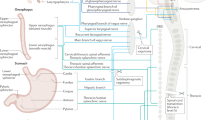
Abstract
Interoception broadly refers to awareness of one’s internal milieu. Although the importance of the body-to-brain communication that underlies interoception is implicit, the vagal afferent signalling and corresponding brain circuits that shape perception of the viscera are not entirely clear. Here, we use mice to parse neural circuits subserving interoception of the heart and gut. We determine that vagal sensory neurons expressing the oxytocin receptor (Oxtr), referred to as NGOxtr, send projections to cardiovascular or gastrointestinal tissues and exhibit molecular and structural features indicative of mechanosensation. Chemogenetic excitation of NGOxtr decreases food and water consumption, and remarkably, produces a torpor-like phenotype characterized by reductions in cardiac output, body temperature and energy expenditure. Chemogenetic excitation of NGOxtr also creates patterns of brain activity associated with augmented hypothalamic–pituitary–adrenal axis activity and behavioural indices of vigilance. Recurrent excitation of NGOxtr suppresses food intake and lowers body mass, indicating that mechanosensation of the heart and gut can exert enduring effects on energy balance. These findings suggest that the sensation of vascular stretch and gastrointestinal distention may have profound effects on whole-body metabolism and, possibly, mental health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data reported in these studies are available in the Source Data files. Source data are provided with this paper.
References
Hsueh, B. et al. Cardiogenic control of affective behavioural state. Nature 615, 292–299 (2023).
Krieger, J. P. et al. Neural pathway for gut feelings: vagal interoceptive feedback from the gastrointestinal tract is a critical modulator of anxiety-like behavior. Biol. Psychiatry 92, 709–721 (2022).
Suarez, A. N. et al. Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat. Commun. 9, 2181 (2018).
Elsaafien, K. et al. A novel organ-specific approach to selectively target sensory afferents innervating the aortic arch. Front. Physiol. 13, 841078 (2022).
Han, W. et al. A neural circuit for gut-induced reward. Cell 175, 665–678.e23 (2018).
Kupari, J., Haring, M., Agirre, E., Castelo-Branco, G. & Ernfors, P. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 27, 2508–2523.e4 (2019).
Bai, L. et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143 (2019).
Jurek, B. & Neumann, I. D. The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev. 98, 1805–1908 (2018).
Tan, Y. et al. Oxytocin receptors are expressed by glutamatergic prefrontal cortical neurons that selectively modulate social recognition. J. Neurosci. 39, 3249–3263 (2019).
Fox, E. A., Phillips, R. J., Martinson, F. A., Baronowsky, E. A. & Powley, T. L. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J. Comp. Neurol. 428, 558–576 (2000).
Berthoud, H. R. & Powley, T. L. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J. Comp. Neurol. 319, 261–276 (1992).
Zagorodnyuk, V. P., Chen, B. N. & Brookes, S. J. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J. Physiol. 534, 255–268 (2001).
Maejima, Y. et al. Impact of sex, fat distribution and initial body weight on oxytocin’s body weight regulation. Sci. Rep. 7, 8599 (2017).
Min, S. et al. Arterial baroreceptors sense blood pressure through decorated aortic claws. Cell Rep. 29, 2192–2201.e3 (2019).
Yang, H. et al. The molecular makeup of peripheral and central baroreceptors: stretching a role for Transient Receptor Potential (TRP), Epithelial Sodium Channel (ENaC), Acid Sensing Ion Channel (ASIC), and Piezo channels. Cardiovasc. Res. 118, 3052–3070 (2021).
Zeng, W. Z. et al. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362, 464–467 (2018).
Weingart, S. Push-dose pressors for immediate blood pressure control. Clin. Exp. Emerg. Med. 2, 131–132 (2015).
Min, S. et al. Control of feeding by Piezo-mediated gut mechanosensation in Drosophila. Elife 10, e63049 (2021).
Phillips, R. J. & Powley, T. L. Gastric volume rather than nutrient content inhibits food intake. Am. J. Physiol. 271, R766–R769 (1996).
Stocker, S. D., Stricker, E. M. & Sved, A. F. Acute hypertension inhibits thirst stimulated by ANG II, hyperosmolality, or hypovolemia in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R214–R224 (2001).
Kaufman, S. The role of the heart in body fluid and electrolyte homeostasis. J. Physiol. (Paris) 79, 542–546 (1984).
Vaughn, A. C. et al. Energy-dense diet triggers changes in gut microbiota, reorganization of gut–brain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp. (Wars.) 77, 18–30 (2017).
Kim, J. S. et al. Gut microbiota composition modulates inflammation and structure of the vagal afferent pathway. Physiol. Behav. 225, 113082 (2020).
Grill, H. J. & Hayes, M. R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 16, 296–309 (2012).
Adriaenssens, A. E. et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab. 30, 987–996.e6 (2019).
Geisler, C. E. et al. Tirzepatide suppresses palatable food intake by selectively reducing preference for fat in rodents. Diabetes Obes. Metab. 25, 56–67 (2023).
Ludwig, M. Q. et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat. Metab. 3, 530–545 (2021).
Zhang, C. et al. Area postrema cell types that mediate nausea-associated behaviors. Neuron 109, 461–472.e5 (2021).
Campos, C. A., Bowen, A. J., Roman, C. W. & Palmiter, R. D. Encoding of danger by parabrachial CGRP neurons. Nature 555, 617–622 (2018).
Chen, J. Y., Campos, C. A., Jarvie, B. C. & Palmiter, R. D. Parabrachial CGRP neurons establish and sustain aversive taste memories. Neuron 100, 891–899.e5 (2018).
de Kloet, A. D. et al. A unique “angiotensin-sensitive” neuronal population coordinates neuroendocrine, cardiovascular, and behavioral responses to stress. J. Neurosci. 37, 3478–3490 (2017).
Daviu, N. et al. Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat. Neurosci. 23, 398–410 (2020).
Iliff, B. W. & Swoap, S. J. Central adenosine receptor signaling is necessary for daily torpor in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R477–R484 (2012).
Swoap, S. J. & Gutilla, M. J. Cardiovascular changes during daily torpor in the laboratory mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R769–R774 (2009).
Matsuo, T. et al. Thiazoline-related innate fear stimuli orchestrate hypothermia and anti-hypoxia via sensory TRPA1 activation. Nat. Commun. 12, 2074 (2021).
Matsuo, T. et al. Artificial hibernation/life-protective state induced by thiazoline-related innate fear odors. Commun. Biol. 4, 101 (2021).
Turbill, C. & Stojanovski, L. Torpor reduces predation risk by compensating for the energetic cost of antipredator foraging behaviours. Proc. R. Soc. B Biol. Sci. 285, 20182370 (2018).
Feng, N. Y., Junkins, M. S., Merriman, D. K., Bagriantsev, S. N. & Gracheva, E. O. Osmolyte depletion and thirst suppression allow hibernators to survive for months without water. Curr. Biol. 29, 3053–3058.e3 (2019).
Cerri, M. The central control of energy expenditure: exploiting torpor for medical applications. Annu. Rev. Physiol. 79, 167–186 (2017).
Bouma, H. R. et al. Induction of torpor: mimicking natural metabolic suppression for biomedical applications. J. Cell. Physiol. 227, 1285–1290 (2012).
Shi, Z. et al. Human torpor: translating insights from nature into manned deep space expedition. Biol. Rev. Camb. Philos. Soc. 96, 642–672 (2021).
Furay, A. R., Bruestle, A. E. & Herman, J. P. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology 149, 5482–5490 (2008).
Harpaz, I. et al. Chronic exposure to stress predisposes to higher autoimmune susceptibility in C57BL/6 mice: glucocorticoids as a double-edged sword. Eur. J. Immunol. 43, 758–769 (2013).
Heller, H. C. & Ruby, N. F. Sleep and circadian rhythms in mammalian torpor. Annu Rev. Physiol. 66, 275–289 (2004).
Covasa, M., Grahn, J. & Ritter, R. C. Reduced hindbrain and enteric neuronal response to intestinal oleate in rats maintained on high-fat diet. Auton. Neurosci. 84, 8–18 (2000).
Daly, D. M., Park, S. J., Valinsky, W. C. & Beyak, M. J. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J. Physiol. 589, 2857–2870 (2011).
How, J. M., Wardak, S. A., Ameer, S. I., Davey, R. A. & Sartor, D. M. Blunted sympathoinhibitory responses in obesity-related hypertension are due to aberrant central but not peripheral signalling mechanisms. J. Physiol. 592, 1705–1720 (2014).
Gong, S. et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003).
McDougle, M. et al. Separate gut–brain circuits for fat and sugar reinforcement combine to promote overeating. Cell Metab. 36, 1431 (2024).
Baumer-Harrison, C. et al. Alleviating hypertension by selectively targeting angiotensin receptor expressing vagal sensory neurons. J. Neurosci. 44, e1154232023 (2024).
Halatchev, I. G. & Cone, R. D. Peripheral administration of PYY3–36 produces conditioned taste aversion in mice. Cell Metab. 1, 159–168 (2005).
Riley, A. L. Conditioned flavor aversions: assessment of drug-induced suppression of food intake. Curr. Protoc. Neurosci. 5, 8.6E.1–8.6E.12 (1998).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Biddinger, J. E. & Fox, E. A. Meal parameters and vagal gastrointestinal afferents in mice that experienced early postnatal overnutrition. Physiol. Behav. 101, 184–191 (2010).
Acknowledgements
This project received funding from National Institutes of Health grants HL150750 (to E.G.K), AT012142 (to E.G.K., A.d.K. and G.d.L.), HL136595 (to A.d.K.), AHA 23POST1020034 (to K.E.) and R01DK116004 (to G.d.L.).
Author information
Authors and Affiliations
Contributions
K.A.S., G.d.L., A.D.d.K. and E.G.K. designed the study, analysed the data and wrote the manuscript. D.N.J., R.M.-H. and M.K.K. analysed the data; Y.T. analysed the data and contributed to study design; K.E. analysed the data and assisted with manuscript writing. All authors were involved with the implementation of the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Ben Jurek, Jessica Yue and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewcz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Impact of bilateral NG injection of OxtrCre mice with the Cre-inducible AAVPHP.S-FLEX-tdTomato or AAV5-hSyn-DIO-Gq-mCherry on fluorophore expression in the hindbrain.
(a) Representative confocal scans of coronal sections spanning the brainstem of OxtrCre mice that received a bilateral NG injection with the Cre-inducible AAVPHP.S-FLEX-tdTomato. (b) Corresponding atlas schematics of representative coronal sections highlighting the area postrema (AP), nucleus of the solitary tract (NTS), dorsal motor nucleus of the vagus (DMNV), and nucleus ambiguous (NA). (c) Representative coronal section depicting tdTomato-labeled fibers in the AP and NTS; however, the DMNV and NA are unlabeled. (d, left) Schematic of bilateral injection of AAVPHP.S-FLEX-tdTomato into the NG. (middle) Representative higher magnification images of the AP, NTS, DMNV, and (right) the NA. (e, left) Schematic of bilateral injection of AAV5-hSyn-DIO-mCherry into the NG. (middle) Representative higher magnification images of the AP, NTS, DMNV, (right) the and NA. CC; Central Canal, 4 V; 4th Ventricle. Scale Bars; 1 mm (a, c), 100 µm (d, e). n = 6-8 sections per 4 mice (tdTom groups) and 2-3 sections per 5 mice (Gq-mCherry groups). Schematics (d) and (e) made with Biorender.com.
Extended Data Fig. 2 Acutely isolated Gq-NGOxtr neurons depolarize during CNO bath application.
(a) Gq-NGOxtr neurons expressing mCherry acutely dissociated from the mouse NG are visualized with DIC and epifluorescence microscopy. (b, non-glowing, top) Representative whole-cell current clamp recordings of control NG neurons or (red glowing, bottom) neurons expressing Gq-mCherry. After a 60-s baseline recording, CNO (10 µM) was bath applied for 5 min. (c, left) Average membrane potentials of neurons at baseline and CNO were significantly different between groups (2-Way ANOVA). Multiple comparisons demonstrate that control neurons exposed to CNO failed to depolarize (Tukey post hoc) while CNO significantly depolarized Gq-DGOxtr neurons (Tukey post hoc). (c, right) The same data as (c, left) expressed as the Δ membrane potential during CNO application. CNO significantly depolarized membrane potential in Gq-NGOxtr compared to control neurons (unpaired, two-tailed t-test). Center line is median, edges show quartiles, and error bars are min/max. Recordings acquired from a total of 6 Gq and 5 Control neurons; n = 2 mice.
Extended Data Fig. 3 Representative graphs of individual temperature traces following CNO administration.
CNO (0.3 mg/kg i.p.) was administered at ZT11. Data expressed in 10 min bins.
Extended Data Fig. 4 Acute chemogenetic activation of NGOxtr results in decreased (a) food intake and (b) core body temperature in female mice.
CNO (0.3 mg/kg i.p.) was administered at ZT11. n = 9 mice. Data represented as mean ± SEM. Within subjects 2-way repeated measures ANOVA, Šídák’s multiple comparisons test, *p < 0.05.
Extended Data Fig. 5 The effects of acute CNO administration are recapitulated in male Gq-NGOxtr mice maintained on high fat diet.
Impact of CNO (0.3 mg/kg i.p.) or saline administered at ZT11 on hourly (a) food and (b) water intake, (c) oxygen consumption (VO2), (d) respiratory exchange ratio (RER), (e) body temperature, (f) systolic blood pressure (SBP), (g) heart rate (HR) and (h) change in body weight in Gq-NGOxtr mice (n = 7). Data represented as mean ± SEM. (a-g) Within subjects two-way repeated measures ANOVA with Šídák’s multiple comparisons test, (h) Paired, two-tailed t-test, *p < 0.05.
Extended Data Fig. 6 Acute activation of NGOxtr promotes vigilance and reduces locomotor activity.
Schematics of the (a) light dark box (LDB), (d) elevated plus maze (EPM) and (g) open field (OF) arena. Gq-NGOxtr made (b) fewer entries into the light and (c) spent less time in the light side of the LDB. Gq-NGOxtr mice (e) made fewer entries and (f) travelled less distance in the EPM. Gq-NGOxtr (h) made fewer center entries and (i) travelled less in the OF. (b,c,e,f) n = 10 CON-NGOxtr, 12 Gq-NGOxtr; (h) n = 14 CON-NGOxtr, 13 Gq-NGOxtr; (i) n = 14 CON-NGOxtr, 12 Gq-NGOxtr. Unpaired, two-tailed t-tests. Schematics (a, d, g) made with Biorender.com.
Extended Data Fig. 7 Cardiometabolic effects of chronic CNO administration in CON-NGOxtr mice.
CNO (0.3 mg/kg, i.p.) was administered (left) every other day for 11 days at ZT11 (1 h before lights off) or (right) ZT23 (1 h before lights on). Impact of chronic CNO administration on (a,c) food intake, (b, d) water intake, (e, g) body temperature, (f, h) VO2, (i, k) RER, (j, l) SBP or (m, n) HR. CON-NGOxtr data are averaged between baseline (2 days), no injection (5 days) and CNO injection days (6 days). (a,b,f,i) n = 15; (c, d, e, g, h, m, n) n = 7; (j,l) n = 6. Within-subject two-way repeated measures ANOVA with Tukey’s multiple comparisons test. *p < 0.05 BASELINE vs CNO INJ, #p < 0.05 NO INJ vs CNO INJ, &p < 0.05 BASELINE vs NO INJ.
Extended Data Fig. 8 Chronic administration of CNO (0.3 mg/kg i.p.) to male Gq-NGOxtr mice results in (a) weightloss attributed to (b) loss in fat mass but not (c) lean mass.
n = 8 mice. Within subject two-way repeated measures ANOVA and Šídák’s multiple comparisons test.
Extended Data Fig. 9 Chronic activation of NGOxtr does not induce anxiety-like behavior or reduce social preference.
Schematics of the (a) light dark box (LDB), (d) elevated plus maze (EPM) and (g) three-chamber social interaction tests. (b) Entries into and (c) duration of time spent in the light side of the LDB did not differ between groups. (e) Open arm entries and (f) distance travelled in the EPM did not differ between groups. Chronic NGOxtr activation did not affect (h) preference for investigating a novel mouse or (i) distance travelled in the three-chamber social interaction test. n = 8 CON-NGOxtr, 7 Gq-NGOxtr. Unpaired, two-tailed t-tests. Schematics (a, d, g) made with Biorender.com.
Supplementary information
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 9
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scott, K.A., Tan, Y., Johnson, D.N. et al. Mechanosensation of the heart and gut elicits hypometabolism and vigilance in mice. Nat Metab 7, 263–275 (2025). https://doi.org/10.1038/s42255-024-01205-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-024-01205-6
This article is cited by
-
Vagal circuits in the heart and gut can regulate cardiometabolism and stress responses
Nature Reviews Cardiology (2025)