Abstract
Germline BRCA1 mutation carriers face a high breast cancer risk; however, the underlying mechanisms for this risk are not completely understood. Using a new genetically engineered mouse model of germline Brca1 heterozygosity, we demonstrate that early tumor onset in a Brca1 heterozygous background cannot be fully explained by the conventional ‘two-hit’ hypothesis, suggesting the existence of inherent tumor-promoting alterations in the Brca1 heterozygous state. Single-cell RNA sequencing and assay for transposase-accessible chromatin with sequencing analyses uncover a unique set of differentially accessible chromatin regions in ostensibly normal Brca1 heterozygous mammary epithelial cells, distinct from wild-type cells and partially mimicking the chromatin and RNA-level changes in tumor cells. Transcription factor analyses identify loss of ELF5 and gain of AP-1 sites in these epigenetically primed regions; in vivo experiments further implicate AP-1 and Wnt10a as strong promoters of Brca1-related breast cancer. These findings reveal a previously unappreciated epigenetic effect of Brca1 haploinsufficiency in accelerating tumorigenesis, advancing our mechanistic understanding and informing potential therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The scRNA-seq and ATAC–seq data generated in this study have been deposited in the GEO under accession no. GSE247454 and are available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE247454. The microarray and RNA-seq gene expression data of the human breast tumor samples were obtained from METABRIC and The Cancer Genome Atlas via the cBioPortal (https://www.cbioportal.org/), whereas the gene expression data of the human breast cancer cell lines were obtained from DepMap (https://depmap.org/portal/). Source data are provided with this paper.
Code availability
No new custom code was developed in this study. All data were processed and analyzed using existing code and software as detailed in the Methods section.
Change history
20 November 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41588-024-02034-9
References
Kuchenbaecker, K. B. et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317, 2402–2416 (2017).
Foulkes, W. D. et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J. Natl Cancer Inst. 95, 1482–1485 (2003).
Sessa, C. et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann. Oncol. 34, 33–47 (2023).
Domchek, S. M. Risk-reducing mastectomy in BRCA1 and BRCA2 mutation carriers: a complex discussion. JAMA 321, 27 (2019).
Couch, F. J., Nathanson, K. L. & Offit, K. Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science 343, 1466–1470 (2014).
Knudson, A. G. Mutation and cancer: statistical study of retinoblastoma. Proc. Natl Acad. Sci. USA 68, 820–823 (1971).
Konishi, H. et al. Mutation of a single allele of the cancer susceptibility gene BRCA1 leads to genomic instability in human breast epithelial cells. Proc. Natl Acad. Sci. USA 108, 17773–17778 (2011).
Pathania, S. et al. BRCA1 haploinsufficiency for replication stress suppression in primary cells. Nat. Commun. 5, 5496 (2014).
Sedic, M. et al. Haploinsufficiency for BRCA1 leads to cell-type-specific genomic instability and premature senescence. Nat. Commun. 6, 7505 (2015).
Lim, E. et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 15, 907–913 (2009).
Sau, A. et al. Persistent activation of NF-κB in BRCA1-deficient mammary progenitors drives aberrant proliferation and accumulation of DNA damage. Cell Stem Cell 19, 52–65 (2016).
Proia, T. A. et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell 8, 149–163 (2011).
Molyneux, G. et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell 7, 403–417 (2010).
Annunziato, S. et al. Comparative oncogenomics identifies combinations of driver genes and drug targets in BRCA1-mutated breast cancer. Nat. Commun. 10, 397 (2019).
Wang, H. et al. Inadequate DNA damage repair promotes mammary transdifferentiation, leading to BRCA1 breast cancer. Cell 178, 135–151 (2019).
Bach, K. et al. Time-resolved single-cell analysis of Brca1 associated mammary tumourigenesis reveals aberrant differentiation of luminal progenitors. Nat. Commun. 12, 1502–1511 (2021).
Nee, K. et al. Preneoplastic stromal cells promote BRCA1-mediated breast tumorigenesis. Nat. Genet. 55, 595–606 (2023).
Caputo, A. et al. Spatial transcriptomics suggests that alterations occur in the preneoplastic breast microenvironment of BRCA1/2 mutation carriers. Mol. Cancer Res. 22, 169–180 (2024).
Pal, B. et al. A single‐cell RNA expression atlas of normal, preneoplastic and tumorigenic states in the human breast. EMBO J. 40, e107333 (2021).
Gray, G. K. et al. A human breast atlas integrating single-cell proteomics and transcriptomics. Dev. Cell 57, 1400–1420 (2022).
Reed, A. D. et al. A single-cell atlas enables mapping of homeostatic cellular shifts in the adult human breast. Nat. Genet. 56, 652–662 (2024).
Liu, X. et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc. Natl Acad. Sci. USA 104, 12111–12116 (2007).
Holstege, H. et al. High incidence of protein-truncating TP53 mutations in BRCA1-related breast cancer. Cancer Res. 69, 3625–3633 (2009).
Manié, E. et al. High frequency of TP53 mutation in BRCA1 and sporadic basal-like carcinomas but not in BRCA1 luminal breast tumors. Cancer Res. 69, 663–671 (2009).
Saal, L. H. et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat. Genet. 40, 102–107 (2008).
Martins, F. C. et al. Evolutionary pathways in BRCA1-associated breast tumors. Cancer Discov. 2, 503–511 (2012).
Palacios, J. et al. Phenotypic characterization of BRCA1 and BRCA2 tumors based in a tissue microarray study with 37 immunohistochemical markers. Breast Cancer Res. Treat. 90, 5–14 (2005).
Lakhani, S. R. et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin. Cancer Res. 11, 5175–5180 (2005).
Bodily, W. R. et al. Effects of germline and somatic events in candidate BRCA-like genes on breast-tumor signatures. PLoS ONE 15, e0239197 (2020).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Dravis, C. et al. Epigenetic and transcriptomic profiling of mammary gland development and tumor models disclose regulators of cell state plasticity. Cancer Cell 34, 466–482 (2018).
Chung, C.-Y. et al. Single-cell chromatin analysis of mammary gland development reveals cell-state transcriptional regulators and lineage relationships. Cell Rep. 29, 495–510 (2019).
Zheng, R. et al. Cistrome Data Browser: expanded datasets and new tools for gene regulatory analysis. Nucleic Acids Res. 47, D729–D735 (2019).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Zhou, J. et al. Elf5 is essential for early embryogenesis and mammary gland development during pregnancy and lactation. EMBO J. 24, 635–644 (2005).
Oakes, S. R. et al. The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Genes Dev. 22, 581–586 (2008).
Choi, Y. S., Chakrabarti, R., Escamilla-Hernandez, R. & Sinha, S. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev. Biol. 329, 227–241 (2009).
Chakrabarti, R. et al. Elf5 inhibits the epithelial–mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nat. Cell Biol. 14, 1212–1222 (2012).
Singh, S. et al. Loss of ELF5–FBXW7 stabilizes IFNGR1 to promote the growth and metastasis of triple-negative breast cancer through interferon-γ signalling. Nat. Cell Biol. 22, 591–602 (2020).
Chakrabarti, R. et al. Elf5 regulates mammary gland stem/progenitor cell fate by influencing notch signaling. Stem Cells 30, 1496–1508 (2012).
Baron, M. et al. The stress-like cancer cell state is a consistent component of tumorigenesis. Cell Syst. 11, 536–546 (2020).
Tam, W. L. et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell 24, 347–364 (2013).
Zhao, C. et al. Genome-wide profiling of AP-1-regulated transcription provides insights into the invasiveness of triple-negative breast cancer. Cancer Res. 74, 3983–3994 (2014).
Xie, X. et al. c-Jun N-terminal kinase promotes stem cell phenotype in triple-negative breast cancer through upregulation of Notch1 via activation of c-Jun. Oncogene 36, 2599–2608 (2017).
Comandante-Lou, N., Baumann, D. G. & Fallahi-Sichani, M. AP-1 transcription factor network explains diverse patterns of cellular plasticity in melanoma cells. Cell Rep. 40, 111147 (2022).
Bejjani, F., Evanno, E., Zibara, K., Piechaczyk, M. & Jariel-Encontre, I. The AP-1 transcriptional complex: local switch or remote command? Biochim. Biophys. Acta Rev. Cancer 1872, 11–23 (2019).
Casalino, L., Talotta, F., Cimmino, A. & Verde, P. The Fra-1/AP-1 oncoprotein: from the ‘undruggable’ transcription factor to therapeutic targeting. Cancers 14, 1480 (2022).
Liu, J. et al. The oncogene c-Jun impedes somatic cell reprogramming. Nat. Cell Biol. 17, 856–867 (2015).
Kendrick, H. et al. Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics 9, 591 (2008).
Oliphant, M. U. J., Akshinthala, D. & Muthuswamy, S. K. Establishing conditions for the generation and maintenance of estrogen receptor-positive organoid models of breast cancer. Breast Cancer Res. 26, 56 (2024).
Yue, F. et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 515, 355–364 (2014).
He, X., Ohba, S., Hojo, H. & McMahon, A. P. AP-1 family members act with Sox9 to promote chondrocyte hypertrophy. Development 143, 3012–3023 (2016).
Link, V. M. et al. Analysis of genetically diverse macrophages reveals local and ___domain-wide mechanisms that control transcription factor binding and function. Cell 173, 1796–1809 (2018).
Yoon, H. et al. p27 transcriptionally coregulates cJun to drive programs of tumor progression. Proc. Natl Acad. Sci. USA 116, 7005–7014 (2019).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576 (2017).
Xu, X. et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat. Genet. 22, 37–43 (1999).
Shakya, R. et al. The basal-like mammary carcinomas induced by Brca1 or Bard1 inactivation implicate the BRCA1/BARD1 heterodimer in tumor suppression. Proc. Natl Acad. Sci. USA 105, 7040–7045 (2008).
McPherson, J. P. et al. Collaboration of Brca1 and Chk2 in tumorigenesis. Genes Dev. 18, 1144–1153 (2004).
McCarthy, A. et al. A mouse model of basal-like breast carcinoma with metaplastic elements. J. Pathol. 211, 389–398 (2007).
Drost, R. et al. BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J. Clin. Invest. 126, 2903–2918 (2016).
Hanasoge Somasundara, A. V. et al. Parity-induced changes to mammary epithelial cells control NKT cell expansion and mammary oncogenesis. Cell Rep. 37, 110099 (2021).
Zhu, Y., Ghosh, P., Charnay, P., Burns, D. K. & Parada, L. F. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science 296, 920–922 (2002).
Yang, F.-C. et al. Nf1-dependent tumors require a microenvironment containing Nf1+/−- and c-kit-dependent bone marrow. Cell 135, 437–448 (2008).
Voutilainen, M. et al. Ectodysplasin regulates hormone-independent mammary ductal morphogenesis via NF-κB. Proc. Natl Acad. Sci. USA 109, 5744–5749 (2012).
Chakrabarti, R. et al. Notch ligand Dll1 mediates cross-talk between mammary stem cells and the macrophageal niche. Science 360, eaan4153 (2018).
Wu, W. et al. Drivers and suppressors of triple-negative breast cancer. Proc. Natl Acad. Sci. USA 118, e2104162118 (2021).
Lane, T. F. & Leder, P. Wnt-10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene 15, 2133–2144 (1997).
Domchek, S. M. & Vonderheide, R. H. Advancing cancer interception. Cancer Discov. 14, 600–604 (2024).
Hu, Y.-F., Hao, Z. L. & Li, R. Chromatin remodeling and activation of chromosomal DNA replication by an acidic transcriptional activation ___domain from BRCA1. Genes Dev. 13, 637–642 (1999).
Ye, Q. et al. BRCA1-induced large-scale chromatin unfolding and allele-specific effects of cancer-predisposing mutations. J. Cell Biol. 155, 911–921 (2001).
Neish, A. S., Anderson, S. F., Schlegel, B. P., Wei, W. & Parvin, J. D. Factors associated with the mammalian RNA polymerase II holoenzyme. Nucleic Acids Res. 26, 847–853 (1998).
Bochar, D. A. et al. BRCA1 is associated with a human SWI/SNF-related complex linking chromatin remodeling to breast cancer. Cell 102, 257–265 (2000).
Hill, D. A., de la Serna, I. L., Veal, T. M. & Imbalzano, A. N. BRCA1 interacts with dominant negative SWI/SNF enzymes without affecting homologous recombination or radiation-induced gene activation of p21 or Mdm2. J. Cell. Biochem. 91, 987–998 (2004).
Harte, M. T. et al. BRD7, a subunit of SWI/SNF complexes, binds directly to BRCA1 and regulates BRCA1-dependent transcription. Cancer Res. 70, 2538–2547 (2010).
Dos Santos, C. O., Dolzhenko, E., Hodges, E., Smith, A. D. & Hannon, G. J. An epigenetic memory of pregnancy in the mouse mammary gland. Cell Rep. 11, 1102–1109 (2015).
Feigman, M. J. et al. Pregnancy reprograms the epigenome of mammary epithelial cells and blocks the development of premalignant lesions. Nat. Commun. 11, 2649 (2020).
Slepicka, P. F., Cyrill, S. L. & Dos Santos, C. O. Pregnancy and breast cancer: pathways to understand risk and prevention. Trends Mol. Med. 25, 866–881 (2019).
Huh, S. J. et al. Age- and pregnancy-associated DNA methylation changes in mammary epithelial cells. Stem Cell Rep. 4, 297–311 (2015).
Pal, B. et al. Global changes in the mammary epigenome are induced by hormonal cues and coordinated by Ezh2. Cell Rep. 3, 411–426 (2013).
Lagarde, C. B. et al. Obesity-associated epigenetic alterations and the obesity-breast cancer axis. Oncogene 43, 763–775 (2024).
Bhardwaj, P. et al. Obesity promotes breast epithelium DNA damage in women carrying a germline mutation in BRCA1 or BRCA2. Sci. Transl. Med. 15, eade1857 (2023).
LaBarge, M. A., Mora-Blanco, E. L., Samson, S. & Miyano, M. Breast cancer beyond the age of mutation. Gerontology 62, 434–442 (2016).
Angarola, B. L. et al. Comprehensive single cell aging atlas of mammary tissues reveals shared epigenomic and transcriptomic signatures of aging and cancer. Preprint at bioRxiv https://doi.org/10.1101/2023.10.20.563147 (2023).
Shalabi, S. F. et al. Evidence for accelerated aging in mammary epithelia of women carrying germline BRCA1 or BRCA2 mutations. Nat. Aging 1, 838–849 (2021).
Song, M.-A. et al. Landscape of genome-wide age-related DNA methylation in breast tissue. Oncotarget 8, 114648–114662 (2017).
Platt, R. J. et al. CRISPR–Cas9 knockin mice for genome editing and cancer modeling. Cell 159, 440–455 (2014).
Xue, W. et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 514, 380–384 (2014).
Sánchez-Rivera, F. J. et al. Rapid modeling of cooperating genetic events in cancer through somatic genome editing. Nature 516, 428–431 (2014).
Li, C. M. et al. Aging-associated alterations in mammary epithelia and stroma revealed by single-cell RNA sequencing. Cell Rep. 33, 108566 (2020).
Butler, A., Hoffman, P., Smibert, P. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Corces, M. R. et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods 14, 959–962 (2017).
Acknowledgements
We thank J. Jonkers (Netherlands Cancer Institute) and the late D. Livingston (Dana-Farber Cancer Institute) for assistance in generating the GEMMs in this study. We thank the Harvard Center for Comparative Medicine and the Rodent Pathology Core for assistance with the mouse work, R. Bronson (Harvard Medical School) for histopathology consultation and the Dana-Farber Flow Cytometry Core for the FACS service. We thank members of the Aviv Regev laboratory, including D. Dionne, L. Nguyen, J. Waldman, M. Cuoco, M. Slyper, C. Rodman and O. Rozenblatt-Rosen (Broad Institute), and members of the Harvard Bauer Core, including C. Bailey Hartmann, N. El-Ali, C. Gerhardinger and C. Daly for assistance with scRNA-seq. We also thank Active Motif (including P. Labhart, W. Doyle, J. Lopez and C. Henry) and M. Brown, H. Long, X. Qiu and A. Sankar (Dana-Farber Cancer Institute) for assistance with the ATAC–seq and TF analyses. We thank D. Akshinthala in the Senthil Muthuswamy laboratory (Beth Israel Deaconess Medical Center) for sharing the organoid culture medium and A. Li, H. Wang, Q. Kong and B. Liu in the David Livingston laboratory (Dana-Farber Cancer Institute) for sharing reagents. We also thank all members of the Brugge laboratory, especially H. J. Kuiken, A. Martinez-Gakidis and K. Gray, as well as M. Bao (Harvard Medical School) for providing technical assistance and feedback on this manuscript. This work was supported in part by a Susan G. Komen Postdoctoral Fellowship (C.M.L.), a Croucher Postdoctoral Fellowship (C.M.L.), a Gray Foundation gift (J.S.B.), a Goldberg Family Research Fund gift (J.S.B.), a Komen Scholar Award no. SAC180002 (J.S.B.) and a National Cancer Institute grant no. R35 CA242428 (J.S.B.).
Author information
Authors and Affiliations
Contributions
J.S.B. provided guidance and funding for the project. C.M.L. conceived the project, designed the experiments, analyzed the data and interpreted the results. The experiments and data analyses were conducted by C.M.L. with assistance from A.C., M.U.J.O., M.T., J.J.Z., H.S., C.T., K.P.G. and S.P., and support from J.S.B. and S.K.M., except for the analysis of the tissue immunofluorescence staining, which was performed by N.G. The scRNA-seq analysis was carried out by C.M.L. with assistance from the laboratory of A.R. The ATAC–seq analyses were carried out by C.M.L. and S.A.Q. with guidance and support from C.K. The computational analyses were carried out by C.M.L. with input from L.M.S., F.S. and G.R. The manuscript was prepared by C.M.L. and J.S.B. with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
A.R. is a cofounder of and equity holder in Celsius Therapeutics and an equity holder in Immunitas; she was a scientific advisory board member for Thermo Fisher Scientific, Syros Pharmaceuticals, Neogene Therapeutics and Asimov until 31 July 2020. A.R. has been an employee of Genentech (a member of the Roche Group) since August 2020 and holds equity in Roche. None of these affiliations represent competing interests regarding the present study. The other authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of WT and Brca1-HET GEMMs of breast cancer.
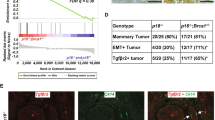
a, Representative immunofluorescence images of mammary glands of the Brca1/p53 (BP) mouse model at 4, 8, 12, and 16 weeks after intraductal Lenti-Cre injection, showing a mosaic distribution of GFP+ cell puncta throughout the glands, as marked by 1-6. LN, lymph node. Scale bars = 500 μm in the overview images and 50 μm in the insets. Results are representative of two experiments. b, Representative immunofluorescence images (top) and quantification (mean ± s.e.m.) (bottom) of GFP+ cells in the luminal (KRT8 + ) layer versus the basal (SMA + ) layer. A total of 1,997 luminal cells and 712 basal cells across 37 regions of interest (ROI) from n = 5 mice were analyzed. Only ROIs containing GFP+ cells were examined. P-value (two-sided) was determined by Fisher’s exact test. Scale bar = 50 μm. c, Genotyping analysis of Brca1 floxed and null alleles in cultured mammary epithelial cells derived from WT and HET mice and infected with a lentiviral vector expressing the Cre recombinase. Cre-infected, GFP-positive cells were isolated by FACS and subjected to DNA purification and genotyping PCR. Each time point is an independent experiment. The gel image is representative of two genotyping PCR analyses. d, Size of tumors (mean ± s.e.m.) detected in WT and Brca1-HET GEMMs in the Brca1/p53 (BP) and Brca1/p53/Pten (BPP) models. (BP, n = 4 WT and 17 HET; BPP, n = 7 WT and 15 HET; only mice with at least three longitudinal measurements are included.). e, Representative H&E and IHC staining showing basal-like features of BP tumors developed in WT and Brca1-HET GEMMs. Scale bars = 100 μm. Insets show ER+ and PR+ normal glands embedded in neighboring ER/PR- tumor cells. f, Quantification (mean ± s.e.m.) of IHC stained area using basal marker KRT14 and luminal marker KRT8/18 in BP tumors as determined by a pathologist. P-values were determined by two-sided unpaired t-test.
Extended Data Fig. 2 scRNA-seq analysis of normal mammary tissues and mammary tumors from WT and Brca1-HET GEMMs.
a, UMAP of scRNA-seq data labeled by sample. b, Heatmap showing levels of differentially expressed genes in BPP tumors in WT versus HET GEMMs at a lenient cutoff of 1.3-fold (FDR < 0.05). All genes show only subtle fold change and/or wide variations across biological replicates. c, Relative abundance (mean ± s.e.m.) of epithelial, stromal, and immune cell types in normal mammary tissues at young age (3-4 months; n = 3 WT and 3 HET) and older age (13-14 months; n = 4 WT and 4 HET) as well as in BPP tumors in WT GEMMs (n = 3 tumors) and Brca1-HET GEMMs (n = 4 tumors). P-values were determined by two-way ANOVA with Bonferroni correction for multiple comparisons. Potential contamination from partially resected lymph nodes in young Brca1-HET samples precluded meaningful analysis of immune cell types in this age group (indicated in gray). d, Venn diagrams showing differentially expressed genes (FC > 1.3-fold, FDR < 0.05) in each Brca1-HET sample compared to all WT samples within the indicated cell types, either in young tissues (3-4 months; n = 3 HET and 3 WT), aged tissues (13-14 months; n = 4 HET and 4 WT), or BPP tumors (n = 3 tumors in WT mice and n = 4 tumors in HET mice). Only cell types with sufficient representation ( > 10 cells per sample) are shown; samples with insufficient cell representation or potential contamination from partially resected lymph nodes in the young Brca1-HET samples were not analyzed (indicated by an x). e, Violin plots showing the expression levels of genes identified in (d) as differentially expressed between WT and Brca1-HET vascular endothelial cells in young mice (3-4 months; n = 3 WT and 3 HET) or between T cells in tumors of WT (n = 3) and Brca1-HET (n = 4) GEMMs. Most of these genes, except for the non-coding RNA (ncRNA) BC018473, showed no striking differential expression across genotypes on a per-sample basis.
Extended Data Fig. 3 Stromal and epithelial cell-cell communications inferred by CellPhoneDB.
Potential interactions between stromal cells and AV cells in normal mammary tissues of (a) young mice (3-4 months; n = 3 WT and 3 HET mice) and (b) aged mice (13-14 months; n = 4 WT and 4 HET mice), as well as (c) interactions between stromal cells and tumor epithelial cells in mammary tumors from WT GEMMs (n = 3 tumors) and Brca1-HET GEMMs (n = 3 tumors) based on CellPhoneDB analyses of ligand-receptor pairs in scRNA-seq data. To investigate as many stromal populations as possible, any stromal cell types with at least 5 cells per sample within a given cohort condition were analyzed. Of note, a fourth HET tumor sample, tuHETc, was excluded in the CellPhoneDB analysis due to low cell yield in order to allow more stromal cell types to pass the filtering criteria of at least 5 cells per sample and be included in this analysis.
Extended Data Fig. 4 ATAC-seq analysis of AV cells and tumor cells.
a, Differentially accessible chromatin regions identified between Gp1-WT-like AV cells and Gp2-HET AV cells (top, more open in Gp2 than Gp1, Gp1 < Gp2; bottom, more closed in Gp2 than Gp1, Gp1 > Gp2) are further categorized by differential accessibility between Gp2-HET AV cells and Gp3-tumor cells. ‘Progressively open’ regions (that is Gp1 < Gp2 < Gp3 and Gp1 < Gp2 ~ Gp3) and ‘progressively closed’ regions (that is Gp1 > Gp2 > Gp3 and Gp1 > Gp2 ~ Gp3) are highlighted. b, Stacked bar graph of distance to transcription start site (TSS) for progressively altered chromatin regions identified between Gp1 vs Gp2 vs Gp3 cells. c, Proportion of genes associated with the indicated differentially accessible chromatin regions in ATAC-seq (that is genes with coding regions located within 10 kb or those with the nearest TSS) that exhibit a higher, lower, or not significantly different expression level in tumor cells relative to AV cells by scRNA-seq. In both groups (Gp1 < Gp2 and Gp1 > Gp2), and for both gene association approaches (within 10 kb or nearest TSS), p = 4.89E-36 (Fisher’s exact test). d, Percent of genes associated with the indicated open or closed chromatin regions in ATAC-seq (that is genes with coding regions located within 10 kb or those with the nearest TSS) that exhibit a higher or lower expression level in tumor cells relative to AV cells in scRNA-seq analysis, respectively. For Tumor > AV (Gp1 < Gp2 vs Gp1 < Gp2 < /~Gp3), p = 1.10E-06 (within 10 kb) or 3.38E-05 (nearest TSS); for Tumor < AV (Gp1 > Gp2 vs Gp1 > Gp2 > /~Gp3), p = 8.30E-05 (within 10 kb) or 1.92E-05 (nearest TSS) (Fisher’s exact test, two-sided).
Extended Data Fig. 5 ELF5 and AP-1 are associated with progressively closed and open chromatin regions.
a, Quantification (mean ± s.e.m.) of ELF5 IHC staining of mammary glands of WT (n = 7) and HET (n = 6) GEMMs. b, Quantification (mean ± s.e.m.) of JUN IHC staining of mammary glands of WT (n = 4) and HET (n = 3) GEMMs. c, Representative IHC staining of total JUN (as shown in Fig. 4d) and pSer73 JUN in normal mammary gland, hyperplasia, and tumor tissues. Scale bars = 100 μm. Results are representative of n = 3 WT and n = 3 HET GEMMs. d, Immunoblot of a GFP-sorted Cre-reporter murine cell line after infection with lentivirus expressing Cre alone (–), or co-expressing the dominant-negative mutant of Jun (Jun-DN), or a non-expressed version where the coding sequence of Jun-DN is missing an ATG start codon (Ctrl). Endogenous expression of Jun is partially inhibited by Jun-DN, but not by the non-expressed control, as a result of a negative feedback loop. HSP90 serves as loading control in each respective blot. Results are representative of two experiments. e, Schematic illustrating that increased chromatin accessibility in Brca1-HET AV cells allows higher sensitivity to JUN-mediated activation of target genes.
Extended Data Fig. 6 Wnt10a is epigenetically altered in Brca1-HET AV cells and is associated with AP-1 and basal differentiation state.
a, Degree of conservation in sequence identity and span between the mouse (mm10) and human (hg38) genomes for the chromatin regions and genes that showed progressively increased accessibility from Gp1 to Gp2 to Gp3 cells and higher expression levels in tumor cells compared to AV cells, respectively. b, Spearman analysis showing genes with significant correlation (two-sided p < 0.05) with Wnt10a expression at a single-cell level within the tumor epithelial cell population in scRNA-seq data. Positively correlated genes in the AP-1 family, Wnt pathway, or the basal differentiation markers are highlighted. c, Violin plot showing expression levels of Wnt10a across the major mammary epithelial cell types and tumor cells in scRNA-seq data. (Abbreviations: AV, alveolar cells; HS, hormone-sensing cells; BA, basal myoepithelial cells).
Extended Data Fig. 7 Characterization of AV and tumor organoid cultures.
a, Left, Pearson correlation (r2 and two-sided p-value) of ATAC-seq profiles of uncultured AV cells versus their derivative organoid culture. Right, chromatin accessibility tracks of representative genes. b, Representative IHC staining of ELF5, JUN, and pSer73-JUN in organoid cultures of normal AV cells and tumor cells. Scale bars = 200 μm. Results are representative of two experiments. c, Bar graphs (mean ± s.e.m.) showing RT-qPCR quantification of expression levels of Wnt10a and Wnt target genes, Axin2 and Tcf7, in organoid cultures of AV cells (n = 3 WT, 7 HET) and BPP tumor cells (n = 2). Each dot represents an independent organoid line, with its gene expression level averaged across 2-8 independent technical replicates. d, Bar graphs (mean ± s.e.m.) showing RT-qPCR quantification of expression levels of dominant-negative mutant Jun-DN upon doxycycline induction (2 μg/mL) in Brca1-HET AV organoids and the corresponding levels of Wnt10a and other Gp2-associated genes. P-values were determined by two-sided unpaired t-test (n = 3 technical replicates). Results are representative of two independent experiments. Similar plots for Jun-DN and Wnt10a are shown Fig. 5f and are included here for completeness. e, Bar graphs (mean ± s.e.m.) showing the effects of doxycycline-induced (0, 0.02, 0.2, and 2 μg/mL) expression of Jun on the levels of Wnt10a and other Gp2-associated genes in AV organoids as measured by RT-qPCR. P-values were determined by two-sided unpaired t-test (n = 3 technical replicates). Results are representative of three independent experiments. Similar plots for Jun and Wnt10a are shown Fig. 5e and are included here for completeness. f, Bar graphs (mean ± s.e.m.) showing RT-qPCR quantification of expression levels of dominant-negative mutant Jun-DN upon doxycycline induction (2 μg/mL) in tumor organoids and the corresponding levels of Wnt10a and other Gp2-associated genes. P-values were determined by two-sided unpaired t-test (n = 3 technical replicates). Results are representative of two independent experiments.
Extended Data Fig. 8 Wnt10a overexpression experiments in vitro and in vivo.
a, Bar graphs (mean ± s.e.m.) showing RT-qPCR quantification of expression levels of Wnt10a and canonical Wnt target genes Axin2 and Tcf7 in AV organoid cultures with or without overexpression of Wnt10a. P-values were determined by two-sided unpaired t-test (n = 3 technical replicates). Results are representative of two independent experiments. b, Immunoblot of a GFP-sorted Cre-reporter murine cell line after infection with Lenti-Cre/sgPten vector expressing Wnt10a or a non-expressed version where the coding sequence of Wnt10a is missing an ATG start codon (Ctrl). HSP90 serves as a loading control. Results are representative of two experiments.
Extended Data Fig. 9 WNT10A expression is associated with triple-negative and basal-type breast cancers in patients.
a, UMAP (left) and dot plot (right) of scRNA-seq data published in Pal et al. 2023, showing expression levels of WNT10A and Wnt target genes in triple-negative breast tumor cells and normal AV cells from human donors who are either non-carriers (WT) or BRCA1 mutation carriers (BRCA1). b, Heatmap of Wnt ligand expression levels across different breast cancer subtypes in the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) dataset (Basal, n = 209; HER2, n = 224; Luminal A, n = 700; Luminal B, n = 475). c, Dot plot showing Spearman correlations (two-sided p and rho values) between the expression levels of Wnt pathway genes and AP-1 family related genes based on RNA-seq analysis of human breast tumor samples (n = 945) in the The Cancer Genome Atlas (TCGA) dataset.
Supplementary information
Supplementary Information
FACS gating strategy.
Supplementary Tables
Supplementary Table 1. Genes differentially expressed in tumor cells versus AV cells. Supplementary Table 2. Gene Set Enrichment Analysis (GSEA) of the tumor gene signature in the scRNA-seq data. Supplementary Table 3. ATAC–seq differentially accessible regions and transcription factor analyses. Supplementary Table 4. Significant regions and genes identified by both ATAC–seq and scRNA-seq analyses. Supplementary Table 5. shRNA and primer sequences.
Source data
Source Data
Statistical source data for Figs. 5e,f and 6a and Extended Data Figs. 7c–f and 8a.
Source Data
Unprocessed immunoblots for Extended Data Figs. 5d and 8b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C.MC., Cordes, A., Oliphant, M.U.J. et al. Brca1 haploinsufficiency promotes early tumor onset and epigenetic alterations in a mouse model of hereditary breast cancer. Nat Genet 56, 2763–2775 (2024). https://doi.org/10.1038/s41588-024-01958-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-024-01958-6
This article is cited by
-
Epigenomic disorder and partial EMT impair luminal progenitor integrity in Brca1-associated breast tumorigenesis
Molecular Cancer (2025)
-
Mutational Selection: Fragile Sites, Replicative Stress, and Genome Evolution
Evolutionary Biology (2025)
-
Epigenetic scars of Brca1 loss point toward breast cancer cell of origin
Nature Genetics (2024)