebrafish vendors or propagated specifically in a laboratory, the WT strains used should be detailed within the methods and made available upon request. It is particularly important to report the background strain of any transgenic lines. Further, if multiple transgenic insertions are bred together, the background strain of each line crossed together should be noted, as well as the WT line used to outcross for propagation. The use of pet store zebrafish poses a particular challenge in scientific reproducibility due to the maintenance of genetic variation alone. Such stocks can become problematic for the researcher due to differences in sequences from the published zebrafish genome. Additionally, providing new WT lines to the corresponding stock center for distribution would also be expected. Consequently, any lower initial cost of using such fish is greatly balanced by the substantive overall costs of using such animals in publications, and the use of established WT lines is almost always the most practical choice for effective zebrafish research. In fact, a method whereby sperm can be stored for as much as 7 days at room temperature117 has been described, thereby making it even easier for labs in remote locations to establish WT strains. Accordingly, any mutant or transgenic lines should have the information on the background lines from which they are generated. All lines should be named using ZFIN nomenclature and given a ZFIN link to connect the paper to previous data.
Critical methodological detail is required for experimental repetition
The “Experimental Information” in Table 4 highlights vital information for those wishing to replicate the experiment and validate the findings reported within the paper. DNA sequences required for identifying the genes are critical to report and include, but are not limited to, plasmids, genomic sequence changes, morpholino sequences, primer sequences, etc. Additionally, the genome coordinates and the genomic assembly version should be stated since the sequences and annotation can differ depending upon the assembly version used. To ensure the specific gene sequence is associated with other information known about the gene, the ENSEMBL identification number or the gene ID should be given. Protocol details should also be reported. It has become a trend to see a paper cited for the full protocol, when in fact that paper does not include the entire protocol. For example, when staining for mRNA with an in situ protocol, it is simple to cite (Thisse et al.118). However, most laboratories do not follow this protocol step by step. Any changes to the cited protocol should be added to the methods section of a paper.
Similarly, behavioral experiments using the zebrafish can be difficult to replicate without adequate details. It is essential to clearly state the details of the behavioral assay system used, whether it is commercially available or developed within the institution, the software used for data analysis, and any possible confounding environmental details (temperature, noise level, possible vibrations, etc.).
Details on data analysis used
The number of zebrafish used, and the replicates done should be reported. Additionally, the statistical method should be justified for the type of data collected. Within the data analysis discussion, each experiment should specify whether the data were scored blind (single or double). Any informatics tools used for data analysis or scripts created to run the analysis should be made available to aid in reproducing the data. Finally, it is becoming common practice to share raw data in an open data repository. While not zebrafish specific, there are many examples of excellent data deposit sites available: https://www.nature.com/sdata/policies/repositories#general, http://oad.simmons.edu/oadwiki/Data_repositories, https://datadryad.org/stash.
Conclusions
In conclusion, the zebrafish is a robust and valuable vertebrate model organism. The large number of animals available for experimentation compensates for and takes advantage of the high genomic diversity within this animal system. However, effective deployment requires rigorous planning for both the number of animals and replicates required for experimentation, as well as the thoughtful use of statistical methods. It is also critical to include methodological details not commonly listed for experiments using isogenic animals to enable experimental repetition and direct comparisons. The guidelines detailed above lay a foundation for understanding the expectations of the zebrafish field to ensure rigor and repeatability of data to fully unlock the potential of this amazing model system.
Methods
Zebrafish husbandry
All zebrafish experiments were ethically carried out and with the approval of the Mayo Clinic and the University of Pennsylvania IACUC boards. We have complied with all relevant ethical regulations for animal use. The adult animals were maintained using standard husbandry conditions31. The zebrafish embryos were raised and maintained at 28 °C before experimentation. The WT larvae were outcrossed TLF line mated with the casper background. Larvae from the WT line (Figs. 1e,f, and 3) were exposed to approximately 0.003% PTU before 24 hpf to prevent pigment formation. The VEGF receptor (kdr-like) florescent line (Tg(kdrl:Hsa.HRAS-mCherry)s916) was used to image the vasculature. All experimental data was obtained from embryos or larval zebrafish prior to any sexual differentiation.
Zebrafish imaging
In situ hybridization was used to assess the mRNA expression pattern of the shox1 gene as previously described118. The mRNA probe was created from the predicted Danio rerio shox homeobox transcript (GeneID: 66448; Zfin-Gene-051030-21). PCR primers (Forward: 5′-CAGCGCCTCGGCGTGTTTTC-3′; Reverse: 5′-AACTCACGGCGTTCGTGTCG-3′) were used to clone into a pCR4 TOPO vector (ThermoFisher: K457501). The dig-labeled probe was made using the reverse strand by digesting using the speI restriction enzyme. The larval images were taken using SCORE imaging119 within a capillary tube. The final image is a z-stack of multiple in-focus images manually stacked.
The Tg(kdrl:Hsa.HRAS-mCherry)s916 was imaged as previously described. Briefly, the embryos were embedded in 1% low-melt agarose in E3 with tricaine to prevent movement. They were imaged using an inverted SP5 Leica Microscope system120.
Zebrafish research analysis
To approximate the number of papers that utilize the zebrafish, we searched PubMed using the keyword zebrafish. The number of papers published was binned into the year they were published and graphed using GraphPad Prism software. Data is available on Dryad: https://doi.org/10.5061/dryad.nvx0k6f22.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The original data is available on dryad data under: Zebrafishology, study design guidelines for rigorous and reproducible data using zebrafish in situ images dataset (https://doi.org/10.5061/dryad.nvx0k6f22).
References
Howe, K. et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503 (2013).
Goldsmith, J. R. & Jobin, C. Think small: zebrafish as a model system of human pathology. J. Biomed. Biotechnol. 2012, 817341 (2012).
Lieschke, G. J. & Currie, P. D. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 8, 353–367 (2007).
Menke, A. L., Spitsbergen, J. M., Wolterbeek, A. P. & Woutersen, R. A. Normal anatomy and histology of the adult zebrafish. Toxicol. Pathol. 39, 759–775 (2011).
Vidan, S. & Snyder, M. Large-scale mutagenesis: yeast genetics in the genome era. Curr. Opin. Biotechnol. 12, 28–34 (2001).
Sugi, T. Genome editing in C. elegans and other nematode species. Int J. Mol. Sci. 17, 295 (2016).
Port, F. & Bullock, S. L. Creating heritable mutations in Drosophila with CRISPR-Cas9. Methods Mol. Biol. 1478, 145–160 (2016).
Venken, K. J. & Bellen, H. J. Chemical mutagens, transposons, and transgenes to interrogate gene function in Drosophila melanogaster. Methods 68, 15–28 (2014).
Fuentes, R., Letelier, J., Tajer, B., Valdivia, L. E. & Mullins, M. C. Fishing forward and reverse: advances in zebrafish phenomics. Mech. Dev. 154, 296–308 (2018).
Rafferty, S. A. & Quinn, T. A. A beginner’s guide to understanding and implementing the genetic modification of zebrafish. Prog. Biophys. Mol. Biol. 138, 3–19 (2018).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 (1995).
Parichy, D. M., Elizondo, M. R., Mills, M. G., Gordon, T. N. & Engeszer, R. E. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev. Dyn. 238, 2975–3015 (2009).
Singleman, C. & Holtzman, N. G. Growth and maturation in the zebrafish, Danio rerio: a staging tool for teaching and research. Zebrafish 11, 396–406 (2014).
White, R. M. et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183–189 (2008).
Antinucci, P. & Hindges, R. A crystal-clear zebrafish for in vivo imaging. Sci. Rep. 6, 29490 (2016).
Holtzman, N. G., Iovine, M. K., Liang, J. O. & Morris, J. Learning to fish with genetics: a primer on the vertebrate model Danio rerio. Genetics 203, 1069–1089 (2016).
Nüsslein-Volhard, C. & Dahm, R. Zebrafish: a Practical Approach 1st edn (Oxford University Press, 2002).
Driever, W. et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123, 37–46 (1996).
Haffter, P. et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123, 1–36 (1996).
Amsterdam, A. et al. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 13, 2713–2724 (1999).
Wu, R. S. et al. A rapid method for directed gene knockout for screening in G0 zebrafish. Dev. Cell 46, 112–125.e114 (2018).
Clark, K. J. et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nat. Methods 8, 506–515 (2011).
Krauss, S., Concordet, J. P. & Ingham, P. W. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75, 1431–1444 (1993).
Flynt, A. S., Rao, M. & Patton, J. G. Blocking zebrafish MicroRNAs with Morpholinos. Methods Mol. Biol. 1565, 59–78 (2017).
Nasevicius, A. & Ekker, S. C. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 26, 216–220 (2000).
Stainier, D. Y. R. et al. Guidelines for morpholino use in zebrafish. PLoS Genet 13, e1007000 (2017).
Bedell, V. M., Westcot, S. E. & Ekker, S. C. Lessons from morpholino-based screening in zebrafish. Brief. Funct. Genom. 10, 181–188 (2011).
Kawakami, K., Shima, A. & Kawakami, N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc. Natl. Acad. Sci. USA 97, 11403–11408 (2000).
Simone, B. W., Martínez-Gálvez, G., WareJoncas, Z. & Ekker, S. C. Fishing for understanding: unlocking the zebrafish gene editor’s toolbox. Methods 150, 3–10 (2018).
Van Slyke, C. E. et al. Using ZFIN: data types, organization, and retrieval. Methods Mol. Biol. 1757, 307–347 (2018).
Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio) 4th edn (University of Oregon Press, 2000).
Smith, S. A. Zebrafish resources on the internet. ILAR J. 53, 208–214 (2012).
Guryev, V. et al. Genetic variation in the zebrafish. Genome Res. 16, 491–497 (2006).
Brown, K. H. et al. Extensive genetic diversity and substructuring among zebrafish strains revealed through copy number variant analysis. Proc. Natl. Acad. Sci. USA 109, 529–534 (2012).
Buth, D. G., Gordon, M. S., Plaut, I., Drill, S. L. & Adams, L. G. Genetic heterogeneity in isogenic homozygous clonal zebrafish. Proc. Natl. Acad. Sci. USA 92, 12367–12369 (1995).
Whiteley, A. R. et al. Population genomics of wild and laboratory zebrafish (Danio rerio). Mol. Ecol. 20, 4259–4276 (2011).
Audira, G., Siregar, P., Strungaru, S. A., Huang, J. C. & Hsiao, C. D. Which zebrafish strains are more suitable to perform behavioral studies? A comprehensive comparison by phenomic approach. Biology 9. https://doi.org/10.3390/biology9080200 (2020).
Taylor, J. S., Braasch, I., Frickey, T., Meyer, A. & Van de Peer, Y. Genome duplication, a trait shared by 22000 species of ray-finned fish. Genome Res. 13, 382–390 (2003).
Postlethwait, J., Amores, A., Cresko, W., Singer, A. & Yan, Y. L. Subfunction partitioning, the teleost radiation and the annotation of the human genome. Trends Genet. 20, 481–490 (2004).
Force, A. et al. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151, 1531–1545 (1999).
Nagy, A. Manipulating the mouse embryo : a laboratory manual. 3rd edn (Cold Spring Harbor Laboratory Press, 2003).
Tuttle, A. H., Philip, V. M., Chesler, E. J. & Mogil, J. S. Comparing phenotypic variation between inbred and outbred mice. Nat. Methods 15, 994–996 (2018).
Abrams, E. W. & Mullins, M. C. Early zebrafish development: it’s in the maternal genes. Curr. Opin. Genet Dev. 19, 396–403 (2009).
Ryu, S., Holzschuh, J., Erhardt, S., Ettl, A. K. & Driever, W. Depletion of minichromosome maintenance protein 5 in the zebrafish retina causes cell-cycle defect and apoptosis. Proc. Natl. Acad. Sci. USA 102, 18467–18472 (2005).
Plaster, N., Sonntag, C., Busse, C. E. & Hammerschmidt, M. p53 deficiency rescues apoptosis and differentiation of multiple cell types in zebrafish flathead mutants deficient for zygotic DNA polymerase delta1. Cell Death Differ. 13, 223–235 (2006).
Bill, B. R., Petzold, A. M., Clark, K. J., Schimmenti, L. A. & Ekker, S. C. A primer for morpholino use in zebrafish. Zebrafish 6, 69–77 (2009).
Robu, M. E. et al. p53 activation by knockdown technologies. PLoS Genet. 3, e78 (2007).
Fricke, T. et al. Targeted RNA knockdown by a Type III CRISPR-Cas complex in zebrafish. CRISPR J. 3, 299–313 (2020).
Kushawah, G. et al. CRISPR-Cas13d induces efficient mRNA knockdown in animal embryos. Dev. Cell 54, 805–817.e807 (2020).
Gunitseva, N., Evteeva, M., Borisova, A., Patrushev, M. & Subach, F. RNA-dependent RNA targeting by CRISPR-Cas systems: characterizations and applications. Int. J. Mol. Sci. 24. https://doi.org/10.3390/ijms24086894 (2023).
de Bruijn, E., Cuppen, E. & Feitsma, H. Highly efficient ENU mutagenesis in zebrafish. Methods Mol. Biol. 546, 3–12 (2009).
Sivasubbu, S., Balciunas, D., Amsterdam, A. & Ekker, S. C. Insertional mutagenesis strategies in zebrafish. Genome Biol. 8, S9 (2007).
Doyon, Y. et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 26, 702–708 (2008).
Huang, P. et al. Heritable gene targeting in zebrafish using customized TALENs. Nat. Biotechnol. 29, 699–700 (2011).
Hwang, W. Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 (2013).
Bedell, V. M. et al. In vivo genome editing using a high-efficiency TALEN system. Nature 491, 114–118 (2012).
DiNapoli, S. E. et al. Synthetic CRISPR/Cas9 reagents facilitate genome editing and homology directed repair. Nucleic Acids Res. 48, e38 (2020).
Varshney, G. K. & Burgess, S. M. Mutagenesis and phenotyping resources in zebrafish for studying development and human disease. Brief. Funct. Genom. 13, 82–94 (2014).
Uribe-Salazar, J. M. et al. Evaluation of CRISPR gene-editing tools in zebrafish. BMC Genom. 23, 12 (2022).
Daniel, J. G., Yu, X., Ferguson, A. C. & Shavit, J. A. CRISPR/Cas9-mediated genome editing in zebrafish. Methods Mol. Biol. 2631, 371–380 (2023).
Varshney, G. K. et al. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 25, 1030–1042 (2015).
Höijer, I. et al. CRISPR-Cas9 induces large structural variants at on-target and off-target sites in vivo that segregate across generations. Nat. Commun. 13, 627 (2022).
Manghwar, H. et al. CRISPR/Cas systems in genome editing: methodologies and tools for sgRNA Design, off-target evaluation, and strategies to mitigate off-target effects. Adv. Sci. 7, 1902312 (2020).
Li, C. et al. Computational tools and resources for CRISPR/Cas genome editing. Genom. Proteom. Bioinforma. 21, 108–126 (2023).
Guo, C., Ma, X., Gao, F. & Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 11, 1143157 (2023).
Kroll, F. et al. A simple and effective F0 knockout method for rapid screening of behaviour and other complex phenotypes. Elife 10. https://doi.org/10.7554/eLife.59683 (2021).
Buglo, E. et al. Genetic compensation in a stable slc25a46 mutant zebrafish: a case for using F0 CRISPR mutagenesis to study phenotypes caused by inherited disease. PLoS ONE 15, e0230566 (2020).
Klatt Shaw, D. & Mokalled, M. H. Efficient CRISPR/Cas9 mutagenesis for neurobehavioral screening in adult zebrafish. G3 11. https://doi.org/10.1093/g3journal/jkab089 (2021).
LaFave, M. C., Varshney, G. K., Vemulapalli, M., Mullikin, J. C. & Burgess, S. M. A defined zebrafish line for high-throughput genetics and genomics: NHGRI-1. Genetics 198, 167–170 (2014).
Butler, M. G. et al. SNPfisher: tools for probing genetic variation in laboratory-reared zebrafish. Development 142, 1542–1552 (2015).
Postlethwait, J. H. et al. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res. 10, 1890–1902 (2000).
Ciruna, B. et al. Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc. Natl. Acad. Sci. USA 99, 14919–14924 (2002).
Moravec, C. E., Voit, G. C., Otterlee, J. & Pelegri, F. Identification of maternal-effect genes in zebrafish using maternal crispants. Development 148. https://doi.org/10.1242/dev.199536 (2021).
Bertho, S. et al. A transgenic system for targeted ablation of reproductive and maternal-effect genes. Development 148. https://doi.org/10.1242/dev.198010 (2021).
Nguyen, V. H. et al. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev. Biol. 199, 93–110 (1998).
Mintzer, K. A. et al. Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development 128, 859–869 (2001).
Keller, P. J., Schmidt, A. D., Wittbrodt, J. & Stelzer, E. H. Reconstruction of zebrafish early embryonic. Dev. Scanned light Sheet Microsc. Sci. 322, 1065–1069 (2008).
Kane, D. A. & Kimmel, C. B. The zebrafish midblastula transition. Development 119, 447–456 (1993).
Schmidt, R., Strähle, U. & Scholpp, S. Neurogenesis in zebrafish—from embryo to adult. Neural Dev. 8, 3 (2013).
Drummond, I. A. et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125, 4655–4667 (1998).
Holmberg, A., Olsson, C. & Hennig, G. W. TTX-sensitive and TTX-insensitive control of spontaneous gut motility in the developing zebrafish (Danio rerio) larvae. J. Exp. Biol. 210, 1084–1091 (2007).
Quinlivan, V. H. & Farber, S. A. Lipid uptake, metabolism, and transport in the larval zebrafish. Front. Endocrinol. 8, 319 (2017).
Kossack, M. E. & Draper, B. W. Genetic regulation of sex determination and maintenance in zebrafish (Danio rerio). Curr. Top. Dev. Biol. 134, 119–149 (2019).
McMenamin, S. K., Chandless, M. N. & Parichy, D. M. Working with zebrafish at postembryonic stages. Methods Cell Biol. 134, 587–607 (2016).
Clark, T. S., Pandolfo, L. M., Marshall, C. M., Mitra, A. K. & Schech, J. M. Body condition scoring for adult zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 57, 698–702 (2018).
Ulloa, P. E., Iturra, P., Neira, R. & Araneda, C. Zebrafish as a model organism for nutrition and growth: towards comparative studies of nutritional genomics applied to aquacultured fishes. Rev. Fish. Biol. Fish. 21, 649–666 (2011).
Wilson, C. Aspects of larval rearing. ILAR J. 53, 169–178 (2012).
Castranova, D. et al. The effect of stocking densities on reproductive performance in laboratory zebrafish (Danio rerio). Zebrafish 8, 141–146 (2011).
Andersson, M. & Kettunen, P. Effects of holding density on the welfare of zebrafish: a systematic review. Zebrafish 18, 297–306 (2021).
Valentine, S. & Kwasek, K. Feeding rate and protein quality differentially affect growth and feeding efficiency response variables of zebrafish. Zebrafish 19, 94–103 (2022).
Kopp, R., Legler, J. & Legradi, J. Alterations in locomotor activity of feeding zebrafish larvae as a consequence of exposure to different environmental factors. Environ. Sci. Pollut. Res. Int. 25, 4085–4093 (2018).
Ord, J. Ionic stress prompts premature hatching of zebrafish (Danio rerio) embryos. Fishes 4, 20 (2019).
Lara, R. A. & Vasconcelos, R. O. Impact of noise on development, physiological stress and behavioural patterns in larval zebrafish. Sci. Rep. 11, 6615 (2021).
Sawant, M. S., Zhang, S. & Li, L. Effect of salinity on development of zebrafish, Brachydanio rerio. Curr. Sci. 81, 1347–1350 (2001).
Olson, H. M. & Nechiporuk, A. V. Using zebrafish to study collective cell migration in development and disease. Front. Cell Dev. Biol. 6, 83 (2018).
Davidson, A. J. & Zon, L. I. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 23, 7233–7246 (2004).
Jing, L. & Zon, L. I. Zebrafish as a model for normal and malignant hematopoiesis. Dis. Model Mech. 4, 433–438 (2011).
Clift, D., Richendrfer, H., Thorn, R. J., Colwill, R. M. & Creton, R. High-throughput analysis of behavior in zebrafish larvae: effects of feeding. Zebrafish 11, 455–461 (2014).
Alsop, D. & Vijayan, M. M. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R711–R719 (2008).
Alderman, S. L. & Bernier, N. J. Ontogeny of the corticotropin-releasing factor system in zebrafish. Gen. Comp. Endocrinol. 164, 61–69 (2009).
Lee, H. B. et al. Novel zebrafish behavioral assay to identify modifiers of the rapid, nongenomic stress response. Genes Brain Behav. 18, e12549 (2019).
Herculano-Houzel, S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc. Natl. Acad. Sci. USA 109, 10661–10668 (2012).
Zhao, Y. J. et al. Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution. Light Sci. Appl. 7, 17153 (2018).
Leung, L. C., Wang, G. X. & Mourrain, P. Imaging zebrafish neural circuitry from whole brain to synapse. Front. Neural Circuits 7, 76 (2013).
Kozol, R. A. et al. Function over form: modeling groups of inherited neurological conditions in zebrafish. Front. Mol. Neurosci. 9, 55 (2016).
Carten, J. D., Bradford, M. K. & Farber, S. A. Visualizing digestive organ morphology and function using differential fatty acid metabolism in live zebrafish. Dev. Biol. 360, 276–285 (2011).
Otis, J. P., Shen, M. C., Caldwell, B. A., Reyes Gaido, O. E. & Farber, S. A. Dietary cholesterol and apolipoprotein A-I are trafficked in endosomes and lysosomes in the live zebrafish intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 316, G350–G365 (2019).
Gotelli, N. J. & Ellison, A. M. A Primer of Ecological Statistics 2nd edn (Oxford University Press, 2012).
Cohen, J. The statistical power of abnormal-social psychological research: a review. J. Abnorm. Soc. Psychol. 65, 145–153 (1962).
Blainey, P., Krzywinski, M. & Altman, N. Points of significance: replication. Nat. Methods 11, 879–880 (2014).
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals 8th edn (The National Academies Press, 2011).
Lumley, T., Diehr, P., Emerson, S. & Chen, L. The importance of the normality assumption in large public health data sets. Annu Rev. Public Health 23, 151–169 (2002).
Schmidt, A. F. & Finan, C. Linear regression and the normality assumption. J. Clin. Epidemiol. 98, 146–151 (2018).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
Baker, D., Lidster, K., Sottomayor, A. & Amor, S. Two years later: journals are not yet enforcing the ARRIVE guidelines on reporting standards for pre-clinical animal studies. PLoS Biol. 12, e1001756 (2014).
Cartner, S. C. et al. (eds). The zebrafish in Biomedical Research: Biology, Husbandry, Diseases, and Research Applications. (Academic Press, 2019).
Takemoto, K. et al. In vitro storage of functional sperm at room temperature in zebrafish and medaka. Zebrafish 20, 229–235 (2023).
Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 (2008).
Petzold, A. M. et al. SCORE imaging: specimen in a corrected optical rotational enclosure. Zebrafish 7, 149–154 (2010).
Sugden, W. W. et al. Endoglin controls blood vessel diameter through endothelial cell shape changes in response to haemodynamic cues. Nat. Cell Biol. 19, 653–665 (2017).
Acknowledgements
National Institutes of Health T32 GM 112596, RM1 GM136511, FAER MRTG (V.M.B.), Austin Lamont Professorship (R.G.E.), National Institutes of Health R01-GM120762 and R35-GM134863 (M.J.F.), National Institutes of Health R01-GM063904 and R24-OD020166 (S.C.E.), National Science Foundation 2300505 (D.B.), National Institutes of Health R35-GM131908 (M.M.).
Author information
Authors and Affiliations
Contributions
V.M.B.: wrote and assembled the manuscript, made the figures, responded to the reviewers, and coordinated the authors. P.D.: helped write and research the manuscript. H.B.L.: wrote the statistics section of the manuscript, provided statistical expertise. D.S.B.: helped write the section on maternal genome manipulation. J.L.A.: helped write the initial manuscript specifically on the maternal genome. A.J.L.: helped write the initial manuscript. R.X.: helped write the statistical section and provided statistical expertise. E.V.L.: created the zebrafish vasculature figure. M.J.F.: edited the manuscript and provided zebrafish expertise to ensure quality guidelines. M.A.P.: edited the manuscript and provided zebrafish expertise to ensure quality guidelines. M.M.: edited the manuscript and provided zebrafish expertise to ensure quality guidelines. S.A.F.: edited the manuscript and provided zebrafish expertise to ensure quality guidelines. R.G.E.: edited the manuscript from a zebrafish novice point of view for clarity. S.C.E.: edited the manuscript and provided zebrafish expertise to ensure quality guidelines.
Corresponding author
Ethics declarations
Competing interests
The Authors declare the following conflict of interests: SCE and Mayo Clinic have a financial interest in the technology used in this research and may gain financially from its successful outcome.
Peer review
Peer review information
Communications Biology thanks John Kimble Frazer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Eirini Trompouki and David Favero. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bedell, V.M., Dubey, P., Lee, H.B. et al. Zebrafishology, study design guidelines for rigorous and reproducible data using zebrafish. Commun Biol 8, 739 (2025). https://doi.org/10.1038/s42003-025-07496-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-07496-z