Abstract
Biomolecular condensates have emerged as an important subcellular organizing principle1. Replication of many viruses, including human respiratory syncytial virus (RSV), occurs in virus-induced compartments called inclusion bodies (IBs) or viroplasm2,3. IBs of negative-strand RNA viruses were recently shown to be biomolecular condensates that form through phase separation4,5. Here we report that the steroidal alkaloid cyclopamine and its chemical analogue A3E inhibit RSV replication by disorganizing and hardening IB condensates. The actions of cyclopamine and A3E were blocked by a point mutation in the RSV transcription factor M2-1. IB disorganization occurred within minutes, which suggests that these molecules directly act on the liquid properties of the IBs. A3E and cyclopamine inhibit RSV in the lungs of infected mice and are condensate-targeting drug-like small molecules that have in vivo activity. Our data show that condensate-hardening drugs may enable the pharmacological modulation of not only many previously undruggable targets in viral replication but also transcription factors at cancer-driving super-enhancers6.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All experimental data shown in Figs. 1–3 and Extended Data Figs. 1–10 are available from the corresponding authors upon reasonable request. Sequences of newly described viruses are accessible from the GenBank under accession numbers MW039434, MT994243 and MT994242. Source data are provided with this paper.
Code availability
No new custom computer code or algorithm was used to generate the results reported in the paper.
References
Wheeler, R. J. & Hyman, A. A. Controlling compartmentalization by non-membrane-bound organelles. Phil. Trans. R. Soc. Lond. B 373, 20170193 (2018).
Wileman, T. Aggresomes and pericentriolar sites of virus assembly: cellular defense or viral design? Annu. Rev. Microbiol. 61, 149–167 (2007).
Rincheval, V. et al. Functional organization of cytoplasmic inclusion bodies in cells infected by respiratory syncytial virus. Nat. Commun. 8, 563 (2017).
Nikolic, J. et al. Negri bodies are viral factories with properties of liquid organelles. Nat. Commun. 8, 58 (2017).
Guseva, S. et al. Measles virus nucleo- and phosphoproteins form liquid-like phase-separated compartments that promote nucleocapsid assembly. Sci. Adv. 6, eaaz7095 (2020).
Sabari, B. R. et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 361, eaar3958 (2018).
Nam, H. H. & Ison, M. G. Respiratory syncytial virus infection in adults. Br. Med. J. 366, l5021 (2019).
Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case–control study. Lancet 394, 757–779 (2019).
Heylen, E., Neyts, J. & Jochmans, D. Drug candidates and model systems in respiratory syncytial virus antiviral drug discovery. Biochem. Pharmacol. 127, 1–12 (2017).
Galloux, M. et al. minimal elements required for the formation of respiratory syncytial virus cytoplasmic inclusion bodies in vivo and in vitro. mBio 11, e01202-20 (2020).
Feric, M. et al. Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697 (2016).
Richard, C. A. et al. RSV hijacks cellular protein phosphatase 1 to regulate M2-1 phosphorylation and viral transcription. PLoS Pathog. 14, e1006920 (2018).
Bailly, B. et al. Targeting human respiratory syncytial virus transcription anti-termination factor M2-1 to inhibit in vivo viral replication. Sci. Rep. 6, 25806 (2016).
Weierstall, U. et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat. Commun. 5, 3309 (2014).
Peluso, M. O. et al. Impact of the Smoothened inhibitor, IPI-926, on smoothened ciliary localization and Hedgehog pathway activity. PLoS ONE 9, e90534 (2014).
Barron, R. M. et al. PrP aggregation can be seeded by pre-formed recombinant PrP amyloid fibrils without the replication of infectious prions. Acta Neuropathol. 132, 611–624 (2016).
Wei, M. T. et al. Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat. Chem. 9, 1118–1125 (2017).
Iserman, C. et al. Genomic RNA elements drive phase separation of the SARS-CoV-2 nucleocapsid. Mol. Cell 80, 1078–1091.e6 (2020).
Rameix-Welti, M. A. et al. Visualizing the replication of respiratory syncytial virus in cells and in living mice. Nat. Commun. 5, 5104 (2014).
Blodörn, K. et al. A bovine respiratory syncytial virus model with high clinical expression in calves with specific passive immunity. BMC Vet. Res. 11, 76 (2015).
Kirsebom, F., Michalaki, C., Agueda-Oyarzabal, M. & Johansson, C. Neutrophils do not impact viral load or the peak of disease severity during RSV infection. Sci. Rep. 10, 1110 (2020).
Buchholz, U. J., Finke, S. & Conzelmann, K. K. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73, 251–259 (1999).
Blockus, S. et al. Labyrinthopeptins as virolytic inhibitors of respiratory syncytial virus cell entry. Antiviral Res. 177, 104774 (2020).
Mullor, J. L., Sánchez, P. & Ruiz i Altaba, A. Pathways and consequences: Hedgehog signaling in human disease. Trends Cell Biol. 12, 562–569 (2002).
McNally, J. G. Quantitative FRAP in analysis of molecular binding dynamics in vivo. Methods Cell Biol. 85, 329–351 (2008).
Haid, S., Grethe, C., Bankwitz, D., Grunwald, T. & Pietschmann, T. Identification of a human respiratory syncytial virus cell entry inhibitor by using a novel lentiviral pseudotype system. J. Virol. 90, 3065–3073 (2016).
Acknowledgements
We thank T. Mitchison for discussions, interpretation of the data and reading the manuscript, H. Rezaei for mouse PrP amyloid fibrils and discussions, D. Descamps and M. Bauducel for help with animal work, C. Sizun for NMR assays, J. Fix for virus amplification, G. Hansen and M. Wetzke for collection of primary RSV isolates, H. Wang for technical assistance in DNA recombination and D. Xu for logistic support and helpful advice; W.-L. (Billy) NG for reviewing the SI chemistry; staff of the MIMA2 platform for access to the IVIS200 station, which was acquired with funds from the Ile de France région (SESAME); and staff of the Cymages platform for access to the Leica SP8 microscope and Olympus FV3000 inverted confocal microscope, which was supported by grants from the region Ile-de-France. This work was supported by grants from the Natural Science Foundation of China Youth Project (31900147) to J.C., Fondation Air Liquide grant to J.-F.E. and R.A., the 111 Project (B16030), ATIP-AVENIR INSERM program and the Fondation Del Duca-Institut de France grant to J.R.-B. and M.-A.R.-W., a Sino-German Helmholtz International Lab grant (10000089395401) to Y.Z. and T.P., and People’s Livelihood Technology Project of Qingdao City (17-3-3-2-nsh) to Z.W.
Author information
Authors and Affiliations
Contributions
J.R.-B., J.C., V.R., M.G., J.-F.E., M.-A.R.-W. and R.A. designed experiments, analysed data and wrote the manuscript. R.A. initiated and designed the overall RSV drug discovery programme and designed the chemical analogues. M.-A.R.-W., J.-F.E., T.P. and R.A. reviewed the manuscript. R.A. and J.C. designed, performed and analysed the experiments, and analysed the data relative to the effects of the drugs on RSV replication and HH antagonism. H.Z., M.D. and X.Z. participated in the antiviral and HH compound testing. T.P., S.H. and S.M.S. designed, performed and analysed the inhibition assay with minimally laboratory-experienced RSV. J.R.-B., V.R., J.-F.E. and M.-A.R.-W. designed the experiments and analysed the data relative to the effects of the drugs on condensates in infected cells. J.R.-B. performed the experiments. V.R. performed experiments and provided expertise to analyse condensate disorganization and A.M.-J. provided support and expertise for the dextran micro-injection experiments. R.A., M.G. and R.L.G. designed and analysed the animal experiments. M.G., R.L.G., A.D. and F.H. infected and handled mice for animal efficacy studies. Y.Z. and Z.W. provided critical logistic and infrastructure support. Z.W., Y.Z., T.P., J.-F.E., M.-A.R.-W. and R.A. obtained funding for the study. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.A. and J.J.C. are inventors of patents 2016100874979.4 (China), 10,035,816 B2 (USA), 6588156 (Japan) and patent application 17749894.6 (EU) held by Shandong University. The other authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Synthetic route of the CPM analogues.
The detailed synthesis protocol is described in the Supplementary Methods. The IDs of the end products are A3M (A-ring, 3′-methoxy-cyclopamine; compound 4), A3E (A-ring, 3′-ethoxy-cyclopamine, compound 6) and A3P (A-ring, 3′-propoxy-cyclopamine, compound 8). The name of A3E (3′-ethoxy-cyclopamine) according to IUPAC nomenclature is 3S,3′R,3a′S,6aS,6bS,6′S,7a′R,9R,11aS,11bR-3-ethoxy-3′,6′,10,11b-tetramethyl-1,2,3,3a′,4,4′,5′,6,6a,6b,6′,7,7′,7a′,8,11,11a,11b-octadecahydro-3′H-spiro[benzo[a]fluorene-9,2′-furo[3,2-b]pyridine].
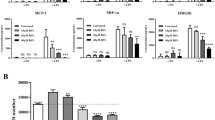
Extended Data Fig. 2 Biological activity of CPM chemical analogues in vitro.
a, c, e, g, Potency of compounds to inhibit SHH signalling was tested in Gli-dependent luciferase-reporter-expressing NIH3T3 cells. Data are expressed as the percentage ± s.d. of the DMSO control. Data are representative of two experiments performed in triplicate. b, d, f, h, Potency of compounds to inhibit RSV replication was tested in RSV–Luc-infected HEp-2 cells. Luciferase activity was measured at 24 h after infection and expressed as the percentage ± s.d. of the DMSO control. The cytotoxicity of compounds was measured in parallel in either HEp-2 or NIH3T3 reporter cells after treatment for 24 h (data not shown) or 72 h. IC50 and CC50 values were calculated with GraphPad software. Data shown are representative of two (b, f) or three (d, h) experiments performed in triplicate.
Extended Data Fig. 3 Ability of A3E and CPM to inhibit minimally laboratory-experienced RSV.
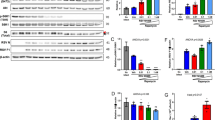
a, Inhibition of minimally laboratory-experienced RSV was tested in HEp-2 cells infected for 24 h with two RSV A or one RSV B isolates and analysed by flow cytometry as described in the Methods. The results are expressed as mean ± s.d. from three independent experiments performed in duplicate. b, c, IC50 values are calculated from three independent experiments performed in duplicate. CC50 values are from a representative experiment out of three experiments performed in triplicate. The selectivity index (SI) is calculated as SI = CC50/IC50.
Extended Data Fig. 4 Disorganization of IB and IBAG architecture in RSV–M2-1–mGFP-infected and compound-treated cells.
HEp-2 cells were infected with RSV–M2-1–mGFP for 24 h and then treated for 1 h with A3E and CPM at the indicated concentrations and stained to detect poly(A) RNA and N protein as described in the Methods. a, Representative images from two independent experiments are shown. Poly(A) RNA (red), N (white), M2-1–mGFP (green) and nuclei (blue) were visualized. A merged image of red, green and blue channels is shown (merge). Scale bars, 10 μm. b, The presence of IBAGs within IBs was quantified and indicated as IBAG (+) when visible and IBAG (−) when absent (described in the Methods) for approximately 50 infected cells per condition from two independent experiments.
Extended Data Fig. 5 RSV IBs lose velocity and the ability to fuse but remain sensitive to 1,6-hexanediol.
a, b, HEp-2 cells infected with RSV–P–BFP for 24 h were treated for 1 h with DMSO, CPM (5 μM) or A3E (25 μM) and then exposed to 10% 1,6-hexanediol (Hex), imaged at 10 min and quantified (see Methods). Data are from 10 acquisitions captured in two independent experiments. Representative images are shown in a. Scale bar, 10 μm. The mean ± s.d. number of IBs per image is expressed as the percentage of the pre-treatment control. **P < 0.01; two-tailed Wilcoxon signed-rank test. c, d, HEp-2 cells were infected with RSV–P–BFP for 24 h and treated for 1 h with DMSO, CPM (5 μM) or A3E (25 μM) and then imaged as described in the Methods. Fusion events were quantified as described in the Methods. Results are expressed as mean ± s.d. from 10 videos (15 min) from two independent experiments (c). Maximum velocities were obtained by automatic tracking of IBs from 10 videos (15 min) from two independent experiments (see Methods). Box plots show the median (centre line) and the first and third quartiles (upper and lower hinges). Statistical analysis of maximum velocities was done using Kruskal–Wallis tests followed by Dunn’s test for multiple comparisons (d). *P < 0.05; ***P < 0.001. e, HEp-2 cells were infected for 24 h with RSV–P–BFP and treated with CPM (5 μM), A3E (25 μM) or DMSO as control for 1 h. IBs shape was analysed as described in the Methods. Box plots indicate the median (centre line) and the first and third quartiles (upper and lower hinges). **P < 0.01; Welch’s ANOVA followed by a Games–Howell post hoc test.
Extended Data Fig. 6 A3E and CPM do not induce the formation of fibrillar solids but increase the mesh size of IBs.
a, HEp-2 cells infected with RSV–P–BFP for 24 h and treated with CPM (5 μM) or A3E (25 μM) for 1 h, then fixed and stained with ThS. Purified mouse PrP amyloid fibrils adsorbed on fixed cells and treated in parallel were used as positive control. IBs are shown by P–BFP fluorescence (blue) and ThS is shown in green. Representative images from two independent experiments are shown. Scale bar, 10 μm. b, c, Fluorescent dextran beads were selected based on their approximate hydrodynamic radius (Rh) and micro-injected into HEp-2 cells that were infected with RSV–P–BFP for 20–24 h and treated for 1 h with A3E (25 μM) or CPM (5 μM). Cells were imaged 2–5 min after micro-injection under a wide-field microscope. Representative images from two independent experiments are shown in c. Scale bar, 2 μm. The fluorescent dextran signal inside IBs relative to the cytoplasm was quantified as described in the Methods. Data are mean ± s.e.m. of the signal ratio from at least 25 IBs from two independent experiments. ****P < 0.0001; ns, not significant; Kruskal–Wallis test followed by Dunn’s test for multiple comparisons.
Extended Data Fig. 7 Variation in the size and shape of IBs in compound-treated RSV-infected cells.
a, b, HEp-2 cells were infected with RSV–P–BFP or RSV–M2-1(R151K)–P–BFP for 24 h and treated for 1 h with A3E (25 μM), CPM (5 μM) or DMSO at 24 h after infection. Cells were fixed, stained and analysed by confocal microscopy as described in the Methods. The size (a) and roundness (b) of IBs were quantified. Results of 50 cells from two independent experiments are shown. Box plots indicate the median (centre line) and the first and third quartiles (upper and lower hinges). Statistical significance analysis of size and roundness data was performed using Welch’s ANOVA followed by a Games–Howell test for multiple comparisons. **P < 0.01.
Extended Data Fig. 8 IBs in RSV–M2-1(R151K)–P–BFP-infected and compound-treated cells are still susceptible to hypotonic shock.
a, b, HEp-2 cells were infected for 24 h and treated with A3E (25 μM), CPM (5 μM) or DMSO for 1 h. Hypotonic shock was applied for 10 min and cells were imaged at the indicated times and the signals were quantified. Measurements labelled ‘Rec’ (recovery period) were taken after 5 min of shock followed by 5 min in culture medium. a, Representative images from two independent experiments and ten acquisitions are shown. Scale bar, 10 μm. b, Data are expressed as the mean ± s.d. number of IBs per image and compared to pre-shock values. Statistical significance analysis was performed using Kruskal–Wallis tests followed by two-sided Dunn’s test for multiple comparisons; **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 9 IB and IBAG architecture is unaffected by compound treatment in cells infected with the RSV–M2-1(R151K) mutant virus.
a, b, HEp-2 cells were infected with RSV–M2-1(R151K)–P–BFP for 24 h and then treated for 1 h with A3E (25 μM) or CPM (5 μM) followed by staining for poly(A) RNA and N protein as described in the Methods. a, Representative images from two independent experiments are shown. Poly(A) RNA, N and P–BFP are shown in red, white and blue, respectively. Merge of poly(A) RNA, N and P–BFP or poly(A) RNA and P–BFP are shown in the fifth and fourth column, respectively. Scale bar, 10 μm. b, The presence of IBAGs within IBs was quantified and scored as IBAG (+) when visible and IBAG (−) when absent from approximately 50 infected cells per condition from two independent experiments (see Methods).
Extended Data Fig. 10 Time course and treatment window for A3E and CPM in RSV–Luc-infected mice.
a, Time course of RSV–Luc infection in compound-treated mice. Mice were infected intranasally with 5 × 104 PFU ml−1 RSV–Luc and treated with the indicated compounds twice daily from 0 to 3 d.p.i. and bioluminescence readings were obtained at 3, 4, 5 and 6 d.p.i. One representative of two time-course experiments performed is shown. The mean ± s.e.m. radiance is expressed as the sum of the photons per second from each pixel inside the region of interest per the number of pixels (p s−1 cm−2 sr−1). Vehicle group, n = 10 mice; all treatment groups, n = 5 mice. b, Experimental design to determine the A3E and CPM treatment window for RSV–Luc-infected mice. Viral replication in lungs was determined by bioluminescence imaging as described in a and the Methods. Mice were infected with 5 × 104 PFU ml−1 RSV–Luc, treated with compounds at 15 mg kg−1 twice daily for the indicated time periods and imaged at 4 d.p.i. c, Results are expressed as the percentage of RSV replication compared to vehicle control and are shown as mean ± s.e.m. for a specified number of RSV–Luc-infected animals. Vehicle group, n = 27 mice, n = 4 experiments; CPM 0–3 d.p.i., n = 12 mice, n = 3 experiments; CPM 1–3 d.p.i., n = 4 mice, n = 1 experiment; CPM 2–3 d.p.i., n = 4 mice, n = 1 experiment; CPM 3 d.p.i., n = 4 mice, n = 1 experiment; A3E 0–3 d.p.i., n = 12 mice, n = 2 experiments; A3E 1–3 d.p.i., n = 4 mice, n = 1 experiment; A3E 2–3 d.p.i., n = 4 mice, n = 1 experiment; A3E 3 d.p.i., n = 4 mice, n = 1 experiment. Statistical significance analysis was performed using Kruskall–Wallis tests followed by two-sided Dunn’s test for multiple comparisons. *P < 0.05, **P < 0.01, ****P < 0.0001.
Supplementary information
Supplementary Information
This file contains details of the chemical synthesis of A3M, A3E and A3P, including a List of compounds, synthesis scheme and images of analytical data.
Supplementary Video 1
IBAG dynamics in compound-treated cells. Time-lapse microscopy of IBAGs in HEp-2 cells infected with RSV-M2-1-mGFP. At 24 hpi, images were taken every 6 s in a CO2-controlled chamber heated at 37 °C, with an Olympus FV3000 inverted confocal microscope. After five minutes, cells were treated with DMSO (mock, Supplementary Video 1), 5 μM CPM (Supplementary Video 2) or 25 μM A3E (Supplementary Video 3) and subsequently images were taken for another 20 min. The resulting videos were visualized using Image J software (10 fps). A representative video from three independent experiments in which 3 videos were acquired is shown. Scale bar 10 μm.
Supplementary Video 2
IBAG dynamics in compound-treated cells. Cells were treated with 5 μM CPM; see legend for Supplementary Video 1.
Supplementary Video 3
IBAG dynamics in compound-treated cells. Cells were treated with 25 μM A3E; see legend for Supplementary Video 1.
Supplementary Video 4
IB dynamics. Time-lapse microscopy of IBs in HEp-2 cells infected with RSV-P-BFP. At 24 hpi, cells were imaged every 30 s in a CO2-controlled chamber heated at 37 °C, with an Olympus FV3000 inverted confocal microscope. The resulting videos were visualized under the Image J software (10 pfs). A representative video from 10 videos from 2 independent experiments is shown. Scale bar 10 μm.
Supplementary Video 5
IB dynamics and mobility. Time-lapse microscopy of IBs in HEp-2 cells infected with RSV-P-BFP. At 24 hpi, cells were treated for 1 h with DMSO (mock, Supplementary Video 5), 5 μM CPM (Supplementary Video 6) or 25 μM A3E (Supplementary Video 7) and subsequently imaged every 30s for 15 min in a CO2-controlled chamber heated at 37 °C, with an Olympus FV3000 inverted confocal microscope. The resulting videos were visualized under Image J and ICY software (10 pfs). A representative video from 10 videos from 2 independent experiments is shown. Scale bar 10 μm.
Supplementary Video 6
IB dynamics and mobility. Cells were treated with 5 μM CPM; see legend for Supplementary Video 5.
Supplementary Video 7
IB dynamics and mobility. Cells were treated with 25 μM A3E; see legend for Supplementary Video 5.
Source data
Rights and permissions
About this article
Cite this article
Risso-Ballester, J., Galloux, M., Cao, J. et al. A condensate-hardening drug blocks RSV replication in vivo. Nature 595, 596–599 (2021). https://doi.org/10.1038/s41586-021-03703-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03703-z
This article is cited by
-
Peptides targeting RAB11A–FIP2 complex inhibit HPIV3, RSV, and IAV replication as broad-spectrum antivirals
Cell & Bioscience (2025)
-
Current perspectives in drug targeting intrinsically disordered proteins and biomolecular condensates
BMC Biology (2025)
-
Emerging regulatory mechanisms and functions of biomolecular condensates: implications for therapeutic targets
Signal Transduction and Targeted Therapy (2025)
-
Functional specificity in biomolecular condensates revealed by genetic complementation
Nature Reviews Genetics (2025)
-
Rational design of phytovirucide inhibiting nucleocapsid protein aggregation in tomato spotted wilt virus
Nature Communications (2025)