Abstract
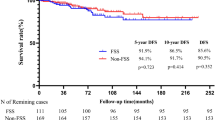
Surgery for platinum-sensitive, relapsed ovarian cancer (PSROC) is widely practiced but had contradictory survival outcomes in previous studies. In this multicenter, open-label, phase 3 trial, women with PSROC, and having had one previous therapy and no platinum-based chemotherapy (platinum-free interval) of 6 months or more, were randomly assigned to either the surgery group (182 patients) or the no-surgery group (control) (175 patients). Patients with resectable diseases were eligible according to the international model (iMODEL), combined with a positron emission tomography-computed tomography imaging. Overall survival (OS) and progression-free survival were coprimary endpoints in hierarchical testing, and a significantly longer progression-free survival with surgery was previously reported. Final analysis of OS was planned at data maturity of 59%. Between 19 July 2012 and 3 June 2019, 357 patients were enrolled. Median follow-up was 82.5 months. Median OS was 58.1 months with surgery and 52.1 months for control (hazard ratio (HR) 0.80, 95% confidence interval (CI) 0.61–1.05, P = 0.11). The predefined threshold for statistical significance was not met, but prespecified sensitivity analysis was performed. Overall, 61 of 175 (35%) patients in control had crossed over to surgery following subsequent relapse, and adjusted HR for death in the surgery group compared with control was 0.76, 95% CI 0.58–0.99. In subgroup analysis of relapse sites by imaging, median survival was not estimable in the surgery group and was 69.5 months in control in patients with <20 sites (HR 0.69, 95% CI 0.46–1.03). Patients with a complete resection had the most favorable outcome, with a median OS of 73.0 months. Twenty-four of 182 (13.2%) patients remained relapse free and alive >60 months in the surgery group as compared with five of 175 (2.9%) patients in the control group. In patients with PSROC, surgery did not increase OS in the intention-to-treat population but resulted in a prolongation of survival following adjustment of crossover.
ClinicalTrials.gov registration: NCT01611766.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The study protocol and statistical analysis plan are available in Supplementary Information. Individual participant-level data that underlie the reported results are not publicly available because this is the first phase 3 study to include detailed postprogression data. Patient-level meta-analyses of the SOC-1, GOG-0213 and DEKTOP III trials were discussed and planned within the Gynecological Cancer InterGroup meta-analysis committee. Other requests for access to patient-level data from this study can be submitted via email to [email protected] with a detailed proposal for approval. In response to any inquiry, please be informed that the timeframe for responding is approximately 2 months.
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Bristow, R. E. et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J. Clin. Oncol. 41, 4065–4076 (2023).
Reuss, A. et al. TRUST: trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). Int. J. Gynecol. Cancer 29, 1327–1331 (2019).
Jiang, R. et al. Study of upfront surgery versus neoadjuvant chemotherapy followed by interval debulking surgery for patients with stage IIIC and IV ovarian cancer, SGOG SUNNY (SOC-2) trial concept. J. Gynecol. Oncol. 31, e86 (2020).
du Bois, A. et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 115, 1234–1244 (2009).
Parmar, M. K. B. et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet 361, 2099–2106 (2003).
Coleman, R. L. et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 18, 779–791 (2017).
Aghajanian, C. et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 30, 2039–2045 (2012).
Poveda, A. et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 22, 620–631 (2021).
Coleman, R. L. et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N. Engl. J. Med. 381, 1929–1939 (2019).
Harter, P. et al. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N. Engl. J. Med. 385, 2123–2131 (2021).
Shi, T. et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 22, 439–449 (2021).
Harter, P. et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann. Surg. Oncol. 13, 1702–1710 (2006).
Armstrong, D. K. et al. NCCN guidelines® insights: ovarian cancer, version 3.2022. J. Natl Compr. Canc. Netw. 20, 972–980 (2022).
Vergote, I. et al. Clinical research in ovarian cancer: consensus recommendations from the Gynecologic Cancer InterGroup. Lancet Oncol. 23, e374–e384 (2022).
Zang, R. Y. et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br. J. Cancer 105, 890–896 (2011).
Tian, W.-J. et al. A risk model for secondary cytoreductive surgery in recurrent ovarian cancer: an evidence-based proposal for patient selection. Ann. Surg. Oncol. 19, 597–604 (2012).
Robins, J. M. & Tsiatis, A. A. Correcting for non-compliance in randomized trials using rank preserving structural failure time models. Commun. Stat. Theory Methods 20, 2609–2631 (1991).
Robins, J. M. & Finkelstein, D. M. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics 56, 779–788 (2000).
Gardner, G. J. & Chi, D. S. Recurrent ovarian cancer – sculpting a promising future with surgery. N. Engl. J. Med. 385, 2187–2188 (2021).
Coleman, R. L. The role of secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer: what do the trials tell us? J. Gynecol. Oncol. 32, e20 (2021).
Regan, M. M. et al. Treatment-free survival: a novel outcome measure of the effects of immune checkpoint inhibition—a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 37, 3350–3358 (2019).
Yang, D. et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 306, 1557–1565 (2011).
Shi, T. et al. Shanghai Gynecologic Oncology Group’s consensus on academic and industry’s clinical trial types. Chin. Med. J. (Engl.) https://doi.org/10.1097/CM9.0000000000003146 (2024).
Rustin, G. J. S. et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int. J. Gynecol. Cancer 21, 419–423 (2011).
Peto, R. et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br. J. Cancer 34, 585–612 (1976).
Acknowledgements
We thank Zhongshan Talent Fund (no. 016, to R.Z.), the National Natural Science Foundation of China (nos. 81972429 and 82273388, to R.Z.) and Shanghai Municipal Science and Technology Major Project (no. 22Y21900300, to R.Z.) for funding this trial. We thank especially members of the Independent Data Monitoring Committee: H. Cui (Chair), X. Chen, X. Wang, Y. Zhu and W. Zhang. We thank J. Zhu (Department of Radiation Oncology, Cancer Hospital of the University of Chinese Academy of Sciences, Hangzhou, China); Z. Chen and T. Zhu (Department of Gynecologic Oncology, Cancer Hospital of the University of Chinese Academy of Sciences, Hangzhou, China); X. Lu (Department of Gynecologic Oncology, Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China); Z. Wu (Department of Biostatistics, School of Public Health, Fudan University, Shanghai, China); Y. Zhang (Department of Gynecologic Oncology, Cancer Hospital of the University of Chinese Academy of Sciences, Hangzhou, China); Y. Cai (Department of Obstetrics and Gynecology, Zhongda Hospital, Jiangsu, China); G. Cai (Department of Gynecologic Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Centre for Cancer Medicine, Sun Yat-sen University Cancer Centre, Guangzhou, China); and S. Yin (Department of Obstetrics and Gynecology, Fudan University Zhongshan Hospital, Shanghai, China) for participation; and F. Liang (Statistical Center, Fudan University Zhongshan Hospital, Shanghai, China) for statistical assistance.
Author information
Authors and Affiliations
Consortia
Contributions
R.Z. conceived and designed the study. R.J., Y.F., Y.C., R.Z., T.S., J.Z., P.Z., J.L., X.C., H.Y., X.H., S.J. and W.G. collected and assembled data. R.Z., Y.C., R.J. and Y.G. conducted data analysis. R.Z. and Y.C. wrote the first draft of the manuscript. H.J., Y.G. and D.T. performed statistical analysis. R.Z., Y.C., R.J. and H.J. accessed and verified data. Y.C., S.J. and R.J. accessed raw data. All authors were involved in data interpretation and writing and revision and critical review of the manuscript. All authors approved the submitted version and vouch for the accuracy of the data reported and adherence to the protocol.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Dennis Chi, Dmitriy Zamarin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Kaplan-Meier estimates of updated progression-free survival in the intention-to-treat population.
A two-sided log-rank test was used to compare the time-to-event end points between groups at a 0.05 significance level with no adjustment of multiplicity. HRs and the corresponding 95% two-sided CIs were estimated using an unstratified Cox proportional hazards model. P < 0.0001.
Extended Data Fig. 2 Prespecified descriptive analysis of updated accumulated treatment-free survival.
a, accumulated treatment-free survival (TFSa) for the surgery arm. b, TFSa for the control arm. c, RMST estimates of TFSa. One patient (No. 45) in the surgery group had negative pathology during the assigned secondary cytoreduction. TFSa is defined as the duration of OS minus each treatment period after randomization including the period of surgery (from the date of randomization or next surgery to the date of the first cycle of chemotherapy), chemotherapy, and radiotherapy, without subtracting the time on targeted maintenance therapy. TFS1 = Treatment-free interval before first subsequent therapy initiation or duration of relapse-free survival. TFSa after third-line therapy = TFSa after first subsequent therapy initiation for those with secondary relapse. RMST = Restricted mean survival time.
Extended Data Fig. 3 Kaplan-Meier estimates of updated TFST and TSST.
a, time to first subsequent therapy (TFST). b, time to second subsequent therapy (TSST) in the intention-to-treat population. Hazard ratio and the corresponding 95%CI were estimated using an unstratified Cox proportional hazards model.
Extended Data Fig. 4 Kaplan-Meier estimates of overall survival by number of relapses.
a, OS by lesions in the overall cohort without separating surgery or control arm. b, OS by lesions in surgery and control arms. Hazard ratio and the corresponding 95%CI were estimated using an unstratified Cox proportional hazards model. In the GOG-0213 trial, 55% of the women had 1-2 sites of relapse. NE = not estimable.
Extended Data Fig. 5 Kaplan-Meier estimates of overall survival depending on treatment arms and surgical outcomes in the surgical arm.
Hazard ratio and the corresponding 95%CI were estimated using an unstratified Cox proportional hazards model. NGR = no gross residual disease/complete resection; gross residual = incomplete resection. NE = not estimable. OS = overall survival.
Extended Data Fig. 6 Crossover-adjusted analysis of overall survival by IPCW model.
a, IPCW curves. P values were two-sided with no adjustment of multiplicity, P = 0.019. HRs and the corresponding 95% two-sided CIs were estimated using a Cox proportional hazards model. b, Covariates selection for inverse-probability-of-censoring weights model. Numbers of patients at risk are not included for the IPCW curve due to the lack of a clear clinical interpretation of the number of patients at risk associated with the weighted methodology. * Selected by a model or by clinical consideration; iMODEL score was not selected due to the collinearity with CA125 at 1st relapse and platinum-free interval before 1st relapse. BMI = Body Mass Index. IPCW = inverse probability censoring weighting. CA125 = Cancer antigen 125. ECOG = Eastern Cooperative Oncology Group. FIGO = International Federation of Gynecology and Obstetrics. iMODEL = the international model.
Supplementary information
Supplementary Information
CONSORT Checklist, SOC-1 trial investigators; Supplementary Figs. 1 and 2, Tables 1–5, Protocol and Statistical analysis plan.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, R., Feng, Y., Chen, Y. et al. Surgery versus no surgery in platinum-sensitive relapsed ovarian cancer: final overall survival analysis of the SOC-1 randomized phase 3 trial. Nat Med 30, 2181–2188 (2024). https://doi.org/10.1038/s41591-024-02981-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-024-02981-0
This article is cited by
-
Secondary cytoreductive surgery in platinum-sensitive relapsed ovarian cancer: a meta-analysis of randomized controlled trials
Archives of Gynecology and Obstetrics (2024)