Abstract
Unipotent basal and luminal stem cells maintain prostate homeostasis, with an intermediate cell population emerging during prostate inflammation or cancer. However, the identities of basal stem cell and intermediate cell population remain unclear. Here we identified a rare intermediate cell population expressing luminal markers (termed Basal-B) with enhanced organoid formation capacity, and a larger basal population (termed Basal-A). Genetic lineage tracing revealed Basal-B cells represented a transient basal stem cell state during prostate homeostasis and androgen-mediated regeneration. Activated JAK/STAT signaling was identified in Basal-B cells, and its inhibition significantly reduced Basal-B markers expression. Inflammation increased Basal-B-to-luminal cell transdifferentiation, but JAK/STAT inhibition notably attenuated this effect. Pten gene deletion increased Nkx3.1-expressing Basal-B-like cell population and led to neoplasia. In humans, h-Basal-B cells were more prevalent in benign prostate hyperplasia. This study reveals the identities of intermediate Basal-B cells and underscores the role of JAK/STAT signaling in prostate cell fate determination.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All raw sequencing data in this study have been uploaded to the Genome Sequence Archive (GSA; https://ngdc.cncb.ac.cn/gsa/) with the accession number CRA018252, https://bigd.big.ac.cn/gsa/browse/CRA018252. Previously published scRNA-seq data reanalyzed in our manuscript are available under accession codes OEP000825 (PMID: 32807988, NODE, https://www.biosino.org/node/project/detail/OEP00000825) and GSE150692 (PMID: 32915138). Source data are provided with this paper.
Code availability
The custom code used is available via GitHub at https://github.com/LinLi-0909/Prostate_Basal and via Zenodo at https://zenodo.org/records/13852658 (ref. 56). Software and tools used are described in the Methods and Reporting Summary.
References
Merrell, A. J. & Stanger, B. Z. Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat. Rev. Mol. Cell Biol. 17, 413–425 (2016).
Blanpain, C. & Simons, B. D. Unravelling stem cell dynamics by lineage tracing. Nat. Rev. Mol. Cell Biol. 14, 489–502 (2013).
Blanpain, C. & Fuchs, E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344, 1242281 (2014).
Shen, M. M. & Abate-Shen, C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 24, 1967–2000 (2010).
Li, J. J. & Shen, M. M. Prostate stem cells and cancer stem cells. Cold Spring Harb. Perspect. Med. 9, a030395 (2019).
Burger, P. E. et al. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc. Natl Acad. Sci. USA 102, 7180–7185 (2005).
Goldstein, A. S., Huang, J., Guo, C., Garraway, I. P. & Witte, O. N. Identification of a cell of origin for human prostate cancer. Science 329, 568–571 (2010).
Leong, K. G., Wang, B. E., Johnson, L. & Gao, W. Q. Generation of a prostate from a single adult stem cell. Nature 456, 804–808 (2008).
Xin, L., Lawson, D. A. & Witte, O. N. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc. Natl Acad. Sci. USA 102, 6942–6947 (2005).
Karthaus, W. R. et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159, 163–175 (2014).
Chua, C. W. et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 16, 951–961 (2014).
Choi, N., Zhang, B., Zhang, L., Ittmann, M. & Xin, L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 21, 253–265 (2012).
Centonze, A. et al. Heterotypic cell–cell communication regulates glandular stem cell multipotency. Nature 584, 608–613 (2020).
Ousset, M. et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat. Cell Biol. 14, 1131–1138 (2012).
Kwon, O. J., Zhang, L., Ittmann, M. M. & Xin, L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc. Natl Acad. Sci. USA 111, E592–E600 (2014).
Sfanos, K. S., Yegnasubramanian, S., Nelson, W. G. & De Marzo, A. M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 15, 11–24 (2018).
de Bono, J. S. et al. Prostate carcinogenesis: inflammatory storms. Nat. Rev. Cancer 20, 455–469 (2020).
Evans, G. S. & Chandler, J. A. Cell proliferation studies in the rat prostate: II. The effects of castration and androgen-induced regeneration upon basal and secretory cell proliferation. Prostate 11, 339–351 (1987).
English, H. F., Santen, R. J. & Isaacs, J. T. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate 11, 229–242 (1987).
Wang, X. et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461, 495–500 (2009).
van Leenders, G. J. et al. Intermediate cells in human prostate epithelium are enriched in proliferative inflammatory atrophy. Am. J. Pathol. 162, 1529–1537 (2003).
Chan, J. M. et al. Lineage plasticity in prostate cancer depends on JAK/STAT inflammatory signaling. Science 377, 1180–1191 (2022).
Deng, S. et al. Author correction: Ectopic JAK-STAT activation enables the transition to a stem-like and multilineage state conferring AR-targeted therapy resistance. Nat. Cancer 3, 1271 (2022).
Mu, P. et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88 (2017).
Labrecque, M. P. et al. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J. Clin. Invest 129, 4492–4505 (2019).
Guo, W. et al. Single-cell transcriptomics identifies a distinct luminal progenitor cell type in distal prostate invagination tips. Nat. Genet. 52, 908–918 (2020).
Karthaus, W. R. et al. Regenerative potential of prostate luminal cells revealed by single-cell analysis. Science 368, 497–505 (2020).
Crowley, L. et al. A single-cell atlas of the mouse and human prostate reveals heterogeneity and conservation of epithelial progenitors. eLife 9, e59465 (2020).
Chen, S. et al. Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nat. Cell Biol. 23, 87–98 (2021).
Song, H. et al. Single-cell analysis of human primary prostate cancer reveals the heterogeneity of tumor-associated epithelial cell states. Nat. Commun. 13, 141 (2022).
Henry, G. H. et al. A cellular anatomy of the normal adult human prostate and prostatic urethra. Cell Rep. 25, 3530–3542.e5 (2018).
Yoo, Y. A. et al. Bmi1 marks distinct castration-resistant luminal progenitor cells competent for prostate regeneration and tumour initiation. Nat. Commun. 7, 12943 (2016).
Wang, Z. A. et al. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat. Cell Biol. 15, 274–283 (2013).
Lu, T. L. et al. Conditionally ablated Pten in prostate basal cells promotes basal-to-luminal differentiation and causes invasive prostate cancer in mice. Am. J. Pathol. 182, 975–991 (2013).
He, L. et al. Proliferation tracing reveals regional hepatocyte generation in liver homeostasis and repair. Science 371, eabc4346 (2021).
He, L. et al. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat. Med. 23, 1488–1498 (2017).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Liu, K. et al. Triple-cell lineage tracing by a dual reporter on a single allele. J. Biol. Chem. 295, 690–700 (2020).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019).
Drost, J. et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 11, 347–358 (2016).
Li, L. et al. Single-cell causal network inferred by cross-mapping entropy. Brief. Bioinform. 24, bbad281 (2023).
Boehm, B. J., Colopy, S. A., Jerde, T. J., Loftus, C. J. & Bushman, W. Acute bacterial inflammation of the mouse prostate. Prostate 72, 307–317 (2012).
Zhao, H. & Zhou, B. Dual genetic approaches for deciphering cell fate plasticity in vivo: more than double. Curr. Opin. Cell Biol. 61, 101–109 (2019).
Deng, S. et al. Ectopic JAK-STAT activation enables the transition to a stem-like and multilineage state conferring AR-targeted therapy resistance. Nat. Cancer 3, 1071–1087 (2022).
Lo, U. G. et al. The driver role of JAK-STAT signalling in cancer stemness capabilities leading to new therapeutic strategies for therapy- and castration-resistant prostate cancer. Clin. Transl. Med. 12, e978 (2022).
Gao, D. et al. A Tmprss2-CreERT2 knock-in mouse model for cancer genetic studies on prostate and colon. PLoS ONE 11, e0161084 (2016).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Shi, J., Teschendorff, A. E., Chen, W., Chen, L. & Li, T. Quantifying Waddington’s epigenetic landscape: a comparison of single-cell potency measures. Brief. Bioinform. 21, 248–261 (2020).
Teschendorff, A. E. & Enver, T. Single-cell entropy for accurate estimation of differentiation potency from a cell’s transcriptome. Nat. Commun. 8, 15599 (2017).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Qu, M. et al. Establishment of intestinal organoid cultures modeling injury-associated epithelial regeneration. Cell Res. 31, 259–271 (2021).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Li, L. JAK/STAT signaling maintains intermediate cell population in prostate basal cell fates determination. Zenodo https://doi.org/10.5281/zenodo.13852657 (2024).
Acknowledgements
We thank B. Wu and G. Chen for their help with animal husbandry and W. Bian for providing technical help at the CEMCS (SIBCB) Core Facility. We would like to thank the Genome Tagging Project (GTP) Center, CEMCS (SIBCB) and CAS for technical support (Shanghai, China). This study was supported by the National Key Research and Development Program of China (2020YFA0509000 and 2023YFC2506400 to D.G., 2022YFA1004800 to L.C., 2019YFA0802000 to B.Z.), the National Science Fund for Distinguished Young Scholars (32125013 to D.G.), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB38040400 to L.C.), the National Natural Science Foundation of China (92253304 to D.G., 82088101 to B.Z., 31930022, 12131020, T2341007 and T2350003 to L.C.), the Shanghai Municipal Science and Technology Major project to D.G. and B.Z., the Basic Frontier Science Research Program of Chinese Academy of Sciences (ZDBS-LY-SM015 to D.G.), the Special Research Assistant Program of the Chinese Academy of Sciences (2022000065 to W.G.), Chinese Academy of Sciences (JCTD-2020-17, D.G.), the Shanghai Science and Technology Committee (21XD1424200 and 21ZR1470100 to D.G.), Innovative Research Team of High-level Local Universities in Shanghai (SHSMUZDCX20211800 to D.G.), the Guangdong Basic and Applied Basic Research Foundation (2023A1515011075 to C.Y.), Shenzhen Bay Laboratory (21250071 to C.Y.), JST Moonshot R&D (JPMJMS2021 to L.C.) and New Cornerstone Science Foundation to B.Z.
Author information
Authors and Affiliations
Contributions
D.G. conceived the project. W.G., B.Z. and D.G. designed the experiments. A.M.D.M. reviewed the human histopathological data. W.G. and X.Z. performed most of the experiments. H.Z., K.L., S.W., Y.P., Y.J. and C.Y. helped with the experiments and provided technical support. J.L. provided assistance in critical evaluation and interpretation of the experiments. P.S. and C.L. provided human prostate samples. L.L. and L.C. performed computational and statistical analyses. B.Z. and K.L. provided the ProTracer and dual recombinases system. C.Y., L.C., B.Z. and D.G. oversaw the project. D.G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
D.G., W.G., X.Z. and H.Z. have filed a patent application regarding targeting JAK/STAT signaling for prostatitis and prostate hyperplasia treatment. The other authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of basal cell subtypes in adult mouse prostates.
a to c, t-SNE plots show the expression levels of basal signature genes: Krt5, Krt14 and Trp63, (a) Basal-A signature genes: Krt17, Dlk2 and Prelp, (b) Basal-B signature genes: Nkx3.1, Pbsn, Fgl1, Mmp7 and C1rb, (c) across basal cells. (d) Heatmap shows the expression levels of the top 30 signature genes across basal cells.
Extended Data Fig. 2 Isolation and characterization of mouse prostate Basal-A and Basal-B cells.
a, FACS plot showing C1rb− Basal-A and C1rb+ Basal-B cells isolated from the basal cells (Lin−/Epcamhigh/CD49fhigh) of WT adult mouse anterior prostates. b to e, Gene expression levels of C1rb (b), Nkx3.1 (c), Ck5 (d) and Trp63 (e) in isolated C1rb− Basal-A and C1rb+ Basal-B cells by qRT‒PCR (n = 3 repeats per group). f, Construction strategy of NT (Nkx3.1-CreER; Rosa26-loxp-stop-loxp-tdTomato) mice. g, Schedule of labeling and tracing Nkx3.1-expressing cells in NT mice. h, Immunofluorescence staining of tdTomato, Trp63, Ck5 and Ck8 in intact NT mouse prostates 2 weeks after Tam administration. i, Percentage of Ck8+ luminal cells, Ck5+ and Trp63+ basal cells among tdTomato+ cells in NT mouse prostates (n = 6). j, FACS plot showing tdTomato− Basal-A and tdTomato+ Basal-B cells isolated from the basal cells (Lin−/Epcamhigh/CD49fhigh) of NT mouse anterior prostates. k, A bar graph shows the percentages of isolated C1rb+ Basal-B cells from WT mouse prostates and tdTomato+ Basal-B cells from NT mouse prostates (n = 3 mice per group). l,m, Top 25 significantly enriched (Methods; p < 0.01; −log10(P value)) gene ontology terms in the gene signature for the Basal-A (l) and Basal-B (m) subtype of bulk RNA analysis (n = 3). n, Bar diagram shows MCE value of Basal-A and Basal-B cells. The P value is from a one-sided t test. o, GSEA of injury-associated regenerative signatures in Basal-A and Basal-B cells. Scale bars, 50 μm. Data are shown as the mean ± SD. Data were analyzed by unpaired two-tailed Student’s t test; ***p < 0.001, ****p < 0.0001 and ns, nonsignificant.
Extended Data Fig. 3 Trp63-expressing and Nkx3.1-expressing cell labeling.
a,b, Construction strategy of T-TLR (Trp63-DreER;R26-TLR) mice (a) and N-TLR (Nkx3.1-CreER;R26-TLR) mice (b) for Trp63+ and Nkx3.1+ cell labeling, respectively. c, Schedule of tamoxifen injection and analysis in T-TLR and N-TLR mouse prostates. d-f, Immunofluorescence staining of Trp63, Nkx3.1, Ck8, ZsGreen and tdTomato in T-TLR (d and e) and N-TLR (f) mouse prostates. g, Percentage of Trp63+ (n = 12,757), Trp63− (n = 0), Ck8+ (n = 0), and Ck8− (n = 15,300) cells among ZsGreen+ cells in T-TLR mice (n = 4). h, Percentage of Nkx3.1+ (n = 14,438) and Nkx3.1− (n = 0) cells among tdTomato+ cells in N-TLR mice (n = 4). i, Immunofluorescence staining of Trp63, Nkx3.1, Ck8 and tdTomato in N-TLR mouse prostates. j, Percentage of Ck8+ luminal cells and Trp63+ basal cells among tdTomato+ cells (n = 27,195) in N-TLR mice (n = 4). k, Percentage of Nkx3.1+ cells (n = 2,649) and Nkx3.1− cells (n = 0) among tdTomato+Trp63+ cells in N-TLR mice (n = 4). Scale bars, 50 μm. Data are shown as the mean ± SD.
Extended Data Fig. 4 The percentage of Basal-B cell population during prostate regeneration.
a, Schedule of Basal-B-cell labeling in TN-TLR mice during prostate regression and regeneration. b, Immunofluorescence staining of Trp63, Ck8, ZsGreen and tdTomato in TN-TLR mouse prostates after 3 cycles of prostate regression and regeneration. c, Bar graph showing the percentage of Basal-B cells (tdTomato+ZsGreen+) among ZsGreen+ basal cells of intact (n = 6 mice) and regenerated TN-TLR mouse anterior prostates after 1 cycle (n = 4 mice) or 3 cycles (n = 4 mice) of regression-regeneration. d,e, FACS plots (d) and a bar graph (e) showing the percentage of tdTomato+ZsGreen+ Basal-B cells among ZsGreen+ basal cells of intact (n = 6 mice) and regenerated TN-TLR mouse anterior prostates after 1 cycle (n = 4 mice) or 3 cycles (n = 4 mice) of prostate regression-regeneration.
Extended Data Fig. 5 Prostate cell proliferation tracing during homeostasis using the ProTracer model.
a, Schedule of tracing proliferated cells during prostate homeostasis in ProTracer mice. b,c, Immunofluorescence staining of GFP and Trp63 for basal cells (b) and GFP and Ck8 for luminal cells (c) of ProTracer mouse prostates at 4 time points (1 week, 3 months, 7 months and 12 months) after tamoxifen injection. d,e, Percentage of GFP+ cells in Trp63+ basal cells (d) or Ck8+ luminal cells (e) of ProTracer mouse prostates at 4 time points (1 week (n = 3 mice), 3 months (n = 4 mice), 7 months (n = 3 mice) and 12 months (n = 3 mice)) after tamoxifen injection. f to i, FACS plots and bar graphs showing the percentage of GFP+ cells among Lin−/Epcamhigh/CD49fhigh basal cells (f and h) or Lin−/Epcamhigh/CD49flow luminal cells (g and i) of ProTracer mouse prostates at 4 time points (1 week (n = 3 mice), 3 months (n = 4 mice), 7 months (n = 3 mice) and 12 months (n = 3 mice)) after tamoxifen injection.
Extended Data Fig. 6 The percentage of Basal-B cells is stable during long-term prostate homeostasis.
a, Schedule of Basal-B-cell labeling in TN-TLR mice during long-term prostate homeostasis. b-e, Immunofluorescence staining of Trp63, ZsGreen and tdTomato in TN-TLR mouse anterior prostates at 1 week (b), 3 months (c), 7 months (d) and 12 months (e) after tamoxifen injection. f,g, FACS plots (f) and bar graphs (g) showing the percentage of Basal-B cells (tdTomato+/ZsGreen+) among basal cells (ZsGreen+) of TN-TLR mouse anterior prostates at 4 time points (1 week (n = 5 mice), 3 months (n = 3 mice), 7 months (n = 5 mice) and 12 months (n = 4 mice)) after tamoxifen injection. h, Immunofluorescence staining of ZsGreen and tdTomato in TN-TLR mouse prostates at 4 time points (1 week, 3 months, 7 months and 12 months) after tamoxifen injection. White arrows indicate ZsGreen+tdTomato+ Basal-B cells. i, Percentage of Trp63+Nkx3.1+ Basal-B cells among Trp63+ basal cells in TN-TLR mouse prostates at 4 time points (1 week (n = 5 mice), 3 months (n = 3 mice), 7 months (n = 5 mice) and 12 months (n = 4 mice)) after vehicle injection. j. immunofluorescence staining of Nkx3.1, ZsGreen and tdTomato of Tam treated 0.25 month, 7 months and 12 months of TN-TLR mouse prostates. White and red arrows especially indicate ZsGreen+/tdTomato+/Nkx3.1+ and ZsGreen+/tdTomato+/Nkx3.1− cells. k. A bar graph showing Nkx3.1+ cells and Nkx3.1− cells in the ZsGreen+tdTomato+ cells of Tam-treated 0.25 month (n = 5 mice), 7 months (n = 5 mice) and 12 months (n = 4 mice) of TN-TLR mouse prostates. Data are shown as the mean ± SD. Scale bars, 50 μm.
Extended Data Fig. 7 Activation of JAK/STAT signaling in Basal-B cells.
a, Immunofluorescence staining of Trp63, p-Stat1, p-Stat3 and Nkx3.1 in WT mouse prostates. White arrows indicate Trp63+Nkx3.1+ Basal-B cells. b, Bar graphs compare the percentages of p-Stat1+ and p-Stat3+ cells among Trp63+Nkx3.1− Basal-A cells and Trp63+Nkx3.1+ Basal-B cells (n = 4 mice per group). c, A timeline illustrates the protocol for ruxolitinib treatment and subsequent analysis in WT mouse prostates. d,e, Immunofluorescence staining of Trp63, p-Stat1 and p-Stat3 in vehicle (d) versus ruxolitinib-treated (e) WT mouse prostates. Red arrows indicate Trp63+p-Stat1+ basal cells, and Trp63+p-Stat3+ basal cells. f, Bar graphs showing the percentage of p-Stat1+ cells and p-Stat3+ cells among Trp63+ basal cells of vehicle and ruxolitinib-treated WT mouse prostates (n = 4 mice per group). g, Immunofluorescence staining of Trp63 and Nkx3.1 in vehicle and ruxolitinib-treated WT mouse prostates. White arrows indicate Trp63+Nkx3.1+ Basal-B cells. h, A bar graph shows the percentage of Nkx3.1+ cells among Trp63+ basal cells in vehicle and ruxolitinib-treated NT mouse prostates (n = 4 mice per group). i,j, Flow cytometry plots (i) and a bar graph (j) display the percentage of C1rb+ basal cells among basal cells in vehicle versus ruxolitinib-treated WT mouse prostates (n = 4 mice per group). k,l, Bar graphs show organoid formation efficiency (Basal-A-cell-derived organoids n = 199 in vehicle groups, n = 192 in ruxolitinib treated group; Basal-B-cell-derived organoids n = 287 in vehicle groups, n = 302 in ruxolitinib treated group) of tdTomato− Basal-A cells (k) and tdTomato+ Basal-B-cells (l), which were isolated from vehicle and ruxolitinib treated NTO mouse prostates. Data are shown as the mean ± SD. Data were analyzed by unpaired two-tailed Student’s t test; *p < 0.05, **p < 0.01 and ***p < 0.001. Scale bars, 50 μm.
Extended Data Fig. 8 Basal-B cells serve as stem cells during bacteria-induced prostate inflammation.
a, The experimental timeline for labeling Basal-B cells and E. coli infection in TN-TLR mouse prostates. b,c, Flow plots showing the percentage of ZsGreen+ tdTomato− Basal-A and ZsGreen+ tdTomato+ Basal-B cells isolating from vehicle (b) and E.coli (c) treated TN-TLR mouse prostates. d, Bar graphs showing ZsGreen+ tdTomato− Basal-A cells in ZsGreen+ basal cells, and ZsGreen+ tdTomato+ Basal-B cells in ZsGreen+ basal cells of TN-TLR mouse prostates with (n = 3 mice) or without (n = 3 mice) E. coli infection. e,f, Flow plots showing the percentage of ZsGreen+ tdTomato− Basal-A-derived luminal cells and ZsGreen+ tdTomato+ Basal-B-derived luminal cells isolating from vehicle (e) and E.coli (f) treated TN-TLR mouse prostates. g, A bar graph showing the differentiation efficiency of Basal-A cells and Bas-B cells to luminal in TN-TLR mouse prostates with or without E. coli infection (n = 5 mice per group). Data are shown as the mean ± SD. Data were analyzed by unpaired two-tailed Student’s t test; ****p < 0.0001. Scale bars, 50 μm.
Extended Data Fig. 9 Significant reduction in Basal-B like cells following diphtheria toxin treatment during inflammation.
a,b, Immunofluorescence staining of Trp63, Ck8, ZsGreen and tdTomato in vehicle-treated (a) and diphtheria toxin-treated (b) Bas-B-DTR mouse prostates. c,d, Flow plots showing the percentage of Basal-B cells (ZsGreen+tdTomato+) among basal cells (ZsGreen+) of vehicle-treated (c) and diphtheria toxin- treated (d) Basal-B-DTR mouse anterior prostates. e. A bar graph showing Basal-B cell in ZsGreen+ basal cells of vehicle-treated (n = 5 mice) and diphtheria toxin-treated (n = 3 mice) Bas-B-DTR prostates. Data are shown as the mean ± SD. Data were analyzed by unpaired two-tailed Student’s t test; **p < 0.01, ***p < 0.001. Scale bars, 50μm.
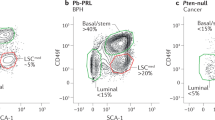
Extended Data Fig. 10 Integration analysis of basal cells from normal human prostate and BPH samples.
a, Visualization of epithelial cell clustering results from normal human prostate samples. b, The expression levels of representative signature genes across all epithelial subtypes in normal human prostate samples. c, Visualization of epithelial cell clustering results from BPH samples. d, The expression levels of representative signature genes across all epithelial subtypes in BPH samples. e, Distribution of Basal-A and Basal-B in normal human prostate and BPH samples, respectively. f,g, UMAP plots showing the expression levels of the basal signature genes KRT5, TP63 and KRT15 (f) and the Basal-B signature genes NKX3.1 and C1R (g) across all basal cells. h, Heatmap showing the expression levels of the top 50 signature genes across basal cells from normal human prostate and BPH samples.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11 and Notes 1 and 2.
Supplementary Table
Table 1, DEGs of basal cells in WT, Bulk RNA-seq, E. coli and Pten. Table 2, GRNs of WT and E. coli; Table 3, Signature genes of basal cell subtypes in human prostates. Table 4, Primers for genome typing and qPCR used in this study. Table 5, Antibodies for IF and FACS used in this study.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Fig. 6
Statistical source data for Fig. 6.
Source Data Fig. 7
Statistical source data for Fig. 7.
Source Data Fig. 8
Statistical source data for Fig. 8.
Source Data Extended Data Fig. 2
Statistical source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Statistical source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Statistical source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Statistical source data for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Statistical source data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Statistical source data for Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Statistical source data for Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Statistical source data for Extended Data Fig 9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, W., Zhang, X., Li, L. et al. JAK/STAT signaling maintains an intermediate cell population during prostate basal cell fate determination. Nat Genet 56, 2776–2789 (2024). https://doi.org/10.1038/s41588-024-01979-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-024-01979-1
This article is cited by
-
Innate immunity and the NF-κB pathway control prostate stem cell plasticity, reprogramming and tumor initiation
Nature Cancer (2025)
-
JAK–STAT signalling and prostate basal cell fate
Nature Reviews Urology (2025)
-
JAK/STAT signaling sustains an intermediate basal cell population in prostate homeostasis and disease
Science China Life Sciences (2025)