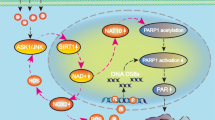
Abstract
Cuproptosis is characterized by lipoylated protein aggregation and loss of iron–sulfur (Fe–S) proteins, which are crucial for a wide range of important cellular functions, including DNA replication and damage repair. Sirt2 and sirt4 are lipoamidases that remove the lipoyl moiety from lipoylated proteins using nicotinamide adenine dinucleotide (NAD+) as a cofactor. However, to date, it is not clear whether nicotinamide mononucleotide (NMN), a precursor of NAD+, affects cellular sensitivity to cuproptosis. Therefore, in the current study, cuproptosis was induced by the copper (Cu) ionophore elesclomol (Es) in HeLa cells. It was also found that Es/Cu treatment increased cellular DNA damage level. On the other hand, NMN treatment partially rescued cuproptosis in a dose-dependent manner, as well as reduced cellular DNA damage level. In addition, NMN upregulated the expression of Fe-S protein POLD1, without affecting the aggregation of lipoylated proteins. Mechanistic study revealed that NMN increased the expression of sirt2 and cellular reduced nicotinamide adenine dinucleotide phosphate (NADPH) level. Overexpression of sirt2 and sirt4 did not change the aggregation of lipoylated proteins, however, sirt2, but not sirt4, increased cellular NADPH levels and partially rescued cuproptosis. Inhibition of NAD+ kinase (NADK), which is responsible for generating NADPH, abolished the rescuing function of NMN and sirt2 for Es/Cu induced cell death. Taken together, our results suggested that DNA damage is a characteristic feature of cuproptosis. NMN can partially rescue cuproptosis by upregulating sirt2, increase intracellular NADPH content and maintain the level of Fe-S proteins, independent of the lipoamidase activity of sirt2.
Similar content being viewed by others
Introduction
A recent study by Tsvetkov and colleagues showed that excess copper (Cu) induces a novel form of regulated cell death (RCD) that is termed “cuproptosis”1. The aggregation of lipoylated mitochondrial enzymes and the loss of Fe–S cluster proteins are the two distinguished characteristics of cuproptosis. Fe-S clusters are conserved cofactors that are essential for redox reactions, enzyme catalysis, protein stability, and regulation of gene expression2. An estimated 60 human proteins contain Fe-S clusters, including key enzymes in DNA replication and repair, protein translation, and energy metabolism3. However, the effect of cuproptosis on DNA damage has not been clearly described.
In human, Fe-S cluster functions as sulfur donor for the biosynthesis of lipoic acid, and the lipoylation modification pathway are controlled by 3 enzymes, including Lipoyl(Octanoyl) Transferase 2 (LIPT2), Lipoic Acid Synthetase (LIAS), and Lipoyltransferase 1 (LIPT1)4. On the other hand, to date, only two lipoamidases, which remove the lipoyl moiety from the lipoylated protein, have been identified. The first one, Sirtuin 4 (Sirt4), predominantly located in mitochondria, was identified by Mathias and colleagues in 20145,while Sirt2, mainly located in cytoplasm, was identified in 2019, which exhibited about 400-fold higher delipoylation activity than Sirt46. The sirtuins were originally identified as a family of nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylases7, interestingly, Sirt2 and Sirt4 also depend on NAD+ for their lipoamidase activity5,6 . Since lipoylated dihydrolipoamide S-acetyltransferase (DLAT) is a key phenomenon of cuproptosis, it is of interest to know whether changes in sirt2 or sirt4 expression had any effect on cuproptosis via their lipoamidase activity. In addition, NAD+ can be converted to nicotinamide adenine dinucleotide phosphate (NADP) catalyzed by NAD+ kinase (NADK), and NADP+ then is reduced to NADPH by several dehydrogenases. In turn, NADPH serves as a donor of hydrogen and electrons, participating in Fe-S cluster biosynthesis8. Such information pointed to a potential regulatory role of NAD+ in cuproptosis. More importantly, besides its lipoamidase activity, sirt2 also play an important role in increasing intracellular NADPH levels through control the pentose phosphate pathway9.
The study of NAD+ metabolism has been a hot topic in recent years. One key reason is that decreased NAD+ levels is associated with aging, and supplementation of NAD+ precursors, such as nicotinamide mononucleotide (NMN), can increase cellular NAD+ levels and showing beneficial effects in cell/animal models, as well as in preliminary clinical trials in human subjects10. However, whether NMN can influence cuproptosis, let alone the underlying molecular mechanism, had not been examined. Thus, in this study, we investigated the impact of NMN on cuproptosis, and the results showed that NMN partially rescued cuproptosis. Further examination revealed that NMN upregulated the expression of lipoamidase sirt2 and elevated intracellular NADPH levels. Nonetheless, the lipoamidase activity of sirt2 was not required for the resistance to cuproptosis.
Materials and methods
Cell lines, plasmids and chemicals
HeLa cells were cultured in Dulbecco modified Eagle medium (DMEM) with 10% (v/v) of FBS, 2 mM glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin and 100 mg/mL streptomycin at 37 °C under 5% CO2. The induction of cuproptosis was conducted as described by Tsvetkov1. In short, cells were grown in DMEM media with 10% (v/v) serum replacement (Gibco #10828028) and challenged with elesclomol (Es) and CuCl2 (ratio 1:1) for 2 h, then cells were washed with PBS and cultured with DMEM with 10% copper free serum replacement before harvest.
The sirt2 (NM_012237.4)- and sirt4 (NM_012240.3)-expression plasmids were constructed using the pEX-1 vector.
Nicotinamide mononucleotide (NMN) (#GC16971), Tetrathiomolybdate (TTM) (#GC25991), Thionicotinamide (thioNA) (#GD22321), glycyrrhizic acid (GA) (#GN10553) were purchased from GlpBio Company (Montclair, CA, USA). CuCl2 was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
RNA interference assay
The commercially synthesized siRNAs specifically targeting sirt2 (5′-CAGAGGCCAUCUUUGAGAUCA-3′), NADK (5′-GGAGAACATGATCGTGTAT-3′), or the non-specific control siRNAs (Riobo, Guangzhou, China) were transfected into HeLa cells using lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). The knockdown efficiency was confirmed by detecting the target genes' mRNA and protein levels.
Cell viability and cytotoxicity assays
Cell viability was determined with the CCK-8 kit (Beyotime, Shanghai, China). Briefly, cells were seeded into a 96-well plate and treated as indicated. At the end of treatment 10 μL CCK-8 reaction solution was added to each well, then the plates were placed in a humidity incubator for 40 min (37 ℃, 5% CO2). The absorbance was measured at 450 nm using a SPARK microplate multimode reader (Tecan, Männedorf, Swiss). Each experiment was conducted at least three times independently.
Quantitative real-time PCR (qPCR)
Total RNA was reverse transcribed using the Thermoscript™ RT-PCR System (Invitrogen, Carlsbad, CA, USA). qPCR was performed following the manufacturer’s instruction (Takara Bio Inc. Otsu, Japan) with specific primers (Table 1). qPCR data were presented using the 2−ΔΔCt method with β-actin as the internal control.
Western blot
Cells were lysed in RIPA lysis buffer supplemented with 1 × HALT protease inhibitor and allowed to homogenize at 4 ℃ for 30 min after lysis. Cell debris was removed by centrifugation for 15 min at 12,000 RPM. Protein concentration was determined by the bicinchoninic acid (BCA) method. Antibodies against β-Actin, GAPDH, γH2AX, sirt2, sirt4, POLD1, DLAT, and DLST were purchased from Abclonal Co., Ltd (Wuhan, China). The protein bands were visualized using an enhanced chemiluminescence kit (Hong Kong Kefit Co., Ltd., Hong Kong) and quantified by Image J software (National Institutes of Health).
Immunofluorescent staining of γH2AX
The expression of γH2AX was evaluated as described before with modifications11. Briefly, cells were fixed in 4% paraformaldehyde for 30 min, and permeabilized in 0.1% Triton-X 100. After blocking with blocking solution for 1 h, samples were incubated with a rabbit monoclonal anti-γH2AX antibody (1:500) for 16 h. After washing, the slides were incubated with a secondary goat anti-rabbit antibody conjugated with Alexa Fluor 633 (1:500) for 2 h. To stain the nuclei, DAPI was added to the cells and incubated for another 15 min. The cover slip was then removed from the plate and mounted onto a glass slide, and observed with a CarlZeiss LSM710 fluorescent microscope (CarlZeiss, German). ImageJ was used to count the γH2AX in each image.
Immunofluorescent staining of DLAT
Cells were seeded on glass bottom plates (Corning, NY, USA) at a density of 5 × 104/mL. After various treatments, cells were washed with 0.1% PBST twice, fixed with 4% paraformaldehyde for 30 min, and then incubated with blocking solution (1% bovine serum albumin (BSA), Sangon Biotech, Shanghai, China) for 30 min at room temperature. The cells were incubated with antibody against DLAT (1:200, ABclonal, Wuhan, China) overnight at 4 °C. After washing with PBST trice, the slides were incubated with Rhodamine Labeled Goat Anti-Rabbit IgG (H + L) (1:200, ZSGB-bio, Beijing, China) for 2 h at room temperature in the dark. Nuclei were stained with DAPI (Beyotime Biotechnology, Shanghai, China) for 5 min. Finally, images were acquired using an Olympus confocal microscope, and ImageJ software was employed to analyze the intensity of immunofluorescence.
Measurement of NAD(H) and NADP(H) levels
NAD(H) and NADP(H) were measured using the NAD+/NADH Assay Kit and NADP+/NADPH Assay Kit, respectively, following the manufacturer’s instructions (Beyotime, Shanghai, China). Briefly, cells were plated in six-well dishes at a density of 3 × 105 cells/well overnight. After various treatments, cells were washed 3 times in ice-cold PBS and were extracted in 200 µL extraction buffer. To quantify the levels of NAD(H), 20 µL of cell lysates were pipetted into a 96-well plate and homogenized with an ethanol dehydrogenase working solution. For the determination of NADP(H) levels, 50 µL of lysates were dispensed into a 96-well plate and thoroughly mixed with a G6PD working solution. Followed by an incubation at 37 ℃ for 10 min, the chromogenic solution was then added to the plate and the mixture was further incubated at 37 ℃ for 30 min. The absorbance was measured at 450 nm and analyzed using SPARK microplate multimode reader. The total concentration of NAD(H) and NADP(H) in the cell samples was calculated according to the standard curve. All measurements were repeated at least thrice in independent experiments.
Statistically analysis
The data were presented as mean ± standard deviation of three independent experimental repeats. In order to exclude systematic errors, results of different batches for the same experiment were standardized based to the results of negative control groups in each group. GraphPadPrism 9.0 software was used for plotting, and One-Way ANOVA and/or Two-way ANOVA were used for multi-group data comparison. Ρ < 0.05 was considered statistically significant (Supplementary file).
Results
NMN partially rescues cuproptosis
Cuproptosis was induced in HeLa cells as described by Tsvetkov et al.1. Dose–response analysis revealed that cell viability was reduced to 45.2% with 1.5 μM Es/Cu treatment (Fig. 1A), therefore, 1.5 μM Es/Cu was chosen in this study. To evaluate the possible protective effect of NMN, cells were pre-treated with 2, 4, or 8 mM nicotinamide (NMN) for 2 h, followed with 1.5 μM Es/Cu for 2 h, while 100 μM TTM (a copper chelator) pre-treatment for 2 h was used as positive control. The results showed that NMN alone did not affect the cell viability (Fig. 1B),on the other hand, it could partially rescue copper-induced death in a dose-dependent manner (Fig. 1C). Aggregation of lipoylated DLAT is the prominent character of cuproptosis. We investigated the effect of NMN on DLAT and DLST expression. While copper decreased the expression of DLAT and DLST, pretreated with NMN increased the expression of DLAT and DLST in copper pulsed cells (Fig. 1D–I). However, NMN only had limited influence on the aggregation of lipoylated DLAT (Fig. 1J).
NMN partially rescues cuproptosis. HeLa cells were cultured in DMEM with 10% copper free serum replacement and pulsed with indicated equimolar concentration of elesclomol and copper chloride (Es/Cu), the cells were harvested 24 h later and the viabilities were determined by CCK-8 assay (A). HeLa cells were treated with indicated concentration of NMN for 24 h, and the cell viabilities were determined by CCK-8 assay (B). HeLa cells were pretreated with indicated dose of NMN and Es/Cu for 2 h and cultured in DMEM with 10% bovine serum supplement, the cell viabilities were determined by CCK-8 assay (C). HeLa cells were cultured in DMEM with 10% copper free serum replacement and pretreated with NMN or TTM before pulsed with 1.5 μM Es/Cu, the expression of DLAT and DLAT protein and mRNA levels were determined by western-blot (D, G) and the bands density were analyzed (E, H), the mRNA level were determined by qPCR (F, I). The aggregation of lipoylated protein were determined by immunofluorescence (J). Results were the mean from 3–5 independent experiments. Data were represented as mean ± SEM. * ns, no significance; * P < 0.05; ** P < 0.01; * P < 0.001; * P < 0.0001.
NMN attenuates cuproptosis-induced DNA damage and loss of Fe-S protein POLD1
Loss of Fe-S protein is an important feature of cuproptosis. Fe-S proteins play crucial roles in various physiological functions including DNA replication and repair. The DNA polymerase POLD1 is such a protein that contains Fe-S clusters and participates in DNA replication and DNA damage repair. In this study, we measured POLD1 protein level as an indicator for Fe-S protein level. As expected, copper decreased the protein level of POLD1, while NMN increased the level of POLD1 protein in Es/Cu treated cells, though less effective than the copper chelator TTM (Fig. 2C, D). We then measured the DNA damage level in cuproptotic cells. The DNA damage marker γH2AX protein levels were determined by Western-blot and immunofluorescence assay, and the results showed that copper significantly increased the levels of γH2AX protein, while NMN significantly attenuated this effect, though less effective than the copper chelator TTM (Fig. 2A–C). These findings indicated that DNA damage is also a characteristic for cuproptosis, and NMN can mitigate cuproptosis-induced DNA damage.
NMN attenuates cuproptosis-induced DNA damage and loss of Fe-S protein POLD1. HeLa cells were cultured in DMEM with 10% copper free serum replacement and pretreated with NMN or TTM before pulsed with 1.5 μM Es/Cu for 24 h, the cells were harvest. The γH2AX Immunofluorescent staining (C). The expression of γH2AX and POLD1 protein levels were determined by western-blot (A, D) and the bands density were analyzed (B, E). Results were the mean from 3 to 5 independent experiments. Data were represented as mean ± SEM. ns, no significance; * P < 0.05; ** P < 0.01; * P < 0.001; * P < 0.0001, the DNA damage levels were assayed.
NMN partially rescues cuproptosis via upregulating the expression of sirt2
Sirtuins are highly conserved nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases, while sirt2 and sirt4 are also recognized as lipoamidases. Thus, it is of interest to know whether there were any changes of expression/activity for Sirt2 and Sirt4. Es/Cu treatment decreased sirt2 expression in HeLa cells, which NMN subsequently increased. More importantly, NMN pretreatment elevated the expression of sirt2 in Es/Cu-treated cells (Fig. 3A–C). On the other hand, the expression level of sirt4 in HeLa cells was too low to be detected by either WB or qPCR. We then investigated the effect of changed expression of sirt2 on cuproptosis. HeLa cells were first transfected with sirt2 overexpression plasmid and cuproptosis was induced by Es/Cu, and the results indicated that overexpression of sirt2 rendered the cells more tolerant to cuproptosis (Fig. 3D–G). Then sirt2 expression was knocked down by transfection with siRNA targeting sirt2 (Fig. 3H–J), and the results showed that sirt2 knock down attenuated the protective effect of NMN on cuproptosis (Fig. 3K).
NMN partially rescues cuproptosis via upregulating the expression of sirt2. HeLa cells were cultured in DMEM with 10% copper free serum replacement and pulsed Es/Cu with or without NMN or only NMN, the sirt2 protein level were determined by western-blot (A) and the band intensity were analyzed (B), the mRNA levels were determined by qPCR (C). HeLa cells were transfected with pEX-1-sirt2 (sirt2) or pEX-1 empty vector (vector) as control, the protein expression of sirt2 was determined by western-blot (D) and the bands density were analyzed (E) and the mRNA levels were determined by qPCR (F). HeLa cells transfected with sirt2 or empty vector for 24 h, and then treated with ES/Cu, the cell viabilities were determined by CCK-8 assay (G). HeLa cells were transfected with siRNA against sirt2 (si-Sirt2) or nonspecific siRNA (si–C), the expression of protein and mRNA were determined (H–J). 24 h after siRNA transfected, cells were treated with ES/Cu, and the cell viabilities were determined by CCK-8 assay (K). Results were the mean from 3 to 5 independent experiments. Data were represented as mean ± SEM. **** P < 0.0001.
sirt2 rescues cuproptosis in lipoamidase independent manner
As the aggregation of lipoylated proteins is an important feature of cuproptosis, and NMN upregulated the expression of Sirt2, we further investigated whether the lipoamidase activity of sirt2 and sirt4 contributed to the protective effect of NMN. HeLa cells were also transfected with sirt4 overexpression plasmid, and the protein and mRNA expression level were determined using Western-blot assay (Fig. 4A). However, unlike sirt2, overexpression sirt4 had no influence on cuproptosis (Fig. 4B). Then the aggregation of DLAT protein was examined by immunofluorescent microscopy, and the results revealed no significant change in aggregated DLAT level in cells overexpressing sirt2 or sirt4 during cuproptosis (Fig. 4C). The results indicated that sirt2 rescued curproptosis in lipoamidase independent manner.
sirt2 rescues cuproptosis in lipoamidase independent manner. HeLa cells were transfected with sirt4 or empty vector, the protein level was determined (A). 24 h after transfected with sirt4, the cells were treated with Es/Cu, and the cell viabilities were determined by CCK-8 assay (B). HeLa cells transfected with sirt2 or sirt4, and were treated with ES/Cu 24 h later, the aggregation of lipoylated protein were determined by immunofluorescence assay (C). Results were the mean from 3 independent experiments. Data were represented as mean ± SEM. ns, no significance; * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001.
NMN and sirt2 partially rescue cuproptosis via increasing intracellular NADPH levels
Apart from its lipoamidase activity, recent studies have shown that sirt2 can increase intracellular NADPH levels by activating the pentose phosphate pathway primarily, mainly through its deacetylation activity9,12,13,14, while there was no evidence suggesting that sirt4 had similar ability. Interestingly, NADPH plays a crucial role in the biosynthesis of Fe-S clusters. We wanted to know whether the rescue of cuproptosis by sirt2 depends on its ability to increase intracellular NADPH levels. Indeed, it was found that Es/Cu treatment decreased NADH and NADPH levels (Fig. 5A, B), while the NAD+ precursor NMN increased intracellular NADH and NADPH levels rapidly as expected (Fig. 5C, D). Also, overexpressing sirt2 increased intracellular NADPH levels, and siRNA knocking down sirt2 expression decreased intracellular NADPH levels (Fig. 5E, F). In contrast, overexpression sirt4 did not influence NADPH levels (Fig. 5G).
NMN and sirt2 partially rescue cuproptosis via increasing intracellular NADPH levels. HeLa cells were treated with ES/Cu for 2 h, and the NADPH (A) and NADH (B) levels were assayed. HeLa cells were treated with 8 mM NMN for 2 h or 8 h and the intracellular NADPH (C) or NADH (D) levels were determined. HeLa cells were transfected with si-sirt2 and the intracellular NADPH level were determined (E). HeLa cells were transfected with sirt2 and the intracellular NADPH levels were determined (F). HeLa cells were transfected with sirt4, and the NADPH levels were determined (G). HeLa cells were treated with indicated doses of thionicotinamide (thioNA) and the cell viabilities were determined by CCK-8 (H). HeLa cells were treated with 100 μM thioNA for 24 h and the intracellular NADPH (I) or NADH (J) levels were determined. 24 h after cells treated with thioNA, the cells then be treated with 1.5 μM Es/Cu with or without NMN, the cell viabilities were determined by CCK-8 assay (K). HeLa cells were transfected with siRNA against NADK (si-NADK) or nonspecific siRNA (si–C), the expression of protein and mRNA of NADK were determined (L–N). 24 h and 48 h after siRNAs been transfected, the cells were harvested and the intracellular NADPH (O, P) or NADH (Q, R) were determined. The cells were treated with 1.5 μM Es/Cu with or without NMN and the cell viabilities were determined by CCK-8 (S). Results were the mean from 3 to 5 independent experiments. Data were represented as mean ± SEM. ns, no significance; * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001.
Thionictotinamide (thioNA), an analog of nicotinamide, is a reversible competitive inhibitor of NAD Kinase (NADK), which catalyzes the transfer of a phosphate group from ATP to NAD+ to generate NADP+. Therefore, cells treated with 100 μM thioNA for 24 h showed significantly decreased intracellular NADH and NADPH levels (Fig. 5I, J) without significant changes in the cell viability (Fig. 5H). HeLa cells were pretreated with thioNA for 12 h also did not change the sensitive to Es/Cu induced cell death, but NMN cannot rescue cuproptosis in thioNA pretreated cells (Fig. 5K).
Since thioNA lowered both the NADH and NADPH concentrations, to further clarify the role of NADPH in cuproptosis. We then knocked down NADK by transfection with si-NADK (Fig. 5L–N). The levels of NADPH were decreased 24 and 48 h post si-NADK transfection, while the NAD levels remained unchanged (Fig. 5O–R). Interestingly, NADK knockdown cells cannot be rescued by NMN from cuproptosis (Fig. 5S). These results indicated that elevated NADPH is essential for NMN and sirt2 to rescue cuproptosis.
Discussion
Cuproptosis is a newly discovered form of RCD, characterized by lipoylation protein oligomerization and loss of Fe-S proteins, distinguishing it from other forms of RCD1. Nonetheless, there are still many questions remain unanswered regarding this new type of cell death. In this study, we reported that increased DNA damage is also characteristic of cuproptosis. Furthermore, our results demonstrated that the NAD+ precursor NMN can partially rescue cuproptosis by decreasing DNA damage, upregulating sirt2, and increasing NADPH levels. In addition, this rescue effect is not cell-type specific, as similar results were also observed in the mice hippocampal neuronal HT22 cells and the human liver carcinoma HepG2 cells (data not shown).
One possible explanation for the DNA damage during cuproptosis is the generation of reactive oxygen species (ROS), which has long been reported to be involved in Es-induced cell death15,16 and since ROS is known to induce DNA damage, it is conceivable to detect DNA damage during cuproptosis. Also, in DDR, distinct signaling pathways involving multiple proteins/molecules are activated to deal with the different types of DNA damage, leading to either cell cycle arrest to repair the damage, or cell death, depending on the degree of the damage17. Interestingly, certain Fe-S proteins play important roles of in DNA replication and repair, such as POLD118,19. During the process of lipoylation, Fe-S cluster is the sulfur donor20,21. Lipoylation modification of DLAT and DLST, which is essential for their acyl transfer function, is triggered by aerobic oxidation22. When aggregated by copper, it leads to increased consumption of Fe-S clusters as the sulfur donor for lipoic acid synthesis, ultimately resulting in Fe-S cluster depletion and the loss of Fe-S proteins. Cells that under aerobic oxidation are nearly 1000-fold more sensitive to cuproptosis than cells undergoing glycolysis1. These findings suggest that the aggregation of lipoylated proteins leads to the loss of Fe-S proteins.
NADPH is the hydrogen and electron donor for the Fe-S cluster biosynthesis process8. Therefore, theoretically, by modulating NAD+ metabolism to increase cellular NADPH level, it should be able to inhibit the loss of Fe-S clusters. A recent study has also found that NADPH affects the sensitivity of cuproptosis23. The NAD+ precursor NMN has been under intensive investigation for the past decade, due to its potent anti-oxidative and anti-aging effects10. Recently, we have reported the strong protective effects of NMN and NR on cisplatin-induced DNA damage as well as the decrease of cell viability of HeLa cells24, therefore, it is of interest to see whether NMN can also protect cells from Es/Cu-induced DNA damage. As expected, Es/Cu-induced DNA damage was significantly inhibited by NMN pretreatment.
To understand how NMN elicits its protective effect on cell viability, we noticed the two lipoamidases5,6, with interest, as they need NAD+ as cofactor25. Examination of these two enzymes revealed that Es/Cu decreased sirt2 expression, while NMN upregulated the expression of sirt2. Furthermore, overexpression of sirt2 also caused a small but significant increase of cell viability after Es/Cu treatment, just as NMN treatment, indicating that sirt2 may play a role in the inhibition cuproptosis. Conversely, knockdown of sirt2 abolished the protective effect of NMN on cuproptosis. Together, such results suggest that NMN could inhibit cuproptosis through the regulation of Sirt2. The two lipoamidases, sirt2 and sirt4, are mainly localized in the cytoplasm and mitochondria, respectively. The lipoylation of DLAT occurs in the mitochondria, and the aggregation of lipoylated DLAT is an important characteristic of cuproptosis. Our results showed that overexpression of sirt2 (should localize in the cytoplasm) rescued copper-induced cell death, while overexpression of sirt4 (should localize in the mitochondria) did not, indicating that the lipoamidase activity of neither sirt2 nor sirt4 is required. These results indicated that sirt2 rescues cuproptosis in lipoamidase independent manner.
Since sirt2 and sirt4 have the lipoamidase activity, we were wondering whether such enzymatic activity is required for the action of NMN. The level of aggregated lipoylated proteins was evaluated by immunofluorescence assay, and our results showed that overexpression of neither sirt2 nor sirt4 affected the level of aggregation of lipoylated protein. These results suggested that sirt2 rescued cuproptosis in a lipoamidase-independent manner. In addition to promoting the deacetylation of G6PD13,14 Sun et al., recently demonstrated that sirt2 also has found that sirt2 can also promote the deacetylation of lactyl moiety from METTL16 protein, a methyltransferase, thereby inhibiting METTL16 activity. They further showed that elevated METTL16 lactylation significantly improves the therapeutic efficacy of Es. More importantly, combining Es with AGK2, a sirt2-specific inhibitor, induce cuproptosis in gastric tumors in vitro and in vivo26.
To further explore the mechanism of rescue cuproptosis by NMN and sirt2, we compared the function of sirt2 and sirt4. Besides its lipoamidase activity, sirt2 also serves as the controller of the pentose phosphate pathway (PPP), the major NADPH- producing process9, in which sirt2 activates Glucose-6-Phosphate Dehydrogenase (G6PD) and enhances NADPH production27,28. Therefore, intracellular NADH and NADPH levels were examined under different conditions. It was found that copper reduced intracellular NADH and NADPH content in HeLa cells. NMN, as expected, increased intracellular NADH and NADPH levels. In contrast, only overexpression of sirt2 increased intracellular NADPH but not sirt4. NADK is important for the generation of NADPH, and thioNA is a NADK inhibitor29. Both the NADH and NADPH levels were decreased by thioNA, and NMN cannot rescue cuproptosis in thioNA pretreated cells, suggesting that either NADH or NADPH might mediate the action of NMN. To determine which one was responsible, we further knockdown NADK by si-NADK, and knockdown of NADK only decreased NADPH level. It turned out that knocking down NADK abolishes the ability of NMN to rescue cuproptosis. These data suggested that NMN and sirt2 rescue cuproptosis via elevating intracellular NADPH level. NADPH provides H and electrons for the synthesis of Fe-S clusters. The elevation of NADPH by sirt2 may be partially responsible for rescuing copper death by counteracting the loss of Fe-S proteins, an important feature of cuproptosis. However, the detailed mechanism of how NADPH protects cells from cuproptosis needs to further investigated.
Taken together, our results demonstrate that increased DNA damage and decreased NADPH are important features of cuproptosis. NMN can partially rescue cuproptosis, and its mechanism probably includes upregulation of POLD1 to counteract copper death-induced DNA damage, as well as upregulation of sirt2 to increase NADPH levels.
Data availability
The data used in this study are available from the corresponding author on reasonable request.
References
Tsvetkov, P. et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375(6586), 1254–1261 (2022).
Lill, R. Function and biogenesis of iron-sulphur proteins. Nature 460(7257), 831–838 (2009).
Andreini, C., Banci, L. & Rosato, A. Exploiting bacterial operons to illuminate human iron-sulfur proteins. J. Proteome Res. 15(4), 1308–1322 (2016).
Rowland, E. A., Snowden, C. K. & Cristea, I. M. Protein lipoylation: an evolutionarily conserved metabolic regulator of health and disease. Curr. Opin. Chem. Biol. 42, 76–85 (2018).
Mathias, R. A. et al. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 159(7), 1615–1625 (2014).
Xie, Y. et al. Chemical probes reveal sirt2’s new function as a robust “eraser” of lysine lipoylation. J. Am. Chem. Soc. 141(46), 18428–18436 (2019).
Covarrubias, A. J., Perrone, R., Grozio, A. & Verdin, E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 22(2), 119–141 (2021).
Maio, N. & Rouault, T. A. Outlining the complex pathway of mammalian Fe-S Cluster Biogenesis. Trends Biochem. Sci. 45(5), 411–426 (2020).
Wu, L. E. & Sinclair, D. A. SIRT2 controls the pentose phosphate switch. EMBO J. 33(12), 1287–1288 (2014).
Song, Q. et al. The safety and antiaging effects of nicotinamide mononucleotide in human clinical trials: An update. Adv. Nutr. 14(6), 1416–1435 (2023).
Zhang, G. et al. Cisplatin treatment leads to changes in nuclear protein and microRNA expression. Mutat. Res. 746(1), 66–77 (2012).
Viswanath, P. et al. Metabolic imaging detects elevated glucose flux through the pentose phosphate pathway associated with TERT expression in low-grade gliomas. Neuro Oncol. 23(9), 1509–1522 (2021).
Geng, S. L., Zhang, X. S. & Xu, W. H. COXIV and SIRT2-mediated G6PD deacetylation modulate ROS homeostasis to extend pupal lifespan. FEBS J. 288(7), 2436–2453 (2021).
Bai, Q. et al. KLF8 promotes the survival of lung adenocarcinoma during nutrient deprivation by regulating the pentose phosphate pathway through SIRT2. Front. Biosci. (Landmark Ed.) 29(1), 27 (2024).
Hasinoff, B. B., Yadav, A. A., Patel, D. & Wu, X. The cytotoxicity of the anticancer drug elesclomol is due to oxidative stress indirectly mediated through its complex with Cu(II). J. Inorg. Biochem. 137, 22–30 (2014).
Kirshner, J. R. et al. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Ther. 7(8), 2319–2327 (2008).
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461(7267), 1071–1078 (2009).
Fuss, J. O., Tsai, C. L., Ishida, J. P. & Tainer, J. A. (2015) Emerging critical roles of Fe-S clusters in DNA replication and repair. Biochim. Biophys. Acta 6, 1253–1271 (1853).
Pijuan, J., Maria, C., Herrero, E. & Belli, G. Impaired mitochondrial Fe-S cluster biogenesis activates the DNA damage response through different signaling mediators. J. Cell Sci. 128(24), 4653–4665 (2015).
Ren, X. et al. The Fe-S cluster assembly protein IscU2 increases alpha-ketoglutarate catabolism and DNA 5mC to promote tumor growth. Cell Discov. 9(1), 76 (2023).
Moseler, A. et al. The function of glutaredoxin GRXS15 is required for lipoyl-dependent dehydrogenases in mitochondria. Plant Physiol. 186(3), 1507–1525 (2021).
Solmonson, A. & DeBerardinis, R. J. Lipoic acid metabolism and mitochondrial redox regulation. J. Biol. Chem. 293(20), 7522–7530 (2018).
Lu, J. et al. FDX1 enhances endometriosis cell cuproptosis via G6PD-mediated redox homeostasis. Apoptosis 28(7–8), 1128–1140 (2023).
Qiu, S. et al. Comparison of protective effects of nicotinamide mononucleotide and nicotinamide riboside on DNA damage induced by cisplatin in HeLa cells. Biochem. Biophys. Rep. 37, 101655 (2024).
Yoshino, J., Baur, J. A. & Imai, S. I. NAD(+) intermediates: The biology and therapeutic potential of NMN and NR. Cell Metab. 27(3), 513–528 (2018).
Sun, L. et al. Lactylation of METTL16 promotes cuproptosis via m(6)A-modification on FDX1 mRNA in gastric cancer. Nat. Commun. 14(1), 6523 (2023).
Wang, Y. P. et al. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J. 33(12), 1304–1320 (2014).
Xu, S. N., Wang, T. S., Li, X. & Wang, Y. P. SIRT2 activates G6PD to enhance NADPH production and promote leukaemia cell proliferation. Sci. Rep. 6, 32734 (2016).
Tedeschi, P. M. et al. Suppression of cytosolic NADPH pool by thionicotinamide increases oxidative stress and synergizes with chemotherapy. Mol. Pharmacol. 88(4), 720–727 (2015).
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 32270186, and 81772168), Zhejiang Provincial Natural Science Foundation (Nos. LY23H190001 and LQ18H190003), the Huadong Medicine Joint Funds of the Zhejiang Provincial Natural Science Foundation (No. LHDMZ24H040001), Hangzhou Bio-medicine and health industry development support science and technology project (Nos. 2021WJCY144, 2022WJC016) and Postgraduate research and innovation promotion program of Hangzhou Normal University (No. 1115B20500437).
Author information
Authors and Affiliations
Contributions
X. T., J.Y. and C. D. conceived and designed the study. Y.Z., S.Q., C.D., carried out the experiments. Y.Z., S.Q., Y.C. analyzed the data. Y.Z. and S.Q. wrote the first draft of the manuscript, X.T., J.Y. and C. D. revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Qiu, S., Shao, S. et al. NMN partially rescues cuproptosis by upregulating sirt2 to increase intracellular NADPH. Sci Rep 14, 19392 (2024). https://doi.org/10.1038/s41598-024-70245-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70245-5
Keywords
This article is cited by
-
Development of a cuproptosis-related prognostic signature to reveal heterogeneity of the immune microenvironment and drug sensitivity in acute lymphoblastic leukemia
European Journal of Medical Research (2025)