Abstract
Torularhodin, a unique carotenoid, confers beneficial effects on nonalcoholic fatty liver disease (NAFLD). However, the precise mechanism underlying its therapeutic effects remains unknown. Here, we report that torularhodin alleviates NAFLD in male mice by modulating the gut microbiota. Additionally, transplanting fecal microbiota from torularhodin-treated mice to germ-free mice also improves NAFLD. Mechanistically, torularhodin specifically enriches the abundance of Akkermansia muciniphila, which alleviates NAFLD by promoting the synthesis of adenosylcobalamin. Utilizing a human gastrointestinal system and a colonic organoid model, we further demonstrate that adenosylcobalamin confers protective effects against NAFLD through reducing ceramides, a well-known liver damaging compound, and this effect is mediated by inhibition of the hypoxia-inducible factor 2α pathway. Notably, we construct electrospun microsphere-encapsulated torularhodin, which facilitates the slow release of torularhodin in the colon. Together, our findings indicate the therapeutic potential of microbial utilization of carotenoids, such as torularhodin, for treating NAFLD.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is characterized by disturbances in blood and liver lipid metabolism. It has been linked to excessive dietary lipid intake1. Recent studies have underscored the crucial role of the colonic microbiota in managing dietary lipid metabolism, and gut microbiota alterations and disruptions are key to this role2. Thus, several studies have focused on repairing gut microbiota imbalances and barrier disruptions as a therapeutic approach for managing NAFLD3. For example, efficacy of Akkermansia muciniphila in treating diet-induced lipid metabolic disorders was established4.
Metabolites produced by the gut microbiota through the degradation of dietary lipids were shown to be vital in regulating host health5. The intestinal endogenous sphingolipid ceramide (Cer) is considered an independent risk factor for fatty liver disease6,7. Cer induces insulin resistance by regulating pathways such as protein kinase B, which ultimately exacerbates NAFLD8. This highlights that regulating sphingolipid signaling associated with the “gut–liver” axis and inhibiting Cer-related synthesis in the gut are key strategies for ameliorating lipid metabolism disorders9. Therefore, manipulating Cer metabolism in the gut microbiota through dietary interventions has emerged as a promising strategy for managing NAFLD.
Torularhodin is a carotenoid from the vinegar fungus Sporidiobolus pararoseus10. It exhibits antioxidant activity11,12 and gut microbiota-modulating properties13. Alleviating NAFLD through nutritional interventions is a promising approach, given that carotenoids have been shown to mitigate NAFLD14. Although carotenoids are primarily absorbed in the small intestine and then enter systemic circulation, they can also enter the colon, where they can be utilized by the gut microbiota15. Furthermore, an electrospun microsphere system has been developed to facilitate the colonic release of torularhodin, thereby enhancing its bioavailability within the colon16. Despite these promising findings, the mechanism through which torularhodin or its metabolites improve NAFLD by targeting the gut microbiota remains to be elucidated.
Here, we found that the carotenoid torularhodin alleviates NAFLD by modulating the gut microbiota in male mice. Through fecal microbial transplantation (FMT) in germ-free mice and pseudo-germ-free mice, and single bacterial transplantation, A. muciniphila was identified as a pivotal bacterium. By employing microbiome functional screening and a human biomimetic gastrointestinal system, we identified adenosylcobalamin as a key metabolite. Lipidomic analysis and metabolite compensation studies revealed that adenosylcobalamin plays a crucial role in inhibiting Cer synthesis. Importantly, utilizing a colonic organoid-lipid coculture model, we demonstrated that adenosylcobalamin inhibits the colonic hypoxia-inducing factor-2α (HIF-2α) pathway, leading to a reduction in Cer levels and subsequent improvements in NAFLD. In summary, our findings revealed a critical pathway involving “colon-targeted torularhodin—A. muciniphila—adenosylcobalamin—HIF-2α/Cer”, highlighting the microbial utilization of special carotenoids as a promising therapeutic strategy for NAFLD treatment. (Fig. 1)
Torularhodin increases Akkermansia muciniphila and promotes adenosylcobalamin synthesis. Adenosylcobalamin inhibits HIF-2α, leading to the decreased Cer level in the colon and liver, subsequently improving NAFLD. Figure created in BioRender. Liu, C. (2025) https://BioRender.com/w60s979.
Results
Compared with small intestine-targeted torularhodin, colon-targeted torularhodin ameliorates HFHCD-induced NAFLD in mice
To demonstrate the slow release of torularhodin encapsulated in electrospun microspheres (EMs-T) in the colon, we assessed the residual torularhodin levels in mouse tissue. Our results demonstrated a notable absence of torularhodin in the serum and liver within the initial 24 h, and its presence persisted in the colon up to 24 h postadministration (Fig. S1A and B). This finding suggests that EMs-T effectively prolonged the release of torularhodin in the colon, indicating its utilization by the gut microbiota. Additionally, we compared the efficacy of colon-targeted torularhodin with that of a sustained small intestine-targeted system for alleviating NAFLD, with the former showing superior results (Fig. S1C–H).
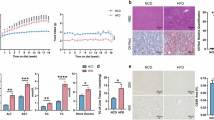
Subsequent investigations focused on determining the detailed effects of the slow release of torularhodin on NAFLD (Fig. 2A and B). Over a 10-week intervention period, body weights were significantly lower in the EMs-T group than in the Mod group, however, food intake of the three groups of mice on the HFHCD diet was almost identical (Fig. 2C, and S2A). EMs-T intervention led to significant improvements in NAFLD symptoms, including decreased levels of triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL-C), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) and increased levels of high-density lipoprotein cholesterol (HDL-C) (Fig. 2D–I). Furthermore, hepatocytes exhibited fewer morphological and structural alterations after EMs-T intervention, with minimal signs of fatty infiltration (Fig. 2J, and S2B). Interestingly, EMs intervention did not result in significant differences, suggesting that torularhodin plays a major role in this process.
A Structure of torularhodin. B Flowchart of the mice experiment. C Trend of body weight in mice (n = 8). D-I Levels of TC, TG, HDL-C, LDL-C, AST, and ALT in mice serum (n = 8). J H&E and Oil Red O sections of mice liver (200×) (n = 3). K Immunofluorescence sections of mice colon (100×) (n = 3). Data expressed as mean± SD, *p < 0.05, **p < 0.01, ***p < 0.001, compared with the Mod group, and p-value was calculated using Dunnet’s post hoc test in one-way ANOVA. Figure B created in BioRender. Liu, C. (2025) https://BioRender.com/z77b287.
Considering the chronic impact of a chronic Western diet on colon compromise17, we investigated the impact of torularhodin on the colonic barrier. Compared with the Mod group, the EMs-T group exhibited relatively unimpaired colonic epithelial cells and crypts, and the expression levels of tight junction proteins, such as occludin (Fig. 2K, and S2C) and mucin 2 (MUC2) (see below, Fig. 5K), were higher in the EMs-T group than in the Mod group. Taken together, our findings indicate the superior efficacy of colon-targeted torularhodin in ameliorating dyslipidaemia in NAFLD mice compared to that of small intestine-targeted torularhodin.
Torularhodin increases A. muciniphila abundance and stimulates adenosylcobalamin metabolism
To assess the impact of torularhodin on the gut microbiota of mice with NAFLD, we analyzed the microbiome in mouse feces. EMs-T administration to mice with NAFLD increased gut microbiota diversity and species richness (Fig. 3A). Principal component analysis (PCA) further revealed a significant alteration in the gut microbial composition of the EMs-T group (Fig. 3B). Specifically, linear discriminant analysis effect size (LEfSe) revealed that the signature microbiota in the EMs-T group included A. muciniphila, Acutalibacter muris, and Ruthenibacteritum lactatiformans. A. muciniphila is known to increase lipid metabolism by restoring the colonic barrier4. Notably, the abundance of A. muciniphila following EMs-T intervention was significantly greater than that of the other identified bacteria (Fig. 3C, D).
A Level of Shannon index and Chao1 index of bacteria in mice. (n = 8). B PCA of bacteria in mice colon. (n = 8). C LEfSe analysis and heatmap of bacterial genus. (n = 8). D Relative abundance of A. muciniphila in mice colon. (n = 8). E LEfSe analysis and heatmap of KEGG functions in mice. (n = 8). F Changes in enzymes of adenosylcobalamin biosynthesis in mice. Enzymes in red are indicated as being significantly enhanced by torularhodin (p < 0.01). (n = 8). G, H Level of adenosylcobalamin in mice serum and feces. (n = 8). I–K Linear regression analysis between LDL-C level and log2 bacteria abundance (A. muciniphila, Ruthenibacterium lactatiformans, and Acutalibacter muris). (n = 8). L Linear regression analysis between log2 A. muciniphila abundance and adenosylcobalamin level. (n = 8). M Interaction network of “bacteria-fungus”, including low-intensity (p < 0.05, correlation >0.85) and high-intensity (p < 0.01, correlation > 0.95) interactions. (n = 8). Data are expressed as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, compared with the Mod group, and p-value was calculated using Dunnet’s post hoc test in one-way ANOVA. p < 0.01 and FDR < 0.05 in LEfSe analysis. For box plots, the midline represents the median, the box spans the interquartile range between the first and third quartiles, and the whiskers extend to the lowest and highest values within 1.5 times the interquartile range from the first and third quartiles. The small icons in (G, H) are created in BioRender. Liu, C. (2025) https://BioRender.com/k07f052.
EMs-T administration primarily promoted two key metabolic pathways: porphyrin metabolism, particularly cobalamin biosynthesis (also known as vitamin B12 synthesis), and sphingolipid metabolism (Fig. 3E). The enzymes in the EMs-T group were identified (compared with those in the Con, Mod, and EMs groups, P < 0.05, FC > 2) and were enriched in the cobalamin biosynthesis pathway. EMs-T administration promoted the expression of 21 enzymes involved in the anaerobic biosynthesis of cobalamin (Fig. 3F). Given that adenosylcobalamin belongs to the cobalamin family18, we sought to examine its levels in both plasma and feces following torularhodin intervention. The levels of adenosylcobalamin in were significantly greater in the EMs-T group than in the control group (P < 0.001) (Fig. 3G, H).
Linear regression analysis of LDL-C and bacteria identified A. muciniphila as the most significant bacteria (Fig. 3I–K). Furthermore, a noteworthy positive correlation between A. muciniphila and adenosylcobalamin levels was detected (Fig. 3L, S2D–S2F). To further elucidate the interactions within the colonic microbiota of mice with NAFLD, we used the SparCC approach to construct interaction networks, with a focus on elucidating the role of torularhodin in bacterial-fungal interactions. Within these networks, key microorganisms, notably A. muciniphila, were identified, both in the EMs-T network (Fig. 3M).
Thus, these findings highlight A. muciniphila as a characteristic bacterium associated with torularhodin metabolism, suggesting that torularhodin has the potential to promote adenosylcobalamin metabolism in NAFLD mice.
Both gut microbiota remodeling by torularhodin and A. muciniphila alleviate NAFLD in mice
We used FMT in antibiotic-induced pseudo-germ-free mice to investigate whether the impact of torularhodin on the gut microbiota contributed to alleviating NAFLD (Fig. 4A). Mice receiving fecal samples from torularhodin-treated donors (EMs-T group) exhibited notable reductions in TC, TG, LDL-C, AST, and ALT levels (Fig. S3A). Moreover, hepatic fatty infiltration was decreased in the FMT-EMs-T group (Fig. S3B), and the expression of colonic occludin tight junction proteins were increased (Figs. S3C). Additionally, in the FMT-EMs-T group, the colonic bacterial diversity and abundance were increased and resembled those of the FMT-Control group (Fig. S3D, E). Notably, A. muciniphila demonstrated noticeable growth in both recipient and donor mice (Fig. 4B, S3F). Remarkably, torularhodin-mediated remodeling of the gut microbiota significantly increased the plasma and fecal levels of adenosylcobalamin in the FMT-EMs-T group (Fig. S3G, H). However, although A. muciniphila tended to increase in the ileum and ceacal of germ-free mice, the increase was not significant (Fig. S3I, J).
A Flowchart of FMT experiment of pseudo-germ-free mice. B LEfSe analysis and heatmap of bacterial genus in pseudo-germ-free mice (n = 8). C Flowchart of FMT experiment of germ-free (GF) mice. D H&E sections of germ-free mice liver (200×) (n = 3). E Weight gain at 20th week in germ-free mice (n = 6). F Level of A. muciniphila in recipient mice colon bacteria in germ-free mice (n = 6). G Levels of TC, TG, HDL-C, LDL-C, and AST in germ-free mice serum (n = 6). H, I Level of adenosylcobalamin in germ-free mice serum and feces (n = 6). J Flowchart of pseudo-germ free mice with EMs-T intervention (n = 8). K Flowchart of the A. muciniphila transplantation experiment. L Trend of body weight in in mice (n = 8). M Levels of TC, TG, HDL-C, LDL-C, AST and ALT in mice serum (n = 8). N H&E and Oil Red O sections of mice liver (200×) (n = 3). O Level of A. muciniphila in mice colon bacteria (n = 8). P, Q Level of adenosylcobalamin in mice serum and feces (n = 8). Data are expressed as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, compared with the Mod or FMT Mod or GF Mod group, and p-value was calculated using Dunnet’s post hoc test in one-way ANOVA. p < 0.01 and FDR < 0.05 in LEfSe analysis. The abbreviations are fecal-microbial transplantation (FMT), antibiotics (Abx), and germ-free (GF). For box plots, the midline represents the median, the box spans the interquartile range between the first and third quartiles, and the whiskers extend to the lowest and highest values within 1.5 times the interquartile range from the first and third quartiles. A, C, J, and K are created in BioRender. Liu, C. (2025) https://BioRender.com/a58c024. The small icons in (H, I, P, Q) are created in BioRender. Liu, C. (2025) https://BioRender.com/k07f052.
To further investigate the role of gut microbiota, we conducted FMT using germ-free (GF) mice (Fig. 4C). The results demonstrated improvements in fatty liver pathology (Fig. 4D, S3K), weight gain (Fig. 4E), and serum indicators (Fig. 4G) in the GF mice following FMT intervention. Notably, compared to the GF Con group, the relative abundance of A. muciniphila was significantly higher (Fig. 4F), and the levels of adenosylcobalamin in both serum and feces were also markedly elevated in the GF EMs-T group (Fig. 4H, I). The FMT results in both pseudo-germ-free and germ-free mice were consistent, highlighting the significant role of A. muciniphila and adenosylcobalamin in NAFLD.
To confirm whether the anti-NAFLD effect of torularhodin was mediated indirectly via the gut microbiota rather than directly via the colon microbiota, we treated antibiotic-induced pseudo-germ free mice with EMs-T (Fig. 4J). After 8 weeks of intervention, mice fed an HFHCD showed no improvement in NAFLD symptoms (Fig. S4A–H), indicating that the anti-NAFLD effect of sustained-release torularhodin in the colon is indeed mediated indirectly by the gut microbiota.
Furthermore, the abundance of A. muciniphila was substantially increased in the colon of both recipient and donor mice. To confirm the role of A. muciniphila, we administered it to mice with NAFLD (Fig. 4K). This led to significant reductions in weight (Fig. 4L) and dyslipidaemia (Fig. 4M) and decreases in hepatic steatosis and lipid droplet accumulation (Fig. 4N, S3L). Additionally, A. muciniphila strengthened the expression levels of tight junction proteins, such as occludin (Fig. S4I) and MUC2 (see below, Fig. 6H), and enhanced the diversity and abundance of colonic bacteria in NAFLD mice (Fig. S4J, K). A. muciniphila was the predominant microbe in both groups, and its relative abundance was significantly greater in Akk-treated mice than that in Mod-treated mice (Fig. 4O, S4L), indicating the stable colonization of A. muciniphila. Importantly, the levels of adenosylcobalamin in the plasma and feces of mice also significantly increased (Fig. 4P, Q).
These findings indicate that the anti-NAFLD effects of torularhodin are largely mediated through its influence on the gut microbiota, particularly A. muciniphila.
Torularhodin reduces Cer levels and inhibits the colonic HIF-2α pathway
To elucidate the impact of biomarkers of torularhodin on lipid metabolism within the gut-liver axis in mice with NAFLD, we conducted lipidomic assays on feces, liver, and serum samples. PCA revealed that torularhodin influenced lipid metabolism in NAFLD mice, which was consistent with the findings in control mice (Fig. 5A–C). Using the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Reactome databases, we identified enriched lipid-related genes in mouse serum, liver, and feces and revealed that the sphingolipid metabolism and the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathway were the major pathways affected by torularhodin (Fig. 5D–F). Notably, sphingolipid metabolism, which is enhanced by torularhodin in the gut, plays a crucial role in NAFLD pathogenesis, as sphingolipids are positively associated with obesity19.
A–C PCA of lipids in mice serum, liver, and feces (n = 8). D–F Lipid metabolism pathways affected by torularhodin in mice serum, liver, and feces (n = 8). G–I Levels of Cer (Glc-Cer, Cer, SM) in mice serum, liver, and feces (n = 8). J Relationship between colonic HIF−2α/Neu3 pathway, Cer metabolism and NAFLD. K Blots and grayscale analysis of MUC2, HIF-2α, Occludin, and Neu3 proteins in mice colon (n = 6). Data are expressed as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, compared with the Mod group, and p-value was calculated using Dunnet’s post hoc test in one-way ANOVA. J created in BioRender. Liu, C. (2025) https://BioRender.com/o32q382. The small icons in (A–I, K) are created in BioRender. Liu, C. (2025) https://BioRender.com/k07f052.
Our findings indicated that the levels of sphingolipids, especially glucosylceramide (Glc-Cer) and Cer, in the liver and serum of mice with NAFLD were reduced by torularhodin treatment (Fig. 5G–I). Given that the gut microbiota can metabolize Cer and other sphingolipids, thereby exacerbating fatty liver conditions20, strategies targeting the gut-liver axis and pathways related to sphingolipids and inhibiting Cer-related synthesis in the colon are crucial for alleviating fatty liver conditions9. Especially, intestinal HIF-2α regulates Cer metabolism by increasing the expression of the neuraminidase 3 (Neu3)21 (Fig. 5J). In our study, torularhodin treatment not only reduced sphingolipid levels but also significantly inhibited the protein expression of intestinal HIF-2α and Neu3, thereby improving the integrity of the colonic barrier (Fig. 5K).
Consequently, torularhodin treatment decreased the levels of C16–C20 long-chain ceramides and suppressed the expression of HIF-2α, a pivotal protein involved in Cer synthesis in NAFLD mice.
Both gut microbiota remodeling by torularhodin and A. muciniphila reduce Cer levels by inhibiting the colonic HIF-2α pathway
For NAFLD-related Cer metabolism, PCA of lipidomic data showed that the lipidomic profiles of the FMT EMs-T group were comparable to those of the FMT Con group (Fig. S5A–C). Lipid enrichment analysis further revealed that sphingolipid metabolism was also one of the most prominent pathways affected (Fig. S5D–F). Notably, gut microbiota remodeling by torularhodin resulted in reduced sphingolipid levels, particularly those of Glc-Cer and Cer, in both the liver and serum both in serum, liver, and feces of germ-free mice (Fig. 6A–C), and pseudo-germ-free mice (Fig. S5G–I). Validation of the colonic HIF-2α/Neu3 pathway in the recipient mice revealed a significant reduction in HIF-2α and Neu3 levels following the remodeling of the gut microbiota by torularhodin (Fig. 6G and S5J).
A–C Levels of Cer (Glc-Cer, Cer, SM) in germ-free mice serum, liver, and feces (n = 6). D–F Levels of Cer (Glc-Cer, Cer, SM) in A. muciniphila transplantation mice serum, liver, and feces (n = 8). G Blots and grayscale analysis of MUC2, HIF-2α, Occludin and Neu3 proteins in germ-free mice colon (n = 6). H Blots and grayscale analysis of MUC2, HIF-2α, Occludin, and Neu3 proteins in A. muciniphila transplantation mice colon (n = 6). Data are expressed as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, compared with the Mod or GF Mod group, and p-value was calculated using Dunnet’s post hoc test in one-way ANOVA. The small icons in (A–H) are created in BioRender. Liu, C. (2025) https://BioRender.com/k07f052.
Furthermore, our findings confirmed that torularhodin increased the abundance of A. muciniphila, and this enrichment notably reduced the accumulation of long-chain (C16–C20) in the liver and serum (Fig. 6D–F). Additionally, A. muciniphila inhibited the expression of HIF-2α and Neu3 (Fig. 6H). Collectively, our results from FMT and single bacterial transplantation experiments indicate the role of torularhodin in inhibiting the intestinal Cer synthesis protein HIF-2α, reducing Cer levels, and alleviating NAFLD through the modulation of the gut microbiota, particularly A. muciniphila.
Adenosylcobalamin reduces Cer levels by inhibiting the HIF-2α pathway, ultimately mitigating NAFLD in mice
To verify that adenosylcobalamin, an intestinal metabolite of torularhodin, reduces Cer levels by inhibiting the HIF-2α pathway, we encapsulated adenosylcobalamin in an electrospinning colon-targeted system and fed it to mice. Moreover, HIF-2a activator and inhibitor groups were established to verify whether the HIF-2a pathway is the pivotal pathway (Fig. 7A). Notably, adenosylcobalamin delivered to the colon significantly attenuated weight gain in mice, mirroring the effects observed with the HIF-2α inhibitor PT238522. However, the HIF-2a activator M1002 + adenosylcobalamin had no discernible effect (Fig. 7B, C). This trend was consistent across measures of blood lipids and liver function (Fig. 7D–H). Furthermore, adenosylcobalamin alone ameliorated hepatic steatosis and lipid droplet accumulation, but its efficacy was compromised when HIF-2α was activated by M1002 (Figs. 7I, S3M).
A Flowchart of the adenosylcobalamin compensation experiment. B Trend of body weight in mice (n = 8). C Weight gain of mice at the 8th week (n = 8). D–H Levels of TC, TG, LDL-C, AST and ALT in mice serum (n = 8). I H&E and Oil Red O sections of mice liver (200×) (n = 3). J–L Levels of Cer (Glc-Cer, Cer, SM) in mice serum, liver, and feces (n = 8). M Blots and grayscale analysis of HIF-2α and Neu3 proteins in mice colon (n = 6). Data are expressed as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, compared with the Mod group, and p-value was calculated using Dunnet’s post hoc test in one-way ANOVA. A created in BioRender. Liu, C. (2025) https://BioRender.com/v54i969. The small icons in J–M are created in BioRender. Liu, C. (2025) https://BioRender.com/k07f052.
Adenosylcobalamin supplementation led to a reduction in sphingolipid accumulation in the colon, liver, and serum, particularly in the presence of Glc-Cer and Cer from C16-C20, and an increase in SM levels. However, under the influence of HIF-2α agonists, adenosylcobalamin failed to lower Cer levels (Fig. 7J–L). Protein expression analysis revealed that adenosylcobalamin inhibited the expression of HIF-2α and Neu3 (Fig. 7M). Notably, when HIF-2α was activated and stabilized by M1002, the ability of adenosylcobalamin to inhibit HIF-2α was markedly diminished. Thus, the anti-NAFLD effect of the intestinal metabolite of torularhodin, adenosylcobalamin, is mediated by inhibiting the expression of the Cer-producing protein HIF-2α and reducing Cer levels.
Torularhodin promotes the growth of A. muciniphila and enhances adenosylcobalamin levels, which subsequently reduces Cer levels by inhibiting the HIF-2α pathway
To elucidate the direct influence of torularhodin on A. muciniphila and adenosylcobalamin, we used the human gastrointestinal system for the coculture of fecal bacteria from NAFLD patients and EMs-T (Fig. 8A). After 7 days of EMs-T intervention, the abundance of A. muciniphila was significantly increased, particularly in the transverse and descending colon (Fig. 8B). Moreover, adenosylcobalamin levels were significantly increased, predominantly in the transverse and descending colon (Fig. 8C). However, when fecal bacteria from healthy individuals were used as negative controls and co-cultured with EMs-T, there was no significant increase in the levels of A. muciniphila and adenosylcobalamin (Fig. S6A, B). This suggests that torularhodin is more effective in individuals with obesity, who typically have lower levels of A. muciniphila. These findings suggest that torularhodin directly stimulates A. muciniphila and adenosylcobalamin production in the middle and distal colon, with subsequent absorption and utilization by colonic cells.
A Flowchart of co-culture flowchart of fecal bacteria and EMs-T. B Changes in A. muciniphila between the beginning and end (n = 3). C Changes in adenosylcobalamin between the beginning and end (n = 3). D Flowchart of co-culture flowchart of colonic organoids and fecal lipids. The model involves co-culturing colonic organoids with fecal lipids from NAFLD mice, and treating with compounds (torularhodin or adenosylcobalamin) to investigate their effects on organoids. E Fluorescent images of colonic organoids, with Edu labeled proliferating cells in green (n = 6). F Fluorescent images of colonic organoids, and green representing MUC2 (n = 6). G Blots and grayscale analysis of MUC2, HIF-2α, Occludin, and Neu3 proteins in colonic organoids. And mRNA levels of Lgr5 gene in colonic organoids (n = 6). H Levels of Cer (Glc-Cer, Cer, SM) in colonic organoids (n = 6). Data are expressed as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, compared with the Mod group, and p-value was calculated using Dunnet’s post hoc test in one-way ANOVA (G, H). Statistical significance was determined using a two-tailed paired t-test (B, C). A, D are created in BioRender. Liu, C. (2025) https://BioRender.com/h37g164. The small icon in (G) is created in BioRender. Liu, C. (2025) https://BioRender.com/k07f052.
To delineate the primary impact of adenosylcobalamin on the colon and its role in mediating Cer metabolism in the host gut, we established a mouse colonic organoid coculture model with fecal lipids and then treated with adenosylcobalamin or torularhodin (Fig. 8D). Intestinal organoids, which serve as reliable models of the intestinal epithelium, offer valuable insights into intestinal physiology and dietary interactions23. Notably, organoids treated with adenosylcobalamin for 7 days exhibited enhanced proliferation of 5-ethynyl-2’-deoxyuridine (EDU)-labeled intestinal epithelial cells (Fig. 8E and S6C) at 7th day. Moreover, adenosylcobalamin treatment effectively reversed the reduction in the fluorescence intensity of MUC2, a specific marker of goblet cells, induced by fecal lipids (Fig. 8F and S6D). Adenosylcobalamin treatment significantly upregulated the expression of the leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) transcription factor in organoids, which is indicative of enhanced proliferation of intestinal stem cells, whereas torularhodin treatment had no significant effect (Fig. 8G). Furthermore, adenosylcobalamin promoted the expression of the intestinal tight junction proteins occludin and MUC2, enhancing the integrity of the colonic barrier (Fig. 8G).
Given our previous findings implicating ceramides as key regulators of torularhodin-mediated lipid metabolism via the HIF-2α pathway, we examined lipid profiles in colonic organoids to determine whether adenosylcobalamin affects Cer metabolism without gut microbiota. Adenosylcobalamin treatment significantly inhibited the expression of HIF-2α and Neu3 proteins in organoids (Fig. 8G). Notably, the levels of C16–C20 Glc-Cer and Cer were more robustly decreased by adenosylcobalamin than by torularhodin, and there were slightly significant alterations in SM levels (Fig. 8H). Thus, as the gut microbiota metabolite of torularhodin, adenosylcobalamin enhances the colonic barrier, suppresses HIF-2α expression, and further decreases long-chain Cer levels.
Discussion
This study investigated the influence of the carotenoid torularhodin on Cer metabolism in NAFLD mice, with a particular focus on the gut microbiota. The sustained release of torularhodin in the colon led to an increase in A. muciniphila abundance and facilitated adenosylcobalamin synthesis. Consequently, adenosylcobalamin treatment suppressed colonic Cer synthesis, reduced its accumulation in the liver, and ultimately alleviated hepatic steatosis in NAFLD mice (Fig. 1).
A previous study has shown that a comparison of the serum cobalamin concentrations in 150 NAFLD patients and 50 healthy individuals revealed a substantial reduction exceeding 30% in its concentration in the NAFLD cohort (Fig. S7A)24. Extensive clinical investigations have consistently demonstrated a significant inverse correlation between cobalamin (vitamin B12) levels and obesity (Fig. S7B, C)25,26,27. Moreover, mice with NAFLD commonly manifest dyslipidaemia alongside imbalances in gut microbiota composition and compromised barrier integrity28. Cobalamin supplements have been shown to expedite intestinal barrier restoration in ulcerative colitis29. Addressing these multifaceted issues presents a therapeutic avenue for NAFLD management.
Our study revealed a notable increase in A. muciniphila abundance in both recipient and donor mice, indicating that torularhodin can promote A. muciniphila proliferation and colonization in NAFLD mice. Furthermore, intestinal adenosylcobalamin levels significantly increased. Previous research has shown that adenosylcobalamin is exclusively synthesized by bacteria and archaea, with gut bacteria possessing the ability to synthesize adenosylcobalamin30. Additionally, the A. muciniphila genome harbors genes associated with adenosylcobalamin biosynthesis31,32, underscoring its role in adenosylcobalamin metabolism33. Therefore, torularhodin can promote adenosylcobalamin production by A. muciniphila.
Given the antioxidant properties of torularhodin11 and the tight regulation of adenosylcobalamin biosynthesis by environmental redox conditions34, it is reasonable to expect that supplementation with this carotenoid could enhance adenosylcobalamin biosynthesis. Previous research has suggested that silymarin contributes to intestinal redox balance, with microbially produced adenosylcobalamin contributing to the lipid-lowering effect of silymarin35. In turn, adenosylcobalamin has been shown to modulate intestinal microbiota function, thereby improving intestinal barrier integrity36, a finding consistent with our observations. Our finding suggests that the increased synthesis of colonic adenosylcobalamin is likely associated with the ability of torularhodin to balance colonic redox reactions and the microbiota.
The consumption of a Western diet has been linked to obesity and alterations in Cer metabolism, with significant changes observed in the levels of various Cer species37. Our lipidomic analyses highlighted the pivotal role of sphingolipid metabolism, particularly Cer metabolism, in NAFLD mice. The positive association between serum and tissue Cer levels and obesity-related complications indicates the importance of inhibiting Cer biosynthesis in alleviating fatty liver conditions38,39. Cer can be synthesized through diverse pathways, including from Glc-Cer. In individuals with obesity, intestinal HIF-2α promotes Cer synthesis by upregulating the expression of Neu321. However, our intervention with torularhodin in NAFLD mice led to a reduction in HIF-2α expression. Furthermore, our colonic organoid coculture model without gut microbiota demonstrated that adenosylcobalamin inhibited intestinal Cer production by suppressing HIF-2α expression, indicating that torularhodin plays an anti-NAFLD role by increasing host colon Cer metabolism through microbial adenosylcobalamin.
In conclusion, supplementation with the carotenoid torularhodin increased A. muciniphila abundance and promoted intestinal adenosylcobalamin synthesis. Subsequently, the increased adenosylcobalamin levels inhibited HIF-2α expression, leading to a reduction in Cer accumulation and alleviation of hepatic steatosis in NAFLD mice. Thus, optimizing the gut microbiota through torularhodin supplementation could represent a promising dietary intervention strategy for NAFLD treatment.
Methods
Ethics statement
The experiments using human samples were approved by the Medical Ethics Committee of Jiangnan University (approval number JNU202312IRB11). All research was conducted in accordance with relevant guidelines and regulations, and written informed consent was obtained from all volunteers. The animal experimental procedures were approved by the Animal Ethics Committee of Jiangnan University (approval number JN. No 20210630c1401201[267]).
Human studies
The feces of volunteers with obesity (with serum total cholesterol levels higher than 5.7 mmol/L and triglyceride levels higher than 1.7 mmol/L) who had not used antibiotics within half a year were collected. Feces from three healthy volunteers (3 males) were used as the negative control.
Preparation of electrospun microspheres encapsulating torularhodin
The preparation process of electrospun microspheres encapsulated torularhodin (EMs-T) followed a previous study16. Sodium alginate (5 mg/mL) and chitosan (5 mg/mL) were dissolved in 0.5 M NaCl solution with a pH adjusted to 6.0. Next, 300 mg of torularhodin was added, and the solution was stirred for 5 min at 5000 rpm in an ice-water bath. The sodium alginate and chitosan mixed solution had a slight viscosity, which facilitated the uniform dispersion of torularhodin, a lipophilic compound, during stirring. The electrospinning device consisted of a high-voltage power supply, a constant-speed syringe pump, and a grounded collector (5% CaCl2 solution). The metal needle was connected to the syringe, which was driven by the constant-speed syringe pump. An electric field was applied between the needle tip (positive polarity) and the grounded collector. The electrospun microspheres were prepared at an ambient temperature of 25 ± 1 °C, with a voltage of 24 kV, a distance of 15 cm between the needle tip and the collector, and a solution flow rate of 1 mL/h. After solidification in CaCl2 solution for 2 h, the electrospun microspheres were collected.
In vivo colonic sustained-release of torularhodin
Thirty male C57BL/6 mice (8 weeks old) were raised with a stable temperature of 25 ± 2 °C and humidity of 60 ± 10%. Mice were orally administered with torularhodin-loaded electrospun microspheres (EMs-T) at a dose of 20 mg/kg. The mice were euthanized with isoflurane (2%–3% concentration). Plasma and tissues (liver, small intestinal contents, and feces) were collected at various time points (1, 2, 4, 8, 16, and 24 h, n = 5). Plasma and tissues were immediately stored in liquid nitrogen and processed together. Then, plasma and tissue homogenate were mixed with 1 mL ice-cold ethanol (containing 30 mg/L butylated hydroxytoluene) and 0.8 mL n-hexane for 10 min. The extraction process was repeated twice and the supernatants were combined and blown dry with nitrogen gas to obtain the residue. Residue was mixed with 1 mL dimethyl sulfoxide and extraction solvent (n-hexane: dichloromethane = 4:1, v/v), shaken and centrifuged at 5000 × g for 10 min at 4 °C to obtain the supernatant. Torularhodin was quantified using an Agilent 1260 HPLC system equipped with a DAD detector. The mobile phase comprised of solvent A (acetonitrile: water: formic acid, 86:10:4, v/v/v) and solvent B (ethyl acetate: formic acid, 96:4, v/v/v) at a flow rate of 1.0 mL/min. The flow rate of solvent A decreased linearly from 100% to 0% during 0–25 min, the flow rate of solvent B increased linearly from 0% to 100% during 25–30 min, and the flow rate of solvent A increased linearly to 100% during 25–32 min. A standard curve was established by preparing torularhodin solutions at concentrations of 0.1–100 μg/mL in n-hexane at 492 nm.
NAFLD mice modeling and treatment
Thirty-two male ApoE−/−C57BL/6J mice, aged 6 weeks, were raised with a stable temperature of 25 ± 2 °C and humidity of 60 ± 10% (Fig. 2B). After 7 days of adaptation period, all mice, except for the control group, were fed a high-fat, high-cholesterol diet (HFHCD) from weeks 1 to 10. From weeks 11 to 20, the control group (Con) and the model group (Mod) were given saline by gavage, while the electrospun microspheres carrier group (EMs), and torularhodin electrospun microspheres group (EMs-T, 20 mg/kg of torularhodin40) were fed samples. The body weight of mice was recorded on the same morning of each week. HFHCD was purchased from Nantong Tefoly Feed Science and Technology Co., Ltd. (Nantong, China), and the formula and calorie ratio are presented in the Supplementary Tables S1, S2. Mice were housed in two cages per group, with 4 mice per cage. Food intake per cage was recorded weekly by measuring the amount of fresh food added and the remaining food at the end of the week. The average food intake per day for each mouse was calculated based on the total food consumed. The mice were euthanized with isoflurane (2–3% concentration), followed by the collection of biological samples.
Detection of histopathology in mice
Histopathological examination of mouse tissues involved prompt excision, fixation in 4% paraformaldehyde for 24 h at 4 °C, dehydration, paraffin embedding, and sectioning. Sections of 4 μm thickness were stained with oil red O and hematoxylin. Sections were incubated in Oil Red O solution for 10-15 minutes at room temperature and then differentiated with 85% isopropanol. Sections were then washed, dehydrated, and mounted. The NAS (Non-Alcoholic Fatty Liver Disease Activity Score) was used to evaluate the pathological sections of the liver for the degree of fatty liver41.
Immunofluorescence staining was performed following antigen retrieval, permeabilization, blocking, and antibody staining. Tissue sections underwent antigen retrieval by heating in a citrate buffer at 95 °C for 20 min, followed by cooling to room temperature. The sections were then permeabilized using 0.3% Triton X-100 in PBS for 10 min and blocked with 5% serum for 1 h at room temperature. Primary antibodies (Muc2, 1:500) were applied overnight at 4 °C, and after washing, the sections were incubated with fluorophore-conjugated secondary antibodies for 1 h at room temperature. The stained tissue sections were mounted with a mounting medium containing DAPI for nuclear counterstaining. Finally, all slides were analyzed using an automated scanner. For quantitative analysis, ImageJ software was used to measure the relevant parameters.
Detection of lipids in mice
After homogenization of 10 mg of mice liver or colonic contents or 30 μL of mice serum and 210 μL cold methanol (containing the internal standard PC17:0 at 10 μg/mL) at 50 Hz for 5 min, 0.7 mL of methyl tert-butyl ether was added to the mixture. Following 60 min of shaking at 25 °C, 175 μL of ultrapure water was introduced, and the mixture was vortexed for 10 s to create a two-phase system. After centrifugation at 14,000 × g for 10 min at 4 °C, the supernatant was dried in vacuo at 30 °C. The dried supernatant residue was reconstituted with 100 μL of isopropanol and then sonicated for 10 min at 4 °C. Following centrifugation at 14,000 × g for 10 min, the supernatant was used for UPLC/QTOF-MS analysis. The instrument parameter settings refer to previous study42.
Data processing and analysis were conducted using MS-DIAL, RaMP-DB, and MetaboAnalyst 5.0. Data processing and analysis were conducted as follows: (1) LC-MS Lipidomics Raw Data: First, raw data from LC-MS lipidomics experiments were collected to obtain the mass spectrometry information of lipids in the samples. (2) Data Processing with MS-DIAL: The raw data were then input into MS-DIAL, where peak identification, noise reduction, and lipid identification were performed, generating comprehensive qualitative and quantitative data for all lipids. (3) Data Filtering: The processed data were filtered based on the criteria that the relative standard deviation of quality control samples was less than 20% and the signal-to-noise ratio was greater than 5. (4) Normalization and Transformation: Following filtering, the data were normalized, log-transformed, and subjected to dimension reduction analysis. (5) Differential Lipid Analysis: The processed data were imported into MetaboAnalyst 5.0 for differential lipid analysis, where univariate analysis of variance (ANOVA) was performed using Fisher’s least significant difference (LSD) method. Lipids with a P-value < 0.05 and false discovery rate (FDR) < 0.05 were considered significantly different. (6) Pathway Enrichment Analysis: The differential lipids were then imported into RaMP-DB for pathway enrichment analysis, using the KEGG and Reactome databases to identify potential biological roles of lipid metabolism in various biological processes. (7) Data Visualization: Finally, data were visualized using RStudio to generate lipid PCA plots, heatmaps, scatter plots, and differential gene pathway bubble plots.
Detection of bacteria and fungus in mice
DNA was extracted from feces for subsequent PCR amplification. Bacterial 16S rRNA sequencing gene (V3-V4 region) and fungal ITS RNA sequencing gene (ITS1-5F region) were amplified from the whole genome using specific primers: 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) for bacteria, and ITS5-1737F (GGAAGTAAAAGTCGTAACAAGG) and ITS2-2043R (GCTGCGTTCTTCATCGATGC) for fungi. The PCR protocol included initial denaturation at 95 °C for 2 min, followed by 25 cycles of amplification at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, with a final extension at 72 °C for 5 min.
Sequencing and data processing involved verification of amplification products through 2% agarose gel electrophoresis, purification, and quantification. Sequencing of qualified libraries was performed on the Novaseq 6000 PE250 (Illumina Inc., San Diego, USA) platform. Raw fastq files were then quality-controlled, denoised, joined, and dereplicated using the DADA2 plugin in QIIME 2.0, resulting in amplicon sequence variants (ASV). Species annotation and visualization utilized representative sequences and feature tables, with RDP classifier and the Greengenes2 database for bacterial 16S rRNA, and the UNITE database for fungal ITS rRNA. Predictions of the microbiota’s functional potential were based on ASV abundance and representative sequences via PICRUSt2. Bacterial classification utilized the upgraded Greengenes2 database (version 2022.10), providing higher resolution results consistent with metagenomic data in principal coordinate space, taxonomy, and phenotypic effect size43.
Fecal-microbial transplantation experiment for pseudo-germ-free and germ-free mice
Fecal-microbial transplantation (FMT) procedure in pseudo-germ-free mice is illustrated in Fig. 4A. Thirty-two male ApoE−/−C57BL/6J mice (8 weeks old) were treated with a two-week course of broad-spectrum antibiotics in water, including vancomycin (0.5 g/L), ampicillin (1 g/L), neomycin (1 g/L), and metronidazole (1 g/L). 100 mg fresh feces were collected from the donor mice and suspended in 1 mL of sterile anaerobic PBS. Samples were stored at 4 °C and processed under anaerobic conditions within 2 hours. After centrifugation at 500 × g for 2 min to remove insoluble materials, supernatant was orally administered to recipient mice at a dose of 0.2 mL per mouse everyday. The fecal samples from torularhodin-treated donor mice (EMs-T group) were transplanted to recipient mice without the supplementation of torularhodin (FMT-EMs-T group). FMT-Con, FMT-Mod, and FMT-EMs group mice were similarly treated, and all groups were fed HFHCD except the FMT-Con group. FMT experiment lasted for 8 weeks with two mice in a cage.
FMT procedure in germ-free mice is illustrated in Fig. 4C. Twenty-four male C57BL/6J mice (8 weeks old) were maintained in a sterile environment, ensuring complete absence of microbes. The above fecal samples from torularhodin-treated donor mice (EMs-T group) were transplanted to recipient mice without the supplementation of torularhodin (GF EMs-T group) at a dose of 0.2 mL three times a week. GF Con, GF Mod, and GF EMs group mice were similarly treated, and all groups were fed HFHCD except the GF-Con group. The experiment lasted for 8 weeks with two mice in a cage.
In addition, twenty-four male mice were assigned to one of three groups to become pseudo-germ free mice after two weeks of antibiotic intervention (Fig. 4J). Antibiotics (Abx)-Con group mice were fed normal diet, Abx-Mod group mice were fed HFHCD, and Abx-EMs-T group mice were fed HFHCD and EMs-T. The experiment lasted for eight weeks with two mice in a cage.
Akkermansia muciniphila transplantation in mice
A. muciniphila isolated from the feces and was identified as CICC 24917. It was cultured anaerobically in medium containing 3.85% brain heart infusion, 0.5% porcine mucin, and 0.05% L-cysteine. Subsequently, it underwent centrifugation, washing, and resuspension in anaerobic PBS to achieve a concentration 1 × 109 cfu/mL. Twenty-four male ApoE−/− C57BL/6J mice (7 weeks old) were fed an HFHCD for 10 weeks. From the 11th to the 20th week, A. muciniphila group (Akk) was given 200 µL of bacterial suspension everyday, while the control (Con) and model (Mod) groups were given saline as shown in Fig. 4K.
Adenosylcobalamin compensation experiment in mice
As shown in Fig. 7A, forty male C57BL/6J mice, aged 6 weeks, were raised for 7 days of adaptation period. All mice, except for the control group, were fed an HFHCD from weeks 1 to 8. At the same time, the control group (Con) and the model group (Mod) were given saline by gavage, while the electrospun microspheres encapsulating adenosylcobalamin group (150 mg/kg)29, electrospun microspheres encapsulating M1002 (50 mg/kg)+adenosylcobalamin (150 mg/kg) group, and electrospun microspheres encapsulating PT2385 group (20 mg/kg)21 were fed corresponding samples. All mice were administered the treatment via oral gavage, with a dose given once daily.
Co-culture of EMs-T and fecal bacteria from patients with NAFLD in vitro
As shown in Fig. 8A. Simulated stomach-small intestine digestion was referred to as the standard INFOGEST in vitro digestion44. Briefly, 5 g EMs-T were mixed with simulated oral fluid, followed by digestion with simulated gastric fluid. The pH was adjusted to 3.0, and the mixture was incubated in a 37 °C water bath. After gastric digestion, simulated small intestinal fluid was added, and the pH was adjusted to 7.0. The mixture was then incubated again at 37 °C in a water bath to simulate small intestinal digestion. It is known that ~10% of the chyme in the small intestine is not absorbed and reaches the colon45. Therefore, to simulate the material that reaches the colon, 10% (v/v) of the soluble fraction of digestive juice, along with the undigested solid residue (4000 × g, 10 min) from the small intestine, was used as the substrate for colonic fermentation.
Simulated colon fermentation system was designed according to previous researches16,46. The feces of volunteers with obesity (with serum total cholesterol levels higher than 5.7 mmol/L and triglyceride levels higher than 1.7 mmol/L) who had not used antibiotics within half a year were collected. Fecal samples from three volunteers with obesity (3 males) were collected, and 5 g from each sample was mixed thoroughly with 120 mL of sterile anaerobic PBS buffer (pH 6.8). The mixture was stirred until fully suspended, then filtered using sterile three-layer gauze to remove solid particles. All procedures were performed in an anaerobic chamber in 1 h.
From the 50 mL simulated small intestinal digestion liquid, 5 mL of the soluble fraction was collected, along with the undigested solid residue. This mixture was then added to 300 mL of intestinal culture medium that had been previously inoculated with 10% intestinal fecal microbiota. The fermentation temperature was maintained at 37 °C, and pH was automatically controlled using a pH regulation system. To maintain an anaerobic environment, nitrogen gas was introduced into each fermentation vessel three times daily to expel air. To support normal microbial growth, fresh medium was replenished daily, and 300 mL of culture medium in total was removed to maintain constant volume.
After inoculating the fecal microbiota, continuous fermentation was carried out for 7 days, during which the microbiota reached a stable state (from day -7 to day 0). From 1st day, the fermentation medium containing EMs-T digestion liquid was introduced. New digestion solution was introduced daily, and old fermentation solution was expelled until day 7. At the same time, fecal microbiota from three healthy volunteers (3 males) were used as the negative control.
Mice colonic organoids and lipids co-culture model
The experimental procedure is outlined in Fig. 8D. Crypts were isolated from the colons of 4–6-week-old C57BL/6 mice47,48. Colon was cut and washed with cold DPBS (PBS without calcium and magnesium). Colon fragments were immersed in 2 mM EDTA/DPBS at 4 °C for 30 min. After removing EDTA, a 1 mL pipette, rinsed with cold 0.1% BSA/DPBS, was employed to cut and disrupt the colon tissue effectively. Tissue fragments were vigorously dispersed with a moistened pipette in 0.1% BSA/DPBS. The initial cleaning solution was discarded, and the last three cleaning solutions were collected and filtered through a dampened 70 μm filter. Filtrate was combined and centrifuged at 200 × g and 4 °C for 3 min. Crypts were resuspended in the matrix glue and plated onto a 24-well plate, with 50 μL of matrix glue containing 200 crypts. The plate was inverted in an incubator at 37 °C for 30 min for the matrix glue to solidify. Then, medium was added refer to study49.
Fecal lipids of Con group mice (control mice) and Mod group mice (NAFLD mice) served as substrates for colonic organoids. 20 mg feces was mixed with 1.5 mL ice chloroform/methanol (1:2, v/v) and centrifuged at 15,000 × g for 10 min at 4 °C50. The supernatant was filtered through a 70 μm filter and mixed with 600 μL distilled water. After a subsequent centrifugation for phase separation, the lipid metabolite layer was collected. The lipids were dissolved in 50 μL DMSO. Finally, the extract was added to the colonic organoid differentiation medium at a 1:100 ratios (containing 100 μmol/L CoCl2, creating a micro-oxygen environment). Once the organoid diameter reaches 150–200 µm, replace the proliferation medium with differentiation medium. Continue culturing the organoids in a 5% CO2 incubator for 7 days, replacing differentiation medium containing extracts daily.
Colonic organoids were divided into 5 groups. Negative group, where DMSO did not contain any ingredients. Con group, where DMSO contained fecal lipid extract from control mice. Mod group, where DMSO contained fecal lipid extract from NAFLD mice. Torularhodin group, where DMSO contained fecal lipid extract from NAFLD mice and torularhodin (10 μmol/L). Adenosylcobalamin group, where DMSO contained fecal lipid extract from NAFLD mice and adenosylcobalamin (10 μmol/L). Organoids were cultured in 24-well cell plates, with 24 wells per group.
Immunofluorescence assay of colonic organoids
The proliferation of colonic organoids was detected using EdU proliferation kit49. Organoids were cultured in the medium containing 10 μmol/L EdU for 24 h on the 7th day of differentiation, and then fixed in 4% paraformaldehyde for 30 min. After permeation (0.5%Triton X-100/PBS) for 10 min, organoids were reacted with the reactants for 30 min. Immunofluorescence experiment referred to the previous study51. First, the medium was aspirated from the wells. Then, 1 mL of cold cell recovery solution was added to each well, and the plates were incubated at 4 °C for 30 min. After incubation, the Matrigel was dissolved and dispersed. Cold PBS with 0.1% BSA-treated tips were used to handle the organoids, preventing adhesion to the tips. The organoids were washed twice with cold PBS containing 0.1% BSA and then resuspended in 4% paraformaldehyde for 30 min for fixation. Following fixation, the organoids were washed twice with organoid wash buffer (1 mL Triton X-100 and 2 g BSA dissolved in 1 L PBS), and incubated with the buffer at 4 °C for 30 min during the third wash for permeabilization. After removing the wash buffer, wash buffer containing the primary antibody was added to each well, and the organoids were incubated overnight at 4 °C with gentle shaking. The Muc2 antibody was diluted at 1:500. Then, the organoids were washed five times with organoid wash buffer, each time for 5 min. Next, fluorescently labeled secondary antibody was added, and the plates were incubated at room temperature for 2 h with gentle shaking. After incubation, the organoids were washed five times with organoid wash buffer, each time for 5 min. Finally, the organoids were stained with DAPI to counterstain the cell nuclei. The organoids were scanned layer-by-layer (thickness of each layer is less than 10 μm) using laser confocal microscope to reconstruct the 3D image.
Western blotting and qRT-PCR analysis
Organoids or mice tissues homogenate were prepared using a lysis buffer containing phosphatase and protease inhibitors. Protein concentration was determined using the BCA protein assay kit. Samples with equal protein concentrations were separated by 10% SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked with 5% skim milk solution for 2 h, and then incubated with primary antibodies overnight at 4 °C. After washing, the membranes were incubated with secondary antibodies for 2 h. Blots were detected using chemiluminescence reagent. Antibodies included MUC2 (1:2000), HIF-2α (1:2000), Occludin (1:5000), Neu3 (1:2000), and GADPH (1:2000).
Total RNA was extracted from mice tissues or organoids using a kit. Samples with an absorbance ratio at 260/280 nm between 1.8 and 2.0 were considered qualified. The RNA was reverse transcribed to cDNA using a cDNA reverse transcription kit. For qRT-PCR experiments, a SYBR qPCR Master Mix kit was used, with β-actin as the reference gene. The relative expression levels of the target genes were calculated using the 2−ΔΔCt method. Primer sequence of β-action is CCACAGCTGAGAGGGAAA, AAGGAAGGCTGGAAAAGAGC. Primer sequence of Lgr5 is CCTACTCGAAGACTTACCCAGT, GCATTGGGGTGAATGATAGCA.
For bacterial DNA extraction and quantitative PCR52, fecal pellets, cecal contents, and whole ileum with content were resuspended in 1.5 mL buffer (Qiagen) and homogenized. DNA was extracted from the entire suspension using the QiaAmp Mini Stool Kit (Qiagen) according to the manufacturer’s protocol. The extracted DNA was quantified using assay to ensure accurate DNA concentration measurement. For quantitative PCR analysis, a total of 10 ng of DNA was used per reaction. The target genes included Akkermansia muciniphila (5′CAGCACGTGAAGGTGGGGAC′, 5′CCTTGCGGTTGGCTTCAGAT′) and total bacteria (5′ACTCCTACGGGAGGCAGCAGT′, 5′ATTACCGCGGCTGCTGGC′)53. qPCR was carried out using the SYBR qPCR Master Mix kit. The cycling conditions included an initial denaturation step at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 seconds, and extension at 72 °C for 30 s. The relative expression levels of the target genes were calculated using the 2−ΔΔCt method.
Data analysis
Data were reported as mean ± standard deviation (SD) and analyzed using GraphPad Prism 7.0 software. For comparisons among multiple groups, Dunnett’s post-hoc testing method in One-way ANOVA was employed. Linear discriminant analysis Effect Size (LEfSe) was conducted on the Galaxy platform (https://huttenhower.sph.harvard.edu/galaxy/) using a non-parametric Kruskal-Wallis rank-sum test to identify significantly different metabolites among groups. Grouping Wilcoxon rank-sum test was applied to analyze differences, with an alpha value of 0.05. Linear discriminant analysis (LDA) was used for data dimensionality reduction and evaluation of differences. An interaction network was constructed using the SparCC method and Spearman’s correlation, with visualization carried out using Gephi (https://gephi.org/). The remaining data were visualized using R 3.6 software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings described in this manuscript are available in the article and in the Supplementary Information and from the corresponding author upon request. Mouse gut microbiota sequencing and assembly: Sequence Read Archive PRJNA861713. The lipidomics data are referenced in figshare https://doi.org/10.6084/m9.figshare.28601780.v1. Source data are provided as a Source Data file. Source data are provided in this paper.
References
Kuang, J. L. et al. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 35, 1752–1766 (2023).
Ko, C. W. et al. Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 17, 169–183 (2020).
Ni, Y. et al. Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. 35, 1530–1547 (2023).
Cani, P. D. et al. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 19, 625–637 (2022).
Brown, E. M., Clardy, J. & Xavier, R. J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 31, 173–186 (2023).
Chen, B. et al. Gut bacteria alleviate smoking-related NASH by degrading gut nicotine. Nature 610, 562–573 (2022).
Wang, X. et al. Candida albicans accelerates atherosclerosis by activating intestinal hypoxia-inducible factor2alpha signaling. Cell Host Microbe (2024).
Green, C. D. et al. Sphingolipids in metabolic disease: the good, the bad, and the unknown. Cell Metab. 33, 1293–1306 (2021).
Wang, K. et al. The role of gut microbiota in host lipid metabolism: an eye on causation and connection. Small Methods 4, 18–24 (2020).
Liu, C. et al. Study on the cellular anti-Inflammatory effect of torularhodin produced by sporidiobolus pararoseus ZQHL isolated from vinegar fungus. Molecules 28, 1436–1441 (2023).
Zhang, W. et al. Torularhodin from sporidiobolus pararoseus attenuates d-galactose/AlCl(3)-induced cognitive impairment, oxidative stress, and neuroinflammation via the Nrf2/NF-kappaB pathway. J. Agric. Food Chem. 68, 6604–6614 (2020).
Liu, C. et al. Torularhodin ameliorates oxidative activity in vitro and d-Galactose-Induced liver injury via the Nrf2/HO-1 signaling pathway in vivo. J. Agric. Food Chem. 67, 10059–10068 (2019).
Liu, C. et al. Role of the gut microbiota in dietary patterns rich in torularhodin via OSA colon-targeted delivery. Food Funct. 13, 11034–11048 (2022).
Zhu, R. et al. Lycopene in protection against obesity and diabetes: a mechanistic review. Pharmacol. Res. 159, 104966 (2020).
Silva Meneguelli, T. et al. Effect of carotenoids on gut health and inflammatory status: a systematic review of in vivo animal studies. Crit. Rev. Food Sci. Nutr. 64, 11206–11221 (2024).
Liu, C. et al. A colon-targeted delivery system of torularhodin encapsulated in electrospinning microspheres, and its co-metabolic regulation mechanism of gut microbiota. Food Hydrocoll. 135, 108189 (2023).
Adolph, T. E. & Tilg, H. Western diets and chronic diseases. Nat. Med. 30, 2133–2147 (2024).
Coelho, D. et al. Gene identification for the cblD defect of vitamin B12 metabolism. N. Engl. J. Med. 358, 1454–1464 (2008).
Fiorenza, M. et al. Weight-loss maintenance is accompanied by interconnected alterations in circulating FGF21-adiponectin-leptin and bioactive sphingolipids. Cell Rep. Med. 5, 101629 (2024).
Le, H. H., Lee, M.-T. & Johnson, E. L. Host hepatic metabolism is modulated by gut microbiota-derived sphingolipids. Cell Host Microbe 30, 798–808 (2022).
Xie, C. et al. Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat. Med. 23, 1298–1232 (2017).
Wu, D. L. et al. Bidirectional modulation of HIF-2 activity through chemical ligands. Nat. Chem. Biol. 15, 367–376 (2019).
Clevers, H. Modeling development and disease with organoids. Cell 165, 1586–1597 (2016).
Sharma, C. & Badyal, A. Association between vitamin B12 and non-alcoholic fatty liver disease: a case-control study in Katra Jammu. Int. J. Basic Clin. Pharmacol. 10, 726–731 (2021).
Al-Musharaf, S. et al. Low serum vitamin B12 levels are associated with adverse lipid profiles in apparently healthy young Saudi women. Nutrients 12, 2395 (2020).
Boachie, J. et al. Low vitamin B12 and lipid metabolism: evidence from pre-clinical and clinical studies. Nutrients 12, 1925 (2020).
Haloul, M. et al. Hyperhomocysteinemia and low folate and vitamin B12 are associated with vascular dysfunction and impaired nitric oxide sensitivity in morbidly obese patients. Nutrients 12, 2014 (2020).
Tilg, H., Burcelin, R. & Tremaroli, V. Liver tissue microbiome in NAFLD: next step in understanding the gut-liver axis?. Gut 69, 1373–1374 (2020).
Kovatcheva, M. et al. Vitamin B12 is a limiting factor for induced cellular plasticity and tissue repair. Nat. Metab. 5, 1911–1930 (2023).
Li, J. et al. Regulating vitamin B12 biosynthesis via the cbiMCbl riboswitch in Propionibacterium strain UF1. Proc. Natl. Acad. Sci. USA 117, 602–609 (2020).
Sokolovskaya, O. M., Shelton, A. N. & Taga, M. E. Sharing vitamins: cobamides unveil microbial interactions. Science 369, 48–56 (2020).
Mok, K. C. et al. Identification of a novel cobamide remodeling enzyme in the beneficial human gut bacterium Akkermansia muciniphila. Mbio 11, 18–24 (2020).
Kirmiz, N. et al. Comparative genomics guides elucidation of Vitamin B12 biosynthesis in novel human-associated Akkermansia strains. Appl. Environ. Microbiol. 86, 16–20 (2020).
Roth, J. R., Lawrence, J. G. & Bobik, T. A. Cobalamin (coenzyme B-12): Synthesis and biological significance. Annu. Rev. Microbiol. 50, 137–181 (1996).
Sun, W. L. et al. Microbially produced vitamin B12 contributes to the lipid-lowering effect of silymarin. Nat. Commun. 14, 1552 (2023).
Qi, X. Z. et al. Vitamin B12 produced by Cetobacterium somerae improves host resistance against pathogen infection through strengthening the interactions within gut microbiota. Microbiome 11, 25–28 (2023).
Kasumov, T. et al. Ceramide as a mediator of insulin resistance-associated atherosclerosis. Arteriosc Thromb. Vasc. Biol. 34, 2–4 (2014).
Chaurasia, B. et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 365, 386–342 (2019).
Summers, S. A. Could ceramides become the new cholesterol? Cell Metab. 27, 276–280 (2018).
Li, J. et al. Determination of the effects of torularhodin against alcoholic liver diseases by transcriptome analysis. Free Radic. Biol. Med. 143, 47–54 (2019).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Shi, J. et al. Lipidome reveals the alleviation of acrylamide-induced impairment by krill oil. Food Funct. 13, 8012–8021 (2022).
Mcdonald, D. et al. Greengenes2 unifies microbial data in a single reference tree. Nat. Biotechnol. 42, 10–20 (2023).
Brodkorb, A. et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 14, 991–1014 (2019).
Perez-Burillo, S. et al. An in vitro batch fermentation protocol for studying the contribution of food to gut microbiota composition and functionality. Nat. Protoc. 16, 3186–3209 (2021).
Xiang, S. et al. Xylitol enhances synthesis of propionate in the colon via cross-feeding of gut microbiota. Microbiome 9, 62–66 (2021).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–267 (2009).
Hou, Q. H. et al. Bacillus subtilis programs the differentiation of intestinal secretory lineages to inhibit Salmonella infection. Cell Rep. 40, 22–26 (2022).
Zhang, J. B. et al. Coculture of primary human colon monolayer with human gut bacteria. Nat. Protoc. 16, 3874–3900 (2021).
Sridharan, G. V. et al. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nat. Commun. 5, 13–18 (2014).
Dekkers, J. F. et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 14, 1756–1771 (2019).
Greer, R. L. et al. Akkermansia muciniphila mediates negative effects of IFNgamma on glucose metabolism. Nat. Commun. 7, 13329 (2016).
Collado, M. C. et al. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 73, 7767–7770 (2007).
Acknowledgements
The gastrointestinal digestion and fermentation work is supported by the National Key Research and Development Program of China (No. 2021YFD2100101, Y.Q.T.). This work is also financially by National Natural Science Foundation of China (U24A20797, X.J.Z. and H.Q.), National Natural Science Foundation of China (32125031, X.L.S.), China Postdoctoral Science Foundation (2024M761182, C.L.), Postdoctoral Fellowship Program of CPSF (No. GZB20240279, C.L.), Basic Research Program of Jiangsu (Grants No. BK20241613, C.L.), and Jiangsu Funding Program for Excellent Postdoctoral Talent (No. 2024ZB209, C.L.).
Author information
Authors and Affiliations
Contributions
Chang Liu designed and performed the experiments, analyzed the data, interpreted the results, and wrote the article. Hoi Leong Xavier Wong, Yaoqi Tian, and He Qian designed the experiments, interpreted the results, and wrote the article. Xiaojiao Zheng, Jian Ji, Xuan Zhu, and Xiaoning Liu designed the experiments and interpreted the results. Lichun Guo, He Liu, and Kun Ye interpreted the results and wrote the article. Shuang Zhang performed the experiments. Yong-jiang Xu, Xiulan Sun, and Weibiao Zhou interpreted the results. All the authors discussed and read the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chongming Wu and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, C., Zheng, X., Ji, J. et al. The carotenoid torularhodin alleviates NAFLD by promoting Akkermanisa muniniphila-mediated adenosylcobalamin metabolism. Nat Commun 16, 3338 (2025). https://doi.org/10.1038/s41467-025-58500-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58500-3