Abstract
Underrepresented populations’ participation in clinical trials remains limited, and the potential impact of genomic variants on drug metabolism remains elusive. This study aimed to assess the pharmacokinetics (PK) and pharmacogenomics (PGx) of ribociclib in self-identified Black women with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2) advanced breast cancer. LEANORA (NCT04657679) was a prospective, observational, multicenter cohort study involving 14 Black women. PK and PGx were evaluated using tandem mass spectrometry and PharmacoScan™ microarray (including CYP3A5*3, *6, and *7). CYP3A5 phenotypes varied among participants: 7 poor metabolizers (PM), 6 intermediate metabolizers (IM), and one normal metabolizer (NM). The area under the curve did not significantly differ between PMs (39,230 h*ng/mL) and IM/NMs (43,546 h*ng/mL; p = 0.38). The incidence of adverse events (AEs) was also similar. We found no association between CYP3A5 genotype and ribociclib exposure. Continued efforts are needed to include diverse populations in clinical trials to ensure equitable treatment outcomes.
Similar content being viewed by others
Introduction
Breast cancers can be subclassified based on receptors on tumor cells; these play a critical role in tumor biology and determining the optimal treatment approach for each patient1. Hormone (estrogen and/or progesterone) receptor-positive (HR+) and human epidermal growth factor receptor 2 negative (HER2-) breast cancer accounts for approximately 70% of all breast cancers1. The cornerstone of treatment for patients with endocrine-sensitive advanced HR+/HER2− breast cancer is endocrine therapy (ET), which consists of selective estrogen receptor modulators (SERM: tamoxifen), aromatase inhibitors (AIs: letrozole, anastrozole, exemestane) or selective estrogen receptor degraders (SERDs: fulvestrant)2,3. However, patients often develop resistance to these treatments, and progression of disease. Novel agents have been added to ET with the goal of improving patient outcomes.
When added to ET, the cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) palbociclib, ribociclib, and abemaciclib improve progression-free survival (PFS)4,5,6,7,8,9,10. In the first-line setting, ribociclib also led to a statistically significant improvement in overall survival (OS), when added to an AI11. These oral agents have had a profound impact on the outcomes of patients with advanced HR+/HER2− disease, improving cancer-related outcomes while maintaining a good quality of life. CDK4/6i is now the preferred first-line treatment for patients with metastatic HR+/HER2− disease.
Ribociclib is an active drug that is metabolized (into inactive metabolites) by CYP3A12. Therefore, the recommended ribociclib dose varies with CYP3A activity. Prior studies identified that ribociclib is metabolized by CYP3A, and concomitant use of strong CYP3A inhibitors increased ribociclib exposure by 3.2-fold13. The FDA recommends the use of alternative therapy or a 50% dose reduction (i.e., from 600 mg to 400 mg) if ribociclib is used with strong CYP3A inhibitors14. However, it is unknown if dose changes are needed based on variations in baseline CYP3A activity, which is constituted by CYP3A4 and CYP3A5.
Variations in the genes encoding these proteins, CYP3A4 and CYP3A5, could mimic the effect of inhibition of these enzymes. There is currently insufficient evidence regarding the potential influence of CYP3A4 and CYP3A5 genotype on ribociclib exposure15. For CYP3A4, outside of rare variants (e.g., CYP3A4*22, CYP3A4*6), there is a paucity of evidence describing the broad impacts of CYP3A4 variants on enzymatic function. In contrast, variations in CYP3A5 genotype are well established with tacrolimus dosing and pharmacokinetic properties16. In brief, patients who express CYP3A5 (i.e., intermediate or normal metabolizers) are more likely to experience subtherapeutic trough levels and require higher doses of tacrolimus. Interestingly, previous evidence, including the FDA label, suggests that African American patients require higher doses of tacrolimus than White patients16,17,18. Although race and genetic ancestry are distinct, these findings align with the fact that patients of African ancestry are likely (~85%) to be expressors (normal or intermediate metabolizers) of CYP3A516,17,19. This is contrary to patients of European ancestry where ~85% are CYP3A5 poor metabolizers (CYP3A5 non-expressors)16
CDK4/6i were approved based on large studies. However, there was limited representation of racial and ethnic minorities; for example, in the pivotal trials studying ribociclib, only 41 of 2066 (<2%) patients identified as Black6,7,8. This is primarily a concern because the lack of underrepresented patients reflects a potential continuation of disparities in care20. Secondly, it also suggests ribociclib dosing was tested in a population that predominantly lacks CYP3A5 expression (poor metabolizers), and other patient populations may require a different dose to achieve similar outcomes in safety and efficacy. Specifically, patients who self-identify as Black or African American were underrepresented in ribociclib studies and there may be differences in ribociclib metabolism and ribociclib exposure based on CYP3A5 genotype, which could lead to altered clinical outcomes. LEANORA was a prospective study that assessed the pharmacogenomics (PGx) and pharmacokinetics (PK) of ribociclib in self-identified Black patients with advanced breast cancer.
Results
Patient population
Between May 2021 and March 2024, 84 patients were reviewed; 63 patients were ineligible (e.g., not metastatic disease, EKG abnormality) or declined to participate, three participants withdrew after providing consent (i.e., participant changed their mind [n = 1], inability to obtain blood samples for analyses [n = 2]), 3 participants enrolled in the non-Hispanic White (NHW) cohort that closed to due low accrual (Supplementary figure 1). Results, including the NHW cohort, are available in Supplementary tables 1–4. Fourteen self-identified Black participants with HR+/HER2− advanced breast cancer completed the study, and their data was included in this analysis. Demographic characteristics are described in Table 1. The median age was 61.5 years, and none of the patients identified as Hispanic. The most common sites of metastatic disease were bone, soft tissue, and lung. All patients received ribociclib and ET (letrozole or fulvestrant) per standard of care. The majority (13) of the patients received letrozole, while one received fulvestrant. Three patients received concurrent ovarian suppression.
CYP3A5 genotype/phenotype
A total of 50% were CYP3A5 intermediate metabolizers (IM)/ normal metabolizers (NM) (n = 1 NM and n = 6 IM) and 50% (n = 7) poor metabolizers (PM). The genotype was CYP3A5*1/*1 for the one NM. The IM phenotype included the CYP3A5*1/*3 (n = 3), CYP3A5*1/*6 (n = 2), and CYP3A5*1/*7 (n = 1) genotypes. The PM phenotype included the CYP3A5*3/*3 (n = 4), CYP3A5*3/*6 (n = 2), and CYP3A5*3/*7 (n = 1) genotypes.
Human liver microsome analysis
Ribociclib metabolism was evaluated in human liver microsomes obtained from three individuals not from this current study, each harboring a different CYP3A5 genotype: CYP3A5*1/*1 (NM), CYP3A5*1/*3 (IM), and CYP3A5*3/*3 (no CYP3A5 function or PM). Consistent with the importance of CYP3A5 in ribociclib metabolism, the formation of major ribociclib metabolites was correlated with CYP3A5 genotype status (Fig. 1). Microsomes from a CYP3A5 NM had the greatest ribociclib metabolite formation followed by IM and PM.
Formation of major ribociclib metabolites over time was correlated with CYP3A5 genotype status. Human liver microsomes from a CYP3A5 NM (CYP3A5 *1/*1) had the greatest ribociclib metabolite formation followed by IM (CYP3A5 *1/*3) and PM (CYP3A5 *3/*3). Graphs represent three technical replicates per datapoint, error bars show standard deviation.
Pharmacokinetic and pharmacogenetic findings
The primary endpoint, area under the curve (AUCTAU), was similar between CYP3A5 PM (39,230 h*ng/mL; IQR: 18,745 to 57,566 h*ng/mL) vs. IM/NM (43,546 h*ng/mL; IQR: 35,298 to 46,647 h*ng/mL; p = 0.38). PK properties by CYP3A5 phenotype are summarized in Table 2. Similarly, there were no statistical differences in maximum concentration (Cmax) or in the time to reach Cmax suggesting that differences in CYP3A5 genotype did not impact ribociclib exposure.
Toxicity profile
Adverse events (AEs) of interest are summarized in Table 3. Overall, the toxicity profile was consistent with previous reports with ribociclib. The most common toxicities were leukopenia, nausea, vomiting, and diarrhea. The reported grade 3 toxicities included neutropenia, nausea, vomiting, transaminitis, and increased creatinine. This study was not powered to assess differences in AEs between PMs and IM/NMs. There was a similar number of all grade toxicities by CYP3A5 phenotype: 100% (7 of 7) vs. 71% (5 of 7) for IM/NM vs. PMs, respectively (p = 0.46). PMs also had a similar number of grade 3+ AEs (29%, 2/7) compared to IM/NMs (29%, 2/7) (p = 1). Table 4 shows a similar change in laboratory values (i.e., transaminases, absolute neutrophil count [ANC]) and QTc by CYP3A5 phenotype between baseline and mid-cycle and end of cycle 1. Supplementary table 3 provides data about the toxicity profile in the entire study, and Supplementary table 4 provides additional AE data related to QTc prolongation, including one case from cohort 2 where a participant experienced grade 1 QTc prolongation and was unknowingly prescribed a prohibited medication that could prolong the QTc (venlafaxine). Univariate analyses of clinical factors did not identify significant associations with ribociclib AUC (Table 5). These findings should be considered with caution due to the small sample size and because the study was not powered to assess these differences.
Exploratory candidate gene analysis
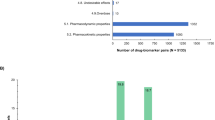
Genetic data were available for all 14 participants in the exploratory candidate gene analysis. Out of 495 variants assessed, 285 variants were identical among all participants. No further evaluation was conducted among those 285 variants. The remaining 210 variants yielded two different genotypes in 106 variants and three different genotypes in 104 variants. This exploratory analysis of 14 participants identified one variant with a p < 0.05 for ribociclib AUCTAU: CYP3A4 rs2246709 (p = 0.0041), with A/G being associated with elevated AUCTAU compared to A/A (Fig. 2A, B).
2A Genetic data were available for all 14 exploratory candidate gene analysis participants. Variants are plotted by p-value with a dotted line representing a p-value of 0.05. This exploratory analysis of 14 participants identified one variant with a p < 0.05 for ribociclib AUCTAU: CYP3A4 rs2246709 (p = 0.0041). 2B. This exploratory analysis identified an association between ribociclib area under the curve (AUC) in participants with a CYP3A4 variant (rs2246709), showing a higher AUC in those with A/G relative to those with A/A (p = 0.0041).
Discussion
In the LEANORA trial, we found no association between CYP3A5 genotype and ribociclib exposure or adverse events among self-identified Black participants. Our findings do not suggest that CYP3A5 screening is warranted to determine the safety of ribociclib. However, correlative studies of larger trials using ribociclib may provide more insight into interindividual exposure and response to this agent. This was seen in the microsome data in which metabolites from a CYP3A5 NM had the greatest ribociclib metabolite formation (inactive) followed by IM and PM, respectively.
These findings are aligned with prior population pharmacokinetics (popPK) and pharmacokinetic/pharmacodynamic (PK/PD) models21. We identified weak associations between age and increased ribociclib AUCTAU and weight and decreased AUCTAU. This small inverse correlation between body weight and ribociclib exposure is aligned with data included in the FDA review of ribociclib but is not expected to be clinically relevant22. Similar to ribociclib, palbociclib is predominantly metabolized by CYP3A15. A case report identified that CYP3A5 expression (CYP3A5*1/*3, IM) was associated with lower palbociclib plasma concentration23. James et al. conducted several studies that identified ~63% of ribociclib metabolism is produced by CYP3A24. There is significant overlap in the substrates of CYP3A4 and CYP3A5, and it is possible that both impact drug metabolism13,24. However, our findings suggest CYP3A5 genotype has a minimal role. Recombinant human enzyme studies suggest CYP3A4 is the primary enzyme responsible for ribociclib metabolism24.
It is possible that genetic variations in CYP3A4 may be associated with ribociclib pharmacokinetics, but variations in CYP3A4 are less common. One pre-identified CYP3A4 variant of interest, CYP3A4*22, was present in one participant in this trial, which limited further investigation of that specific variant. Additionally, the exploratory candidate gene analysis identified one variant of CYP3A4, rs2246709, potentially associated with ribociclib AUCTAU, which happened to be located in CYP3A4. However, this is an intronic variant with no obvious impact on enzyme expression or function. A query in the National Institutes of Health (NIH) LDLink did not identify linkage disequilibrium with other variants (highest R2 was 0.36)25. Future steps could include exploring the impact of rare variants on ribociclib exposure in larger samples or may also explore genetic associations (e.g., KCNH2, SCN5A, SNTA1) with QTc prolongation secondary to ribociclib therapy26.
This study was focused on identifying a potential association between CYP3A5 genotype and ribociclib PKs. Therefore, concomitant medications were strictly regulated. In the one case where a patient was unknowingly prescribed a prohibited medication (venlafaxine), administration of this agent was associated with an adverse event (Grade 1 QTc prolongation). The retrospective AB-ITALY study, included 173 patients treated with abemaciclib and endocrine therapy and revealed that software-predicted CDK4/6 drug interactions were an independent predictor of worse PFS27. The findings of this study are hypothesis-generating and serve as an important reminder for clinicians and pharmacists to monitor patient’s concurrent medications when prescribing a CDK4/6 inhibitor.
In terms of ribociclib dosing, the current recommended dose for patients with mBC is 600 mg daily for 21 days, followed by 7-day off treatment. There are prespecified dose reductions based on tolerance. An analysis of the MONALEESA 2, 3, and 7 studies revealed that 42% of patients required a dose reduction, which did not affect the efficacy of the drug28. The AMALEE (NCT03822468) study compared the standard 600 mg dose of ribociclib to 400 mg with the same schedule; after a median follow-up of 14 months, the study did not meet its primary endpoint of non-inferiority for the low dose based on objective response rate29. However, the study showed a favorable toxicity profile of the lower dose, particularly for neutropenia and QTc prolongation. The approval of cancer-directed therapies has traditionally been based on the maximum tolerated dose, and in recent years, there has been a shift to consider optimal biological doses. The latter is not based on toxicities but rather on other cancer-specific endpoints such as AMALEE30. We encourage researchers to rethink study endpoints to improve patient outcomes while limiting toxicity when possible.
Although no difference was found in ribociclib pharmacokinetics by CYP3A5 genotype, this trial has important implications for clinical practice. It is reassuring for patients and clinicians that the standard of care is not affected by the CYP3A5 genotype. Additionally, this trial represents the largest known cohort of ribociclib PK data in patients who self-identify as Black.
It is critical to understand the differences between race and ancestry when conducting and interpreting research. Race and ethnicity are social constructs that can be self-ascribed and based on shared physical or social qualities31. They do not necessarily reflect genetic ancestry; therefore, using race or ethnicity as proxies for genetic ancestry can be inaccurate19. Genetic ancestry refers to people in the past to whom an individual is biologically connected32,33. Genetic ancestry and genealogy can influence frequencies of genetic variants (e.g., carrier of HLA-B*15:02 or CYP3A5*1). Recommending genetic testing based on race or ethnicity remains controversial33,34,35. It is likely that race, ethnicity, and genetic ancestry all contribute to patient outcomes. This trial studied an underrepresented population based on race as well as genetic variants more prevalent among those of African ancestry.
Strengths of this trial include that it was a PK and PGx study conducted in participants with cancer and to our knowledge, this is the largest cohort with ribociclib pharmacokinetic data in Black participants. Lastly, the robust sample collection (prior to the ribociclib dose, and 0.5 h, 1 h, 2 h, 4 h, and 6 h) allows for calculation of AUC over a 24-hour period given the reported Tmax is one to four hours per the FDA label14.
Limitations of this study include limited sample size for secondary analyses (e.g., adverse events) and for the investigation of rare variants on ribociclib pharmacokinetics. Secondly, it only followed participants through the first cycle, data on long term toxicities and the impact of dose reductions in PKs are not available.
In conclusion, we found no association between CYP3A5 genotype and ribociclib exposure or adverse events through cycle 1. Ensuring diverse patient representation in clinical trials is critical results that are applicable to the population, so we can continue to improve outcomes of all patients.
Methods
Study design
LEANORA (NCT04657679) was a prospective, observational, multicenter cohort study that assessed the pharmacokinetics and pharmacogenomics of ribociclib. The trial was opened in four sites in the United States (Georgetown Lombardi Comprehensive Cancer Center, Washington DC; MedStar Washington Hospital Center, Washington DC; Tufts Medical Center, Boston, MA; MedStar Franklin Square, Baltimore MD). All participants were followed for the first cycle and prescribed ribociclib 600 mg daily (3 weeks on, 1 week off) plus letrozole 2.5 mg daily or fulvestrant 500 mg on days 1 and 15. Ovarian suppression was required for premenopausal patients, with either goserelin (3.6 mg every 28 days or 10.8 mg every 12 weeks) or leuprolide depot (3.75 mg every 28 days or 11.25 mg every 12 weeks). Dose modifications were provided for ribociclib based on adverse events; however, none of the participants had dose reductions prior to the PGx and PK studies. The institutional review boards (IRBs) approved the study at Georgetown University -and affiliated sites- (study ID: STUDY00003100) and Tufts Medical Center (study ID: STUDY00002025). Written informed consent was obtained from all participants. All procedures were in accordance with the ethical standards of the Declaration of Helsinki.
Patient population
Eligible patients included women with previously untreated HR+/HER2− metastatic or locally advanced breast cancer who self-identified as NHW or African American/Black. Documentation of estrogen receptor (ER) positive and/or progesterone receptor (PR) positive tumor (≥1% positive stained cells) based on the most recent tumor biopsy utilizing an assay consistent with local standards. HER2 negative was defined by the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines36. Patients were excluded if they were receiving other ET (given for concern for potential drug interaction or increased risk for toxicity) or if they were receiving concurrent medications that could impact CYP3A, other proteins related to ribociclib pharmacokinetics, or risk of adverse events (Supplementary tables 5-6).
Participants were considered postmenopausal if (i) they have had prior bilateral oophorectomy; (ii) age ≥ 60 years; (iii) age <60 years and have had amenorrhea for 12 or more months (in the absence of chemotherapy, tamoxifen, toremifene, or ovarian suppression) and follicle-stimulating hormone (FSH) and estradiol in the postmenopausal range per local normal ranges.
The study initially enrolled participants into two independently powered cohorts based on self-identified race: cohort 1) African American or Black participants and cohort 2) NHW participants. However, the planned cohort 2 only enrolled three patients, and due to difficulty enrolling patients, this cohort was closed. In this manuscript, we present the data about cohort 1. However, the results of the patients enrolled in cohort 2 are described in the Supplementary material.
Human liver microsome analysis
Human Liver Microsomes (20 pmol/mL CYP/reaction; 37°C, Seksui Xenotech, Kansas City, KS) were added to preincubated reaction buffer containing 0.5 M potassium phosphate buffer (pH 7.4), NADPH solutions A and B according to the manufacturer’s instructions (Corning, Corning NY), and ribociclib (Selleckchem, Houston, TX) or tacrolimus (MedChemExpress, Monmouth Junction, NJ) dissolved in acetonitrile at a final concentration of 25 µm (0.1% acetonitrile). Aliquots (100 μL) were removed from the reaction immediately after the addition of HLMs (0 min) and 60 min later. Reactions were terminated by the addition of 100 μL of ice-cold acetonitrile followed by 1 min of vortexing. Tubes were then centrifuged at maximum speed for 10 min at 4°C and harvested supernatant was stored (−20°C) until pharmacokinetic analysis. Bioanalytical measurements of ribociclib and metabolite concentrations were made using an LC-MS/MS assay described in the next section.
Pharmacokinetic and pharmacogenetic testing
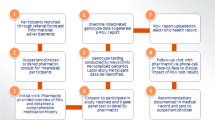
Serial blood samples were collected for PK and PGx analyses (Fig. 3). The FDA label reports that ribociclib has an elimination half-life of 32 h and a maximum plasma concentration (Cmax) of 1 to 4 h14. For sampling to occur at steady-state concentrations, the collection day was scheduled between days 8 and 16 of therapy during cycle 1. Blood samples were collected immediately prior to the ribociclib dose, and 0.5 h ± 5 min, 1 h ± 5 min, 2 h ± 15 min, 4 h ± 15 min, and 6 h ± 15 min after the daily dose of ribociclib, processed to plasma and stored at −80 °C until bioanalysis.
During screening, a swab CYP3A5 genotype was obtained to ensure adequate power. On C1D1, C1D8-16, and C2D1, blood counts and chemistries were obtained, as well as an electrocardiogram per standard of care. The list of concurrent medications was also obtained, and patients received a drug diary and patient-reported outcomes (CTCAE-PRO) questionnaires. Created with BioRender.com.
For in vitro metabolism experiments and clinical PK plasma samples, ribociclib total concentrations (i.e. protein bound+unbound) were measured using a validated and robust LC-MS/MS assay using palbociclib as an internal standard. Briefly, samples were mixed with acetonitrile containing palbociclib as an internal standard and run alongside calibration and quality control (QC) standards, ranging from 0.25 to 50 μM. The analyte (ribociclib) and internal standard were chromatographically separated using a Phenomenex Polar Omega C18 column (5 μm, 2.1 × 100 mm) on a Shimadzu Prominence HPLC system (Shimadzu, Columbia, MD). A gradient mobile phase consisting of 0.1% formic acid (aq) and 0.1% formic acid in acetonitrile was flowed at a rate of 0.4 mL/min; the injection volume was 5 μL. Following chromatographic separation and elution, the compounds were detected using tandem mass spectrometric detection in the positive ion mode. MRM transitions were m/z 435.3 → 322.2 and m/z 448.4 → 380.2 for ribociclib and palbociclib, respectively. Additionally, ribociclib metabolites were also detection and quantitated based off the parent ribociclib calibration: m/z 421.3 → 322.2 (Demethyl); m/z 407.3 → 322.2 (Di-demethyl); m/z 451.3 → 338.3 (Hydroxyl); m/z 449.3 → 336.3 (Oxidation).
A non-compartmental approach to clinical plasma PK analysis was employed using Phoenix WinNonlin v8.3 (Certara Corp, Cary, NC) that was validated per FDA 21CFR Part 11 regulations. The maximum observed plasma concentration (Cmax) and the time of Cmax (Tmax) were recorded as observed values. The area under the concentration-time curve over the first 6 h post-dose and over the dosing interval during steady-state (AUC0-6 h and AUCtau, respectively) was calculated using the linear-up/log-down trapezoidal method.
Ten milliliters of frozen whole blood was used to isolate DNA via the Wizard® Genomic DNA Purification Kit (Promega) according to manufacturer instructions. PGx data were obtained from the PharmacoScan™ (ThermoFisher) microarray per manufacturer instructions, which tests 4,627 pharmacogenetic markers in 1,191 genes. This test included the three variants known to correspond with no function (i.e., CYP3A5*3, *6, and *7). In accordance with Clinical Pharmacogenetics Consortium (CPIC) guidelines, Phenotypes assigned: poor metabolizers (PM, 2 variant alleles), intermediate metabolism (IM; 1 variant allele), NM (0 variant alleles)16.
Endpoints
The primary endpoint was ribociclib AUCtau (visit between days 8-16 of cycle 1) CYP3A5 PM and CYP3A5 IM/NM. Secondary endpoints included additional ribociclib pharmacokinetic properties and adverse events (AEs). Pharmacokinetic properties were maximum concentration (Cmax), AUC0-6 h, the time to reach Cmax (Tmax), clearance, volume of distribution(vd), and elimination half-life. Secondary safety endpoints included change in QTc interval and occurrence of neutropenia or aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevations per Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. An appearance or worsening of an undesirable sign, symptom, or medical condition after initiation of ribociclib per the CTCAE was considered an adverse event regardless of attribution to ribociclib.
Sample size calculation
Sample size accounted for CYP3A5 allelic frequencies for CYP3A5 from CPIC and PharmGKB, which were translated to phenotypes via the Hardy-Weinberg equation16. Based on known frequencies for African Americans (normal metabolism: 35%; intermediate metabolism: 48%; poor metabolism: 16%), a sample size of 18 would provide 80% power at an α = 0.05 to detect a 2-fold change in AUC (SD is 0.56) between CYP3A5 PM vs. CYP3A5 expressors (i.e., CYP3A5 IM/NM). After the enrollment of 14 participants who self-identified as African American or Black, the distribution of CYP3A5 results was a 1:1 split for CYP3A5 IM/NM vs. PM. A second sample size calculation identified this cohort would provide 86.5% power to detect a 2 vs 1 difference in AUC pending the SD is 0.56. Alpha set at 0.05. The cohort that enrolled self-identified non-Hispanic White participants was terminated prematurely due to low accrual (n = 3). A buccal swab for CYP3A5 genotyping (Kailos Genetics) was obtained during the screening period for each patient to ensure adequate sample sizes of each phenotype per cohort.
Statistical analysis
The area under the curve (AUCtau) between CYP3A5 PMs vs. IM/NMs was compared with the exact Wilcoxon rank-sum test due to small sample sizes, with alpha set to 0.05. Secondly, a multiple regression analysis was intended to identify variables associated with ribociclib AUC. Covariates to be assessed include CYP3A5 metabolism status, CYP3A4 metabolism status, renal function, liver function, age, race, weight, sex, use of “medication not recommended”, AI, number of ribociclib doses taken. However, the multiple linear regression was not conducted due to the inability to provide valid inference with the small sample size. Fisher’s exact test assessed the AEs and grade 3+ AEs to day 28. Descriptive statistics were used to characterize the data profile, frequency, and percentages for categorical variables and mean (SD) or median [IQR] for continuous variables based on the data normality. Laboratory and electrocardiographic assessments were done per the current standard of care at baseline, close to C1D15, and at the end of the first cycle14. The QT interval was corrected using the Bazett method. Analysis was performed using SAS™ software (v 9.1; Cary, NC, USA) and R Statistical Software (v4.3.1; R Core Team 2023).
Exploratory candidate gene analysis
We conducted an exploratory candidate gene analysis among measured variants in genes that were previously linked to ribociclib pharmacokinetics. The objective of this analysis was to assess if there was a strong genetic effect on ribociclib AUCTAU. A literature review in May 2024 (search terms “ribociclib AND pharmacokinetics” in PubMed) identified 80 articles. Articles were screened for content related to genes or proteins related to ribociclib metabolism. Seventeen articles met inclusion and led to the identification of 15 genes of interest: ABCB1, ABCG2, CYP1A2, CYP2C19, CYP2C9, CYP3A4, CYP3A5, FMO1, FMO3, NR1/2, PPARα, POR, SLCO1B1, SLCO1B3, SULT2A1. Genetic data were available for 14 of 15 genes, with it unavailable for PPARα. The list of variants interrogated is available in Supplementary Table 7. The association of each of the variants with ribociclib AUCTAU was evaluated by an exact Wilcoxon rank sum test or a Kruskal-Wallis test if there are two or three types of genotype within each variant, respectively. All the statistical results should be interpreted as exploratory and descriptive with two-tailed p-values unadjusted for multiple comparisons.
Data availability
Deidentified data used for this analysis are publicly available at dbGAP (https://www.ncbi.nlm.nih.gov/gap/) with data accession code phs003770.v1.p1.
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA: A Cancer J. Clin. 74, 12–49 (2024).
Mouridsen, H. et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J. Clin. Oncol. 19, 2596–2606 (2001).
Robertson, J. F. R. et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 388, 2997–3005 (2016).
Finn, R. S. et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J. Clin. Oncol. 40, LBA1003–LBA1003 (2022).
Cristofanilli, M. et al. Overall survival (OS) with palbociclib (PAL) + fulvestrant (FUL) in women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (ABC): Updated analyses from PALOMA-3. J. Clin. Oncol. 39, 1000–1000 (2021).
Lu, Y. S. et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2-advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin. Cancer Res. 28, 851–859 (2022).
Hortobagyi, G. N. et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 29, 1541–1547 (2018).
Slamon, D. J. et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann. Oncol. 32, 1015–1024 (2021).
Johnston, S. et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5, 5 (2019).
Sledge, G. W. Jr. et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35, 2875–2884 (2017).
Hortobagyi, G. N. et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N. Engl. J. Med. 386, 942–950 (2022).
Alsubi, T. A. et al. In silico and in vitro metabolism of ribociclib: a mass spectrometric approach to bioactivation pathway elucidation and metabolite profiling. RSC Adv. 10, 22668–22683 (2020).
Samant, T. S. et al. Ribociclib drug-drug interactions: clinical evaluations and physiologically-based pharmacokinetic modeling to guide drug labeling. Clin. Pharm. Ther. 108, 575–585 (2020).
Novartis. HIGHLIGHTS OF PRESCRIBING INFORMATION. These highlights do not include all the information needed to use KISQALI safely and effectively. See full prescribing information for KISQALI (2023).
Zhu, Z. & Zhu, Q. Differences in metabolic transport and resistance mechanisms of Abemaciclib, Palbociclib, and Ribociclib. Front. Pharm. 14, 1212986 (2023).
Birdwell, K. A. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin. Pharm. Ther. 98, 19–24 (2015).
Beermann, K. J., Ellis, M. J., Sudan, D. L. & Harris, M. T. Tacrolimus dose requirements in African-American and Caucasian kidney transplant recipients on mycophenolate and prednisone. Clin. Transpl. 28, 762–767 (2014).
Prograf [Package insert]. Northbrook, I. A. P. I.
Feero, W. G. et al. Guidance on use of race, ethnicity, and geographic origin as proxies for genetic ancestry groups in biomedical publications. JAMA https://doi.org/10.1001/jama.2024.3737 (2024).
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines) Breast Cancer. V 2.2024 ed. Accessed 5 July 2024. Available from https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
Lu, Y., Yang, S., Ho, Y. Y. & Ji, Y. Ribociclib Population Pharmacokinetics and Pharmacokinetic/Pharmacodynamic Analysis of Neutrophils in Cancer Patients. J. Clin. Pharm. 61, 1054–1068 (2021).
Food and Drug Administration. Center for Drug Evaluation and Research. Ribociclib Multi-discipline Review. (2017). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209092Orig1s000MultidisciplineR.pdf. Accessed 5 July 2024.
Roncato, R. et al. An integrated pharmacological counselling approach to guide decision-making in the treatment with CDK4/6 inhibitors for metastatic breast cancer. Front. Pharm. 13, 897951 (2022).
James, A. D. et al. An integrated assessment of the ADME properties of the CDK4/6 Inhibitor ribociclib utilizing preclinical in vitro, in vivo, and human ADME data. Pharm. Res Perspect. 8, e00599 (2020).
Machiela, M. J. & Chanock, S. J. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31, 3555–3557 (2015).
Santoni, M. et al. Different cardiotoxicity of palbociclib and ribociclib in breast cancer: gene expression and pharmacological data analyses, biological basis, and therapeutic implications. BioDrugs 33, 613–620 (2019).
Scagnoli, S. et al. Clinical impact of drug-drug interactions on abemaciclib in the real-world experience of AB-ITALY study. npj Breast Cancer 10, 58 (2024).
Burris, H. A. et al. Safety and impact of dose reductions on efficacy in the randomised MONALEESA-2, -3 and -7 trials in hormone receptor-positive, HER2-negative advanced breast cancer. Br. J. Cancer 125, 679–686 (2021).
Cardoso, F. et al. Abstract PD17-12: Primary efficacy and safety results from the AMALEE trial evaluating 600 mg vs 400 mg starting doses of first-line ribociclib in patients with HR+/HER2− advanced breast cancer. Cancer Res. 83, PD17-12–PD17-12 (2023).
Marshall, J. L. Maximum-tolerated dose, optimum biologic dose, or optimum clinical value: dosing determination of cancer therapies. J. Clin. Oncol. 30, 2815–2816 (2012).
Mohsen, H. Race and genetics: somber history, troubled present. Yale J. Biol. Med. 93, 215–219 (2020).
Borrell, L. N. et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N. Engl. J. Med. 384, 474–480 (2021).
Ali-Khan, S. E., Krakowski, T., Tahir, R. & Daar, A. S. The use of race, ethnicity and ancestry in human genetic research. Hugo J. 5, 47–63 (2011).
Bhopalwala, A. M., Hong, R. A., Khan, Z. R., Valentin, M. R. & Badawi, R. A. Routine screening for CYP2C19 polymorphisms for patients being treated with clopidogrel is not recommended. Hawaii J. Med. Public Health 74, 16–20 (2015).
Goodman, C. W. & Brett, A. S. Race and pharmacogenomics-personalized medicine or misguided practice? JAMA 325, 625–626 (2021).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 36, 2105–2122 (2018).
Acknowledgements
This project has been funded and/or supported in whole or in part with BCRF-20-156, Conquer Cancer-ASCO Young Investigator Award, Georgetown Lombardi Comprehensive Cancer Center - Core grant (P30CA051008), Grant # UL1TR000101 (previously UL1RR031975) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through the Clinical and Translational ScienceAwards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise. The authors thank Dr. Asma Dilawari for her help with study design and operational considerations, Hiwot Guebre-Xabiher, Terry Jeffs, Ramamohana Jonnala, Meghan Mavredes, Dionyssia Clagett, Ryan P. Brown, and Jude Uwah for operational support, Ricki Fairley for patient advocacy input. We thank all of the patients who participated in the trial and their caregivers.
Author information
Authors and Affiliations
Contributions
I.S. and D.M.S. contributed equally as co-first authors. Conception and Design: I.S., D.M.S., W.D.F., S.M.S. Provision of study materials or patients: I.S., A.C., C.G., N.A., K.W., C.M., S.T., and C.I. Collection and assembly of data: I.S., D.M.S., N.S., and S.M.S. Data analysis and interpretation: I.S., D.M.A., C.P., T.S., K.T.S., M.T., S.S., H.C.W., G.N., W.D.F., and S.M.S. Manuscript writing and final approval of manuscript: All authors. Accountable for all aspects of work all authors.
Corresponding author
Ethics declarations
Competing interests
D.M.S. reports research funding to institution from Kailos Genetics, Inc. MTT reports receiving honoria from AstraZeneca, Incyce, Otsuka, Sanofi Pasteur, consulting for American Gene Technologies, and receiving research funding to institution from Genentech. N.H.B. reports they or an immediate family member have consulted for Catena, a leadership role for Seagen, stock or ownership in Seagen, Lilly, Gilead Sciences, and Pfizer. C.G. reports consulting for Daiichi Sankyo, Illy, Biotheranostics, and Pfizer and Speakers’ Bureau for Daiichi Sankyo/UCB Japan. K.D.W. reports consults for Biotheranostics and receiving honoraria from MHJ Life Sciences. C.B.M. reports receiving research funding to institution from Pfizer and Cantex Pharmaceuticals. C.I. reports consulting for Pfizer, Novartis, Puma Biotechnology, Seagen, Ion Solutions, AstraZeneca/MedImmune, Gilead Sciences, receiving travel support from Pfizer, holding patents, royalties, or other intellectual property from McGraw Hill Publishing, UpToDate (Wolters Kluwer), Elsevier, receiving honoraria from Pfizer, receiving research funding to institution from Tesaro, Merck, Seagen, Pfizer, GlaxoSmithKline, AstraZeneca, Novartis, Genentech/Rosche, Bristol-Myers Squibb/Celgene, and other relationships with Side-Out Foundation, M.J.H./P.E.R., Curio/Vaniam Group, and Medscape. W.D.F. reports research funding to institution from Celgene, Astellas Pharma, Nerviano Medical Sciences, Pfizer, NovaRX, TRACON Pharma, Biocompatibles, and Propella Therapeutics. SM Swain reports consulting for Genetech/Roche, Daiichi Sankyo, Molecular Templates, AstraZeneca, Aventis Pharma, Jaguar Health, a leadership role at Seagen, receiving travel support from Daiichi Sankyo, Aventis Pharma, Genentech/Roche, and Chugai/Roche, stock or ownership in Seagen, receiving honoraria from Chugai/Roche, and other relationships with Roche, AstraZeneca, and Genentech/Roche. I.S., C.P., T.S., K.T.S., A.C., S.S., H.C.W., N.A., S.R.T., N.S., G.C.N., S.K.M.: Nothing to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schlam, I., Smith, D.M., Peer, C. et al. Pharmacokinetics and pharmacogenomics of ribociclib in black patients with metastatic breast cancer the LEANORA study. npj Breast Cancer 10, 84 (2024). https://doi.org/10.1038/s41523-024-00692-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-024-00692-w