Abstract
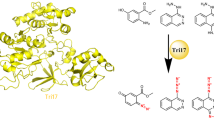
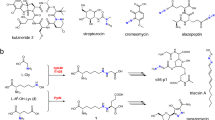
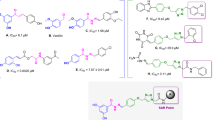
Triacsins are an intriguing class of specialized metabolites possessing a conserved N-hydroxytriazene moiety not found in any other known natural products. Triacsins are notable as potent acyl-CoA synthetase inhibitors in lipid metabolism, yet their biosynthesis has remained elusive. Through extensive mutagenesis and biochemical studies, we here report all enzymes required to construct and install the N-hydroxytriazene pharmacophore of triacsins. Two distinct ATP-dependent enzymes were revealed to catalyze the two consecutive N–N bond formation reactions, including a glycine-utilizing, hydrazine-forming enzyme (Tri28) and a nitrite-utilizing, N-nitrosating enzyme (Tri17). This study paves the way for future mechanistic interrogation and biocatalytic application of enzymes for N–N bond formation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper, Supplementary information and the Extended data, and/or from the corresponding author on reasonable request.
References
Tanaka, H., Yoshida, K., Itoh, Y. & Imanaka, H. Structure and synthesis of a new hypotensive vasodilator. Tetrahedron Lett. 22, 1809–1812 (1981).
Hartman, E. J., Omura, S., Laposata, M. & Triacsin, C. A differential inhibitor of arachidonoyl-CoA synthetase and nonspecific long chain acyl-CoA synthetase. Prostaglandins 37, 62–73 (1989).
Omura, S., Tomoda, H., Xu, M. Q., Takahashi, Y. & Iwai, Y. T. Triacsins, new inhibitors of acyl-CoA synthetase produced by Streptomyces sp. J. Antibiot. (Tokyo) 39, 1211–1218 (1986).
Hiroshi, T., Kazuaki, I. & Satoshi, O. Inhibition of acyl-CoA synthetase by triacsins. Biochim. Biophys. Acta 921, 595–598 (1987).
Tomoda, H., Igarashi, K., Cyong, J. C. & Omura, S. Evidence for an essential role of long chain acyl-CoA synthetase in animal cell proliferation: inhibition of long chain acyl-CoA synthetase by triacsins caused inhibition of Raji cell proliferation. J. Biol. Chem. 266, 4214–4219 (1991).
Kim, J. H., Lewin, T. M. & Coleman, R. A. Expression and characterization of recombinant rat acyl-CoA synthetases 1, 4, and 5: selective inhibition by triacsin C and thiazolidinediones. J. Biol. Chem. 276, 24667–24673 (2001).
Matsuda, D., Namatame, I., Ohshiro, T., Ishibashi, S. & Tomoda, H. J. Anti-atherosclerotic activity of triacsin C, an acyl-CoA synthetase inhibitor. J. Antibiot. (Tokyo) 61, 318–321 (2008).
Nakayama, T. et al. Enhanced lipid metabolism induces the sensitivity of dormant cancer cells to 5-aminolevulinic acid-based photodynamic therapy. Sci. Rep. 11, 7290 (2021).
Guo, F. et al. Amelioration of cryptosporidium parvum infection in vitro and in vivo by targeting parasite fatty acyl-coenzyme a synthetases. J. Infect. Dis. 209, 1279–1287 (2014).
Ernst, A. M. et al. S-palmitoylation sorts membrane cargo for anterograde transport in the Golgi. Dev. Cell 47, 479–493 (2018).
Silvas, J. A. et al. Inhibitors of VPS34 and lipid metabolism suppress SARS-CoV-2 replication. Preprint at https://www.biorxiv.org/content/10.1101/2020.07.18.210211v1 (2020).
Blair, L. M. & Sperry, J. Natural products containing a nitrogen-nitrogen bond. J. Nat. Prod. 76, 794–812 (2013).
Le Goff, G. & Ouazzani, J. Natural hydrazine-containing compounds: biosynthesis, isolation, biological activities and synthesis. Bioorg. Med. Chem. 22, 6529–6544 (2014).
Waldman, A. J., Ng, T. L., Wang, P. & Balskus, E. P. Heteroatom-heteroatom bond formation in natural product biosynthesis. Chem. Rev. 117, 5784–5863 (2017).
Du, Y. L., He, H. Y., Higgins, M. A. & Ryan, K. S. A heme-dependent enzyme forms the nitrogen-nitrogen bond in piperazate. Nat. Chem. Biol. 13, 836–838 (2017).
Matsuda, K. et al. Discovery of unprecedented hydrazine-forming machinery in bacteria. J. Am. Chem. Soc. 140, 9083–9086 (2018).
Ng, T. L., Rohac, R., Mitchell, A. J., Boal, A. K. & Balskus, E. P. An N-nitrosating metalloenzyme constructs the pharmacophore of streptozotocin. Nature 566, 94–99 (2019).
Kawai, S. et al. Complete biosynthetic pathway of alazopeptin, a tripeptide con‐sisting of two molecules of 6‐diazo‐5‐oxo‐L-norleucine and one molecule of alanine. Angew. Chem. Int. Ed. Engl. 60, 10319–10325 (2021).
Waldman, A. J. & Balskus, E. P. Discovery of a diazo-forming enzyme in cremeomycin biosynthesis. J. Org. Chem. 83, 7539–7546 (2018).
Twigg, F. F. et al. Identifying the biosynthetic gene cluster for triacsins with an N-hydroxytriazene moiety. ChemBioChem 20, 1145–1149 (2019).
White, M. D. et al. UbiX is a flavin prenyltransferase required for bacterial ubiquinone biosynthesis. Nature 522, 502–506 (2015).
Payne, K. A. P. et al. New cofactor supports α,β-unsaturated acid decarboxylation via 1,3-dipolar cycloaddition. Nature 522, 497–501 (2015).
Tian, G. & Liu, Y. Mechanistic insights into the catalytic reaction of ferulic acid decarboxylase from Aspergillus niger: a QM/MM study. Phys. Chem. Chem. Phys. 19, 7733–7742 (2017).
Marshall, S. A., Payne, K. A. P. & Leys, D. The UbiX-UbiD system: the biosynthesis and use of prenylated flavin (prFMN). Arch. Biochem. Biophys. 632, 209–221 (2017).
Hegedus, H., Gergely, A., Veress, T. & Horváth, P. Novel derivatization with Sanger’s reagent (2,4-dinitrofluorobenzene [DNFB]) and related methodological developments for improved detection of amphetamine enantiomers by circular dichroism spectroscopy. Analusis 27, 458–463 (1999).
Garg, R. P., Qian, X. L., Alemany, L. B., Moran, S. & Parry, R. J. Investigations of valanimycin biosynthesis: elucidation of the role of seryl-tRNA. Proc. Natl Acad. Sci. USA 105, 6543–6547 (2008).
Zhao, G. et al. The biosynthetic gene cluster of pyrazomycin—a C-nucleoside antibiotic with a rare pyrazole moiety. ChemBioChem 21, 644–649 (2020).
Wang, K. K. A. et al. Glutamic acid is a carrier for hydrazine during the biosyntheses of fosfazinomycin and kinamycin. Nat. Commun. 9, 3687 (2018).
Huang, Z., Wang, K. K. A. & Van Der Donk, W. A. New insights into the biosynthesis of fosfazinomycin. Chem. Sci. 7, 5219–5223 (2016).
Zhang, W., Bolla, M. L., Kahne, D. & Walsh, C. T. A three enzyme pathway for 2-amino-3-hydroxycyclopent-2-enone formation and incorporation in natural product biosynthesis. J. Am. Chem. Soc. 132, 6402–6411 (2010).
Jones, B. N. & Gilligan, J. P. O-phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid chromatography of polypeptide hydrolysates and physiological fluids. J. Chromatogr. A 266, 471–482 (1983).
Sugai, Y., Katsuyama, Y. & Ohnishi, Y. A nitrous acid biosynthetic pathway for diazo group formation in bacteria. Nat. Chem. Biol. 12, 73–75 (2016).
Nofiani, R., Philmus, B., Nindita, Y. & Mahmud, T. 3-Ketoacyl-ACP synthase (KAS) III homologues and their roles in natural product biosynthesis. MedChemComm 10, 1517–1530 (2019).
Masschelein, J. et al. A dual transacylation mechanism for polyketide synthase chain release in enacyloxin antibiotic biosynthesis. Nat. Chem. 11, 906–912 (2019).
Saridakis, V. et al. Crystal structure of Methanobacterium thermoautotrophicum conserved protein MTH1020 reveals an NTN-hydrolase fold. Proteins 48, 141–143 (2002).
Quadri, L. E. N. et al. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carder protein domains in peptide synthetases. Biochemistry 37, 1585–1595 (1998).
Yoshida, K. et al. Studies on new vasodilators, WS-1228 A and B I. Discovery, taxonomy, isolation and characterization. J. Antibiot. (Tokyo) XXXV, 157–163 (1981).
Hertweck, C., Luzhetskyy, A., Rebets, Y. & Bechthold, A. Type II polyketide synthases: gaining a deeper insight into enzymatic teamwork. Nat. Prod. Rep. 24, 162–190 (2007).
Chen, A., Re, R. N. & Burkart, M. D. Type II fatty acid and polyketide synthases: deciphering protein-protein and protein-substrate interactions. Nat. Prod. Rep. 35, 1029–1045 (2018).
Szu, P. H. et al. Analysis of the ketosynthase-chain length factor heterodimer from the fredericamycin polyketide synthase. Chem. Biol. 18, 1021–1031 (2011).
Grammbitter, G. L. C. et al. An uncommon type II PKS catalyzes biosynthesis of aryl polyene Ppgments. J. Am. Chem. Soc. 141, 16615–16623 (2019).
Du, D., Katsuyama, Y., Shin-ya, K. & Ohnishi, Y. Reconstitution of a type II polyketide synthase that catalyzes polyene formation. Angew. Chem. Int. Ed. Engl. 57, 1954–1957 (2018).
Zhang, J. et al. Reconstitution of a highly reducing type II PKS system reveals 6π-electrocyclization is required for O-dialkylbenzene biosynthesis. J. Am. Chem. Soc. 143, 2962–2969 (2021).
Sacco, E. et al. The missing piece of the type II fatty acid synthase system from Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 104, 14628–14633 (2007).
Rui, Z. et al. Biochemical and genetic insights into asukamycin biosynthesis. J. Biol. Chem. 285, 24915–24924 (2010).
Dunwell, J. M., Purvis, A. & Khuri, S. Cupins: the most functionally diverse protein superfamily? Phytochemistry 65, 7–17 (2004).
Stipanuk, M. H., Simmons, C. R., Karplus, P. A. & Dominy, J. E. Thiol dioxygenases: unique families of cupin proteins. Amino Acids 41, 91–102 (2011).
Ng, T. L. et al. The l-alanosine gene cluster encodes a pathway for diazeniumdiolate biosynthesis. ChemBioChem 21, 1155–1160 (2020).
Wang, M., Niikura, H., He, H. Y., Daniel-Ivad, P. & Ryan, K. S. Biosynthesis of the N–N-bond-containing compound l-alanosine. Angew. Chem. Int. Ed. Engl. 59, 3881–3885 (2020).
Jenul, C. et al. Biosynthesis of fragin is controlled by a novel quorum sensing signal. Nat. Commun. 9, 1297 (2018).
Jonnalagadda, R. et al. Biochemical and crystallographic investigations into isonitrile formation by a non-heme iron-dependent oxidase/decarboxylase. J. Biol. Chem. 296, 100231 (2021).
Winkler, R. ESIprot: a universal tool for charge state determination and molecular weight calculation of proteins from electrospray ionization mass spectrometry data. Rapid Commun. Mass Spectrom. 24, 285–294 (2010).
Acknowledgements
We thank T.-Y. Huang and B. McCloskey at UC Berkeley for their assistance with initial GC–MS experiments and consultation in designing experiments. We thank M. Zhang from the UC Berkeley Catalysis Center for training with the GC–MS apparatus and aiding with data interpretation, J. Pelton for helping with NMR spectroscopic analysis and W. Yang for helping with ICP–OES analysis. The GC–MS work was made possible by the Catalysis Facility of Lawrence Berkeley National Laboratory, supported by the Director, Office of Science, of the US Department of Energy (contract no. DE-AC02-05CH11231). This research was financially supported by grants to W.Z. from the NIH (nos. R01GM136758 and DP2AT009148) and the Chan Zuckerberg Biohub Investigator Program. We thank Z. Hu for helpful discussions regarding the complex biosynthesis of triacsins. Lastly, we thank Y. Shen for assistance in protein purification and kinetic assays.
Author information
Authors and Affiliations
Contributions
A.D.R.F. designed the experiments, performed all in vitro experiments with purified enzymes, analyzed the data and wrote the manuscript. F.F.T. designed experiments to detect the biosynthetic intermediate, constructed plasmids for protein expression in E. coli and analyzed data. Y.D. analyzed all NMR data. W.C. performed GC–MS experiments and helped analyze NMR data. D.Q.A. helped purify the biosynthetic intermediate. M.S. isolated triacsin A. M.J.D., M.N. and J.G. aided in protein purification, construction of plasmids and repeating biochemical assays for this study. N.A.Z. performed comparative metabolomics work. R.Z. performed FPLC purification for Tri22, Tri28 and individual domains. W.Z. designed the experiments, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemical Biology thanks Jonathan Caranto, Yohei Katsuyama and Jurgen Rohr for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The decarboxylation event in triacsin biosynthesis.
a) Feeding of unlabeled and labeled glycine to the triacsin producing cultures. HRMS analysis of 3 from a S. aureofaciens culture provided with 10 mM 1-13C-glycine demonstrated that the carboxylate carbon of glycine was retained in triacsin C (3). b) Detection of CO2 production from the Tri109 biochemical assay. GC-MS chromatograms extracted with m/z = 44, demonstrating CO2 production from a Tri109 assay containing 5 as compared with an authentic standard. A 10-ppm mass error tolerance was used for each trace. At least two independent replicates were performed for each assay, and representative results are shown.
Extended Data Fig. 2 In vitro reconstitution of Tri26 and Tri28.
a) EICs showing the production of 7 in an assay containing Tri26, lysine, and NADPH. Tri26 selectively hydroxylates lysine and not ornithine. The calculated mass for 7: m/z = 163.1078 ([M + H]+) is used for each trace, except for the ornithine assay in which the calculated mass for N-OH-Orn was used (m/z = 149.0921 ([M + H]+)). b) EICs demonstrating that the production of 8 is dependent on Tri28, glycine, lysine, and ATP, along with the Tri26 assay components. The calculated mass for 8: m/z = 220.1292 ([M + H]+) is used for each trace. A 10-ppm mass error tolerance was used for each trace. At least three independent replicates were performed for each assay, and representative results are shown.
Extended Data Fig. 3 Characterization of the Tri22-catalyzed reaction product, 11.
a) Schematic of a biochemical assay performed containing HAA, succinyl-CoA, Tri29-31, and Tri22 that is subjected to base hydrolysis, followed by OPTA derivatization to yield 2-HYAA-D. b) EICs showing the generation of 2-HYAA-D from the biochemical assay previously described in comparison with a derivatized 2-HYAA synthesized standard. Omission of Tri22, HAA, or succinyl-CoA from the couple enzymatic reaction resulted in abolishment of 2-HYAA-D. The calculated mass for 2-HYAA-D: m/z = 265.0642 ([M + H]+) is used for each trace. A 10-ppm mass error tolerance was used for each trace. At least three independent replicates were performed for each assay, and representative results are shown.
Extended Data Fig. 4 In vitro analysis of Tri14, a putative thioesterase.
a) EICs demonstrating hydrolysis of lauroyl-S-Tri20 catalyzed by Tri14, resulting in production of lauric acid when compared to an authentic standard. Tri14 was shown to be Tri20 specific and imparted no activity on lauroyl-S-Tri30. The calculated mass for lauric acid: m/z = 199.1701 ([M-H]−) is used for each trace. b) EIC demonstrating inability of Tri14 to hydrolyze hexanoyl-S-Tri20, thus suggesting that Tri14 activity is chain length specific. The calculated mass for hexanoic acid: m/z = 115.0764 ([M-H]−) is used for each trace. A 10-ppm mass error tolerance was used for each trace. At least three independent replicates were performed for each assay, and representative results are shown.
Extended Data Fig. 5 AMP Formation from Tri17 assay and proposed reaction mechanism.
LC-UV detection of AMP at 260 nm from an assay containing 15, nitrite, ATP, and Tri17. Controls lacking enzyme or nitrite result in undetectable amounts of AMP, while the production of AMP was slightly increased in the presence of 15. ATP is proposed to activate nitrite to form a nitrite-AMP intermediate that undergoes a subsequent nucleophilic attack by 15 and tautomerization to yield 1. At least three independent replicates were performed for each assay, and representative results are shown.
Supplementary information
Supplementary Information
Supplementary Tables 1–3, Figs. 1–16 and Notes 1–5.
Rights and permissions
About this article
Cite this article
Del Rio Flores, A., Twigg, F.F., Du, Y. et al. Biosynthesis of triacsin featuring an N-hydroxytriazene pharmacophore. Nat Chem Biol 17, 1305–1313 (2021). https://doi.org/10.1038/s41589-021-00895-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00895-3
This article is cited by
-
Structure and mechanism of haem-dependent nitrogen–nitrogen bond formation in piperazate synthase
Nature Catalysis (2025)
-
Enzymatic synthesis of azide by a promiscuous N-nitrosylase
Nature Chemistry (2024)
-
Expanding chemistry through in vitro and in vivo biocatalysis
Nature (2024)
-
O-methyltransferase-like enzyme catalyzed diazo installation in polyketide biosynthesis
Nature Communications (2023)
-
Characterization of three succinyl-CoA acyltransferases involved in polyketide chain assembly
Applied Microbiology and Biotechnology (2023)