Abstract
Phosphodiesterase-5 (PDE5) inhibitors have shown promise as anti-cancer agents in malignancies. However, their specific effects on gastric cancer (GC) and the underlying mechanisms remain elusive. Our aim was to investigate this by combining evidence from population-based studies with data obtained from in vivo and in vitro experiments. By combing a couple of nationwide Swedish registers, GC patients who received PDE5 inhibitors were compared to matched controls while adjusting for confounding factors. The anti-tumor effect and mechanism of the PDE5 inhibitor sildenafil were evaluated via using tumor cells, patient-derived tumor organoids and xenograft animal models in GC. A total of 161 Swedish GC patients from a nationwide population-based cohort who received post-diagnostic PDE5 inhibitors demonstrated lower cancer-specific mortality compared to the controls (HR = 0.66, 95% CI = 0.47-0.92, P = 0.016). Functionally, the PDE5 inhibitor sildenafil exhibited the suppressive ability to prevent oncogenic growth in GC. Mechanistically, sildenafil restrained GC growth by directly activating PKG through PDE5 inhibition for regulating c-MYC expression via its phosphorylation and ubiquitination degradation, thereby suppressing c-MYC stability for IL-6 transcription within the downstream IL-6/JAK/STAT3 signalling pathway. The PDE5 inhibitor sildenafil may serve as a promising adjuvant for GC therapy if further randomized clinical trials confirm its efficacy.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the most frequently diagnosed gastrointestinal cancer globally, with more than one million new cases and ~783,000 estimated deaths each year. Consequently, there is an urgent need to enhance our understanding of the molecular targets associated with GC.
Phosphodiesterase (PDEs) are a common class of metallohydrolases that catalyze the hydrolysis of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). They play a crucial role in regulating various physiological processes, including visual transduction, cell proliferation and differentiation, cell cycle regulation, gene expression, inflammation, cell apoptosis, and metabolic function1,2. Phosphodiesterase type-5 (PDE5) is a cGMP-specific hydrolytic enzyme that has been reported to be expressed in smooth muscle, the cerebellum, the brain, the retina, and platelets3. PDE5 is up-regulated in the bladder, lung, thyroid, cervical and prostate cancer4,5,6,7,8,9,10. PDE5 inhibitors, such as sildenafil, vardenafil, and tadalafil, are FDA-approved and PDE5-specific targeted therapies commonly used to treat patients with erectile dysfunction, pulmonary arterial hypertension, and other non-malignant diseases11,12. However, emerging evidence indicates that PDE5 inhibitors may also suppress tumor growth in various cancers, including those of the colorectum, breast, prostate, pancreas, lung, thyroid, and liver. The anti-tumor mechanisms of PDE5 inhibitors in cancer involve mitotic arrest, induction of tumor apoptosis, improvement of therapeutic sensitivity, reversal of immune exclusion, and induction of PD-L1 upregulation13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34. Sildenafil increases intracellular levels of cGMP by inhibiting PDE5, impacting various cellular processes such as proliferation, apoptosis, and angiogenesis. This modulation affects cell signaling pathways associated with cancer cell growth and survival, as well as enhancing blood flow to tumor by regulating VEGF expression, thereby improving the delivery of therapeutic agents35,36,37. Sildenafil enhances the tumor hypoxia response and stimulates the immune response, thereby modulating the tumor microenvironment. It alleviates hypoxia by promoting vasodilation and improving oxygen delivery, which can inhibit cancer progression and potentially enhance the efficacy of concurrent therapies. Additionally, sildenafil induces the infiltration of immune cells, potentially strengthening anti-tumor immunity38,39. Sildenafil exhibits synergistic effects when used in conjunction with chemotherapy and other targeted or immunotherapeutic agents. Its ability to enhance blood flow may improve the therapeutic efficacy by ensuring adequate delivery and distribution throughout the tumor. Combining sildenafil with certain therapies can inhibit signaling pathways involved in oncogenesis, resulting in a multifaceted treatment approach that can lead to improved outcomes40,41. Sildenafil has a well-established safety profile, with known side effects that are generally mild and manageable. This characteristic makes it a suitable candidate for combination therapies without introducing significant additional risks. Early-phase clinical trials have started to investigate the safety and efficacy of sildenafil in conjunction with standard anticancer treatments42,43,44. Therefore, the rationale for using sildenafil in cancer therapy is anchored in its pharmacological effects, which modulate key cell signaling pathways involved in tumor growth, improve the tumor hypoxic response and immune response within the tumor microenvironment, and enhance the efficacy of existing therapies, and maintain a favorable safety profile.
Nevertheless, the potential effects of PDE5 inhibitors in GC are still unknown. Several studies have observed that sildenafil offers protective effects against gastric damage induced by nonsteroidal anti-inflammatory drugs, ethanol, or acetic acid45,46,47,48,49. Notably, sildenafil’s inhibition in Helicobacter-infected stomachs coincides with the blockage of IFN-α induction of GTP, SLFN4, and NOS2 in myeloid-derived suppressor cells, reversing T-cell suppression and mitigating the development of spasmolytic polypeptide-expressing metaplasia50. Therefore, this study combines evidence from different sources, including a nationwide population-based GC cohort, GC cell lines, GC patient-derived tumor organoids, and xenograft animal models both in vitro and in vivo. This finding tremendously supports further randomized clinical trials (RCT) to assess the clinical feasibility of sildenafil as a promising adjuvant for the treatment of GC.
Results
GC patients who administrated with PDE5 inhibitors exhibit lower cancer-specific mortality
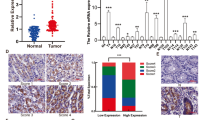
A total of 161 patients from a nationwide Swedish cohort were prescribed PDE5 inhibitors following a diagnosis of GC (Supplementary Table 1). The mortality rate was 93.50 per 1000 person–years among patients using PDE5 inhibitors, compared to 179.67 per 1,000 person-years among matched controls. GC patients who received post-diagnostic PDE5 inhibitors had significantly lower cancer-specific mortality (adjusted HR = 0.66, 95% CI = 0.47–0.92, P = 0.016), particularly those treated with combination use of PDE5 inhibitors (adjusted HR = 0.46, 95% CI = 0.25–0.87, P = 0.016). Furthermore, the negative association was stronger in GC patients diagnosed at an earlier stage (stage I or II) compared to those with advanced stage (stage III or IV) (adjusted HR: 0.55 vs. 0.83) and in patients diagnosed after the age of 66 compared to younger patients (adjusted HR: 0.61 vs. 0.80). The protective effect of PDE5 inhibitors was also more pronounced in patients with gastric adenocarcinoma of the cardia compared to other types (adjusted HR: 0.54 vs. 0.70) (Table 1). The results suggested that PDE5 inhibitors may have anti-cancer potential in GC patients. Additionally, an analysis of PDE5 expression in public GC datasets indicated that PDE5 levels were significantly elevated in tumor tissues compared to paired normal tissues, and overall survival was shorter in the PDE5-high group compared to the PDE5-low group (Supplementary Fig. 1). Moreover, clinical relevance analysis demonstrated a positive association between high PDE5 expression levels and both invasive subtype and diffuse classification subtype, which are defined as more malignant tumor types in GC patients (Supplementary Table 2).
Sildenafil prevents oncogenic growth of GC depending on the PDE5 expression level
To explore the effectiveness of various PDE5 inhibitors in inhibiting tumor growth of GC, we evaluated proliferation and apoptosis in GC cells treated with sildenafil, tadalafil, and vardenafil respectively. The results indicated that sildenafil exhibited a lower IC50 value and a higher apoptosis rate compared to tadalafil and vardenafil in GC cells (Supplementary Fig. 2). Consequently, we focused on sildenafil for further exploration.
PDE5 is the specific target of sildenafil, we examined the expression levels of PDE5 in GC cell lines. We found that PDE5 expression was higher in HGC-27, MKN-45, and NCI-N87 cells, which are classified as relatively PDE5-high GC cells, whereas AGS cells were identified as relatively PDE5-low GC cells. Viability analysis indicated that sildenafil exerted a stronger inhibitory effect on the relatively PDE5-high GC cells compared to the relatively PDE5-low GC cells (Supplementary Fig. 3). So, the relatively PDE5-high cells HGC-27 and MKN-45 were selected for further experiments. CCK-8 assays demonstrated that sildenafil inhibited the proliferation of HGC-27 and MKN-45 cells (Supplementary Fig. 4A). The cell apoptosis analysis revealed that sildenafil induced an increase in total apoptosis rates in both HGC-27 and MKN-45 cells in a dose-dependent manner (Supplementary Fig. 4B). Cell cycle assays also showed that sildenafil treatment led to a higher accumulation of cells in the G0/G1 phase and inhibited the transition from G1 to S phase (Supplementary Fig. 4C). Western blotting assays indicated that the pro-apoptotic proteins Caspase-3 and BAX were increased, while the anti-apoptotic protein BCL2 was reduced in a dose-dependent manner. In addition, the expression levels of the cell cycle-related proteins Cyclin D3 and CDK4 were decreased, whereas the G1 gatekeeper protein p21 was increased following sildenafil treatment (Supplementary Figs. 4D-4E). To investigate the effect of sildenafil in combination with chemotherapy, we observed that sildenafil enhanced the chemotherapeutic sensitivity of relatively PDE5-high GC cells by comparing their IC50 values and the drug combination index (CI) (Supplementary Fig. 5). Collectively, these results suggested that sildenafil inhibits the growth of GC cells in relation to the expression level of PDE5.
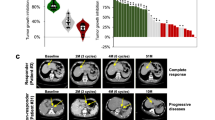
Patient-derived tumor organoids (PDTOs) accurately represent the characteristics of primary cancer in vitro. We successfully cultured and established human GC patient-derived tumor organoids from primary GC tissues (Fig. 1A). The viability of PDTOs significantly decreased in a dose-dependent manner following treatment with sildenafil (Fig. 1B). We evaluated PDE5 expression level in primary GC tumor tissues and the corresponding PDTOs, demonstrating a positive correlation between PDE5 expression in primary GC tumor tissues and PDTOs (Pearson R = 0.79, P = 0.020) (Fig. 1C). Additionally, we explored the relationship between PDE5 expression in PDTOs and sildenafil concentration, revealing a negative correlation between PDE5 expression and the corresponding IC50 values of sildenafil in PDTOs (Pearson R = −0.77, P = 0.025) (Fig. 1D). These findings suggest that sildenafil suppresses the growth of PDTOs depending on PDE5 expression level, indicating that GC patients with higher PDE5 expression may benefit more from sildenafil treatment.
A Patient-derived tumor organoids (PDTOs) were successfully cultured and established from primary GC tissues in vitro, maintaining the characteristics of the primary tumor as presented by H&E staining. Moreover, IHC staining of PDE5 expression in both primary cancer tissues and corresponding PDTOs, with data represented from biological replications (n = 8). Scale bar: 20 μm. B The viability of PDTOs was tested using ATP cell viability assays following sildenafil treatment in 48 h. The IC50 values for PDTOs treated with sildenafil were calculated. Results are presented as the mean ± SD from three independent experiments (n = 3). C, D The association between PDE5 expression in PDTOs and corresponding primary GC tumor tissues, along with the IC50 values of sildenafil, was analyzed based on IHC staining results for PDE5 levels. Statistical significance is indicated as follows: ns, P value > 0.05; *, P-value < 0.05; **, P-value < 0.01; ***, P-value < 0.001.
To further evaluate the in vivo effects of sildenafil, we conducted GC cell-derived and patient-derived xenograft models treated with sildenafil (Fig. 2A, B). The data of H&E, PDE5, Ki-67, and TUNEL staining are presented as representative images (Fig. 2C). The results indicated that both tumor weight and volume of xenografts were reduced following sildenafil treatment (Fig. 2D, E). Additionally, Ki-67 expression levels in GC xenografts decreased, while the number of TUNEL-positive cells increased (Fig. 2F, G). Tumor burdens were lower in GC xenograft models that received two doses of sildenafil (Fig. 2H). Interestingly, we observed a positive correlation between PDE5 expression levels and the response to sildenafil treatment (Fig. 2I). Collectively, these results supported that sildenafil inhibits tumor growth of GC depending on PDE5 expression, suggesting that PDE5 may serve as a potential therapeutic target for sildenafil in anti-cancer treatments for GC.
A Representative photographs illustrated the establishment of cancer cell-derived xenograft (CDX) and patient-derived xenograft (PDX) models treated with sildenafil in GC. This image and every element of this image were originally created and draw by using the BioRender online drawing website (https://biorender.com) after obtaining published permissions. B Tumor sizes in the CDX models (n = 8) and PDX models (n = 6) following sildenafil treatment at doses of 5 mg/kg and 15 mg/kg, respectively, with DMSO serving as a vehicle control. C H&E, PDE5, Ki-67, and TUNEL staining of xenograft sections. Scale bar: 20 μm. D, E Tumor weights of the xenografts were recorded after sildenafil treatment, and tumor volumes were measured on the indicated days. F Analysis of Ki-67 expression levels in the xenograft sections. G Analysis of TUNEL-positive cells in the xenograft sections. H Evaluation of tumor burden in CDX and PDX models treated with sildenafil. I Analysis of PDE5 expression in the xenograft sections. Statistical significance is indicated as follows: ns, P-value > 0.05; *, P-value < 0.05; **, P-value < 0.01; ***, P-value < 0.001.
Sildenafil inhibits PDE5 to suppress the growth of GC through downstream IL-6/JAK/STAT3 signaling pathway
Given that PDE5 is a cellular hydrolytic enzyme expressed in normal gastric epithelial cells, we investigated whether the PDE5 inhibitor sildenafil has side effects on normal gastric epithelial cells. GES-1 cells were used as a cell model for normal gastric epithelial cells in vitro. The results from cell viability and apoptosis assays indicated that no significant differences in cell proliferation and apoptotic ability were observed in GES-1 cells treated with different concentrations of sildenafil for 24 and 48 h (Fig. 3A, B). Therefore, sildenafil does not exhibit an inhibitory effect on normal gastric epithelial cells. We subsequently investigated the relationship between sildenafil and PDE5 in GC tumor tissues, establishing PDE5-knockdown and PDE5-overexpressing GC cells (Supplementary Fig. 6). PDE5 exerts its oncogenic effects by inhibiting apoptosis and promoting cell cycle progression in GC cells (Supplementary Fig. 7). To elucidate whether sildenafil suppresses tumor growth through PDE5 inhibition, IC50 analysis demonstrated that the IC50 value in PDE5-knockdown GC cells treated with sildenafil was increased, while the IC50 value in PDE5-overexpressing GC cells was decreased (Fig. 3C). To ensure that sildenafil’s inhibitory effects are due to PDE5 inhibition rather than general cytotoxicity, different concentrations of sildenafil were applied to these cells. The results indicated that sildenafil suppresses GC cell growth through PDE5 inhibition, with GC cells exhibiting higher PDE5 expression showing a better response to sildenafil treatment (Fig. 3D, E). It is widely known that cGMP serves as an effector in PDE5 inhibition, with phosphodiesterase-5A1 (PDE5A1) recognized as one of the isoforms of PDE5. We directly measured the inhibitory activity against PDE5A1 and the levels of cGMP in both PDE5-knockdown and PDE5-overexpressing GC cells. The results indicated that sildenafil reduced PDE5A1 activity while increasing cGMP levels through PDE5 inhibition (Fig. 3F, G). 8-Bromo-cGMP, an exogenous and cell-permeable analog of cGMP, directly inhibits PDE5 and activates PKG. We evaluated the effects of both sildenafil and 8-bromo-cGMP on cell proliferation and apoptosis in the indicated cells. The results indicated that 8-bromo-cGMP exerted inhibitory effects on GC cells, like those observed with sildenafil (Fig. 3H, I), suggesting that sildenafil prevents GC cell growth through PDE5 inhibition. In vivo studies using subcutaneous xenograft models further examined the function of sildenafil. The results revealed that GC cells with PDE5 overexpression promoted tumor growth compared to control cells, and sildenafil significantly inhibited tumor growth in xenografts derived from PDE5-overexpressing cells, leading to a significant reduction in tumor burden (Fig. 3J–M). Collectively, these observations supported that sildenafil suppresses tumor growth of GC through inhibiting PDE5.
A Cell viability of GES-1 cells was analyzed after sildenafil treatment for 24 h and 48 h, with data represented from technical replicates (n = 3). B Total apoptosis rates of GES-1 cells treated with sildenafil for 24 h and 48 h were assessed, with data represented from technical replicates (n = 3). C IC50 analysis of PDE5-knockdown and PDE5-overexpressing GC cells treated with sildenafil was performed after 48 h, with results presented as the mean ± SD from three independent experiments. D Cell viability of PDE5-knockdown and PDE5-overexpressing GC cells was analyzed following 48 h of sildenafil treatment. E Total apoptosis rates of the indicated cells treated with sildenafil for 48 h were assessed. F Inhibitory activity against PDE5A1 in the indicated cells was measured by using PDE5A1 assay kit. G cGMP level was measured by using cGMP ELISA kit. H Cell viability of PDE5-overexpressing GC cells was analyzed following treatment with either sildenafil (150 μM) or 8-Bromo-cGMP (150 μM) for 48 h. I Total apoptosis rates of the indicated cells treated with sildenafil or 8-Bromo-cGMP for 48 h were assessed. J Tumor sizes of xenografts derived from PDE5-overexpressing GC cells treated with sildenafil (15 mg/kg), with DMSO used as the vehicle control. Data are presented from biological replicates (n = 5). K, L Tumor volumes of xenografts were measured on indicated days, and tumor weights were calculated after sildenafil treatment (15 mg/kg). M Tumor burden of xenografts was evaluated. Statistical significance is indicated as follows: ns, P-value > 0.05; *, P-value < 0.05; **, P-value < 0.01; ***, P-value < 0.001.
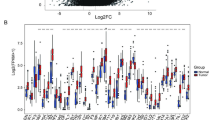
To investigate the potential mechanisms of sildenafil in GC cells, transcriptomic sequencing (RNA-seq) was performed on PDE5-overexpressing cells treated with sildenafil. Hallmark gene signatures were obtained from The Molecular Signatures Database (MSigDB) hallmark gene set collection (https://www.gsea-msigdb.org/gsea/msigdb/human). These hallmark gene sets represent well-defined biological states and processes. The results from the Gene Set Enrichment Analysis (GSEA) revealed that only the IL-6/JAK/STAT3 signaling pathway was significantly enriched in the PDE5-overexpressing cells treated with sildenafil (Fig. 4A, B). The results from GSEA in GC datasets revealed that the IL-6/JAK/STAT3 signaling pathway was enriched in groups with high PDE5 expression and in GC tumor tissues (Supplementary Fig. 8). Western blot analysis showed that overexpression of PDE5 increased the levels of IL-6, phospho-JAK, JAK, phospho-STAT3, and STAT3. Conversely, sildenafil partially reversed these effects by inhibiting PDE5 (Fig. 4C). We further investigated whether the inhibitory effect of sildenafil is dependent on IL-6/JAK/STAT3 signaling pathway through PDE5. PDE5-overexpressing GC cells were treated with an IL-6 pathway inhibitor (LMT28) and a recombinant human IL-6 protein (rHuIL-6). The results demonstrated that the combination of LMT28 and sildenafil reduced cell viability and increased apoptosis rates in PDE5-overexpressing cells compared to control cells (Fig. 4D, E). However, rHuIL-6 partially reversed the proliferative and apoptotic effects of sildenafil (Fig. 4F, G). Thus, sildenafil regulates the IL-6/JAK/STAT3 signaling pathway to inhibit GC cell growth. Together, these findings confirm that sildenafil suppresses GC growth by inhibiting PDE5 and affecting downstream IL-6/JAK/STAT3 signaling pathway.
A, B GSEA was performed on PDE5-overexpressing GC cells after treatment with sildenafil (150 μM) for 48 h, based on results from RNA-Seq data (GSEA: gene set enrichment analysis; NES: normalized enrichment score; NOM: nominal). C Western blotting analysis was conducted to evaluate the levels of pathway-associated proteins involved in the IL-6/JAK/STAT3 signaling pathway in PDE5-overexpressing GC cells following treatment with sildenafil (150 μM) for 48 h. D Cell viability of PDE5-overexpressing GC cells was evaluated after treatment with sildenafil (150 μM) and the IL-6 pathway inhibitor LMT28 (30 μM) for 48 h. Data are presented from technical replicates (n = 3). E Total apoptosis rates of the indicated cells treated with sildenafil (150 μM) and LMT28 (30 μM) for 48 h were analyzed, with data presented from technical replicates (n = 3). F Cell viability of PDE5-overexpressing GC cells was assessed after sildenafil treatment (150 μM) and recombinant human IL-6 protein (rHuIL-6) (10 ng/mL) for 48 h, with data presented from technical replicates (n = 3). G Total apoptosis rates of the indicated cells treated with sildenafil (150 μM) and rHuIL-6 (10 ng/mL) for 48 h were analyzed, with results presented as mean ± SD from three independent experiments. Statistical significance is indicated as follows: ns, P-value > 0.05; *, P-value < 0.05; **, P-value < 0.01; ***, P-value < 0.001.
Sildenafil inhibits PDE5 to directly activate PKG for regulating c-MYC expression to suppress its stability for IL-6 transcription
Previous findings have verified the PDE5-mediated effects of sildenafil via the suppression of IL-6/JAK/STAT3 signaling pathway, we further explored the pathway-enriched genes in PDE5-overexpressing GC cells treated with sildenafil. The results indicated that IL-6 was the most significantly downregulated gene within the IL-6 /JAK /STAT3 signaling pathway (Fig. 5A). We then analyzed the correlation between IL-6 expression and the prognosis of GC patients. The findings showed that high levels of IL-6 mRNA were present in GC tumor tissues and were associated with poorer overall survival in GC patients (Supplementary Fig. 9). Consequently, we focused on the IL-6 gene and performed a knockdown of IL-6 expression in PDE5-overexpressing GC cells treated with sildenafil. As a result, the inhibition of IL-6 partially restored the proliferative ability of GC cells with PDE5 overexpression. However, cell viability was significantly suppressed following the combination of IL-6 knockdown and sildenafil treatment (Fig. 5B and Supplementary Fig. 10). Collectively, these results revealed that the PDE5-mediated inhibitory effects of sildenafil are dependent on IL-6 in GC.
A A heatmap illustrated differentially expressed genes in the IL-6/JAK/STAT3 signaling pathway in PDE5-overexpressing GC cells after sildenafil treatment (150 μM) compared to vehicle control for 48 h. Red indicates upregulated expression, while blue indicates downregulated expression. B Western blotting analysis was performed on the indicated proteins in PDE5-overexpressing GC cells transfected with IL-6-knockdown siRNA and treated with sildenafil (150 μM) for 48 h. β-actin was used as a loading control. C The correlation among PDE5, IL-6, and c-MYC gene expression was analyzed by using the TCGA-STAD database. D, E Western blotting analysis assessed c-MYC and IL-6 expressions in PDE5-knockdown and PDE5-overexpressing GC cells treated with c-MYC-knockdown siRNA and sildenafil (150 μM) for 48 h respectively. F Primer sequences for the canonical c-MYC binding site. G A schematic representation of the IL-6 promoter shows the translational start site, with the regions indicated for luciferase reporter assays. H Luciferase activity analysis was conducted on the mutant fragments of the IL-6 promoter reporter vector after transfecting into GC cells and treated with sildenafil (150 μM) for 48 h. Data are presented from technical replicates (n = 3). I Chromatin immunoprecipitation using specific antibodies demonstrated the binding of c-MYC to the IL-6 promoter, with data shown from technical replicates (n = 5). J Western blotting analysis of the indicated proteins was conducted in PDE5-overexpressing GC cells treated with the PDE5 inhibitor sildenafil (150 μM), the PKG inhibitor KT5823 (20 μM), and the proteasome inhibitor MG132 (20 μM) for 48 h. K IHC staining of PDE5, c-MYC, and IL-6 expression in human GC tumor tissues, with scale bars of 200 μm (10×) and 20 μm (40×). L The correlation among PDE5, c-MYC, and IL-6 expression in human GC tumor tissues (n = 30) was analyzed. M A schematic diagram illustrated the key roles of the PDE5 inhibitor sildenafil in GC. This image and every element of this image were originally created and draw by using the BioRender online drawing website (https://biorender.com) after obtaining published permissions. Statistical significance is indicated as follows: ns, P-value > 0.05; *, P-value < 0.05; **, P-value < 0.01; ***, P-value < 0.001.
To determine the relationship between sildenafil and IL-6, we investigated the upstream transcription factors of IL-6 and identified c-MYC as a potential transcription factor based on prediction and co-expression analysis (Supplementary Fig. 11). We analyzed the correlations among the gene expressions of PDE5, IL-6, and c-MYC in the stomach adenocarcinoma patients using data from the TCGA database. The results revealed a positive correlation between the mRNA levels of PDE5 and IL-6 (r = 0.162, P = 7.37e-04), as well as a positive correlation between the mRNA levels of IL-6 and c-MYC (r = 0.202, P = 3.38e-05) (Fig. 5C). Western blotting analysis showed that sildenafil inhibited PDE5, leading to downregulation of the expression levels of IL-6 and MYC. Furthermore, inhibition of c-MYC partially reversed the increase in IL-6 expression observed in the PDE5-overexpressing GC cells (Fig. 5D, E). We constructed three mutant fragments of the IL-6 promoter reporter vector to analyze the effect of c-MYC on IL-6 transcription. By using the luciferase reporter assays, we found that c-MYC expression significantly enhanced the luciferase activity driven by the full-length IL-6 promoter. In contrast, sildenafil decreased c-MYC-driven luciferase activity of IL-6. Targeted mutations in the promoter region revealed that the -845 and -843 bp sub-regions were critical for c-MYC-enhanced IL-6 promoter activity (Fig. 5F–H). Furthermore, we performed chromatin immunoprecipitation (ChIP) assays, which indicated that c-MYC binds to the IL-6 promoter region, while sildenafil inhibits this binding (Fig. 5I). Thus, c-MYC acts as a transcription factor that directly activates IL-6 gene transcription by binding to a specific region of the IL-6 promoter in cells treated with sildenafil.
Previous studies reported that sildenafil inhibits PDE5 to activate PKG directly. Activated PKG phosphorylates the transcription factor c-MYC, leading to a decrease in c-MYC expression through ubiquitination degradation51,52. To further investigate whether sildenafil inhibits PDE5 to reduce the stability of c-MYC and the expression levels of c-MYC and IL-6 via PKG, PDE5-overexpressing GC cells were treated with the PDE5 inhibitor sildenafil, the PKG inhibitor KT5823, and the proteasome inhibitor MG132. The results indicated that the protein levels of c-MYC, phospho-c-MYC (p-Thr58), and IL-6 were significantly reduced, while the levels of phospho-c-MYC (p-Ser62) showed no significant changes. Besides, inhibition of PKG partially restored the expression levels of c-MYC and IL-6 in the cells treated with sildenafil. However, the proteasome inhibitor MG132 eliminated the differences in c-MYC and IL-6 protein levels observed in PDE5-overexpressing GC cells treated with sildenafil and KT5823 (Fig. 5J). Sildenafil inhibited PDE5 to restrain c-MYC stability for IL-6 transcription. These findings demonstrated that sildenafil inhibits PDE5 to directly activate PKG for reducing c-MYC expression through phosphorylation and ubiquitination degradation, thereby suppressing c-MYC stability for IL-6 transcription within the downstream IL-6/JAK/STAT3 signaling pathway. Thus, sildenafil prevents oncogenic growth of GC by suppressing c-MYC stability for IL-6 transcription.
Clinically, we evaluated the correlations among the expressions of PDE5, c-MYC, and IL-6 in human GC tumor specimens (n = 30) by using IHC staining. Correlation analysis revealed positive relationships between PDE5 and c-MYC expression (Pearson R = 0.375; P = 0.041) as well as between PDE5 and IL-6 expression (Pearson R = 0.389; P = 0.034). Additionally, a positive correlation was observed between c-MYC and IL-6 expression (Pearson R = 0.498; P = 0.0051) (Fig. 5K, L). Collectively, these findings highlighted the mechanism by which sildenafil suppresses the oncogenic growth of GC by inhibiting PDE5 to directly activate PKG for the regulation of c-MYC and IL-6 in the downstream IL-6/JAK/STAT3 signaling pathway (Fig. 5M). Therefore, PDE5 inhibitor sildenafil might be used as a promising adjuvant for GC therapy in the future.
Discussion
PDE5 inhibitors are commonly used in clinical therapy to treat erectile dysfunction, pulmonary arterial hypertension, and other non-malignant urological disorders. They primarily target intracellular cGMP levels by the nitric oxide (NO)-driven activation of soluble guanylyl cyclase. Recently, there has been growing interest in the role of PDE5 inhibitors in oncology. This study focuses on the potential of PDE5 inhibitors and their significant roles in GC. As previously mentioned, our study, which comprehensively combines evidence from a nationwide population-based GC cohort with in vivo and in vitro experiments, supports the hypothesis that PDE5 inhibitors may be repurposed as therapeutic agents in oncology and could effectively suppress tumor growth in GC. Using a nationwide Swedish population-based cohort, we found that post-diagnostic use of PDE5 inhibitors was significantly associated with a decreased risk of cancer-specific mortality in Swedish patients with GC, suggesting that these inhibitors may have protective and anti-cancer potentials in GC patients. In vitro and in vivo assays indicated that sildenafil inhibited tumor growth in GC, with effectiveness contingent upon PDE5 expression levels. Functionally, sildenafil suppressed tumor growth in GC cells by acting on PDE5. Mechanistically, we demonstrated that sildenafil restrained tumor growth of GC by directly activating PKG through PDE5 inhibition for regulating c-MYC expression via its phosphorylation and ubiquitination degradation, thereby suppressing c-MYC stability for IL-6 transcription within the downstream IL-6/JAK/ STAT3 signaling pathway. These findings provided the possibility of sildenafil may serve as a potential anti-tumor drug for GC.
Currently, the potential anti-cancer effects of PDE5 inhibitors in oncology are under investigation; however, most findings are still in the preclinical study phase15,16,18,19,20,22,30,31,53,54,55. The therapeutic potential of PDE5 inhibitors in GC remains limited. Consistent with previous in vitro and in vivo experiments conducted on other types of cancer, our study supports that the anti-cancer effects of PDE5 inhibitors might be related to their ability to inhibit tumor growth by reducing cell proliferation, inducing cell apoptosis, and inducing cell cycle arrest in tumor cells18,21,56. In addition to their universal potential anti-cancer effects on solid tumor, GC patients may experience additional benefits from PDE5 inhibitor treatment. This is because the nitric oxide (NO)/cGMP-dependent signaling pathway activated by PDE5 inhibitors plays a crucial role in regulating gastric blood flow, mucus secretion, and gastric sensorimotor function13,32,45,46,47,48,49. Additionally, previous studies have reported that key pathways, including the NO/cGMP/PDE5, JNK/c-JUN, Hippo/TAZ, and WNT/β-catenin signaling pathways, are modulated by PDE5 inhibitors to exert anti-tumor effects in various cancers32,57,58,59,60. In the present study, our findings demonstrated that sildenafil mediates the inhibition of downstream IL-6/JAK/STAT3 signaling pathway activation, suppressing tumor growth of GC. The IL-6/JAK/STAT3 signaling pathway is significantly enriched and aberrantly hyperactivated in many cancers, driving the tumor proliferation, survival, invasiveness, and metastasis, while also suppressing the immune response in the tumor microenvironment61. Targeting IL-6/JAK/STAT3 signaling pathway in GC patients treated with sildenafil is likely to provide therapeutic benefits by directly inhibiting tumor cell growth. IL-6 is an indispensable molecule that mediates the regulation of downstream IL-6/JAK/STAT3 signaling by sildenafil. The expression level of IL-6 is associated with prognosis in GC patients. Based on a single-center population-based cohort from the CLASS-01 study in China, our previous research reported that elevated plasma IL-6 levels in GC patients were significantly associated with poor prognosis following surgical radical resection62. In this study, Swedish patients with GC who administrated with PDE5 inhibitors exhibited lower risk of cancer-specific death, potentially linked to the reduction of IL-6 expression level in the body after using these inhibitors.
To the best of our knowledge, this is a novel and prospective study exploring the potential therapeutic effect of the PDE5 inhibitor sildenafil in GC. The primary strength of this study lies in its innovative design, which combines findings from a nationwide population-based cohort with molecular experimental evidence to investigate the potential anti-cancer effects of sildenafil. Evidence from the population-based cohort further corroborated preclinical data, demonstrating that male patients who prescribed PDE5 inhibitors following diagnosis of GC faced lower risk of cancer-specific death compared to matched patients without a prescription. The protective effect appeared to be more pronounced in cancer patients diagnosed at an earlier stage compared to those diagnosed at an advanced stage55. It is worthwhile to further explore this association as well as the optimal dosage and usage in RCTs. Moreover, the nationwide coverage of the population-based cohort study allowed us to investigate the dose-response relationship, strongly supporting the observed negative association by excluding indication bias and providing a reference for the potential effective dose related to its anti-cancer effects. The population-based data were sourced from multiple databases, including TCGA, GEO, Nanfang Hospital cohorts, and Swedish registers, significantly enhancing the feasibility of generalizing our findings from this study. Additionally, novel experimental models, including patient-derived tumor organoids (PDTOs) and patient-derived tumor xenograft (PDX) models, were used to further evaluate the anti-cancer effects of sildenafil in vivo and in vitro. Several limitations should be acknowledged in this study. The Swedish national population-based cohort was limited to male patients, as few females were prescribed PDE5 inhibitors. It would be valuable to conduct RCTs involving female patients, as they may exhibit better tolerance to these drugs. Furthermore, our study focused solely on the drug effects of sildenafil on GC cells, leaving the potential role of sildenafil in stromal immune cells unclear. Notably, PDE5 is highly expressed in cancer-associated fibroblasts (CAFs) and promotes breast tumor progression10. Sildenafil decreased the accumulation and ARG1 expression of PMN-MDSCs after irradiation to abolish the MDSC-mediated immunosuppression caused by PDE563. Sildenafil elicited an effective cellular immune response by increasing levels of granzyme B and IFN-γ, elevating the proportion of splenic cytotoxic T cells and T helper cells, while decreasing the proportion of splenic regulatory T-cells64. The combination of sildenafil and curcumin enhanced the efficacy of 5-FU and anti-PD1 immunotherapy in vivo against CT26 colorectal tumor in mice65. PDE5 is expressed in both tumor cells and stromal immune cells. PDE5 inhibitors impact tumor cells and stromal immune cells within the tumor microenvironment by inhibiting PDE5. Since tumor cells are the most abundant cellular components in this tumor microenvironment, they are more susceptible to the effects of PDE5 inhibitors. In this study, we observed no significant differences in the proliferative and apoptotic abilities of normal gastric epithelial cells after treating with different concentrations of sildenafil. To effectively explain the observation that sildenafil does not affect normal gastric epithelial cells, we propose three possible reasons. Firstly, our findings indicate that the effectiveness of the PDE5 inhibitor sildenafil in preventing oncogenic growth in GC is dependent on the level of PDE5 expression. While both normal gastric epithelial cells and GC cell lines express PDE5, the expression levels in most GC cell lines are higher than those in normal gastric epithelial cells. In the analysis of diverse public GC datasets, GC tumor tissues exhibited significantly higher PDE5 expressions compared to normal gastric epithelial tissues. Additionally, GC cell lines with elevated PDE5 expression levels demonstrated stronger inhibitory effects than those with lower PDE5 expression levels. These results suggest that GC cells have more targets of PDE5 for sildenafil than normal gastric epithelial cells, indicating that most GC cells with higher PDE5 expression show better inhibitory responses and are more susceptible to the effects of sildenafil, while normal gastric epithelial cells are less affected. Secondly, our results demonstrated that sildenafil inhibits tumor growth of GC through the downstream IL-6/JAK/STAT3 signaling pathway. This signaling pathway was significantly enriched in GC tumor tissues compared to normal gastric epithelial tissues. IL-6 was identified as a key immune modulator by sildenafil in its role in inhibiting tumor growth in GC. IL-6 was a key target for sildenafil to exert anti-tumor effects in GC. Our findings indicate that the anti-cancer effect of sildenafil is dependent on IL-6. Notably, IL-6 was significantly upregulated in GC tumor tissues in comparison to paired normal gastric epithelial tissues. IL-6 is rarely expressed in normal gastric epithelial cells and its levels are extremely low in healthy stomach tissues66. Due to the increased signaling pathway enrichment and higher expression levels of IL-6, sildenafil is more likely to impact the biological functions of GC cells rather than those of normal gastric epithelial cells. The PDE5 inhibitor sildenafil does not affect normal gastric epithelial cells without IL-6 expression, even though it expresses PDE5. Thirdly, previously published studies have reported a protective effect of the PDE5 inhibitor sildenafil against gastric damage induced by nonsteroidal anti-inflammatory drugs (NSAIDs), ethanol, or acetic acid45,46,47,67,68,69. Sildenafil may have beneficial effects on normal gastric epithelial cells and does not impair or suppress their biological activity, despite their expression of PDE5. Therefore, within the same GC patient, sildenafil may selectively affect GC cells while sparing normal gastric epithelial cells, even though both cells express PDE5. Therefore, our study focuses primarily on the roles of sildenafil in GC tumor cells. In clinical practice, it remains unclear whether the PDE5 inhibitor sildenafil interacts with existing immune checkpoint blockade-based tumor immunotherapy strategies, which requires further investigation. Additional evidence is necessary to fully elucidate the underlying molecular mechanisms of sildenafil, which may serve as a potential anti-cancer therapeutic agent for inhibiting growth of GC within the complex tumor immune microenvironment.
In summary, our study offered new insights into the underlying mechanisms of the PDE5-specific inhibitor sildenafil, providing a novel theoretical foundation for targeted interventions in GC.
Methods
Nationwide Swedish population-based cohort
The nationwide cohort study in Sweden was approved by the Ethics Committee at Lund University. By using the 10th International Classification of Disease (ICD-10) code (C16), all male patients diagnosed with GC as primary cancer from July 2005 to December 2015 were totally extracted from the Swedish Cancer Registry. Information regarding prescriptions for PDE5 inhibitors was retrieved from the Swedish Prescribed Drug Register, utilizing the Anatomical Therapeutic Chemical (ATC) codes G04BE03, G04BE08, and G04BE09. The outcome was death due to GC, occurring between July 2005 and March 2017, and this data was sourced from the Cause of Death Register using the ICD-10 code C16. We used a matched cohort design for this study, randomly selecting five control patients with GC who did not have a prescription for PDE5 inhibitors for each patient who did use PDE5 inhibitors after post-diagnosis, based on nearest-neighbour propensity score matching.
Tissue specimens
This study received an approval from the Institutional Research Medical Ethics Committee of Nanfang Hospital in Guangzhou, P.R. China. Informed consent was obtained from all patients involved in the study. Tissue samples were collected from GC patients who underwent surgical resection for subsequent mRNA and protein analysis.
The quantitative real-time PCR, western blotting, immunohistochemistry, and immunofluorescence assays
Quantitative real-time PCR (qRT-PCR), western blotting, immunohistochemistry, and immunofluorescence assays were performed orderly, following the manufacturer’s instructions for each protocol. The primer sequences and antibody information are listed in Supplementary Table 3 and Supplementary Table 4.
Culture of patient-derived tumor organoids
Human GC patient-derived tumor organoids were successfully cultured according to the methods described in a previous study70. Resected GC tissues were washed with phosphate-buffered saline, cut into 1 mm3 pieces, and digested in a mixed medium (DMEM/F12, 2% FBS, Pen/Strep, 100 U/mL collagenase XI, and 125 μg/mL Dispase II) at 37 °C for 40 min. After digestion, Tryp-LE Express and DNase I were added for an additional 10 min. The resulting samples were then embedded in Matrigel, cultured, filtered through a 70 μm cell strainer, centrifuged at 300 × g for 5 min, and isolated to obtain tumor organoids. These organoids exhibited high viability and were passaged twice a week at a splitting ratio of 1:2 or 1:3. The viability of the tumor organoids was assessed after a 48-h incubation with sildenafil.
Establishment of patient-derived tumor xenograft models
Animal experiments were approved by the Institutional Animal Care and Use Committee and the Institutional Research Medical Ethics Committee of Nanfang Hospital in Guangzhou, China. All experiments were conducted in accordance with the ARRIVE1 guidelines71. This study used immunocompromised male NOD-SCID mice and male athymic nude mice, following methods established in prior in vivo xenograft studies29,58. The mice were randomly divided into various groups once the tumor reached approximately 0.3 × 0.32 cm3 (length × width2). The control groups received DMSO mixed with 0.9% saline, whereas the treatment groups were administered sildenafil every 2 days for a duration of 22 days. The two perpendicular diameters of the tumor were measured to calculate tumor weight and volume by using the formula: Volume = 0.5 × length × width². Tumor burden was calculated by tumor volume.
Cell transfection and RNA sequencing
A lentivirus with PDE5 overexpression was successfully constructed (GeneChem, Shanghai, China). Following the manufacturer’s instructions, GC cells were transfected with either the PDE5-overexpressing lentivirus vectors (LV-PDE5) or the negative control lentivirus vectors (LV-NC) to further establish PDE5-overexpressing cell lines. Additionally, small interfering RNA (siRNA) constructs were prepared and transfected into GC cells in accordance with the manufacturer’s instructions (GeneChem, Shanghai, China). The sequences of the PDE5-siRNA are listed in Supplementary Table 5. Subsequently, these sequences were used to design short hairpin RNA (shRNA) for the establishment of PDE5-shRNA cells. Finally, the efficiency of PDE5 overexpression and knockdown in GC cells was validated at both the mRNA and protein levels.
Based on the construction of PDE5-overexpressing (LV-PDE5) GC cells and negative control (LV-NC) GC cells, RNA sequencing (RNA-seq) was conducted on these cell lines after treatment with either sildenafil or DMSO for 48 h, with DMSO serving as the vehicle control. The cell samples comprised four groups: LV-NC GC cells treated with DMSO, LV-PDE5 GC cells treated with DMSO, LV-NC GC cells treated with sildenafil, and LV-PDE5 GC cells treated with sildenafil. Each group was independently replicated in three biological experiments. All RNA-seq data were collected and analyzed using a comprehensive and high-throughput platform (Illumina NovaSeq 6000, Applied Protein Technology, Shanghai, China).
Measurement of PDE5A1 activity and human cGMP level
The PDE5A1 Assay Kit (#60350, BPS Biosciences, USA) was used to evaluate the PDE5 inhibitory effects of sildenafil, following the manufacturer’s instructions. Stock solutions of all test compounds (500 mM IBMX; 10 mM dipyridamole; 10 mM TM-dipyridamole) were prepared in DMSO and then diluted to 10x working concentrations in 10% DMSO within the PDE assay buffer (500 μM IBMX; 30 μM and 100 μM dipyridamole; 30 μM and 100 μM TM-dipyridamole). The fluorescent polarization of each sample was measured by using a Multimode Plate Reader (PerkinElmer EnVision 2105). Milli-polarization values were calculated by using EnVision Workstation v1.12 software, with the PDE5A1 enzymatic activity of each reaction normalized against the enzymatic positive control sample.
A human cGMP ELISA kit (#581021, Cayman Chemical, USA) was used to measure intracellular cGMP levels. According to the manufacturer’s protocol, all cell samples were treated with trichloroacetic acid (TCA) at a final concentration of 5% (w/v) to precipitate proteins. Following centrifugation, the supernatant was extracted with ether to eliminate any residual TCA. An acetylation step was performed on the cell samples before setting up the final assay, luminescence measurement, and analysis. Data acquisition was conducted using a Fluostar Omega fluorescence reader (BMG Labtech, Germany), and the concentration level of cGMP was calculated based on the absorbance readings at 405 nm.
Chromatin immunoprecipitation and Luciferase reporter assay
Chromatin immunoprecipitation (ChIP) assays were conducted by using the Plus Enzymatic Chromatin IP Kit (Magnetic Beads) (#9005, Cell Signaling Technology, USA) in accordance with the manufacturer’s protocols. Five pairs of primers for the IL-6 promoter used in ChIP qRT-PCR assays are provided in Supplementary Table 6. Luciferase reporter assays were performed using the Luciferase Assay Kit (GeneCopoeia, China, #LF004) following the manufacturer’s instructions. Luciferase enzyme activity was measured for standard analysis.
Statistics and reproducibility
The student’s t-test and Chi-square test were used for continuous and categorical variables respectively. A multivariate Cox regression model with a time-varying variable was employed to examine the association between death caused by GC and post-diagnostic use of PDE5 inhibitors. Covariates used for calculating propensity scores were included in the adjusted model. The log-rank test was utilized for survival analysis. Error bars represent the mean ± SD. Statistical analyses were performed using SPSS 23.0, Image.J, or SAS 9.4 software, while figures were created using GraphPad Prism 8.0. ANOVA and Student’s t-tests were conducted in this study (ns, P-value > 0.05; *, P-value < 0.05; **, P-value < 0.01; ** *, P-value < 0.001).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All other data generated or analyzed during this study are included in the article and/or supplemental files, or available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Peng, T. et al. Inhibitors of phosphodiesterase as cancer therapeutics. Eur. J. Med. Chem. 150, 742–756 (2018).
Samidurai, A. et al. Beyond erectile dysfunction: cGMP-specific phosphodiesterase 5 inhibitors for other clinical disorders. Annu. Rev. Pharm. Toxicol. 63, 585–615 (2023).
Karami-Tehrani, F. et al. Evaluation of PDE5 and PDE9 expression in benign and malignant breast tumors. Arch. Med. Res. 43, 470–475 (2012).
Liu, P. et al. Sildenafil inhibits the growth and epithelial-to-mesenchymal transition of cervical cancer via the TGF-β1/Smad2/3 pathway. Curr. Cancer Drug Targets 23, 145–158 (2023).
Whitehead, C. M. et al. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol. Cancer Ther. 2, 479–488 (2003).
Piazza, G. A. et al. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res 61, 3961–3968 (2001).
Hankey, W. et al. Prostate Cancer Cell Phenotypes Remain Stable Following PDE5 Inhibition in the Clinically Relevant Range. Transl. Oncol. 13, 100797 (2020).
Chang, Y. C. et al. Discovery of novel agents on spindle assembly checkpoint to sensitize vinorelbine-induced mitotic cell death against human non-small cell lung cancers. Int. J. Mol. Sci. 21, (2020).
Catalano, S. et al. Phosphodiesterase 5 (PDE5) is highly expressed in cancer-associated fibroblasts and enhances breast tumor progression. Cancers 11, 1740 (2019).
Pantziarka, P. et al. Repurposing drugs in oncology (ReDO)-selective PDE5 inhibitors as anti-cancer agents. Ecancermedicalscience 12, 824 (2018).
Das, A. et al. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharm. Ther. 147, 12–21 (2015).
Stehle, D. et al. Heterogeneity of cGMP signalling in tumour cells and the tumour microenvironment: Challenges and chances for cancer pharmacology and therapeutics. Pharm. Ther. 242, 108337 (2023).
Cruz-Burgos, M. et al. New approaches in oncology for repositioning drugs: the case of PDE5 inhibitor sildenafil. Front. Oncol. 11, 627229 (2021).
Li, Q. & Shu, Y. Pharmacological modulation of cytotoxicity and cellular uptake of anti-cancer drugs by PDE5 inhibitors in lung cancer cells. Pharm. Res. 31, 86–96 (2014).
Di, X. et al. Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res. Treat. 124, 349–360 (2010).
Karakhanova, S. et al. Characterization of myeloid leukocytes and soluble mediators in pancreatic cancer: importance of myeloid-derived suppressor cells. Oncoimmunology 4, e998519 (2015).
Mei, X. L. et al. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am. J. Cancer Res. 5, 3311–3324 (2015).
Booth, L. et al. Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol. Pharm. 85, 408–419 (2014).
Sponziello, M. et al. PDE5 expression in human thyroid tumors and effects of PDE5 inhibitors on growth and migration of cancer cells. Endocrine 50, 434–441 (2015).
El-Naa, M. M., Othman, M. & Younes, S. Sildenafil potentiates the antitumor activity of cisplatin by induction of apoptosis and inhibition of proliferation and angiogenesis. Drug Des. Dev. Ther. 10, 3661–3672 (2016).
Booth, L. et al. PDE5 inhibitors enhance celecoxib killing in multiple tumor types. J. Cell Physiol. 230, 1115–1127 (2015).
Huang, W. et al. Use of phosphodiesterase 5 inhibitors is associated with lower risk of colorectal cancer in men with benign colorectal neoplasms. Gastroenterology 157, 672–681.e4 (2019).
Das, A. et al. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc. Natl. Acad. Sci. USA 107, 18202–18207 (2010).
Huang, W. et al. Phosphodiesterase-5 inhibitors use and risk for mortality and metastases among male patients with colorectal cancer. Nat. Commun. 11, 3191 (2020).
Sutton, S. S. et al. The association between phosphodiesterase-5 inhibitors and colorectal cancer in a national cohort of patients. Clin. Transl. Gastroenterol. 11, e00173 (2020).
Hsu, J. L. et al. Phosphodiesterase type 5 inhibitors synergize vincristine in killing castration-resistant prostate cancer through amplifying mitotic arrest signaling. Front. Oncol. 10, 1274 (2020).
Chang, J. F. et al. Phosphodiesterase type 5 (PDE5) inhibitors sensitize topoisomerase II inhibitors in killing prostate cancer through PDE5-independent impairment of HR and NHEJ DNA repair systems. Front. Oncol. 8, 681 (2018).
Yu, S. J. et al. Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma. J. Hepatol. 70, 449–457 (2019).
Kniotek, M. & Boguska, A. Sildenafil can affect innate and adaptive immune system in both experimental animals and patients. J. Immunol. Res. 2017, 4541958 (2017).
Marques, J. G. et al. Co-delivery of sildenafil (Viagra((R))) and crizotinib for synergistic and improved anti-tumoral therapy. Pharm. Res. 31, 2516–2528 (2014).
Peak, T. C. et al. The role of PDE5 inhibitors and the NO/cGMP pathway in cancer. Sex. Med. Rev. 4, 74–84 (2016).
Chen, L. et al. Sildenafil triggers tumor lethality through altered expression of HSP90 and degradation of PKD2. Carcinogenesis 41, 1421–1431 (2020).
Weed, D. T. et al. The Reversal of immune exclusion mediated by tadalafil and an anti-tumor vaccine also induces PDL1 upregulation in recurrent head and neck squamous cell carcinoma: interim analysis of a phase I clinical trial. Front. Immunol. 10, 1206 (2019).
Paronetto, M. P. & Crescioli, C. Rethinking of phosphodiesterase 5 inhibition: the old, the new and the perspective in human health. Front. Endocrinol. 15, 1461642 (2024).
Sigler, S. et al. Novel celecoxib derivative, RF26, blocks colon cancer cell growth by inhibiting PDE5, activating cGMP/PKG signaling, and suppressing β-catenin-dependent transcription. Anticancer Agents Med. Chem. Published online 2 September 2, 2024).
Joshi, P. et al. Role of curcumin in ameliorating hypertension and associated conditions: a mechanistic insight. Mol. Cell Biochem. 477, 2359–2385 (2022).
Ammirante, M., Shalapour, S., Kang, Y., Jamieson, C. A. M. & Karin, M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc. Natl. Acad. Sci. USA 111, 14776–14781 (2014).
Gross, N. E. et al. Phosphodiesterase-5 inhibition collaborates with vaccine-based immunotherapy to reprogram myeloid cells in pancreatic ductal adenocarcinoma. JCI Insight 9, e179292 (2024).
Iratni, R. & Ayoub, M. A. Sildenafil in combination therapy against cancer: a literature review. Curr. Med. Chem. 28, 2248–2259 (2021).
ElHady, A. K., El-Gamil, D. S., Abdel-Halim, M. & Abadi, A. H. Advancements in phosphodiesterase 5 inhibitors: unveiling present and future perspectives. Pharmaceuticals 16, 1266 (2023).
Wang, Y., Zhao, B., Yang, H. & Wan, Z. A real-world pharmacovigilance study of FDA adverse event reporting system events for sildenafil. Andrology 12, 785–792 (2024).
Poklepovic, A. S. et al. A phase 1 study of regorafenib and sildenafil in adults with advanced solid tumors. Anticancer Drugs 35, 450–458 (2024).
Califano, J. A. et al. Tadalafil augments tumor-specific immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 21, 30–38 (2015).
Aydinli, B. et al. The role of sildenafil citrate in the protection of gastric mucosa from nonsteroidal anti-inflammatory drug-induced damage. Ulus. Travma Acids. Cerrahi Derg. 13, 268–273 (2007).
Kalayci, M. et al. Comparison of the therapeutic effects of sildenafil citrate, heparin and neuropeptides in a rat model of acetic acid-induced gastric ulcer. Life Sci. 186, 102–110 (2017).
Medeiros, J. V. et al. Role of the NO/cGMP/K(ATP) pathway in the protective effects of sildenafil against ethanol-induced gastric damage in rats. Br. J. Pharm. 153, 721–727 (2008).
Santos, C. L. et al. Sildenafil prevents indomethacin-induced gastropathy in rats: role of leukocyte adherence and gastric blood flow. Br. J. Pharm. 146, 481–486 (2005).
Sawatzky, D. A., Megson, I. L. & Rossi, A. G. Sildenafil offers protection against NSAID-induced gastric injury. Br. J. Pharm. 146, 477–478 (2005).
Ding, L. et al. Schlafen4+-MDSC in Helicobacter-induced gastric metaplasia reveals role for GTPases. Front. Immunol. 14, 1139391 (2023).
Gu, Y. et al. Stabilization of the c-Myc protein by CAMKIIγ promotes T-cell lymphoma. Cancer Cell 32, 115–128 (2017).
Devaiah, B. N. et al. MYC protein stability is negatively regulated by BRD4. Proc. Natl Acad. Sci. USA 117, 13457–13467 (2020).
Islam, B. N. et al. Sildenafil suppresses inflammation-driven colorectal cancer in mice. Cancer Prev. Res (Philos.) 10, 377–388 (2017).
Wang, R. et al. Phosphodiesterase type 5 inhibitor Tadalafil increases Rituximab treatment efficacy in a mouse brain lymphoma model. J. Neurooncol 122, 35–42 (2015).
Tai, L. H. et al. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid-derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity. Oncoimmunology 7, e1431082 (2018).
Karakoyun, B. et al. The effect of phosphodiesterase-5 inhibition by sildenafil citrate on inflammation and apoptosis in rat experimental colitis. Life Sci. 89, 402–407 (2011).
Tuttle, T. R. et al. The cyclic GMP/protein kinase G pathway as a therapeutic target in head and neck squamous cell carcinoma. Cancer Lett. 370, 279–285 (2016).
Muniyan, S. et al. Sildenafil potentiates the therapeutic efficacy of docetaxel in advanced prostate cancer by stimulating NO-cGMP signaling. Clin. Cancer Res. 26, 5720–5734 (2020).
Piazza, G. A. et al. PDE5 and PDE10 inhibition activates cGMP/PKG signaling to block Wnt/β-catenin transcription, cancer cell growth, and tumor immunity. Drug Discov. Today 25, 1521–1527 (2020).
Liu, N. et al. Phosphodiesterase 5/protein kinase G signal governs stemness of prostate cancer stem cells through Hippo pathway. Cancer Lett. 378, 38–50 (2016).
Johnson, D. E. et al. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Zhang, Z. et al. Effect of perioperative interleukin-6 and tumor necrosis factor-α on long-term outcomes in locally advanced gastric cancer: results from the CLASS-01 trial. J. Immunol. Res. 2022, 7863480 (2022).
Zhang, J. et al. Polymorphonuclear-MDSCs facilitate tumor regrowth after radiation by suppressing CD8+ T cells. Int J. Radiat. Oncol. Biol. Phys. 109, 1533–1546 (2021).
Morsi, D. S. et al. Immunomodulatory, apoptotic and anti-proliferative potentials of sildenafil in Ehrlich ascites carcinoma murine model: In vivo and in silico insights. Int. Immunopharmacol. 119, 110135 (2023).
Dent, P. et al. (Curcumin+sildenafil) enhances the efficacy of 5FU and anti-PD1 therapies in vivo. J. Cell Physiol. 235, 6862–6874 (2020).
Fagerberg, L. et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 13, 397–406 (2014).
El-Sisi, A. E., Sokar, S. S., Abu-Risha, S. E. & Khira, D. Y. The potential beneficial effects of sildenafil and diosmin in experimentally-induced gastric ulcer in rats. Heliyon 6, e04761 (2020).
Moustafa, Y. M., Khoder, D. M., El-Awady, E. E. & Zaitone, S. A. Sildenafil citrate protects against gastric mucosal damage induced by indomethacin in rats. Eur. Rev. Med. Pharm. Sci. 17, 179–188 (2013).
Maziero Alves, G. et al. Sildenafil attenuates nonsteroidal anti-inflammatory-induced gastric ulceration in mice via antioxidant and antigenotoxic mechanisms. Clin. Exp. Pharm. Physiol. 48, 401–411 (2021).
Bartfeld, S. et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148, 126–136.e6 (2015).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Acknowledgements
This work was supported by these grants awarded to H.L. by the Natural Science Foundation of Guangdong Province (2021A1515011146 and 2023A1515010785), to Z.Z.Z. by the President Foundation of Nanfang Hospital, Southern Medical University (2022B003), to G.X.L by the Key Areas Research and Development Programs of Guangdong Province (2023B1111050009), to J.G.J by the by University of Macau Development Foundation UMDF-TISF/2025/001/FHS and to W.Q.H by the National Natural Science Foundation of China (82304213).
Author information
Authors and Affiliations
Contributions
Z.Z.Z., J.G.J., H.L. and G.X.L. were responsible for the study concept and design. Z.Z.Z, H.L., G.X.L. and W.Q.H. obtained funding. W.Q.H, D.H.H. and Z.Z.Z acquired the data. G.X.L., Z.X., Q.F.X, X.T., W.J.H. and W.H.Y. helped to analyze and interpret the data. J.G.J., Z.Z.Z., W.Q.H. and H.L. drafted the manuscript, and all authors revised it for important intellectual content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Mauricio Rodríguez-Dorantes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christina Karlsson Rosenthal. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Z., Huang, W., Huang, D. et al. Repurposing of phosphodiesterase-5 inhibitor sildenafil as a therapeutic agent to prevent gastric cancer growth through suppressing c-MYC stability for IL-6 transcription. Commun Biol 8, 85 (2025). https://doi.org/10.1038/s42003-025-07519-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-07519-9
This article is cited by
-
Therapeutic potential of tadalafil in acetic acid-induced gastric ulcer in rats: mechanisms and outcomes
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)